Abstract
Patas monkeys (Erythrocebus patas) living in African savanna woodlands and grassland habitats have a locomotor system that allows them to run fast, presumably to avoid predators. Long fore- and hindlimbs, long foot bones, short toes, and a digitigrade foot posture were proposed as anatomical correlates with speed. In addition to skeletal proportions, soft tissue and whole body proportions are important components of the locomotor system. To further distinguish patas anatomy from other Old World monkeys, a comparative study based on dissection of skin, muscle, and bone from complete individuals of patas and vervet monkeys (Cercopithecus aethiops) was undertaken. Analysis reveals that small adjustments in patas skeletal proportions, relative mass of limbs and tail, and specific muscle groups promote efficient sagittal limb motion. The ability to run fast is based on a locomotor system adapted for long distance walking. The patas' larger home range and longer daily range than those of vervets give them access to highly dispersed, nutritious foods, water, and sleeping trees. Furthermore, patas monkeys have physiological adaptations that enable them to tolerate and dissipate heat. These features all contribute to the distinct adaptation that is the patas monkeys' basis for survival in grassland and savanna woodland areas.
1. Introduction
Patas monkeys (Erythrocebus [Cercopithecus] patas) live in dry, seasonal habitats in grass and woodland savannas across northern Africa between the equator and Sahara, from Ethiopia to Senegal and southwards into northern Tanzania [1]. Their adaptation to these landscapes contrasts with that of sympatric vervet monkeys (Cercopithecus aethiops) and baboons (Papio cynocephalus) [2]. The discovery that patas monkeys run at high speeds was interpreted as a unique locomotor adaptation to avoid predators [3, 4]. Skeletal features—lengthened limbs, short digits, and digital foot postures—have been cited as the anatomical bases (e.g., [3, 5–7]). Compared to New World monkeys (Ceboidea) and apes (Hominoidea) Old World monkeys (Cercopithecoidea) are remarkably uniform in body dimensions and share a “generalized quadrupedal” locomotor pattern [8]. Due to anatomical similarities, we incorporate soft tissue associated with the locomotor system to tease out potential species-specific patterns.
Two studies stand out in adding new details to patas locomotor function. Based on dissections of the leg and foot and radiography and cine-film, Wood [9] linked the musculoskeleton of the hindlimb with locomotor biomechanics. Compared to baboons, patas monkeys have reduced motion at the ankle joint, longer tarsals and metatarsals, shorter phalanges, and fore- and hindlimbs oriented in the parasagittal plane. Wood interpreted this anatomy as convergent with cursorial mammals. In a later study, Hurov [10] compared patas and vervet monkey vertebral columns. Using cine-film, he measured the flexibility of the back during fast running and found that the patas vertebral column has limited flexion-extension compared to that of vervets. He found that vervets have thicker intervertebral discs and a broader transverse rib cage that promotes greater back mobility and a significantly increased stride length. Patas monkeys have thinner intervertebral discs and a narrower and deeper rib cage that decreases sagittal bending; patas monkeys rely on lengthened fore- and hindlimbs for increased stride length. These regional studies clarify the role of leg, foot, and back structures in locomotor function. To date there have been no studies of complete patas locomotor anatomy, including forelimbs as well as hindlimbs, and musculature as well as the skeleton.
The research presented here adds to previous studies and provides new data on functional anatomy of patas with comparisons to vervet monkeys. Dissections of entire animals give holistic information on limbs, back, tail, and musculoskeletal system. Vervet monkeys serve as a useful comparison because (1) they represent a generalized quadruped; 15 million year old fossil monkeys, Victoriapithecus, most closely resemble vervet body size and limb bone traits [11, 12]; (2) they are the closest relatives to patas with a recent divergence of 2–4 million years ago [13–15]; (3) they are sympatric with patas at study sites so that behavioral ecologies can be compared.
We address the following questions: how unique are patas monkeys and how have they diverged from vervet monkeys in limb proportions, muscle distribution, and muscle groups? How well do these data support the concept that patas monkeys converge with cursorial mammals? Given the close evolutionary relationship of patas and vervet monkeys, we expect patas to show overall anatomical similarity to vervet monkeys. We also expect that differences that do exist will be slight, but when taken together will show a distinct functional pattern.
2. Materials and Methods
2.1. Animal Sample
Two patas monkeys from the University of California, Berkeley colony for research on social behavior died of natural causes and became available for postmortum study. The vervet monkeys were acquired for teaching and donated to this study.All animals were dissected unpreserved (Table 1).
Table 1.
Sample.
| Animal | Age/sex | Body mass (g) | Trunk length (cm) | Tail length (cm) |
|---|---|---|---|---|
| Erythrocebus patas | Adult male | 11260.0 | 65.5 | 59.0 |
| Erythrocebus patas | Immature female | 4088.5 | na | na |
| Cercopithecus aethiops | Adult male | 6450.0 | 49.0 | 71.0 |
| Cercopithecus aethiops | Adult female | 3500.0 | 42.0 | 60.0 |
The male patas was a well-muscled adult with fully erupted canine teeth and all long bones fused; he showed no sign of illness or disease. His body weight at 11.3 kg is in the range reported for captive and wild males (e.g., 7.4–12.6 kg, [3, 10, 16, 17]). The female patas was immature (1.5 yr old) and at 4.1 kg is close to but not yet at adult weight (e.g., 4.4–7.6 kg, [10, 17]), an indicator of how quickly female patas monkeys mature [18].
The vervet monkeys were well-muscled adults. The male weight at 6.4 kg is in the range of published reports (4.3–6.6 kg, [10, 17, 19]) and the female at 3.5 kg similarly (2.9–5.8 kg, [17, 19]). Only the males are compared in muscle distribution and proportions to avoid the confounding variable of sex, and in the case of the patas female, age differences [19, 20].
2.2. Methods of Dissection and Data Collection
Prior to dissection, photographs and external measurements (after [21]) are taken on head/trunk length (vertex to ischium) and tail length. Standardized dissection methods provide quantitative information on body segments, body composition, and muscle groups [22–24].
One side of the body is dissected by segments. The forelimb is detached from the trunk at the shoulder joint, the hindlimb, and at the hip joint. The tail is weighed as a unit. Each limb is then separated into arm, forearm, and hand and thigh, leg, and foot segments. Each is weighed and further divided into muscle, bone, and skin; each tissue is weighed separately and recorded with its associated segment (Figure 1).
Figure 1.
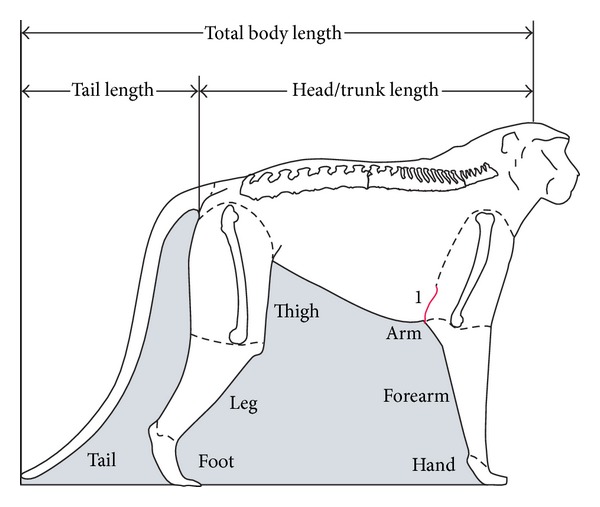
External measurements; body and limb segments; 1 indicates the axial skin fold relative to distal humerus on the patas monkey.
On the other side, individual muscles are dissected with tendon intact and weighed immediately. Skin, bone, and other tissues are separated and weighed.
Similarly, the head/trunk muscles, including the back extensors and tail muscles, are separated and weighed.
After the skeleton is cleaned using dermestid beetles, the bones are measured.
2.3. Methods of Analysis
(1) Segment mass analysis on the forelimb, hindlimb, and tail masses are each calculated relative to total body mass; segments within each limb are calculated relative to total limb mass.
(2) Body composition is determined by adding all the muscle, bone, and skin tissues from the entire dissection. Each is then calculated as a percentage of total body mass.
(3) Total body bone is taken as 100%. Its distribution to the body is determined as follows. Forelimb: humerus, radius, ulna, and hand bones from both sides of the body are taken as a percent of total body bone; similarly in the hindlimb, femur, patella, tibia, fibula, and foot bones are taken as percent of total bone. Bone from the tail is calculated separately. Skull, trunk, pectoral, and pelvic girdles comprise the head/trunk segment.
Total body muscle is taken as 100%. Its distribution to limb and tail segments is determined as follows. Forelimb: all forelimb muscles plus those that attach to the humerus (latissimus dorsi, pectoralis major, teres major, teres minor, supraspinatus, infraspinatus, and subscapularis) are added together, doubled, and taken as a percent of total body muscle. Similarly, all hindlimb muscles plus hip muscles that attach to the femur (the gluteals, iliopsoas, obturator internus and externus, and gemelli) are taken as a percent of total body muscle. Tail muscle is taken as a percent of total body muscle.
(4) Muscles are grouped according to their relationship to movement, and a ratio of antagonists is determined, for example, flexors to extensors. Relative mass of individual muscles or muscle groups serves as a gross approximation of functional importance at the joint. Only the adult male patas and male vervet are compared in limb segment, muscle distribution, and muscle mass due to possible confounding variables of age and sex in these dimensions (e.g., [20, 25, 26]).
(5) Indices are calculated from bone lengths: claviculohumeral index (clavicle as percent of humerus length, after [27]); humerofemoral (humerus to femur length); intermembral (humerus plus radius as a percent of femur plus tibia lengths); brachial (humerus to radius length); crural (femur to tibia length). Foot bones are measured, and relative lengths are calculated after Schultz [28]. Relative tail length is taken as a percentage of total body length (trunk length plus tail length).
3. Results
External assessment of the body is made prior to dissection. The patas chest is narrow and deep which is reflected in the lowest chest index (84) reported for Old World monkeys by Schultz [29]. The skin fold at the axillary region attaches low on the arm segment to bring the forelimb close to the trunk (Figure 1).
The male patas tail is 47.4% of total body length, the male vervet is 59.2% and female vervet 58.8%. These measurements were not available for the female patas.
The contribution of muscle, bone, and skin, components of the locomotor system is reported in Table 2. Both the female patas and the female vervet have relatively less muscle mass than do the males, a sex difference noted in some Old World monkey species [19, 20]. These three tissues together comprise 68.1 to 74.7% of total body mass.
Table 2.
Body composition. Percent contribution of major tissues to total body mass.
| Animal | Body mass (g) | Muscle | Bone | Skin |
|---|---|---|---|---|
| Patas male | 11260.0 | 44.2 | 15.2 | 11.3 |
| Patas female | 4088.5 | 39.7 | 14.7 | 13.7 |
| Vervet male | 6450.0 | 48.0 | 11.1 | 12.3 |
| Vervet female | 3500.0 | 42.9 | 12.4 | 12.9 |
In relative mass of body segments, the two species are similar, although the patas monkeys have a slightly heavier head/trunk segment and lighter tail. The relatively lighter forelimb of the female patas may be due to her younger age or to being female (Figure 2). Within the segments, patas monkey limbs are slightly more tapered, with heavier arm and thigh segments and lighter hand and foot (Figure 3).
Figure 2.
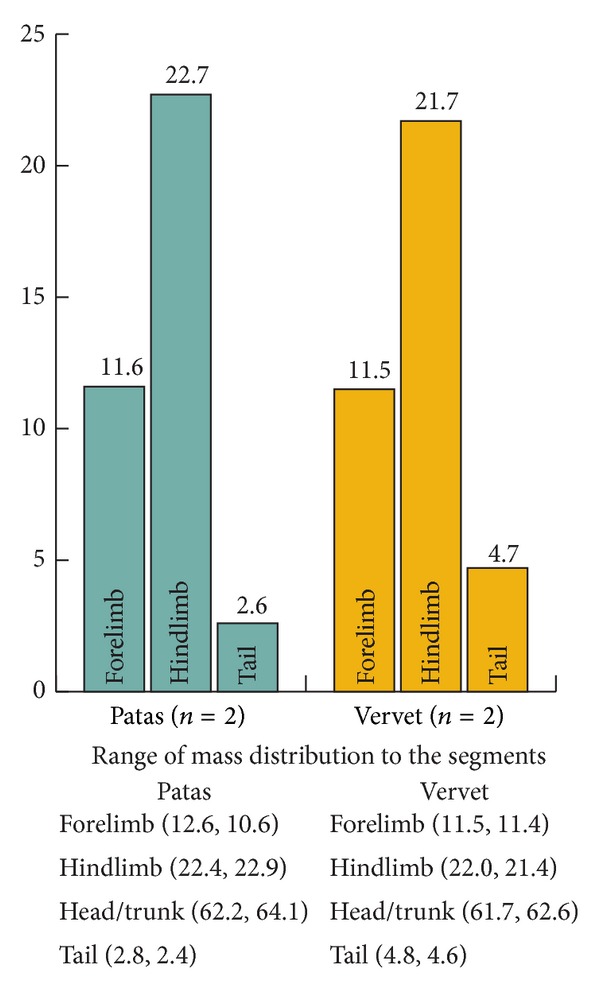
Average mass distribution as % total body mass.
Figure 3.
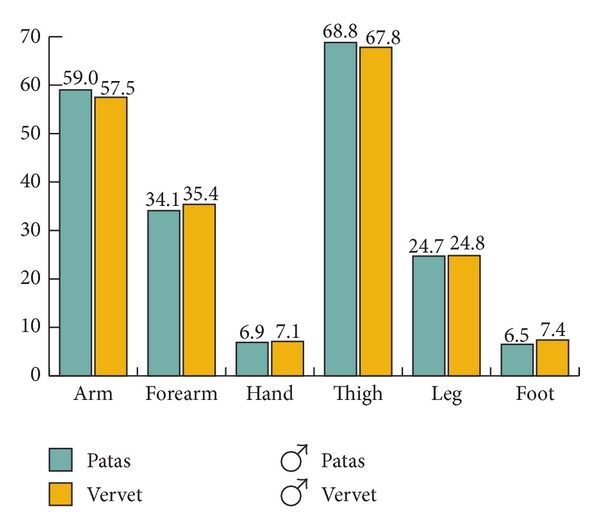
Mass distribution as % total limb mass; males only.
Distribution of bone to the segments is the same in the female and male of each species, and the two species differ in that patas monkeys have relatively heavier bones (Table 2, Figure 4).
Figure 4.
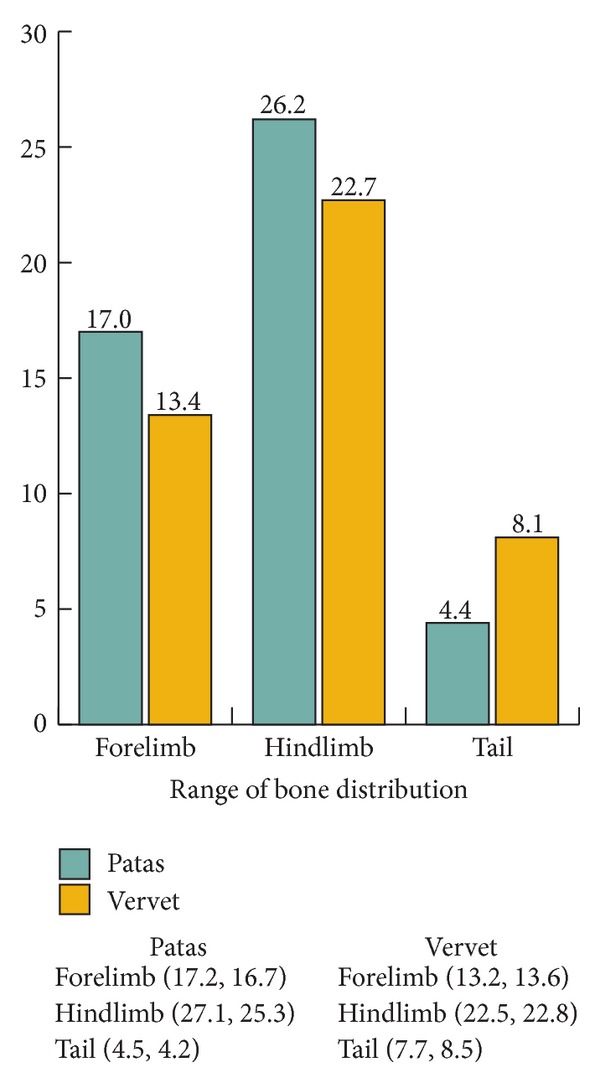
Average bone distribution as a % of forelimb, hindlimb, and tail.
Distribution of muscle to the segments is similar in the male patas and male vervet, though the patas has slightly more muscle in the hindlimb and less in the back and tail (Figure 5).
Figure 5.
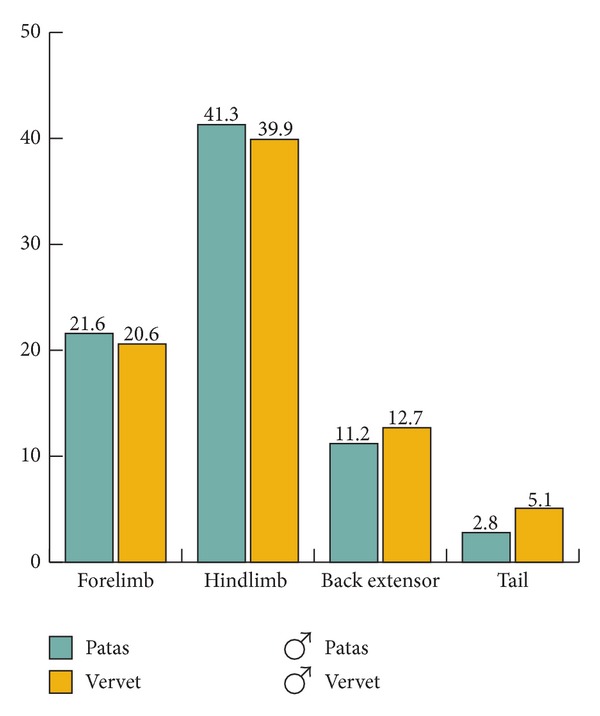
Distribution of muscle to segments as a % of total body muscle; males only.
Muscle groups at key joints show differences. Patas and vervet differ in relative proportions of extensors to flexors at the elbow joint and extrinsic digital extensors and in the hindlimb, plantar flexors (ankle extensors), subtalar invertors, and extrinsic digital extensors (Table 3, Figure 6).
Table 3.
Individual muscle weights to the nearest tenth of a gram.
| Forelimb muscles | Patas | Vervets | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Trunk to humerus | ||||
| Pectoralis major | 44.5 | 17.0 | 32.6 | 13.1 |
| Latissimus dorsi | 55.6 | 19.0 | 46.0 | 18.9 |
| Teres major | 17.9 | 8.0 | 12.7 | 5.0 |
| Teres minor | 4.5 | 1.7 | 2.3 | 1.3 |
| Subscapularis | 29.8 | 11.0 | 21.5 | 10.6 |
| Infraspinatus | 23.5 | 8.5 | 13.1 | 7.5 |
| Supraspinatus | 20.9 | 7.4 | 11.6 | 6.1 |
| Arm | ||||
| Deltoid | 34.3 | 12.2 | 23.6 | 10.9 |
| Biceps brachii: long | 35.1 | 15.1 | 28.5 | 9.9 |
| Biceps brachii: short | w/long hd | w/long hd | w/long hd | 2.2 |
| Coracobrachialis | 1.6 | 1.5 | 1.0 | 0.5 |
| Brachialis | 18.0 | 8.2 | 6.9 | 4.0 |
| Triceps: long | 49.2 | 20.6 | 32.7 | 15.8 |
| Triceps: lateral | 39.8 | 18.6 | 24.2 | 12.3 |
| Triceps: medial | 28.0 | 11.5 | 12.8 | 7.7 |
| Dorsoepitrochlearis | 5.8 | 3.4 | 6.4 | 3.0 |
| Forearm | ||||
| Brachioradialis | 6.0 | 2.9 | 12.9 | 4.5 |
| Palmaris longus | 4.4 | 2.0 | 1.4 | |
| Flexor carpi radialis | 6.4 | 1.9 | 3.2 | 1.6 |
| Flexor carpi ulnaris | 14.0 | 5.5 | 8.7 | 6.1 |
| Flexor digitorum superficialis | 8.8 | 3.1 | 7.1 | 3.7 |
| Flexor digitorum profundus | 29.2 | 11.7 | 18.0 | 9.8 |
| Flexor pollicis longus | w/profundis | 0.1 | w/profundus | w/profundus |
| Supinator | 2.6 | 1.6 | 1.4 | 1.1 |
| Pronator teres | 7.6 | 3.3 | 5.3 | 2.4 |
| Pronator quadratus | 1.6 | 0.5 | 0.9 | 0.5 |
| Extensor carpi radialis longus | 6.8 | 2.8 | 5.6 | 2.8 |
| Extensor carpi radialis brevis | 7.8 | 3.2 | 4.9 | 2.3 |
| Extensor carpi ulnaris | 4.8 | 2.1 | 2.4 | 1.5 |
| Extensor digiti minimi | 1.6 | 0.9 | 1.3 | 0.5 |
| Extensor digiti communis | 6.8 | 3.0 | 2.7 | 1.7 |
| Extensor indicis | 0.7 | 0.6 | 0.4 | |
| Anconeus | 0.3 | 0.4 | ||
| Abductor pollicis longus | 4.0 | 1.6 | 1.5 | 0.5 |
| Extensor pollicis longus | 0.9 | 0.5 | 0.5 | 0.3 |
| Extensor pollicis brevis | 0.9 | 1.0 | ||
|
| ||||
| Hind limb muscles | Patas | Vervets | ||
| Male | Female | Male | Female | |
|
| ||||
| Hip | ||||
| Gluteus maximus | 44.8 | 15.0 | 23.4 | 9.5 |
| Tensor fascia latae | w/glut max | 7.0 | 6.8 | 3.1 |
| Gluteus medius | 114.3 | 42.6 | 50.0 | 27.9 |
| Piriformis | w/glut med | 1.9 | w/glut med | w/glut med |
| Gluteus minimus | 9.4 | 4.1 | 5.0 | 3.2 |
| Gemelli | 3.4 | 1.6 | 1.8 | 1.0 |
| Obturator externus | 16.5 | 3.6 | 6.9 | 4.6 |
| Obturator internus | 12.6 | 5.6 | 7.3 | 5.0 |
| Quadratus femoris | 2.9 | 3.5 | 5.1 | 2.8 |
| Iliopsoas | 68.9 | 25.4 | 57.1 | 20.1 |
| Psoas minor | 11.0 | w/iliopsoas | w/iliopsoas | |
| Tail muscles total | 136.5 | 59.0 | 157.8 | 90.7 |
| Thigh | ||||
| Sartorius | 5.0 | 1.6 | 4.9 | 2.4 |
| Gracilis | 37.2 | 16.5 | 20.9 | 9.8 |
| Pectineus | 7.0 | w/mag | 5.9 | 2.8 |
| Adductor magnus | 108.9 | 37.9 | 66.3 | 40.9 |
| Adductor longus | 7.0 | w/mag | 5.6 | w/mag |
| Adductor brevis and minimus | 20.8 | w/mag | 7.7 | w/mag |
| Rectus femoris | 31.5 | 12.7 | 28.7 | 14.5 |
| Vastus lateralis | 92.7 | 60.0 | 54.9 | 31.6 |
| Vastus intermedius | 26.6 | w/lat | 10.8 | 4.6 |
| Vastus medialis | 23.6 | w/lat | 19.0 | 10.3 |
| Biceps femoris: long | 119.8 | 56.6 | 71.8 | 31.7 |
| Biceps femoris: short | w/long hd | w/long hd | w/long hd | w/long hd |
| Semimembranosus | 32.3 | 42.8 | 29.3 | 12.3 |
| Semitendinosus | 34.9 | 12.9 | 20.9 | 7.9 |
| Leg | ||||
| Gastrocnemius | 49.3 | 20.6 | 29.7 | 16.1 |
| Plantaris | 10.7 | 1.6 | 4.9 | 2.3 |
| Soleus | 19.7 | 7.0 | 9.1 | 5.6 |
| Popliteus | 4.0 | 4.1 | 3.3 | 2.3 |
| Tibialis posterior | 4.2 | 2.3 | 3.5 | 2.0 |
| Flexor digitorum fib/hal | 14.7 | 7.1 | 11.2 | 3.7 |
| Flexor digitoru tibialis/long | 4.3 | 3.2 | 4.5 | 6.1 |
| Peroneals (longus and brev) | 9.9 | 5.3 | 11.3 | 5.9 |
| Tibialis anterior | 15.6 | 7.0 | 13.5 | 8.6 |
| Extensor digitorum longus | 6.7 | 3.6 | 4.3 | 2.7 |
| Extensor hallucis longus | 6.6 | 1.9 | 1.2 | |
| Abductor hallucis longus | 1.7 | 5.2 | ||
Blank: not available.
Figure 6.
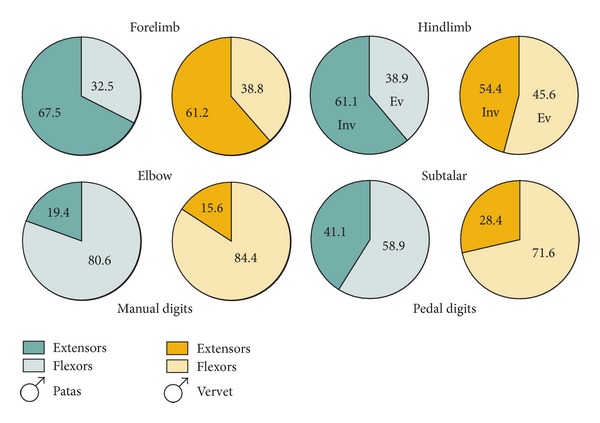
Ratios of the most distinctive muscle groups within the limbs, males only.
The indices calculated from bone lengths are shown in Table 4. The high humero-femoral index indicates that the forelimb and hindlimb are similar in length, whereas the lower index in vervets reflects a shorter humerus and longer femur. The intermembral shows a pattern similar to that of the humero-femoral index. The brachial and crural indices indicate that the radius and tibia are somewhat longer relative to the humerus and femur, respectively, than in the vervets. The claviculohumeral index reflects the narrow chest breadth and the closer approximation of the shoulder joints in patas.
Table 4.
Lengths and indices.
| Bones | Patas male (mm) | Vervet male (mm) | Vervet female (mm) |
|---|---|---|---|
| Clavicle | 58 | 54 | 41 |
| Humerus | 190 | 134 | 110 |
| Radius | 201 | 125 | 107 |
| Femur | 219 | 165 | 132 |
| Tibia | 215 | 149 | 129 |
| Foot | 167 | 130 | 118 |
| Hand | 114 | 85 | 76 |
| Indices | |||
| Intermembral | 90 | 83 | 83 |
| Brachial | 106 | 93 | 97 |
| Crural | 98 | 90 | 98 |
| Humerofemoral | 87 | 81 | 83 |
| Claviculohumeral | 31 | 40 | 37 |
Female patas not available.
Patas hands and feet have longer carpal and tarsal regions, longer metacarpals and metatarsals, and shorter digits compared to vervets (Figure 7).
Figure 7.
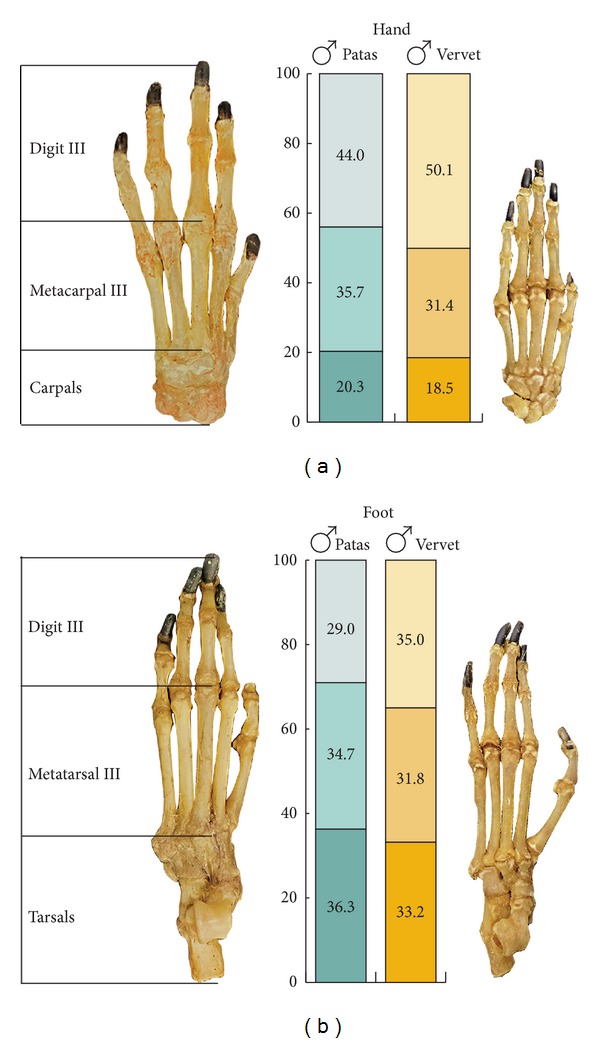
Hand segments as % total hand length (a); foot segments as % total length (b). Hands and feet scaled.
4. Discussion
What does this comparative approach with the addition of soft tissue and body proportions reveal about the uniqueness of patas monkey locomotor anatomy and behavior? Remarkably, even with notable differences in body mass, patas monkeys are built on the same plan as other Old World monkeys. Body proportions, for example, forelimb mass (11–13%) and hindlimb mass (20–24%), are not only characteristic of vervets [30] but also of macaques (Macaca fuscata and M. mulatta, [22, 25]), baboons (Papio cynocephalus, [9]), and langurs (Semnopithecus entellus, [31]), with variation among species in tail proportions. The similar body plans reflect a shared anatomy that underpins Old World monkey quadrupedal locomotion. Patas and vervet monkeys also share similar body compositions and distributions of bone and muscle mass. Hence, the addition of soft tissue data supports and adds to Schultz's general conclusions on structural uniformity of Old World monkeys.
However, small differences in the patas body shape affect locomotor function. Patas limbs are aligned under the trunk to ensure movement in the parasagittal plane. This alignment is achieved by a combination of vertically oriented scapulae against a narrow and deep chest, shoulder joints close together indicated by short clavicles, and attachment of the skin that binds the arm segment close to the trunk. These features reduce abduction of the limbs and keep them functioning in the fore and aft plane.
Limb length proportions also affect body shape. Patas locomotion depends upon a long stride and relatively efficient gait. The relatively longer radius and carpals and metacarpals and a digitigrade hand posture lengthen the forelimb. The slightly lengthened tibia and tarsals and metatarsals, and a digitigrade foot posture, all contribute to a longer hindlimb. The combination of forelimb and hindlimb lengths function to increase stride. The lighter hand and foot segments reduce the force needed during recovery phase of the stride.
The distribution of limb mass and muscle proportions affects power and mobility. The proximal shift of muscle to the shoulder and hip joints is reflected in a somewhat greater mass of arm and thigh segments, compared to vervets. The shorter and lighter tail in patas also keeps body and muscle mass closer to the hip joints. Proportions of muscle mass suggest greater propulsive action. Heavier elbow extensors, triceps brachii, and manual digital extensors underscore extension and deemphasize flexion during recovery phase of gait. Lighter back extensor muscles correlate with the less flexible back in patas [10].
Plantar flexors, or ankle extensors, (gastrocnemius, soleus, and plantaris) are five times the mass of the dorsiflexors (tibialis anterior) in patas compared to vervets' ratio of three. In addition to power at the knee joint, the plantar-flexors assist in maintaining a digitigrade foot posture and along with heavier digital extensors of the toes reflect the absence of a plantigrade foot posture during walking [9]. By cine-film and radiography Wood showed that the patas foot moves little when the lower limb is suspended during recovery, unlike the baboon's foot which inverts during recovery. Patas foot invertor muscles are relatively heavy and may compensate for the bony configuration at the ankle by maintaining joint mobility for climbing. The slender digits are straight and short, and the sole narrow with the thickest cushion of fat over the metatarsal heads [9].
How well does patas monkey anatomy fit with the notion of convergence with cursorial mammals? Cursors are generally defined by speed [32], and it was the ability of patas monkeys to run fast that first called attention to this aspect of their locomotion. Features of cursorial mammals include a deep thorax to accommodate a long vertically oriented scapula, narrow shoulder breadth often with reduced or absent clavicles, long limbs relative to trunk length, long and light distal segments, digitigrade posture, and muscle mass concentrated at the shoulder and hip joints [22, 32]; however, animals that have these features are not necessarily cursorial [33].
Patas monkey anatomical features have shifted somewhat in this direction. The digitigrade foot posture is perhaps the most noted. However, the hand is also digitigrade in walking but becomes palmigrade in running [34, 35]. The convergence of patas with cursorial mammals is slight because of the need to retain limb joint mobility, particularly in the forelimb, which is needed for climbing, manipulation during foraging and catching insect prey, and grooming. Overall, the comparison is superficial, and what predominates is the primate heritage of patas as Old World monkeys and particularly as guenons (e.g., [36, 37]).
The locomotor behavior of patas monkeys has been of interest since Hall's field study in Uganda called attention to their ability to run fast, presumably to avoid predators. Later research at sites in Cameroon (e.g., [38–40]) and Kenya (e.g., [41–43]) provided a detailed picture of behavioral ecology of patas monkeys and comparisons with sympatric vervet monkeys. Patas monkeys have a variety of means to cope with predators in addition to running. In the grasslands, with predators such as jackals, hyenas, lions, cheetahs, and wild dogs, patas monkeys depend upon vigilance from tall trees, giving and responding to alarm calls, crypticity, and rapid flight into the safety of woodlands; at night when leopards hunt, patas monkeys disperse into several sleeping trees and avoid using the same trees in consecutive nights when arboreal predators are present [3, 18, 39–41, 44].
Patas monkeys utilize grasslands and open acacia woodlands but avoid riverine woodlands, whereas vervet monkeys and baboons prefer woodlands and riverine edges [3, 39–41]. The majority of patas daily activities are spent on the ground in search of food. Field researchers emphasize the long distances patas monkeys travel on the ground and characterize their locomotion as one of continual walking rather than running [40, 45–47]. Patas monkeys cannot digest fiber-rich foods of herbaceous plants as most species do that inhabit the savanna. Their grassland foods are small, highly nutritious items like grasshoppers, ants, acacia gums, and seeds which are dispersed with low density and therefore require more time to cover a wide area each day [42, 47–50]. Patas groups are mobile throughout the day and forage for insects as they walk. They stand bipedally to gnaw and scrape gum and collect ants from tree trunks and branches [41]. Their diet yields substantial amounts of energy, protein, and minerals from acacia gum and Crematogaster ants [43, 48]. In the dry season patas monkeys can expand their range to include areas with water and therefore are not limited to where water is readily available [40]. Furthermore, patas monkeys have enhanced heat tolerance due to physiological properties of the skin. A higher density of eccrine sweat glands on the chest and larger though less numerous glands on the lateral thigh than rhesus monkeys result in higher sweat rates, which are more efficient at dissipating heat [51–53].
In summary, compared to vervet monkeys and baboons, which overlap in some areas of the patas habitat, patas monkeys have larger home ranges and longer daily travel and spend more time moving and foraging. Vervets spend less time moving, more time resting, eat less animal matter, focus more on fruits, flowers, leaves, and lipid-rich seeds. They spend more time feeding in trees than on the ground and in the dry season are confined to readily available water sources in their immediate home range. The way that patas monkeys live, move, and exploit resources that occur in savanna habitats is unique among African monkeys and contrasts with the behavior of the smaller vervets and the larger baboons.
5. Conclusions
The patas adaptation involves anatomical structures of muscle, bone, and skin devoted to the locomotor system, which together make up about 70% of body mass. Their skin is multifunctional. Not only does the skin cover the musculoskeleton, but it mechanically restricts the forelimbs to the parasagittal plane. Physiologically it contains sweat glands that dissipate heat and contribute to heat tolerance in environments with less continual tree cover where patas monkeys travel.
Efficient long distance walking is enhanced through a mosaic of musculoskeletal characters, including body shape, limb length, joint orientation and movement, and muscle function. Long legs and digitigrade foot posture are key features but so are changes in the forelimb. Their locomotor abilities underpin and support their adaptation to an unusual Old World monkey ecology and allow patas to forage effectively for insects, seeds, and gums as they move widely across their range between these small packets of dispersed food and water sources.
The emphasis on patas locomotion—or baboon or vervet locomotion—is not just one of “terrestriality” but of utilizing life on the ground in a particular, species-specific way. Taking into account the entire animal, its structures, and their functions as they are integrated into the totality of their behavior in the environmental context provides a more complete analysis of the unique adaptation of this unusual monkey species.
Acknowledgments
The authors thank Lynda (Brunker) LaBrecque for the data on the female patas and Thelma Rowell's laboratory, University of California, Berkeley, for the patas specimens. Debra Bolter provided valuable comments and Jerold Lowenstein editorial assistance. John Hudson, Melissa Fukui, and Will Aguado assisted in dissections, and Richard Baldwin provided laboratory support. They appreciate the support of the Anthropology Department and Division of Social Sciences, University of California, Santa Cruz.
References
- 1.Lernould JM. Classification and geographical distribution of guenons: a review. In: Gautier-Hion A, Bourliere F, Gautier J, Kingdon J, editors. A Primate Radiation: Evolutionary Biology of the African Guenons. Cambridge, UK: Cambridge University Press; 1988. pp. 54–78. [Google Scholar]
- 2.Hall KRL. Ecology and behavior of baboons, patas, and vervet monkeys in Uganda. In: Vagtborg H, editor. The Baboon in Medical Research. Austin, Tex, USA: University of Texas Press; 1965. pp. 43–61. [Google Scholar]
- 3.Hall KRL. Behaviour and ecology of the wild patas monkey, Erythrocebus patas, in Uganda. Journal of Zoology. 1965;148:15–87. [Google Scholar]
- 4.Washburn SL, Hamburg D. The study of primate behavior. In: deVore I, editor. Primate Behavior. Field Studies of Monkeys and Apes. New York, NY, USA: Holt, Rinehart and Winston; 1965. pp. 1–13. [Google Scholar]
- 5.Strasser E. Hindlimb proportions, allometry, and biomechanics in Old World monkeys (primates, cercopithecidae) American Journal of Physical Anthropology. 1992;87(2):187–213. doi: 10.1002/ajpa.1330870207. [DOI] [PubMed] [Google Scholar]
- 6.Kingdon JK. Comparative morphology of hands and feet in the genus Cercopithecus . In: Gautier-Hion A, Bourliere F, Gautier J, Kingdon J, editors. A Primate Radiation: Evolutionary Biology of the African Guenons. Cambridge, UK: Cambridge University Press; 1988. pp. 184–193. [Google Scholar]
- 7.Gebo DL, Sargis EJ. Terrestrial adaptations in the postcranial skeletons of guenons. American Journal of Physical Anthropology. 1994;93(3):341–371. doi: 10.1002/ajpa.1330930306. [DOI] [PubMed] [Google Scholar]
- 8.Schultz AH. The comparative uniformity of the Cercopithecoidea. In: Napier JR, Napier PH, editors. Old World Monkeys. New York, NY, USA: Academic Press; 1970. pp. 39–51. [Google Scholar]
- 9.Wood CD. Morphology and biomechanical adaptations in the hindlimb of Erythrocebus patas for high speed terrestrial locomotion [PhD Dissertation] Seattle, Wash, USA: University of Washington; 1973. [Google Scholar]
- 10.Hurov JR. Terrestrial locomotion and back anatomy in vervets (Cercopithecus aethiops) and patas monkeys (Erythrocebus patas) American Journal of Physical Anthropology. 1987;13:297–311. doi: 10.1002/ajp.1350130307. [DOI] [PubMed] [Google Scholar]
- 11.Benefit BR. Victoriapithecus: the key to Old World monkey and catarrhine origins. Evolutionary Anthropology. 1999;7(5):155–174. [Google Scholar]
- 12.Harrison T. New postcranial remains of Victoriapithecus from the middle Miocene of Kenya. Journal of Human Evolution. 1989;18(1):3–54. [Google Scholar]
- 13.Cronin JE, Sarich VM. Molecular evidence for dual origin of mangabeys among Old World monkeys. Nature. 1976;260(5553):700–702. doi: 10.1038/260700a0. [DOI] [PubMed] [Google Scholar]
- 14.van der Kuyl AC, Kuiken CL, Dekker JT, Goudsmit J. Phylogeny of African monkeys based upon mitochondrial 12S rRNA sequences. Journal of Molecular Evolution. 1995;40(2):173–180. doi: 10.1007/BF00167111. [DOI] [PubMed] [Google Scholar]
- 15.Disotell TR. The phylogeny of Old World monkeys. Evolutionary Anthropology. 1996;5(1):18–24. [Google Scholar]
- 16.Napier JR, Napier PH. A Handbook of Living Primates. New York, NY, USA: Academic Press; 1967. [Google Scholar]
- 17.Smith RJ, Jungers WL. Body mass in comparative primatology. Journal of Human Evolution. 1997;32(6):523–559. doi: 10.1006/jhev.1996.0122. [DOI] [PubMed] [Google Scholar]
- 18.Chism J, Rowell T, Olson D. Life history patterns of female patas monkeys. In: Small MF, editor. Female Primates: Studies by Women Primatologists. NewYork, NY, USA: Alan R. Liss; 1984. pp. 175–190. [Google Scholar]
- 19.Bolter DR, Zihlman AL. Morphometric analysis of growth and development in wild-collected vervet monkeys (Cercopithecus aethiops), with implications for growth patterns in Old World monkeys, apes and humans. Journal of Zoology. 2003;260(1):99–110. [Google Scholar]
- 20.Grand TI. The anatomy of growth and its relation to locomotor capacity in Macaca . In: Eisenberg JF, Kleiman DG, editors. Advances in the Study of Mammalian Behavior. Vol. 7. American Society of Mammalogists; 1983. pp. 5–23. [Google Scholar]
- 21.Schultz AH. The technique of measuring the outer body of human fetuses and of primates in general. Contributions to Embryology. 1929;117:213–257. [Google Scholar]
- 22.Grand TI. Body weight: its relation to tissue composition, segment distribution, and motor function. I. Interspecific comparisons. American Journal of Physical Anthropology. 1977;47(2):211–239. doi: 10.1002/ajpa.1330470204. [DOI] [PubMed] [Google Scholar]
- 23.Zihlman AL, McFarland RK, Underwood CE. Functional anatomy and adaptation of male gorillas (Gorilla gorilla gorilla) with comparison to male orangutans (Pongo pygmaeus) Anatomical Record. 2011;294(11):1842–1855. doi: 10.1002/ar.21449. [DOI] [PubMed] [Google Scholar]
- 24.Zihlman AL, Underwood CE. Profiling primates: anatomical methods for data collection, analysis and comparison. American Journal of Physical Anthropology. 2012;147(supplement 54):p. 35. [Google Scholar]
- 25.Grand TI. Body weight: its relation to tissue composition, segment distribution, and motor function. II. Development of Macaca mulatta . American Journal of Physical Anthropology. 1977;47(2):241–248. doi: 10.1002/ajpa.1330470205. [DOI] [PubMed] [Google Scholar]
- 26.Zihlman AL, McFarland RK. Body mass in lowland gorillas: a quantitative analysis. American Journal of Physical Anthropology. 2000;113:61–78. doi: 10.1002/1096-8644(200009)113:1<61::AID-AJPA6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 27.Schultz AH. Proportions, variability and asymmetries of the long bones of the limbs and the clavicles in man and apes. Human Biology. 1937;9:281–328. [Google Scholar]
- 28.Schultz AH. Relations between the lengths of the main parts of the foot skeleton in primates. Folia Primatologica. 1963;1:150–171. [Google Scholar]
- 29.Schultz AH. Postembryonic age changes. In: Hofer H, Schultz AH, Starck D, editors. Primatologia I: Handbook of Primatology. Basel, Switzerland: Karger; 1956. pp. 887–964. [Google Scholar]
- 30.Zihlman AL, Underwood CE, Bolter DR. Locomotor anatomy of patas monkeys (Erythrocebus patas) American Journal of Physical Anthropology. 2013;150(supplement 56):p. 45. [Google Scholar]
- 31.Underwood CE, Bolter DR, Zihlman AL. Locomotor anatomy of gray langurs (Semnopithecus entellus) American Journal of Physical Anthropology. 2013;150(supplement 56):p. 45. [Google Scholar]
- 32.Howell AB. Speed in Animals. Chicago, Ill, USA: University of Chicago Press; 1944. [Google Scholar]
- 33.Gregory WK. Notes on the principles of quadrupedal locomotion and on the mechanism of the limbs in hoofed animals. Annals of the New York Academy of Sciences. 1912;221:267–294. [Google Scholar]
- 34.Patel BA. Not so fast: speed effects on forelimb kinematics in Cercopithecine monkeys and implications for digitigrade postures in primates. American Journal of Physical Anthropology. 2009;140(1):92–112. doi: 10.1002/ajpa.21039. [DOI] [PubMed] [Google Scholar]
- 35.Patel BA, Polk JD. Distal forelimb kinematics in Erythrocebus patas and Papio anubis during walking and galloping. International Journal of Primatology. 2010;31(2):191–207. [Google Scholar]
- 36.Gautier-Hion A, Bourliere F, Gautier J, Kingdon J, editors. A Primate Radiation: Evolutionary Biology of the African Guenons. Cambridge, U.K: Cambridge University Press; 1988. [Google Scholar]
- 37.Glenn ME, Cords M, editors. The Guenons. Diversity and Adaptation in African Monkeys. New York, NY, USA: Kluwer Academic/Plenum; 2002. [Google Scholar]
- 38.Struhsaker TT, Gartlan JS. Observations on the behaviour and ecology of the patas monkey (Erythrocebus patas) in the Waza Reserve, Cameroon. Journal of Zoology. 1970;161(1):49–63. [Google Scholar]
- 39.Nakagawa N. Activity budget and diet of patas monkeys in Kala Maloue National Park, Cameroon: a preliminary report. Primates. 1989;30(1):27–34. [Google Scholar]
- 40.Nakagawa N. Differential habitat utilization by patas monkeys (Erythrocebus patas) and tantalus monkeys (Cercopithecus aethiops tantalus) living sympatrically in northern Cameroon. American Journal of Primatology. 1999;49:243–264. doi: 10.1002/(SICI)1098-2345(199911)49:3<243::AID-AJP3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 41.Chism J, Rowell T. The natural history of patas monkeys. In: Gautier-Hion A, Bourliere F, Gautier J, Kingdon J, editors. A Primate Radiation: Evolutionary Biology of the African Monkeys. Cambridge, UK: Cambridge University Press; 1988. pp. 412–438. [Google Scholar]
- 42.Isbell LA. Diet for a small primate: insectivory and gummivory in the (large) patas monkey (Erythrocebus patas pyrrhonotus) American Journal of Primatology. 1998;45:381–398. doi: 10.1002/(SICI)1098-2345(1998)45:4<381::AID-AJP5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 43.Isbell LA, Pruetz JD, Young TP. Movements of vetvets (Cercopithecus aethiops) and patas monkeys (Erythrocebus patas) as estimators of food resource size, density, and distribution. Behavioral Ecology and Sociobiology. 1998;42(2):123–133. [Google Scholar]
- 44.Enstam KL, Isbell LA. Microhabitat preference and vertical use of space by patas monkeys (Erythrocebus patas) in relation to predation risk and habitat structure. Folia Primatologica. 2004;75(2):70–84. doi: 10.1159/000076265. [DOI] [PubMed] [Google Scholar]
- 45.Isbell LA, Pruetz JD, Lewis M, Young TP. Locomotor activity differences between sympatric patas monkeys (Erythrocebus patas) and vervet monkeys (Cercopithecus aethiops): implications for the evolution of long hindlimb length in Homo . American Journal of Physical Anthropology. 1998;105:199–207. doi: 10.1002/(SICI)1096-8644(199802)105:2<199::AID-AJPA7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 46.Isbell LA, Pruetz JD, Nzuma BM, Young TP. Comparing measures of travel distances in primates: methodological considerations and socioecological implications. American Journal of Primatology. 1999;48:887–898. doi: 10.1002/(SICI)1098-2345(1999)48:2<87::AID-AJP1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 47.Nakagawa N. Difference in food selection between patas monkeys (Erythrocebus patas) and tantalus monkeys (Cercopithecus aethiops tantalus) in Kala Maloue National Park, Cameroon, in relation to nutrient content. Primates. 2003;44(1):3–11. doi: 10.1007/s10329-002-0001-0. [DOI] [PubMed] [Google Scholar]
- 48.Isbell LA, Rothman JM, Young PJ, Rudolph K. Nutritional benefits of Crematogaster mimosae ants and Acacia drepanolobium gum for patas monkeys and vervets in Laikipia, Kenya. American Journal of Physical Anthropology. 2013;150:286–300. doi: 10.1002/ajpa.22205. [DOI] [PubMed] [Google Scholar]
- 49.Nakagawa N. Seasonal, sex, and interspecific differences in activity time budgets and diets of patas monkeys (Erythrocebus patas) and tantalus monkeys (Cercopithecus aethiops tantalus), living sympatrically in northern Cameroon. Primates. 2000;41(2):161–174. [Google Scholar]
- 50.Nakagawa N. Foraging energetics in patas monkeys (Erythrocebus patas) and tantalus monkeys (Cercopithecus aethiops tantalus): implications for reproductive seasonality. American Journal of Primatology. 2000;52:169–185. doi: 10.1002/1098-2345(200012)52:4<169::AID-AJP2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 51.Mahoney SA. Cost of locomotion and heat balance during rest and runninng from 0 to 55°C in a patas monkey. Journal of Applied Physiology. 1980;49(5):789–800. doi: 10.1152/jappl.1980.49.5.789. [DOI] [PubMed] [Google Scholar]
- 52.Gisolfi CV, Sato K, Wall PT, Sato F. In vivo and in vitro characteristics of eccrine sweating in patas and rhesus monkeys. Journal of Applied Physiology. 1982;53(2):425–431. doi: 10.1152/jappl.1982.53.2.425. [DOI] [PubMed] [Google Scholar]
- 53.Kolka MA, Elizondo RS. Thermoregulation in Erythrocebus patas: a thermal balance study. Journal of Applied Physiology. 1983;55(5):1603–1608. doi: 10.1152/jappl.1983.55.5.1603. [DOI] [PubMed] [Google Scholar]


