Abstract
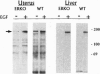
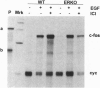
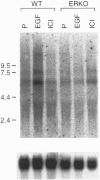
Past studies have shown that epidermal growth factor (EGF) is able to mimic the uterotropic effects of estrogen in the rodent. These studies have suggested a "cross-talk" model in which EGF receptor (EGF-R) signaling results in activation of nuclear estrogen receptor (ER) and its target genes in an estrogen-independent manner. Furthermore, in vitro studies have indicated the requirement for ER in this mechanism. To verify the requirement for ER in an in vivo system, EGF effects were studied in the uteri of ER knockout (ERKO) mice, which lack functional ER. The EGF-R levels, autophosphorylation, and c-fos induction were observed at equivalent levels in both genotypes indicating that removal of ER did not disrupt the EGF responses. Induction of DNA synthesis and the progesterone receptor gene in the uterus were measured after EGF treatment of both ERKO and wild-type animals. Wild-type mice showed increases of 4.3-fold in DNA synthesis, as well as an increase in PR mRNA after EGF treatment. However, these responses were absent in ERKO mice, confirming that the estrogen-like effects of EGF in the mouse uterus do indeed require the ER. These data conclusively demonstrate the coupling of EGF and ER signaling pathways in the rodent reproductive tract.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S., Metzger D., Bornert J. M., Chambon P. Modulation of transcriptional activation by ligand-dependent phosphorylation of the human oestrogen receptor A/B region. EMBO J. 1993 Mar;12(3):1153–1160. doi: 10.1002/j.1460-2075.1993.tb05756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica S. M., Katzenellenbogen B. S. Progesterone receptor regulation in uterine cells: stimulation by estrogen, cyclic adenosine 3',5'-monophosphate, and insulin-like growth factor I and suppression by antiestrogens and protein kinase inhibitors. Endocrinology. 1991 Apr;128(4):2045–2052. doi: 10.1210/endo-128-4-2045. [DOI] [PubMed] [Google Scholar]
- Bunone G., Briand P. A., Miksicek R. J., Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 1996 May 1;15(9):2174–2183. [PMC free article] [PubMed] [Google Scholar]
- Cho H., Aronica S. M., Katzenellenbogen B. S. Regulation of progesterone receptor gene expression in MCF-7 breast cancer cells: a comparison of the effects of cyclic adenosine 3',5'-monophosphate, estradiol, insulin-like growth factor-I, and serum factors. Endocrinology. 1994 Feb;134(2):658–664. doi: 10.1210/endo.134.2.7507831. [DOI] [PubMed] [Google Scholar]
- Couse J. F., Curtis S. W., Washburn T. F., Lindzey J., Golding T. S., Lubahn D. B., Smithies O., Korach K. S. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol Endocrinol. 1995 Nov;9(11):1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- Das S. K., Tsukamura H., Paria B. C., Andrews G. K., Dey S. K. Differential expression of epidermal growth factor receptor (EGF-R) gene and regulation of EGF-R bioactivity by progesterone and estrogen in the adult mouse uterus. Endocrinology. 1994 Feb;134(2):971–981. doi: 10.1210/endo.134.2.7507841. [DOI] [PubMed] [Google Scholar]
- Denner L. A., Weigel N. L., Maxwell B. L., Schrader W. T., O'Malley B. W. Regulation of progesterone receptor-mediated transcription by phosphorylation. Science. 1990 Dec 21;250(4988):1740–1743. doi: 10.1126/science.2176746. [DOI] [PubMed] [Google Scholar]
- DiAugustine R. P., Petrusz P., Bell G. I., Brown C. F., Korach K. S., McLachlan J. A., Teng C. T. Influence of estrogens on mouse uterine epidermal growth factor precursor protein and messenger ribonucleic acid. Endocrinology. 1988 Jun;122(6):2355–2363. doi: 10.1210/endo-122-6-2355. [DOI] [PubMed] [Google Scholar]
- Gannon F., Katzenellenbogen B., Stancel G., Gorski J. Estrogen-receptor movement to the nucleus: discussion of a cytoplasmic-exclusion hypothesis. Symp Soc Dev Biol. 1976;(34):137–149. [PubMed] [Google Scholar]
- Gardner R. M., Verner G., Kirkland J. L., Stancel G. M. Regulation of uterine epidermal growth factor (EGF) receptors by estrogen in the mature rat and during the estrous cycle. J Steroid Biochem. 1989 Mar;32(3):339–343. doi: 10.1016/0022-4731(89)90205-7. [DOI] [PubMed] [Google Scholar]
- Gibson M. K., Nemmers L. A., Beckman W. C., Jr, Davis V. L., Curtis S. W., Korach K. S. The mechanism of ICI 164,384 antiestrogenicity involves rapid loss of estrogen receptor in uterine tissue. Endocrinology. 1991 Oct;129(4):2000–2010. doi: 10.1210/endo-129-4-2000. [DOI] [PubMed] [Google Scholar]
- Heldin C. H. Dimerization of cell surface receptors in signal transduction. Cell. 1995 Jan 27;80(2):213–223. doi: 10.1016/0092-8674(95)90404-2. [DOI] [PubMed] [Google Scholar]
- Hill C. S., Treisman R. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell. 1995 Jan 27;80(2):199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- Huet-Hudson Y. M., Chakraborty C., De S. K., Suzuki Y., Andrews G. K., Dey S. K. Estrogen regulates the synthesis of epidermal growth factor in mouse uterine epithelial cells. Mol Endocrinol. 1990 Mar;4(3):510–523. doi: 10.1210/mend-4-3-510. [DOI] [PubMed] [Google Scholar]
- Ignar-Trowbridge D. M., Nelson K. G., Bidwell M. C., Curtis S. W., Washburn T. F., McLachlan J. A., Korach K. S. Coupling of dual signaling pathways: epidermal growth factor action involves the estrogen receptor. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4658–4662. doi: 10.1073/pnas.89.10.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignar-Trowbridge D. M., Teng C. T., Ross K. A., Parker M. G., Korach K. S., McLachlan J. A. Peptide growth factors elicit estrogen receptor-dependent transcriptional activation of an estrogen-responsive element. Mol Endocrinol. 1993 Aug;7(8):992–998. doi: 10.1210/mend.7.8.8232319. [DOI] [PubMed] [Google Scholar]
- Kato S., Endoh H., Masuhiro Y., Kitamoto T., Uchiyama S., Sasaki H., Masushige S., Gotoh Y., Nishida E., Kawashima H. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995 Dec 1;270(5241):1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- Lahooti H., White R., Danielian P. S., Parker M. G. Characterization of ligand-dependent phosphorylation of the estrogen receptor. Mol Endocrinol. 1994 Feb;8(2):182–188. doi: 10.1210/mend.8.2.8170474. [DOI] [PubMed] [Google Scholar]
- Le Goff P., Montano M. M., Schodin D. J., Katzenellenbogen B. S. Phosphorylation of the human estrogen receptor. Identification of hormone-regulated sites and examination of their influence on transcriptional activity. J Biol Chem. 1994 Feb 11;269(6):4458–4466. [PubMed] [Google Scholar]
- Lees J. A., Fawell S. E., Parker M. G. Identification of constitutive and steroid-dependent transactivation domains in the mouse oestrogen receptor. J Steroid Biochem. 1989;34(1-6):33–39. doi: 10.1016/0022-4731(89)90063-0. [DOI] [PubMed] [Google Scholar]
- Lees J. A., Fawell S. E., Parker M. G. Identification of two transactivation domains in the mouse oestrogen receptor. Nucleic Acids Res. 1989 Jul 25;17(14):5477–5488. doi: 10.1093/nar/17.14.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingham R. B., Stancel G. M., Loose-Mitchell D. S. Estrogen regulation of epidermal growth factor receptor messenger ribonucleic acid. Mol Endocrinol. 1988 Mar;2(3):230–235. doi: 10.1210/mend-2-3-230. [DOI] [PubMed] [Google Scholar]
- Loose-Mitchell D. S., Chiappetta C., Stancel G. M. Estrogen regulation of c-fos messenger ribonucleic acid. Mol Endocrinol. 1988 Oct;2(10):946–951. doi: 10.1210/mend-2-10-946. [DOI] [PubMed] [Google Scholar]
- Lubahn D. B., Moyer J. S., Golding T. S., Couse J. F., Korach K. S., Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z. Q., Santagati S., Patrone C., Pollio G., Vegeto E., Maggi A. Insulin-like growth factors activate estrogen receptor to control the growth and differentiation of the human neuroblastoma cell line SK-ER3. Mol Endocrinol. 1994 Jul;8(7):910–918. doi: 10.1210/mend.8.7.7984152. [DOI] [PubMed] [Google Scholar]
- Marshall C. J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995 Jan 27;80(2):179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Metzger D., Losson R., Bornert J. M., Lemoine Y., Chambon P. Promoter specificity of the two transcriptional activation functions of the human oestrogen receptor in yeast. Nucleic Acids Res. 1992 Jun 11;20(11):2813–2817. doi: 10.1093/nar/20.11.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukku V. R., Stancel G. M. Regulation of epidermal growth factor receptor by estrogen. J Biol Chem. 1985 Aug 15;260(17):9820–9824. [PubMed] [Google Scholar]
- Nelson K. G., Takahashi T., Bossert N. L., Walmer D. K., McLachlan J. A. Epidermal growth factor replaces estrogen in the stimulation of female genital-tract growth and differentiation. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):21–25. doi: 10.1073/pnas.88.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power R. F., Lydon J. P., Conneely O. M., O'Malley B. W. Dopamine activation of an orphan of the steroid receptor superfamily. Science. 1991 Jun 14;252(5012):1546–1548. doi: 10.1126/science.2047861. [DOI] [PubMed] [Google Scholar]
- Power R. F., Mani S. K., Codina J., Conneely O. M., O'Malley B. W. Dopaminergic and ligand-independent activation of steroid hormone receptors. Science. 1991 Dec 13;254(5038):1636–1639. doi: 10.1126/science.1749936. [DOI] [PubMed] [Google Scholar]
- Rubin R. A., Earp H. S. Solubilization of EGF receptor with Triton X-100 alters stimulation of tyrosine residue phosphorylation by EGF and dimethyl sulfoxide. J Biol Chem. 1983 Apr 25;258(8):5177–5182. [PubMed] [Google Scholar]
- Sewall C. H., Clark G. C., Lucier G. W. TCDD reduces rat hepatic epidermal growth factor receptor: comparison of binding, immunodetection, and autophosphorylation. Toxicol Appl Pharmacol. 1995 Jun;132(2):263–272. doi: 10.1006/taap.1995.1107. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Conneely O. M., O'Malley B. W. Modulation of the ligand-independent activation of the human estrogen receptor by hormone and antihormone. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6120–6124. doi: 10.1073/pnas.90.13.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancel G. M., Chiapetta C., Gardner R. M., Kirkland J. L., Lin T. H., Lingham R. B., Loose-Mitchell D. S., Mukku V. R., Orengo C. A. Regulation of the uterine epidermal growth factor receptor by estrogen. Prog Clin Biol Res. 1990;322:213–226. [PubMed] [Google Scholar]
- Stancel G. M., Gardner R. M., Kirkland J. L., Lin T. H., Lingham R. B., Loose-Mitchell D. S., Mukku V. R., Orengo C. A., Verner G. Interactions between estrogen and EGF in uterine growth and function. Adv Exp Med Biol. 1987;230:99–118. doi: 10.1007/978-1-4684-1297-0_6. [DOI] [PubMed] [Google Scholar]
- Sumida C., Pasqualini J. R. Antiestrogens antagonize the stimulatory effect of epidermal growth factor on the induction of progesterone receptor in fetal uterine cells in culture. Endocrinology. 1989 Feb;124(2):591–597. doi: 10.1210/endo-124-2-591. [DOI] [PubMed] [Google Scholar]
- Tora L., White J., Brou C., Tasset D., Webster N., Scheer E., Chambon P. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell. 1989 Nov 3;59(3):477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]