Abstract

Amphiphilic polypeptide-containing hybrid dual brush block copolymers with controlled molecular weights and narrow molecular weight distributions were synthesized in one pot via ring-opening metathesis polymerization of sequentially added norbornyl-PEG and N-(2-((trimethylsilyl)amino)ethyl)-5-norbornene-endo-2,3-dicarboximide (M1) followed by ring-opening polymerization of amino acid N-carboxyanhydrides. Polylactide nanoparticles coated with these am phiphilic dual brush block copolymers showed significantly improved stability in PBS solution compared to those coated with amphiphilic linear block copolymers such as PEG-polylactide and PEG-polypeptides.
Keywords: N-Carboxyanhydride, NCA, ROMP, ring-opening polymerization, self-assembly, nanoparticle, polypeptide, PEG, PEGylation, brush polymer
The synthesis and self-assembly of amphiphilic linear block copolymers with well-defined compositions and structures have been an important research topic in polymer science over the past several decades.1 Numerous well-organized structures have been developed,2 some of which have been widely adopted for drug delivery and controlled release applications.3 Block copolymers bearing more complex segments than linear polymers (e.g., dendritic,4 brush5 and cyclic polymers6), however, are not as actively reported due in part to their relatively challenging synthesis. Controlled synthesis of these complex block copolymers would significantly expand the library of polymeric materials and make it possible to explore their physicochemical properties for potential applications.
Brush-like polymers are useful materials in nanoscience, either as standalone materials or as building blocks for self-assembly.7 Brush block copolymers are particularly interesting materials because of their peculiar topological and large domain structures.8 Most brush polymers developed so far contain flexible brushes made of polyolefins, polyesters and polyethers.9 Brush block copolymers containing rigid polymer brushes would yield materials with unique properties, but such materials with controlled molecular weights (MWs) and narrow molecular-weight distributions (MWDs) remain synthetically challenging. Polypeptides have been frequently used as rigid building blocks in the design of homo- and hybrid copolymers for their unique, protein-like conformations (e.g., α-helix).10 Incorporating polypeptides with intrinsic secondary structures into the brush side chains may significantly expand the horizon of brush-like macromolecules by providing materials with unprecedented properties. We have previously reported the synthesis and self-assembly of a series of polypeptide-brush polymers in the past few years.11 Here, we report the first synthesis of amphiphilic poly(ethylene glycol)(PEG)-polypeptide dual brush block copolymers with controlled MWs and narrow MWDs via one-pot ring-opening metathesis polymerization (ROMP) of norbornyl-PEG (NPEG) and inimer N-(2-((trimethylsilyl)amino)ethyl)-5-norbornene-endo-2,3-dicarboximide (M1), followed by trimethylsilyl amine (N-TMS) mediated ring-opening polymerization (ROP) of amino acid N-carboxyanhydrides (NCAs) (Scheme 1). We further demonstrated that these amphiphilic polypeptide-containing brush block copolymers were remarkable materials for coating polylactide (PLA) nanoparticles and preventing nanoparticles from aggregation in PBS solution for extended period of time.
Scheme 1.
We first prepared the hydrophilic poly(norbornene diimide) with pendant PEG polymer (denoted as PNEM/I where M/I is the monomer/initiator ratio) by ROMP of NPEG using Grubbs’ catalyst C1 in DCM, (Scheme 1). The WMs of PNE20 and PNE40 obtained through ROMP of NPEG at M/I ratios of 20 and 40 were 2.34 × 104 and 4.84× 104 g/mol, agreeing well with the expected Mn of 2.60 × 104 and 5.20 × 104 g/mol, respectively (Table 1). The MWDs of both PNE20 and PNE40 were less than 1.2. The 1H NMR analysis indicated that NPEG monomers were completely consumed in 3 h (Figure S2b).
Table 1.
PNE-Poly(norbornene diimide) prepared by ROMP.
| entry | polymer | initiator | monomer | [M]tot/[I] | conv (%)b | dn/dc (mL/g)c | Mn (Mn*) (× 10−4)d | MWDd |
|---|---|---|---|---|---|---|---|---|
| 1 | PNE20 | – | NPEG | 20 | >98 | 0.032 | 2.34 (2.60) | 1.19 |
| 2 | PNE40 | – | NPEG | 40 | >98 | 0.032 | 4.84 (5.20) | 1.20 |
| 3 | P1 | PNE20 | M1+M2a | 80 | >98 | 0.056 | 6.21 (6.94) | 1.12 |
| 4 | P2 | PNE20 | M1+M2a | 160 | >98 | 0.060 | 9.85 (11.08) | 1.21 |
| 5 | P3 | PNE40 | M1+M2a | 160 | >98 | 0.052 | 11.24 (13.68) | 1.17 |
Random copolymerization, M2/M1 molar ratio was fixed at 3/1
The conversion of monomer was determined by 1H NMR in CDCl3
The dn/dc values were calculated offline by using the internal calibration system processed by the ASTRA V software
The MW obtained by GPC (the expected MW).
We next attempted to synthesize polypeptide-containing dual brush block copolymers via a “grafting-from” strategy by growing polypeptide brushes from a ROMP backbone polymer derivatized with the initiation groups for the NCA polymerization. To facilitate controlled NCA polymerization and prevent inactivation of ROMP catalyst by free amine, the polymer backbone derived from ROMP were designed to have N-TMS amine groups for the subsequent controlled NCA polymerization.11eN-(2-((Trimethylsilyl)amino)ethyl)-5-norbornene-endo-2,3-dicarboximide (M1), an inimer containing a ROMP polymerizable endo-norbornene functional group and an N-TMS amine group, was selected in this study (Scheme 1). M2 was used as a co-monomer to tune the density of N-TMS group along the polynorbornene backbone. Random block co-polynorbornene containing N-TMS groups, denoted as Px (x = 1, 2, and 3), were prepared by adding a mixture of M1 and M2 (M2:M1 = 3:1) to PNE at a (M1+M2)/PNE molar ratio ([Mtot]/[I]) of 80 and 160 (entries 3-5, Table 1 and Scheme 1). The resulting P1-P3 thus contain a hydrophilic PNE block and a hydrophobic random poly(M1/M2) block. As shown in Table 1, the synthesis of P1-P3 was well controlled with expected MWs and narrow MWDs (entries 3-5).
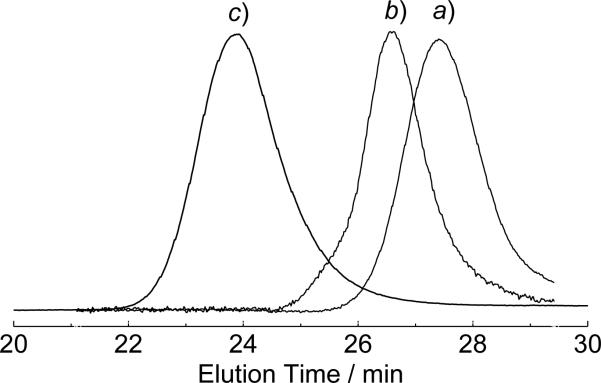
1H NMR analysis showed that the TMS groups on M1 were well preserved in P1-P3 (Figure S2c), similar to what was reported previously.11e P1-P3 were then used as initiators for polymerization of NCAs after DCM (solvent for ROMP) was removed under vacuum and replaced with anhydrous DMF. ROP of the (S)-γ-benzyl-L-glutamate N-carboxyanhydride (Glu-NCA) initiated by P1-P3 proceeded in a similar fashion to those initiated by N-TMS amine-containing small molecules,11f,12 and yielded poly(γ-(benzyl)-L-glutamate) (PBLG)-containing hybrid dual brush block copolymers with controlled MWs and narrow MWDs (Table 2).11a The dual brush block copolymers were denoted as Px-g-Gluz, where “Px” corresponds to the P1-P3 in Table 1 and “z” is the Glu-NCA/M1 ratio. When P1 was used as the macro-initiator (containing ~20 N-TMS groups per chain) and the [Glu-NCA]/[N-TMS] ratio was controlled at 20, the Mn of the dual brush block copolymer P1-g-Glu20 was 14.3 × 104 g/mol, agreeing well with the calculated Mn (15.7 × 104 g/mol) (entry 1, Table 2). GPC analysis revealed a monomodal peak for P1-g-Glu20, the elution time of which shifted to the higher MW region compared to its precursor P1 (Figure 1b and 1c). The actual DP of the PBLG brush was determined to be ~19 by 1H NMR analysis (Figure S3), which agreed well with the expected DP (20). Following the same methods, a series of dual brush block copolymers Px-g-Gluz with controlled MWs were prepared (entries 2-5, Table 2). When the polymerization of (S)-ε-carbobenzoxy-L-lysine-N-carboxyanhydride (Lys-NCA) was mediated by P1 at a Lys-NCA/N-TMS ratio of 20, P1-g-Lys20 with the expected MW was also obtained (entry 6, Table 2). The actual DP of PLys block was determined to be 20 by 1H NMR analysis, which is in perfect agreement with the expected DP of PLys. All Px-g-Glu(or Lys)z dual brush block copolymers reported in Table 2 have very narrow MWDs (< 1.1), substantiating that both ROMP and NCA ROP were highly controlled. We have previously reported a short ROMP polymer (11mer poly(M1)) grafted with 200mer PBLG (Glu200) with excellent controlled MW and narrow MWD.11e ROMP of functional norbornene monomers to give polymers with DP up to 400 has been reported.7e Although dual brush block copolymers with very long backbones or very long brushes are not the focus of this study, dual brush copolymers with a wide range of backbone and brush lengths should be attainable.
Table 2.
Synthesis of PEG-polypeptide dual brush block copolymers
| entry | polymer | micro-initiator | monomer [M]/[I] | conv (%)a | dn/dc (mL/g)b | Mn (Mn*) (× 10−4)c | MWDc | |
|---|---|---|---|---|---|---|---|---|
| 1 | P1-g-Glu20 | P1 | Glu-NCA | 20 | >95 | 0.080 | 14.3 (15.7) | 1.05 |
| 2 | P1-g-Glu40 | P1 | Glu-NCA | 40 | >95 | 0.086 | 20.6 (24.4) | 1.04 |
| 3 | P2-g-Glu20 | P2 | Glu-NCA | 20 | >95 | 0.083 | 25.0 (28.5) | 1.08 |
| 4 | P2-g-Glu40 | P2 | Glu-NCA | 40 | >95 | 0.088 | 38.7 (46.0) | 1.09 |
| 5 | P3-g-Glu20 | P3 | Glu-NCA | 20 | >95 | 0.081 | 26.4 (31.1) | 1.06 |
| 6 | P1-g-Lys20 | P1 | Lys-NCA | 20 | >95 | 0.082 | 13.3 (16.9) | 1.09 |
The conversion of monomer was determined by FT-IR
The dn/dc values were calculated offline by using the internal calibration system processed by the ASTRA V software
The MW obtained by GPC (the expected MW).
Figure 1.
GPC traces (light scattering signal) of PNE20 (a), P1 (b), and P1-g-Glu20 (c).
Modification of the surface of nanoparticles (NPs) with PEG, termed “PEGylation, is a well-established approach to reduce the non-specific protein/tissue binding to NPs and to enhance NP stability under physiological condition.13 The PEG density on the NP surface plays a significant role in enhancing the stability of NPs.14 PEGylating NPs with hydrophobic surface can be achieved via precipitation of amphiphilic PEG-containing block copolymers, such as PEG-polylactide (PEG-PLA), taking advantage of the hydrophobic interaction between NP and PLA. While this approach is simple and effective, several drawbacks are also noted.15 For instance, linear PEG may not provide enough surface coating and thus often cannot offer sufficient stealth effect and render completely inert surface. PEGylated NPs, especially those with low MW PEG, can still be subject to aggregation in biological media, non-specific protein binding, and undesirable recognition by reticuloendothelial system.16 Moreover, single block of hydrophobic polymer may not provide sufficient force to hold PEG, in particular high MW PEG, on the NP surface via its hydrophobic interaction with hydrophobic NP surface. Specially designed block copolymers, such as PLA-PEG-PLA, need to be used to provide more stable NP surface PEGylation by increasing hydrophobic interaction with NP surface.15 It has been reported that branched PEG17 and brush PEG18 can provide more surface stealth effect. To this end, an amphiphile could give rise to stronger hydrophobic interaction and better stealth effect than conventional linear PEG-PLA is of great value. We reason that Px-g-Gluz, with its ordered packing of the rigid rod-like hydrophobic polypeptide domains19 and the bottle brush-like PEG,20 may offer strong hydrophobic interaction and sufficient surface stealth simultaneously.
To examine the stabilization effect of Px-g-Gluz on NPs, we prepared paclitaxel-polylactide (Ptxl-PLA) conjugates through paclitaxel-initiated lactide polymerization at M/I (lactide/paclitaxel) ratio of 100 in the presence of (β-diimine)ZnN(TMS)2 (TMS = trimethylsilyl) as the catalyst.21 The resulting Ptxl-PLA has a Mn of 12.7 × 103 g/mol and MWD (Mw/Mn) of 1.03. It was then used to prepare NPs to evaluate various surface PEGylation methods.22 As expected, co-precipitation of Ptxl-PLA and mPEG5k (PEG) in phosphate buffered saline (PBS) solution could not provide sufficient surface PEGylation and therefore Ptxl-PLA NP aggregation was observed almost instantaneously (Figure 2a). Ptxl-PLA/PEG-PLA co-precipitation formed stable NP in water, as reported previously.15 However, when a 1:1 mixture of mPEG113-PLA194 (PEG-PLA) and Ptxl-PLA was precipitated directly in PBS solution, effective NP surface PEGylation could not be achieved. NP size increased from 101 nm to 195 nm within 4 min based on dynamic light scattering (DLS) analysis. Large aggregates (~ 870 nm) were also observed (Figure 2b). As large aggregates would have substantially changed pharmacological profiles as compared to original NPs and the presence of large aggregates with micrometer diameter sizes would result in undesired death of animals based on our previous studies,22 the NPs prepared via co-precipitation of Ptxl-PLA/PEG-PLA in PBS solution cannot be used for in vivo systemic drug delivery. In contrary, P1-g-Glu20 provided excellent coating and surface PEGylation to Ptxl-PLA NPs in PBS. When P1-g-Glu20 and Ptxl-PLA (1:1, wt/wt) were co-precipitated directly in PBS solution, the obtained particle was 95.9 nm and its size remained unchanged for at least 24 h (Figure 2c and entry 11, Table S1). Other dual brush block copolymer reported in Table 2 could also stably coat Ptxl-PLA NP surface and prevent NPs from aggregation in PBS solution for at least 24 h (entry 12-22, Table S1). To determine whether the bottle brush structure is crucial to the observed NP stabilization effect, we synthesized a linear PEG-PBLG (mPEG226-PBLG215) with an Mn of 57.1 × 103 g/mol (Mw/Mn of 1.18) and a PEG weight percent (17%) similar to that of P1-g-Glu20 (14%). PEG-PBLG was the used for the surface coating of the Ptxl-PLA NPs via co-precipitation method. We found that the size of PEG-PBLG coated Ptxl-PLA NPs increased from 124 nm to 209 nm within just 4 min (Figure 2a). Very large aggregates (~7.5 μm) were observed 24 h later (entry 8, Table S1), demonstrating that linear PEG-PBLG could not afford high density of PEG on the surface of NPs and provide excellent stealth effect.
Figure 2.
(a) Stability of Ptxl-LA NPs in PBS (1×) after coated with mPEG5k (PEG), mPEG5k-PLA (PEG-PLA), PEG-PBLG, or P1-g-Glu20; (b) DLS spectra of Ptxl-LA/PEG-PLA NP in PBS buffer after 0 and 4 min; (c) DLS spectra of Ptxl-LA/P1-g-Glu20 NP in PBS buffer after 0 and 60 min.
In conclusion, a series of amphiphilic PEG-polypeptide dual brush block copolymers with controlled molecular weights and narrow molecular weights distributions were synthesized via a one-pot reaction of ring-opening metathesis polymerization and ring-opening polymerization of amino acid N-carboxyanhydrides. This novel polymerization technique allows easy access to hybrid bottlebrush-like polymeric materials with some unprecedented properties and functions, such as offering excellent surface PEGylation for the formulation of polymeric NPs for drug delivery applications.
Supplementary Material
ACKNOWLEDGMENT
J.C. acknowledges the support from the NSF (CHE-1153122) and the NIH Director's New Innovator Award (1DP2OD007246). Y.L. acknowledges the support of NSF (DMR-1150742).
Footnotes
Supporting Information.
Experimental details, including monomers synthesis and characterizations, polymerization and polymer characterizations. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Zhou JF, Wang L, Chen T, Wang W. Prog. Chem. 2005;17:1102. [Google Scholar]
- 2.a Robb MJ, Connal LA, Lee BF, Lynd NA, Hawker C. J. Polym. Chem. 2012;3:1618. doi: 10.1039/C2PY20131C. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Al-Badri ZM, Maddikeri RR, Zha YP, Thaker HD, Dobriyal P, Shunmugam R, Russell TP, Tew GN. Nat. Commun. 2011;2 doi: 10.1038/ncomms1485. [DOI] [PubMed] [Google Scholar]; c Bates FS, Hillmyer MA, Lodge TP, Bates CM, Delaney KT, Fredrickson GH. Science. 2012;336:434. doi: 10.1126/science.1215368. [DOI] [PubMed] [Google Scholar]; d Cui HG, Chen ZY, Zhong S, Wooley KL, Pochan DJ. Science. 2007;317:647. doi: 10.1126/science.1141768. [DOI] [PubMed] [Google Scholar]
- 3.a Yu YS, Eisenberg A. J. Am. Chem. Soc. 1997;119:8383. [Google Scholar]; b Wang HB, Wang HH, Urban VS, Littrell KC, Thiyagarajan P, Yu LP. J. Am. Chem. Soc. 2000;122:6855. [Google Scholar]; c Hickey RJ, Haynes AS, Kikkawa JM, Park SJ. J. Am. Chem. Soc. 2011;133:1517. doi: 10.1021/ja1090113. [DOI] [PubMed] [Google Scholar]; d Elsabahy M, Wooley KL. Chem. Soc. Rev. 2012;41:2545. doi: 10.1039/c2cs15327k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a Wurm F, Frey H. Prog. Polym. Sci. 2011;36:1. [Google Scholar]; b Zhou ZY, D'Emanuele A, Lennon K, Attwood D. Macromolecules. 2009;42:7936. [Google Scholar]; c Xie C, Ju ZH, Zhang C, Yang YL, He JP. Macromolecules. 2013;46:1437. [Google Scholar]; d Urbani CN, Bell CA, Lonsdale D, Whittaker MR, Monteiro MJ. Macromolecules. 2008;41:76. [Google Scholar]; e Klok HA, Rodriguez-Hernandez J. Macromolecules. 2002;35:8718. [Google Scholar]; f Gillies ER, Jonsson TB, Frechet JMJ. J. Am. Chem. Soc. 2004;126:11936. doi: 10.1021/ja0463738. [DOI] [PubMed] [Google Scholar]; g Wong AD, DeWit MA, Gillies ER. Adv. Drug Delivery Rev. 2012;64:1031. doi: 10.1016/j.addr.2011.09.012. [DOI] [PubMed] [Google Scholar]; h Wang Y, Grayson SM. Adv. Drug Delivery Rev. 2012;64:852. doi: 10.1016/j.addr.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 5.a Miyake GM, Weitekamp RA, Piunova VA, Grubbs RH. J. Am. Chem. Soc. 2012;134:14249. doi: 10.1021/ja306430k. [DOI] [PubMed] [Google Scholar]; b Du JZ, Chen DP, Wang YC, Xiao CS, Lu YJ, Wang J, Zhang GZ. Biomacromolecules. 2006;7:1898. doi: 10.1021/bm051003c. [DOI] [PubMed] [Google Scholar]; c Berezkin AV, Guseva DV, Kudryavtsev YV. Macromolecules. 2012;45:8910. [Google Scholar]; d Cheng ZP, Zhu XL, Fu GD, Kang ET, Neoh KG. Macromolecules. 2005;38:7187. [Google Scholar]; e Stepanyan R, Subbotin A, ten Brinke G. Macromolecules. 2002;35:5640. [Google Scholar]
- 6.a Baba E, Honda S, Yamamoto T, Tezuka Y. Polym Chem-Uk. 2012;3:1903. [Google Scholar]; b Zhu YQ, Gido SP, Latrou H, Hadjichristidis N, Mays JW. Macromolecules. 2003;36:148. [Google Scholar]; c Touris A, Hadjichristidis N. Macromolecules. 2011;44:1969. [Google Scholar]; d Pitet LM, Hillmyer MA. Macromolecules. 2009;42:3674. [Google Scholar]; e Poelma JE, Ono K, Miyajima D, Aida T, Satoh K, Hawker CJ. ACS Nano. 2012;6:10845. doi: 10.1021/nn304217y. [DOI] [PubMed] [Google Scholar]
- 7.a Li CM, Gunari N, Fischer K, Janshoff A, Schmidt M. Angew. Chem. Int. Edit. 2004;43:1101. doi: 10.1002/anie.200352845. [DOI] [PubMed] [Google Scholar]; b Djalali R, Li SY, Schmidt M. Macromolecules. 2002;35:4282. [Google Scholar]; c Mullner M, Yuan JY, Weiss S, Walther A, Fortsch M, Drechsler M, Muller AHE. J. Am. Chem. Soc. 2010;132:16587. doi: 10.1021/ja107132j. [DOI] [PubMed] [Google Scholar]; d Yuan JY, Xu YY, Walther A, Bolisetty S, Schumacher M, Schmalz H, Ballauff M, Muller AHE. Nat. Mater. 2008;7:718. doi: 10.1038/nmat2232. [DOI] [PubMed] [Google Scholar]; e Johnson JA, Lu YY, Burts AO, Lim YH, Finn MG, Koberstein JT, Turro NJ, Tirrell DA, Grubbs RH. J. Am. Chem. Soc. 2011;133:559. doi: 10.1021/ja108441d. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Zhang MF, Muller AHE. J. Polym. Sci. Pol. Chem. 2005;43:3461. [Google Scholar]; g Ruhe J, Ballauff M, Biesalski M, Dziezok P, Grohn F, Johannsmann D, Houbenov N, Hugenberg N, Konradi R, Minko S, Motornov M, Netz RR, Schmidt M, Seidel C, Stamm M, Stephan T, Usov D, Zhang HN. Polyelectrolytes with Defined Molecular Architecture I. 2004;165:79. [Google Scholar]; h Yuan WZ, Zhang JC, Wei JR. Prog. Chem. 2011;23:760. [Google Scholar]; i Lee HI, Pietrasik J, Sheiko SS, Matyjaszewski K. Prog. Polym. Sci. 2010;35:24. [Google Scholar]; j Neiser MW, Okuda J, Schmidt M. Macromolecules. 2003;36:5437. [Google Scholar]; k Rzayev J. Acs Macro Lett. 2012;1:1146. doi: 10.1021/mz300402x. [DOI] [PubMed] [Google Scholar]
- 8.a Xia Y, Olsen BD, Kornfield JA, Grubbs RH. J. Am. Chem. Soc. 2009;131:18525. doi: 10.1021/ja908379q. [DOI] [PubMed] [Google Scholar]; b Rzayev J. Macromolecules. 2009;42:2135. [Google Scholar]; c Sun GR, Cho SH, Clark C, Verkhoturov SV, Eller MJ, Li A, Pavia-Jimenez A, Schweikert EA, Thackeray JW, Trefonas P, Wooley KL. J. Am. Chem. Soc. 2013;135:4203. doi: 10.1021/ja3126382. [DOI] [PubMed] [Google Scholar]; d Bolton J, Bailey TS, Rzayev J. Nano Lett. 2011;11:998. doi: 10.1021/nl103747m. [DOI] [PubMed] [Google Scholar]; e Zhang XJ, Zhong ZL, Zhuo RX. J. Controlled Release. 2011;152:E118. doi: 10.1016/j.jconrel.2011.08.162. [DOI] [PubMed] [Google Scholar]; f Chang Y, Kwon YC, Lee SC, Kim C. Macromolecules. 2000;33:4496. [Google Scholar]
- 9.a Li L, Zheng SX. J. Polym. Sci. Pol. Phys. 2008;46:2296. [Google Scholar]; b Vivek AV, Dhamodharan R. J. Polym. Sci. Pol. Chem. 2007;45:3818. [Google Scholar]; c Cerit N, Cakir N, Dag A, Sirkecioglu O, Durmaz H, Hizal G, Tunca U. J. Polym. Sci. Pol. Chem. 2011;49:2850. [Google Scholar]; d Chen JK, Hsieh CY, Huang CF, Li PM. J. Colloid. Interf. Sci. 2009;338:428. doi: 10.1016/j.jcis.2009.06.040. [DOI] [PubMed] [Google Scholar]; e Ishizu K, Takano S, Ochi K. J. Appl. Polym. Sci. 2007;104:3994. [Google Scholar]; f Shi Y, Zhu W, Chen YM. Macromolecules. 2013;46:2391. [Google Scholar]; g Teuchert C, Michel C, Hansen F, Park DY, Beckham HW, Wenz G. Macromolecules. 2013;46:2. [Google Scholar]; h Li CH, Ge ZS, Fang J, Liu SY. Macromolecules. 2009;42:2916. [Google Scholar]; i Kang EH, Lee IH, Choi TL. Acs Macro Lett. 2012;1:1098. doi: 10.1021/mz3002897. [DOI] [PubMed] [Google Scholar]; j Han DH, Tong X, Zhao Y. Macromolecules. 2011;44:5531. [Google Scholar]; k Tang HY, Li YC, Lahasky SH, Sheiko SS, Zhang DH. Macromolecules. 2011;44:1491. [Google Scholar]; l Li Z, Ma J, Lee NS, Wooley KL. J. Am. Chem. Soc. 2011;133:1228. doi: 10.1021/ja109191z. [DOI] [PMC free article] [PubMed] [Google Scholar]; m Le D, Montembault V, Soutif JC, Rutnakornpituk M, Fontaine L. Macromolecules. 2010;43:5611. [Google Scholar]; n Liu Y, Chen P, Li ZB. Macromol. Rapid Commun. 2012;33:287. doi: 10.1002/marc.201100649. [DOI] [PubMed] [Google Scholar]
- 10.a Blasco E, del Barrio J, Sanchez-Somolinos C, Pinol M, Oriol L. Polym. Chem. 2013;4:2246. [Google Scholar]; b Shi ZH, Lu HJ, Chen ZC, Cheng RS, Chen DZ. Polymer. 2012;53:359. [Google Scholar]; c Yun JP, Faust R, Szilagyi LS, Keki S, Zsuga M. Macromolecules. 2003;36:1717. [Google Scholar]; d Chang YK, Kim CH. Abstr. Pap. Am. Chem. Soc. 2001;221:U411. [Google Scholar]; e Zhu LY, Toug XF, Li MZ, Wang E. J. Phys. Chem. B. 2001;105:2461. [Google Scholar]; f Chang YY, Kim C. J. Polym. Sci. Pol. Chem. 2001;39:918. [Google Scholar]
- 11.a Wang J, Lu H, Ren Y, Zhang YF, Morton M, Cheng JJ, Lin Y. Macromolecules. 2011;44:8699. [Google Scholar]; b Zhang YF, Lu H, Lin Y, Cheng JJ. Macromolecules. 2011;44:6641. doi: 10.1021/ma201678r. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Lu H, Wang J, Bai YG, Lang JW, Liu SY, Lin Y, Cheng JJ. Nat. Commun. 2011;2 doi: 10.1038/ncomms1209. [DOI] [PubMed] [Google Scholar]; d Lu H, Cheng JJ. J. Am. Chem. Soc. 2008;130:12562. doi: 10.1021/ja803304x. [DOI] [PubMed] [Google Scholar]; e Lu H, Wang J, Lin Y, Cheng JJ. J. Am. Chem. Soc. 2009;131:13582. doi: 10.1021/ja903425x. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Lu H, Cheng JJ. J. Am. Chem. Soc. 2007;129:14114. doi: 10.1021/ja074961q. [DOI] [PubMed] [Google Scholar]
- 12.Lemmouchi Y, Perry MC, Amass AJ, Chakraborty K, Schacht E. J. Polym. Sci. Pol. Chem. 2007;45:3975. [Google Scholar]
- 13.Stokes KK, Hammond PT. Abstr. Pap. Am. Chem. Soc. 2004;227:U358. [Google Scholar]
- 14.a Han CC, Yao YH, Dong X. Abstr. Pap. Am. Chem. Soc. 2006;232:88. [Google Scholar]; b Yun JP, Faust R, Szilagyi LS, Keki S, Zsuga M. J. Macromol. Sci. Pure. 2004;A41:613. [Google Scholar]
- 15.Tong R, Yala LD, Fan TM, Cheng JJ. Biomaterials. 2010;31:3043. doi: 10.1016/j.biomaterials.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanomedicine. 2011;6:715. doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prencipe G, Tabakman SM, Welsher K, Liu Z, Goodwin AP, Zhang L, Henry J, Dai HJ. J. Am. Chem. Soc. 2009;131:4783. doi: 10.1021/ja809086q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutz JF. J. Polym. Sci. Pol. Chem. 2008;46:3459. [Google Scholar]
- 19.a Holowka EP, Pochan DJ, Deming TJ. J. Am. Chem. Soc. 2005;127:12423. doi: 10.1021/ja053557t. [DOI] [PubMed] [Google Scholar]; b Bellomo EG, Wyrsta MD, Pakstis L, Pochan DJ, Deming TJ. Nat. Mater. 2004;3:244. doi: 10.1038/nmat1093. [DOI] [PubMed] [Google Scholar]
- 20.a Gillich T, Acikgoz C, Isa L, Schluter AD, Spencer ND, Textor M. ACS Nano. 2013;7:316. doi: 10.1021/nn304045q. [DOI] [PubMed] [Google Scholar]; b Imbesi PM, Gohad NV, Eller MJ, Orihuela B, Rittschof D, Schweikert EA, Mount AS, Wooley KL. ACS Nano. 2012;6:1503. doi: 10.1021/nn204431m. [DOI] [PubMed] [Google Scholar]; c Knop K, Hoogenboom R, Fischer D, Schubert US. Angew. Chem. Int. Edit. 2010;49:6288. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]; d Obermeier B, Wurm F, Mangold C, Frey H. Angew. Chem. Int. Edit. 2011;50:7988. doi: 10.1002/anie.201100027. [DOI] [PubMed] [Google Scholar]; e Kainthan RK, Brooks DE. Bioconjugate Chem. 2008;19:2231. doi: 10.1021/bc800090v. [DOI] [PubMed] [Google Scholar]; f Pang Y, Liu JY, Wu JL, Li GL, Wang RB, Su Y, He P, Zhu XY, Yan DY, Zhu BS. Bioconjugate Chem. 2010;21:2093. doi: 10.1021/bc100325a. [DOI] [PubMed] [Google Scholar]; g Hussain H, Mya KY, He CB. Langmuir. 2012;28:12690. [Google Scholar]; h Chen CC, Borden MA. Biomaterials. 2011;32:6579. doi: 10.1016/j.biomaterials.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Du JZ, Tang LY, Song WJ, Shi Y, Wang J. Biomacromolecules. 2009;10:2169. doi: 10.1021/bm900345m. [DOI] [PubMed] [Google Scholar]
- 21.a Tong R, Cheng JJ. Angew. Chem. Int. Edit. 2008;47:4830. doi: 10.1002/anie.200800491. [DOI] [PubMed] [Google Scholar]; b Tong R, Cheng JJ. J. Am. Chem. Soc. 2009;131:4744. doi: 10.1021/ja8084675. [DOI] [PubMed] [Google Scholar]; c Tong R, Cheng JJ. Bioconjugate Chem. 2010;21:111. doi: 10.1021/bc900356g. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Tong R, Cheng JJ. Chemical Science. 2012;3:2234. [Google Scholar]
- 22.a Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW, Richie JP, Langer R. Proc. Natl. Acad. Sci. U. S. A. 2006;103:6315. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Cheng J, Teply BA, Sherifi I, Sung J, Luther G, Gu FX, Levy-Nissenbaum E, Radovic-Moreno AF, Langer R, Farokhzad OC. Biomaterials. 2007;28:869. doi: 10.1016/j.biomaterials.2006.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.