Abstract
During spermatogenesis, spermatids derived from meiosis simultaneously undergo extensive morphological transformation, to become highly specialized and metabolically quiescent cells, and transport across the seminiferous epithelium. Spermatids are also transported back-and-forth across the seminiferous epithelium during the epithelial cycle until they line up at the luminal edge of the tubule to prepare for spermiation at stage VIII of the cycle. Spermatid transport thus requires the intricate coordination of the cytoskeletons in Sertoli cells since spermatids are non-motile cells, lacking the ultrastructures of lamellipodia and filopodia, as well as the organized components of the cytoskeletons. In the course of preparing this brief review, we were surprised to see that, except for some earlier eminent morphological studies, little is known about the regulation of the microtubule (MT) cytoskeleton and the coordination of MT with the actin-based cytoskeleton to regulate spermatid transport during the epithelia cycle, illustrating that this is a largely neglected area of research required in the field. Herein, we summarize recent findings in the field regarding the significance of actin- and tubulin-based cytoskeletons in the Sertoli cell that support spermatid transport; we also highlight specific areas of research that deserve attentions in future studies.
Keywords: Testis, MARKs, microtubule, spermatogenesis, seminiferous epithelial cycle, spermiogenesis, spermatid adhesion
Introduction
Eukaryotic cells, including those in health (e.g., spermatogenesis) and in disease (e.g., cancer cells during tumorigenesis), are structurally supported by three extensive cytoskeletal networks, which are composed of the actin-, intermediate filament-, and microtubule-based cytoskeletons. It is conceivable that these three cytoskeletons must be precisely coordinated and tightly regulated in order to maintain cellular homeostasis at all levels, encompassing cell movement, metabolism, cell proliferation, synthesis, secretion, endocytic vesicle-mediated protein trafficking and others (Matsuuchi and Naus 2013, Mooren, et al. 2012, Stehbens and Wittmann 2012, Vignaud, et al. 2012). Filamentous-actin (F-actin), vimentin, and tubulin proteins comprise the actin-, intermediate filament-, and microtubule-based cytoskeletons, respectively. These three cytoskeletal proteins are essential for maintaining the integrity of a cell and performing a variety of functions in addition to providing structural support, such as cell movement, maintenance of cell junctions, and intracellular trafficking (Lie, et al. 2010, Su, et al. 2013, Vogl, et al. 2008). Microtubules (MTs) in particular, which are made up of α-tubulin and β-tubulins, are crucial for cellular processes such as mitosis, cell polarization, cell motility, neuronal differentiation, and organelle transport (Drewes, et al. 1995, Etienne-Manneville 2010, Meunier and Vernos 2012, Su, et al. 2012).
Microtubules are regulated by a variety of factors and proteins, including one class of proteins called the microtubule-associated proteins (MAPs). Since MAPs found in the vertebrate brain have been widely studied, so too have their regulators. One regulator, MAP/microtubule-affinity regulating kinase (MARK), was first discovered in the brain for its role in phosphorylating tau, a type of MAP protein (Drewes, et al. 1997). MARK was found to phosphorylate the LXGS motifs of tau, causing detachment of the MAP from MTs. A balance between attachment and detachment of tau from MTs is necessary for normal functioning of neurons. However, in Alzheimer’s disease, this balance is offset and tau becomes hyperphosphorylated (Timm, et al. 2006). Hyperphosphorylation of tau is associated with its own abnormal protein aggregation, a hallmark of Alzheimer’s disease (Marx, et al. 2010). The aggregation of tau leads to the development of neurofibrillary tangles in the nerve cells of the brain (Drewes 2004). Studies have shown that MARK co-localizes with these neurofibrillary tangles in the brain (Matenia and Mandelkow 2009).
Though there is much interest in uncovering the role of MARKs in the brain, there is a dearth of information currently available on the function of MARKs in the testis, since virtually all the studies conducted in the last several decades on the role of cytoskeletons, in particular MTs in spermatogenesis are non-functional but morphologically based studies (Russell 1993, Vogl, et al. 2008). Studying the regulation of the three cytoskeletons in the testis is of great relevance for the development of novel male contraceptives and new approaches in dealing with the problem of male infertility, a global concern. Thus it is pertinent to study the function of MARKs during spermatogenesis, such as the process of spermatid development during spermiogenesis and spermiation. Spermatogenesis takes place in the seminiferous epithelium of the seminiferous tubules in the mammalian testis (Cheng and Mruk 2009, de Kretser and Kerr 1988, Hess and de Franca 2008). The Sertoli cell (SC) in the seminiferous epithelium, a specialized microenvironment devoid of blood vessels and nerves, provides nourishment and structural support for the germ cells that will develop into spermatozoa. As mentioned, the seminiferous tubule is the functional unit where spermatozoa are produced; and in order for this highly regulated process to occur, the tubule must be protected from harmful substances that are found in the systemic circulation in the host body. The blood-testis-barrier (BTB), which is constituted by tight junctions coexisting with basal ectoplasmic specialization (basal ES), as well as gap junctions and desmosome between adjacent Sertoli cells near the basement membrane, thus segregates the events of meiosis I/II and post-meiotic spermatid development from systemic circulation (Cheng and Mruk 2012, Franca, et al. 2012, Pelletier 2011, Wong and Cheng 2005). Sertoli cells (SCs) have been deemed the “nurse cells,” since they provide germ cells in the seminiferous epithelium with the proper environment for development. The Sertoli cells themselves are comprised of a very extensive network of the actin-, vimentin-, and tubulin-based cytoskeletons, and thus serve a unique structural and supporting role during spermatogenesis (Lie, et al. 2010, Vogl, et al. 2008). In this brief review, we present an overview of MARK proteins and their possible function in regulation of the cytoskeleton dynamics in the testis.
Overview of microtubules and MAPs
The dynamic nature of microtubules (MTs) gives rise to the multitude of functions they serve in any mammalian cell. MTs are polarized cylindrical structures comprised of heterodimers of α-tubulin and β-tubulin which polymerize to form protofilament strands (Desai and Mitchison 1997, Mandelkow and Mandelkow 1995). A single MT is usually formed by the association of 13 protofilaments (Kueh and Mitchison 2009, Tilney, et al. 1973). A tubulin heterodimer is made up of an α-tubulin subunit and a β-tubulin subunit; tubulin dimers interact with each other in a head-to-tail manner where the β-subunit of one dimer contacts the α-subunit of another dimer. Thus, in a single protofilament, there will be 1 α-subunit exposed, designated as the plus-end, and 1 β-subunit, termed the minus-end (Etienne-Manneville 2010). MTs have an intrinsic polarity due to the nature of the ends of the MTs. The minus end of a MT undergoes slow growth and is commonly anchored to cellular structures such as the MT organizing center (MTOC) (Lie, et al. 2010) (Figure 1). The plus, or growing, end of a MT undergoes phases of growth and shrinkage; this occurrence is regarded as dynamic instability (Erickson and O’Brien 1992, Mitchison and Kirschner 1984). Some of the biological functions of dynamic instability include: rapid reorganization of cytoskeleton, mitosis, and cell motility (Erickson and O’Brien 1992, Etienne-Manneville 2010).
Figure 1. A schematic drawing illustrating the likely mechanism of spermatid transport across the seminiferous epithelium during spermatogenesis utilizing the microtubule-based cytoskeleton and the intricate relationship between MT and F-actin-rich apical and basal ES.
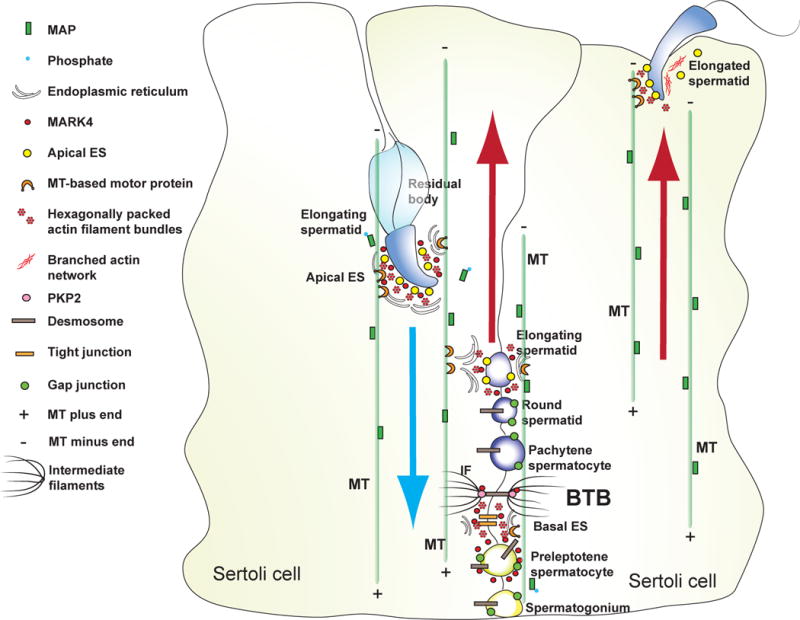
Microtubules are orientated with their plus (+) and minus (−) ends in the Sertoli cell of the seminiferous epithelium and stabilized by MAP, which are being used as the track for spermatids (cargoes) to transport across the epithelium, involving motor proteins (e.g., myosin VIIa), at different stages of the epithelial cycle, moving “up” and “down” the epithelium until fully developed elongated spermatids detach from the epithelium at spermiation. The precise mechanism of spermatid transport remains unknown since many of the crucial molecules involved in this event have yet to be identified and studied in the testis. Abbreviations used:
Due to the inherently unstable nature of MTs, there are a host of proteins that interact with MTs to regulate them. There are three classes of proteins that interact with MTs: microtubule-associated proteins (MAPs), motor proteins [e.g., myosin VII, dynein and kinesin in the testis (Lee and Cheng 2004, Vaid, et al. 2007b)], and non-MAP proteins which associate with MTs, but are not considered MAPs such as glycolytic enzymes and kinases (Mandelkow and Mandelkow 1995). Such regulators of MT dynamic instability include, destabilizing factors, severing proteins, plus- and minus-end capping proteins, and stabilizing factors like MAPs (de Forges, et al. 2012) (see Table 1 for a list of proteins that interact with MTs). MAPs play an important role in regulating the dynamic nature of MTs by attaching to the sides of MTs, which can slow down or even reverse the shrinkage of MTs (Burbank and Mitchison 2006). MAPs have been shown to control MT dynamics both in vitro and in vivo (Drechsel, et al. 1992, Illenberger, et al. 1996).
Table 1.
Functional proteins that interact with microtubules
| Protein Type | Function | Examples | Reference(s) |
|---|---|---|---|
| Stabilizing MAPs | Stabilize MTs; known for regulatory role of MTs in axons and dendrites | MAP2, Tau | (Drewes 2004, Mandelkow, et al. 1995) |
| Plus-end tracking proteins (+TIPs) | Functionally diverse: regulate MT dynamics, favor MT assembly, can link MT ends to actin | EB1, CLIP-170 | (Dixit, et al. 2009, Kumar and Wittmann 2012, Wade 2009) |
| Assembly proteins | Promote growth of MTs | Dis1/XMAP215 | (Al-Bassam and Chang 2011, Brouhard, et al. 2008) |
| Severing proteins | Cut MTs into fragments for reorganization of MT cytoskeleton | Katanin, spastin | (Lumb, et al. 2012, Smith, et al. 2012, Wade 2009) |
| Disassembly proteins | MT disassembly, depolymerization | MCAK, stathmin | (Belletti and Baldassarre 2011, Nakamura, et al. 2007, Wade 2009) |
| Motor proteins | ATP-dependent movement along MTs | Dynein, kinesin | (Endow 1995, Su, et al. 2012, Wu, et al. 2006) |
A number of different MAPs have been identified, with the most studied MAPs found in the vertebrate nervous system (Illenberger, et al. 1996). Tau, MAP2 and MAP4 are some of the best studied MAPs; tau is localized in axons, MAP2 in dendrites, and MAP4 is found in various cell and tissue types (Illenberger, et al. 1996). MAPs are good substrates for many protein kinases and are regulated through phosphorylation. Phosphorylation of MAPs causes them to detach from MTs, resulting in in MT destabilization (Illenberger, et al. 1996). However, detachment of MAPs from MTs also allows for other classes of proteins to interact with MTs. Thus, a regulatory system must be in play in order to allow other MT proteins to interact with MTs without compromising MT integrity (Matenia and Mandelkow 2009). For example, in neuronal axons the binding of MAPs to MTs stabilizes MTs, which is necessary to ensure MT-dependent axonal transport of cargo, like organelles and vesicles, by motor proteins (Matenia and Mandelkow 2009). Though MT stability is required for axonal transport, it must be noted that MAPs are also competing with motor proteins for MT binding. The concept that MTs serve as the track for the translocation of developing spermatids as “cargoes” across the seminiferous epithelium has been proposed for years following the discovery of several MT-based motor proteins (Guttman, et al. 2000, Vaid, et al. 2007a, Vaid, et al. 2007b, Vogl, et al. 2008), however, many other important players that are crucial to microtubule dynamics have yet to be identified and studied in the testis. In short, the model depicted in Figure 1 is rather preliminary, yet it serves as a helpful guide for the design of functional experiments in future studies.
MARK, a regulator of MTs via MAPs
Structure
MARKs were first discovered in studies to uncover the pathogenesis of Alzheimer’s disease (Drewes, et al. 1995, Matenia and Mandelkow 2009). There are four MARK isoforms (MARK1-4) and they belong to the AMPK (adenonsine-monophosphate activated protein kinase) subfamily of CaMK (calcium/calmodulin-dependent protein kinase) kinases (Kemphues 2000, Marx, et al. 2010, Timm, et al. 2008b). CaMKs are ubiquitous and multifunctional protein kinases that phosphorylate a host of substrates upon activation by calcium-bound calmodulin (Braun and Schulman 1995). In addition to MARK1-4, other isoforms exist due to alternative splicing (Matenia and Mandelkow 2009)(Braun, 1995 #29). MARK1-4 exhibit the following conserved functional regions: N-terminal header, catalytic kinase domain (KD), common docking domain (CD), ubiquitin-associated domain (UBA), spacer region, and kinase associated domain 1 (KA1) motif containing tail domain (Figure 2). The spacer region is the least conserved and the most variable region among the four MARK isoforms (Matenia and Mandelkow 2009, Timm, et al. 2008b).
Figure 2. Functional domains of MARK.

MARKs have 6 distinctive functional domains: N-terminal header (N), catalytic kinase domain (KD), common docking domain (CD), ubiquitin-associated domain (UBA), spacer region, and tail domain at the C-terminus.
Regulation of MARKs
Like many kinases, MARKs are involved in multiple signaling pathways and are regulated by a variety of mechanisms. Phosphorylation of the catalytic kinase domain (KD) by upstream kinases like MARKK (MARK kinase) can lead to activation of MARK. Activated MARK can phosphorylate tau protein and other related MAP proteins such as MAP2 and MAP4, which have affinities for stabilizing microtubules, and also can phosphorylate other proteins involved in cell signaling and 14-3-3 (also known as Par 5, partitioning defective protein 5) binding (Drewes 2004, Timm, et al. 2008b). However, phosphorylation of the KD by a kinase such as GSK3β (glycogen synthase kinase 3 β) can lead to MARK inhibition. MARKs can also be activated in a phosphorylation-independent manner through binding of regulatory proteins, such as the AP-2 protein complex to the KD (Schmitt-Ulms, et al. 2009). Likewise, MARKs also can be inhibited through other mechanisms. For example, a conformational change where the tail domain binds to the KD can lead to MARK inhibition (Elbert, et al. 2005, Matenia and Mandelkow 2009). Table 2 illustrates some of the known regulators of MARK in mammalian cells.
Table 2.
Regulators of MARK in mammalian cells*
| Name of Regulator | Method of Regulation | Effect on MARK | Additional Information | Reference(s) |
|---|---|---|---|---|
| MARKK/TAO-1 | Phosphorylation of Thr in catalytic kinase domain (KD) (T208 in MARK2) | Activation | -Upstream activating kinase -TESK1 (a kinase related LIM) can inactivate MARKK |
(Johne, et al. 2008, Matenia and Mandelkow 2009, Timm, et al. 2008b, Timm, et al. 2006) |
| LKB1 | Phosphorylation of Thr in KD (T208 in MARK2) | Activation | Upstream activating kinase | (Brajenovic, et al. 2004, Kojima, et al. 2007, Tanwar, et al. 2012) |
| CAMK1 | Phosphorylation of KD | Activation | Phosphorylation sites differ from MARKK and LKB1 | (Uboha, et al. 2007) |
| GSK-3β | Phosphorylation of Ser (S212 in MARK2) | Inhibition | Can override activation by MARKK or LKB1 | (Timm, et al. 2008a) |
| PAK5 | Binds to KD | -Inhibition | -Also independently affects actin cytoskeleton by activating cofilin | (Timm, et al. 2006) |
| AP-2 protein complex | Binds to KD | Regulation of MT-dependent trafficking of CCVs | (Schmitt-Ulms, et al. 2009) | |
| aPKC | Phosphorylation of spacer domain | Inhibition | -Creates 14-3-3 binding motif -Involved in conferring cell polarity |
(Hurov, et al. 2004, Matenia and Mandelkow 2009, Watkins, et al. 2008) |
| Adaptor protein 14-3-3 (Par5) | Binds to spacer domain (phosphorylation dependent) or interacts with KD (phosphorylation independent) | Inhibition | -Involved in conferring cell polarity | (Benton and St Johnston 2003, Matenia and Mandelkow 2009) |
| Tail Domain | Binds to KD or N-terminal head | Inhibition | Causes conformational change | (Elbert, et al. 2005, Matenia and Mandelkow 2009) |
| Cofactors | Bind to CD site | Multiple interactions with upstream and downstream effectors | -MARK CD site resembles CD site of MAP kinases, which interacts with cofactors such as MAPKKs, phosphatases and substrates | (Tanoue and Nishida 2003, Timm, et al. 2008b) |
| Ubiquitin | Polyubiquitination of UBA domain | Intracellular signaling | This is only a proposed regulatory role based on similarity to UBA domain of MAP kinases | (Panneerselvam, et al. 2006) |
AP-2, activating proteins-2, a mammalian transcription factor; CAMK1, calcium/calmodulion-dependent protein kinase type 1; GSK-3β; glycogen synthase kinase 3β; KD, catalytic kinase domain; MARKK, MARK kinase, a Ste20-like kinase; Ste20, sterile 20 protein; PAK5, p21-activated kinase 5; TAO-1, thousand and one amino acid protein kinase; T, Thr; S, Ser.
Function of MARKs
Sertoli cell MTs play an indispensable role during spermatogenesis. MTs are involved in maintenance of Sertoli cell structure, vesicle transport, tubule fluid secretion, and transport of maturing spermatids (Redenbach and Vogl 1991, Smith, et al. 2012, Vogl, et al. 2008). These events are possible only because of the dynamic nature of MTs. Throughout spermatogenesis MTs are never in a static state and must be regulated by a host of factors. For example, KATNAL1, a recently identified MT severing protein, is one such regulator of MTs in the Sertoli cell (Smith, et al. 2012). MAPs are another class of proteins that regulate MTs; however much of the current information regarding the regulation of MAPs is not specific to the testis. Interestingly, the testis is an abundant source of MTs (Loveland, et al. 1996). MT is intimately associated with the cycle of events that take place in Sertoli cells with regard to the progression of spermatogenesis (Vogl, et al. 2008). Thus, discovering new information regarding MT regulation in the testis may not only lead to a better understanding of processes such as spermatogenesis, but may also contribute to what is known about MT regulation as a whole across all cell types. The following will highlight one type of MAP regulator, MARK protein, and its proposed role in the testis.
MARKs and their homologs are functionally diverse protein kinases, possessing functional roles in cell polarity, microtubule stability, cell cycle control and intracellular signaling (Tassan and Le Goff 2004). Par (partitioning defective) kinases are essential for cytoplasmic partitioning, asymmetric cell division, and establishment of cell polarity (Kemphues 2000, Marx, et al. 2010). MARK is the mammalian homolog of Par-1 (partition-defective kinase 1), which is a serine/threonine kinase first identified in Caenorhabditis elegans, and subsequently identified in Drosophila melanogaster, for its role in antero-posterior (A/P) axis development during embryogenesis (Kemphues, et al. 1988, Marx, et al. 2010, Matenia and Mandelkow 2009, Tassan and Le Goff 2004). The diverse roles of MARKs are likely due to the nature of the microtubules in which they regulate. For instance, cell polarization is possible because the dynamic instability of microtubules is regulated by an interplay between stabilizing and destabilizing events (Kaverina and Straube 2011).
MARKs in the testis
Given that MARKs are involved in a diverse array of cellular processes, it is conceivable that they also serve important roles in the testis. Among all the MARKs, MARK4 is the more prominent MARK isoform found in the testis (Tang, et al. 2012, Trinczek, et al. 2004). Thus our recent study focused on the possible localization and role of MARK4 in the Sertoli cell. MARK4 was detected at the apical ES, BTB, and basement membrane, suggesting that the protein has a functional role in the apical ES-BTB-hemidesmosome/basement membrane axis, which coordinates cellular events like degeneration of apical ES and restructuring of the BTB during spermatogenesis (Yan, et al. 2008). Below are some proposed roles of MARK4 in facilitating crosstalk and cell polarity in the testis based on current information from literature and findings from our own study.
MARK4 and cell polarity
MARKs play a role in cell polarization by regulating the activity of MAPs through phosphorylation. The MAPs that MARKs regulate serve as a bridge between signal transduction cascades and MTs (Etienne-Manneville 2010). However, the exact functional role of MARK in cell polarization in the testis has not been fully elucidated. Cell polarity proteins have recently been identified in the testis, specifically in Sertoli and germ cells, most notably spermatids. Since polarity protein complexes, such as the Par- and the Scribble-based protein complex, can each recruit its binding partners and these two protein complexes also display mutually exclusive distribution pattern, their presence in Sertoli cells and spermatids thus confer cell polarity (Iden and Collard 2008, Wong and Cheng 2009b). For example, the partitioning-defective3/partitoning-defective6/atypical protein kinase C (Par3/Par6/aPKC) protein complex was shown to regulate spermatid adhesion and conferred spermatid polarity during spermiogenesis (Wong and Cheng 2009a). As summarized in Table 2, aPKC can inhibit MARK by phosphorylating the kinase domain. Studies in C. elegans have shown that aPKC of the Par3/Par6/aPKC complex, found at the anterior cortex, can phosphorylate Par1, which is located in the posterior cortex (Hurov, et al. 2004). Studies in epithelial cells also reveal that the Par3/Par6/aPKC complex and Par1, a homolog of MARK, are located in different areas of the cell, at tight junctions (TJs) and laterally beneath TJs, respectively. These findings suggest that Par1 must be physically sequestered from the Par3/Par6/aPKC complex in order to establish cell polarity (Watkins, et al. 2008). In the testis, the Par3/Par6/aPKC complex is found at the apical ES (Wong and Cheng 2009a). Interestingly, MARK4 was also detected at the apical ES, but its spatiotemporal expression and localization at the apical ES (Tang, et al. 2012) is not identical to the Par6-based polarity complex (Wong, et al. 2008). For instance, Par6 is limited mostly to the convex side of the elongating/elongated spermatids in stage VII-VIII tubules until it is considerably diminished by stage VIII (Wong, et al. 2008), whereas MARK4 is found surrounding the entire head of the developing spermatids in stage IV-VI tubules; however, MARK4 is limited almost exclusively to the concave side of the elongated spermatid heads in stage VII tubules and it becomes dispersed to the entire tip of the spermatid head until it is rapidly diminished by stage VIII of the epithelial cycle (Tang, et al. 2012). This distinctive spatiotemporal expression and distribution pattern during the epithelial cycle between MARK4 and Par6 thus illustrates these two groups of proteins are working synergistically to confer spermatid polarity during spermatogenesis.
14-3-3 protein has been shown to regulate cell adhesion at the apical ES in the testis and at the BTB to facilitate preleptotene spermatocyte transit (Wong, et al. 2009). Studies in C. elegans have shown that phosphorylation by aPKC on MARK can lead to the creation of a 14-3-3 protein binding motif to recruit other binding partners to a specific cellular domain. Thus, it is plausible that MARK in the testis may play a similar role in cell polarity through 14-3-3 protein.
In our study, we used the adjudin model to examine MARK4 expression and localization in the seminiferous epithelium. Adjudin is known to induce germ cell loss, most notably spermatids by disrupting the apical ES (Cheng, et al. 2011). Treatment of adult rats with adjudin that induced premature loss of elongating/elongated spermatids from the epithelium was found to coincide with a down-regulation of MARK4 expression and its mis-localization at the apical ES. In short, the premature release of spermatids from the epithelium is associated with a considerable loss of MARK4 at the apical ES (Tang, et al. 2012), similar to the disappearance of Par6 and 14-3-3 at the apical ES in departing spermatids in adjudin-treated rats (Wong, et al. 2009, Wong, et al. 2008). It is likely that MARK4 exerts its effects, at least in part, by modulating the Par3/Par6/aPKC complex and 14-3-3 at the apical ES to induce premature spermatid loss, analogous to spermiation that takes place at stage VIII of the epithelial cycle. This possibility must be carefully elucidated in future studies.
MARK4 and crosstalk between cytoskeletons
In eukaryotic cells, actin-, MT-, and IF-based cytoskeletons work in a concerted manner to maintain cell integrity. Though these cytoskeletons are unique and consist of different proteins, they are perpetually in a state of communication with each other. This crosstalk has been observed in multiple epithelia, but what remains to be uncovered is the mechanism(s) of crosstalk among the cytoskeletal elements in Sertoli cells during spermatogenesis. There is evidence of crosstalk between actin and MTs in Sertoli cells. During spermatogenesis, developing germ cells are translocated from the basal to the apical compartment of the seminiferous epithelium as they mature into spermatids. This translocation process is thought to be the result of a communicative effort among different Sertoli cell elements. One such element is known as the ectoplasmic specialization (ES), which is a tripartite complex found at both Sertoli cell-Sertoli cell and spermatid-Sertoli cell interface in the seminiferous epithelium (Vogl, et al. 2000). It is comprised of F-actin bundles sandwiched in-between cisternae of endoplasmic reticulum and the cytoplasmic side of the Sertoli cell (Vogl, et al. 2000, Vogl, et al. 2008). The ES has been typified as an atypical, testis-specific, adherens junction (AJ) (Mruk and Cheng 2004a). AJs are ubiquitously found at cell junctions, but the ES, in contrast to other AJ, contains protein complexes comprised of nectins, cadherins, connexins, JAM-C, and integrins (Mruk and Cheng 2004a, b), in which connexins, JAMs, and integrins are usually restricted to gap junctions, tight junctions, focal adhesion complex (or focal contact) at the cell-extracellular matrix interface, respectively. It has been proposed that since F-actin found at the apical ES is non-contractile, and spermatids are non-motile cells per se, longitudinal movement of elongating spermatids is mediated by MT-based motors such as dynein and kinesin (Guttman, et al. 2000, Lie, et al. 2010, Vogl, et al. 2000).
In addition to providing a transport mechanism for elongating/elongated spermatids in the Sertoli cells, MTs may also play a role in apical ES restructuring (Lie, et al. 2010). A function of the apical ES is to serve as an anchor for developing spermatids. However, prior to spermiation, the apical ES must be deconstructed in order for mature spermatids to be released into the lumen at spermiation (O’Donnell, et al. 2011). Disassembly of the apical ES occurs from late VII through stage VIII of the epithelial cycle (Lie, et al. 2010). As previously discussed, MTs have an intrinsic polarity. Unlike most motile cells (e.g., fibroblasts, macrophages), the MTs of Sertoli cells do not exhibit centrosomal organization in which MT polymerization is initiated at the centrosome (Dammermann, et al. 2003). As a result, MTs are oriented longitudinally, rather than radially, with their minus ends pointing apically like in other polarized epithelial cells (Dammermann, et al. 2003). Minus ends of MTs are normally sites of anchorage and disassembly (Akhmanova, et al. 2009).
We have shown that the localization of MARK4 in the seminiferous epithelium is stage-specific during the epithelial cycle. When the apical ES begins to degenerate at stage VII or early stage VIII of the seminiferous epithelial cycle, MARK4 is strongly expressed at the concave side of the apical ES (Tang, et al. 2012), which is also the site where extensive endocytic vesicle-mediated protein trafficking takes place. It is now known that as the apical ES begins to degrade at late stage VII, it degenerates into an ultrastructure formerly known as apical tubulobulbar complexes (TBCs) where extensive protein endocytosis takes place (Upadhyay, et al. 2012), so that apical ES proteins can be transcytosed and recycled to assemble “newly” developed step 8 spermatids in the seminiferous epithelium. Indeed, because ES has been shown to associate with motor proteins at the cytoplasmic face of the ER component of the ES (Guttman, et al. 2000), the localization of MARK4 at this site suggests that MARK4 may be facilitating these protein trafficking events. MARK4 expression at the apical ES during the subsequent late stage VIII was greatly reduced. The almost non-detectable level of MARK4 at this stage, when spermiation occurs, seems to be correlated with spermatid loss. Taken collectively, these findings suggest that the actin filaments of the apical ES and MTs localized in close vicinity at the apical ES may be involved in crosstalk regulated in part by MARK4, such that the reorganization of these two cytoskeletons can be coordinated to facilitate spermatid movement and spermiation.
There are ultrastructures of the Sertoli cell that resemble components of motile cells. In motile cells, focal adhesions (FAs) relay regulatory signals and physically participate in cell migration. The dynamic nature of FAs can be attributed to their rapid growth and disassembly. However, FAs per se are not found in the seminiferous epithelium such as by electron microscopy, and MTs in Sertoli cells are not as dynamically unstable as in motile cells. Because minus ends do not polymerize in vivo, they require stabilization and can attach to cell junctions, such as adherens junctions (AJs) (Dammermann, et al. 2003, Lie, et al. 2010). In a recent review (Lie, et al. 2010), it was suggested that the MT minus ends may participate in the assembly of apical ES, and/or may play a role in apical ES restructuring. Since the ES is defined as an AJ, MT interaction with the ES may result in transporting the proper signals for restructuring and disassembly of the cell junction during spermatogenesis (Lie, et al. 2010). In addition, while there are no FAs in the seminiferous epithelium, they are similar to apical ES in makeup. As both contain similar structural proteins, it is plausible that apical ES disassembly may follow a mechanism similar to FA disassembly.
Crosstalk in the testis between MTs and IFs is another area that requires much research. It has already been shown that the interactions between MTs and IFs are important in maintaining the structure of Sertoli cells (Amlani and Vogl 1988). Sertoli cell MTs are parallel in orientation with their minus ends apparently positioned apically and plus ends directed basally; IFs which are concentrated basally in the Sertoli cell anchor the plus ends of MTs (Neely and Boekelheide 1988, Vogl, et al. 1995). A previous co-immunoprecipitation experiment showed that actin-related adaptor proteins, such as zyxin, axin, and Wiskott-Aldrich syndrome protein (WASP) can interact with both vimentin and tubulin (Lee, et al. 2004, Lie, et al. 2010). This suggests that these adaptor proteins play a role in regulating MTs and desmosomes in the testis, they also support the notion of crosstalk among all three cytoskeletons within the Sertoli cell.
Our initial findings suggest that MARK4 plays a role in regulating desmosome at the BTB (Tang, et al. 2012). Desmosomes are intercellular junctions in which IFs are anchored to integral desmosomal cadherin proteins via cytolinker desmoplakin (Delva, et al. 2009, Lie, et al. 2011). Armadillo proteins, such as the plakophilins, reinforce cadherin-desmoplakin interactions (Cowin and Burke 1996). Using co-immunoprecipitation, MARK4 was shown to structurally associate with plakophilin-2 (PKP2) (Tang, et al. 2012). A previous study sought to examine the physiological significance of the desmosome at the BTB in the testis, and reported that a protein complex comprised of connexin 43 and PKP2 regulates BTB dynamics (Li, et al. 2009). The findings from this study suggest that the desmosome facilitates movement of preleptotene spermatocytes at the BTB while maintaining the BTB integrity, and since MARK4 structurally associates PKP2, it is likely that MARK4 plays a functional role in BTB regulation and crosstalk between MTs and IFs.
Concluding remarks and future perspectives
This review briefly summarizes current knowledge of MT regulation in the testis. Results from our recent study on MARK4 suggest that this MAP/MT regulating protein may play a critical role in the regulation of MTs during spermatogenesis. The expression of MARK4 in the seminiferous epithelium is highly spatiotemporal during the epithelial cycle, and MARK4 is found in close vicinity of actin filament bundles at the apical ES, coexisting with actin-regulatory proteins at the apical ES (e.g., Arp3), illustrating this MT-regulatory protein may mediate crosstalk between the actin- and the MT-based cytoskeletons. Further studies should examine the role of MARK4, and other actin regulatory proteins (e.g., the Arp2/3-N-WASP protein complex, filamins) and polarity protein complexes (e.g., Scribble/Lgl/Dlg complex, Par-based complex) in coordinating changes in the organization of the actin filament bundles and the MTs at the apical ES to affect spermatid transport and movement during spermatogenesis. Furthermore, much work is needed to identify other players in regulating MT dynamics in the testis and many questions are open to be addressed. For instance, what are the involving molecules that “direct” the transport of spermatids? This likely involves small GTPases and ATPs, and perhaps other non-receptor proteins kinases, such as FAK, c-Src, and c-Yes.
Footnotes
This work was supported by grants from the National Institutes of Health (NICHD R01 HD056036 to C.Y.C., U54 HD029990 Project 5 to C.Y.C.).
DISCLOSURE STATEMENT: The authors have nothing to disclose.
References
- Akhmanova A, Stehbens SJ, Yap AS. Touch, grasp, deliver and control: functional cross-talk between microtubules and cell adhesions. Traffic. 2009;10:268–274. doi: 10.1111/j.1600-0854.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- Al-Bassam J, Chang F. Regulation of microtubule dynamics by TOG-domain proteins XMAP215/Dis1 and CLASP. Trends Cell Biol. 2011;21:604–614. doi: 10.1016/j.tcb.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlani S, Vogl AW. Changes in the distribution of microtubules and intermediate filaments in mammalian Sertoli cells during spermatogenesis. Anat Rec. 1988;220:143–160. doi: 10.1002/ar.1092200206. [DOI] [PubMed] [Google Scholar]
- Belletti B, Baldassarre G. Stathmin: a protein with many tasks. New biomarker and potential target in cancer. Expert Opin Ther Targets. 2011;15:1249–1266. doi: 10.1517/14728222.2011.620951. [DOI] [PubMed] [Google Scholar]
- Benton R, St Johnston D. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell. 2003;115:691–704. doi: 10.1016/s0092-8674(03)00938-3. [DOI] [PubMed] [Google Scholar]
- Brajenovic M, Joberty G, Kuster B, Bouwmeester T, Drewes G. Comprehensive proteomic analysis of human Par protein complexes reveals an interconnected protein network. The Journal of biological chemistry. 2004;279:12804–12811. doi: 10.1074/jbc.M312171200. [DOI] [PubMed] [Google Scholar]
- Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annual review of physiology. 1995;57:417–445. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- Brouhard GJ, Stear JH, Noetzel TL, Al-Bassam J, Kinoshita K, Harrison SC, Howard J, Hyman AA. XMAP215 is a processive microtubule polymerase. Cell. 2008;132:79–88. doi: 10.1016/j.cell.2007.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbank KS, Mitchison TJ. Microtubule dynamic instability. Curr Biol. 2006;16:R516–517. doi: 10.1016/j.cub.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Lie PP, Wong EW, Mruk DD, Silvestrini B. Adjudin disrupts spermatogenesis via the action of some unlikely partners: Eps8, Arp2/3 complex, drebrin E, PAR6 and 14-3-3. Spermatogenesis. 2011;1:291–297. doi: 10.4161/spmg.1.4.18393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. An intracellular trafficking pathway in the seminiferous epithelium regulating spermatogenesis: a biochemical and molecular perspective. Critical reviews in biochemistry and molecular biology. 2009;44:245–263. doi: 10.1080/10409230903061207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. The blood-testis barrier and its implication in male contraception. Pharmacol Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowin P, Burke B. Cytoskeleton-membrane interactions. Current opinion in cell biology. 1996;8:56–65. doi: 10.1016/s0955-0674(96)80049-4. [DOI] [PubMed] [Google Scholar]
- Dammermann A, Desai A, Oegema K. The minus end in sight. Curr Biol. 2003;13:R614–624. doi: 10.1016/s0960-9822(03)00530-x. [DOI] [PubMed] [Google Scholar]
- de Forges H, Bouissou A, Perez F. Interplay between microtubule dynamics and intracellular organization. The international journal of biochemistry & cell biology. 2012;44:266–274. doi: 10.1016/j.biocel.2011.11.009. [DOI] [PubMed] [Google Scholar]
- de Kretser DM, Kerr JB. The cytology of the testis. In: Knobil E, Neill JB, Ewing LL, Greenwald GS, Markert CL, Pfaff DW, editors. The Physiology of Reproduction. Vol. 1. New York: Raven Press; 1988. pp. 837–932. [Google Scholar]
- Delva E, DK Tucker, Kowalczyk AP. The desmosome. Cold Spring Harb Perspect Biol. 2009;1:a002543. doi: 10.1101/cshperspect.a002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A, Mitchison TJ. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- Dixit R, Barnett B, Lazarus JE, Tokito M, Goldman YE, Holzbaur EL. Microtubule plus-end tracking by CLIP-170 requires EB1. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:492–497. doi: 10.1073/pnas.0807614106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsel DN, Hyman AA, Cobb MH, Kirschner MW. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Molecular biology of the cell. 1992;3:1141–1154. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes G. MARKing tau for tangles and toxicity. Trends Biochem Sci. 2004;29:548–555. doi: 10.1016/j.tibs.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Drewes G, Ebneth A, Preuss U, Mandelkow EM, Mandelkow E. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell. 1997;89:297–308. doi: 10.1016/s0092-8674(00)80208-1. [DOI] [PubMed] [Google Scholar]
- Drewes G, Trinczek B, Illenberger S, Biernat J, Schmitt-Ulms G, Meyer HE, Mandelkow EM, Mandelkow E. Microtubule-associated protein/microtubule affinity-regulating kinase (p110mark). A novel protein kinase that regulates tau-microtubule interactions and dynamic instability by phosphorylation at the Alzheimer-specific site serine 262. The Journal of biological chemistry. 1995;270:7679–7688. doi: 10.1074/jbc.270.13.7679. [DOI] [PubMed] [Google Scholar]
- Elbert M, Rossi G, Brennwald P. The yeast par-1 homologs kin1 and kin2 show genetic and physical interactions with components of the exocytic machinery. Molecular biology of the cell. 2005;16:532–549. doi: 10.1091/mbc.E04-07-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endow SA. Determinants of motor polarity in the kinesin proteins. Biophys J. 1995;6827:1S–274S. [PMC free article] [PubMed] [Google Scholar]
- Erickson HP, O’Brien ET. Microtubule dynamic instability and GTP hydrolysis. Annual review of biophysics and biomolecular structure. 1992;21:145–166. doi: 10.1146/annurev.bb.21.060192.001045. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S. From signaling pathways to microtubule dynamics: the key players. Current opinion in cell biology. 2010;22:104–111. doi: 10.1016/j.ceb.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Franca LR, Auharek SA, Hess RA, Dufour JM, Hinton BT. Blood-tissue barriers: Morphofunctional and immunological aspects of the blood-testis and blood-epididymal barriers. Adv Exp Med Biol. 2012;763:237–259. [PubMed] [Google Scholar]
- Guttman JA, Kimel GH, Vogl AW. Dynein and plus-end microtubule-dependent motors are associated with specialized Sertoli cell junction plaques (ectoplasmic specializations) J Cell Sci. 2000;113(Pt 12):2167–2176. doi: 10.1242/jcs.113.12.2167. [DOI] [PubMed] [Google Scholar]
- Hess RA, de Franca LR. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 2008;636:1–15. doi: 10.1007/978-0-387-09597-4_1. [DOI] [PubMed] [Google Scholar]
- Hurov JB, Watkins JL, Piwnica-Worms H. Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Curr Biol. 2004;14:736–741. doi: 10.1016/j.cub.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization. Nature Rev Mol Cell Biol. 2008;9:846–859. doi: 10.1038/nrm2521. [DOI] [PubMed] [Google Scholar]
- Illenberger S, Drewes G, Trinczek B, Biernat J, Meyer HE, Olmsted JB, Mandelkow EM, Mandelkow E. Phosphorylation of microtubule-associated proteins MAP2 and MAP4 by the protein kinase p110mark. Phosphorylation sites and regulation of microtubule dynamics. The Journal of biological chemistry. 1996;271:10834–10843. doi: 10.1074/jbc.271.18.10834. [DOI] [PubMed] [Google Scholar]
- Johne C, Matenia D, Li XY, Timm T, Balusamy K, Mandelkow EM. Spred1 and TESK1--two new interaction partners of the kinase MARKK/TAO1 that link the microtubule and actin cytoskeleton. Molecular biology of the cell. 2008;19:1391–1403. doi: 10.1091/mbc.E07-07-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaverina I, Straube A. Regulation of cell migration by dynamic microtubules. Seminars in cell & developmental biology. 2011;22:968–974. doi: 10.1016/j.semcdb.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemphues K. PARsing embryonic polarity. Cell. 2000;101:345–348. doi: 10.1016/s0092-8674(00)80844-2. [DOI] [PubMed] [Google Scholar]
- Kemphues KJ, Priess JR, Morton DG, Cheng NS. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell. 1988;52:311–320. doi: 10.1016/s0092-8674(88)80024-2. [DOI] [PubMed] [Google Scholar]
- Kojima Y, Miyoshi H, Clevers HC, Oshima M, Aoki M, Taketo MM. Suppression of tubulin polymerization by the LKB1-microtubule-associated protein/microtubule affinity-regulating kinase signaling. The Journal of biological chemistry. 2007;282:23532–23540. doi: 10.1074/jbc.M700590200. [DOI] [PubMed] [Google Scholar]
- Kueh HY, Mitchison TJ. Structural plasticity in actin and tubulin polymer dynamics. Science. 2009;325:960–963. doi: 10.1126/science.1168823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Wittmann T. +TIPs: SxIPping along microtubule ends. Trends Cell Biol. 2012;22:418–428. doi: 10.1016/j.tcb.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NP, Mruk DD, Conway AM, Cheng CY. Zyxin, axin, and Wiskott-Aldrich syndrome protein are adaptors that link the cadherin/catenin protein complex to the cytoskeleton at adherens junctions in the seminiferous epithelium of the rat testis. J Androl. 2004;25:200–215. doi: 10.1002/j.1939-4640.2004.tb02780.x. [DOI] [PubMed] [Google Scholar]
- Lee NPY, Cheng CY. Ectoplasmic specialization, a testis-specific cell-cell actin-based adherens junction type: is this a potential target for male contraceptive development. Human Reprod Update. 2004;10:349–369. doi: 10.1093/humupd/dmh026. [DOI] [PubMed] [Google Scholar]
- Li MW, Mruk DD, Lee WM, Cheng CY. Connexin 43 and plakophilin-2 as a protein complex that regulates blood-testis barrier dynamics. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:10213–10218. doi: 10.1073/pnas.0901700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PP, Mruk DD, Lee WM, Cheng CY. Cytoskeletal dynamics and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1581–1592. doi: 10.1098/rstb.2009.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PPY, Cheng CY, Mruk DD. The biology of the desmosome-like junction: A versatile anchoring junction and signal transducer in the seminiferous epithelium. Int Rev Cell Mol Biol. 2011;286:223–269. doi: 10.1016/B978-0-12-385859-7.00005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveland KL, Hayes TM, Meinhardt A, Zlatic KS, Parvinen M, de Kretser DM, McFarlane JR. Microtubule-associated protein-2 in the rat testis: a novel site of expression. Biol Reprod. 1996;54:896–904. doi: 10.1095/biolreprod54.4.896. [DOI] [PubMed] [Google Scholar]
- Lumb JH, Connell JW, Allison R, Reid E. The AAA ATPase spastin links microtubule severing to membrane modelling. Biochim Biophys Acta. 2012;1823:192–197. doi: 10.1016/j.bbamcr.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Mandelkow E, Mandelkow EM. Microtubules and microtubule-associated proteins. Current opinion in cell biology. 1995;7:72–81. doi: 10.1016/0955-0674(95)80047-6. [DOI] [PubMed] [Google Scholar]
- Mandelkow EM, Biernat J, Drewes G, Gustke N, Trinczek B, Mandelkow E. Tau domains, phosphorylation, and interactions with microtubules. Neurobiol Aging. 1995;16:355–362. doi: 10.1016/0197-4580(95)00025-a. [DOI] [PubMed] [Google Scholar]
- Marx A, Nugoor C, Panneerselvam S, Mandelkow E. Structure and function of polarity-inducing kinase family MARK/Par-1 within the branch of AMPK/Snf1-related kinases. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:1637–1648. doi: 10.1096/fj.09-148064. [DOI] [PubMed] [Google Scholar]
- Matenia D, Mandelkow EM. The tau of MARK: a polarized view of the cytoskeleton. Trends Biochem Sci. 2009;34:332–342. doi: 10.1016/j.tibs.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Matsuuchi L, Naus CC. Gap junction proteins on the move: Connexins, the cytoskeleton and migration. Biochim Biophys Acta. 2013;1828:94–108. doi: 10.1016/j.bbamem.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Meunier S, Vernos I. Microtubule assembly during mitosis - from distinct origins to distinct functions? J Cell Sci. 2012;125:2805–2814. doi: 10.1242/jcs.092429. [DOI] [PubMed] [Google Scholar]
- Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Mooren OL, Galletta BJ, Cooper JA. Roles of actin assembly in endocytosis. Annu Rev Biochem. 2012;81:661–686. doi: 10.1146/annurev-biochem-060910-094416. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Cell-cell interactions at the ectoplasmic specialization in the testis. Trends Endocrinol Metab. 2004a;15:439–447. doi: 10.1016/j.tem.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004b;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Tanaka F, Haraguchi N, Mimori K, Matsumoto T, Inoue H, Yanaga K, Mori M. Clinicopathological and biological significance of mitotic centromere-associated kinesin overexpression in human gastric cancer. Br J Cancer. 2007;97:543–549. doi: 10.1038/sj.bjc.6603905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely MD, Boekelheide K. Sertoli cell processes have axoplasmic features: an ordered microtubule distribution and an abundant high molecular weight microtubule-associated protein (cytoplasmic dynein) The Journal of cell biology. 1988;107:1767–1776. doi: 10.1083/jcb.107.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell L, Nicholls PK, O’Bryan MK, McLachlan RI, Stanton PG. Spermiation: the process of sperm release. Spermatogenesis. 2011;1:14–35. doi: 10.4161/spmg.1.1.14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneerselvam S, Marx A, Mandelkow EM, Mandelkow E. Structure of the catalytic and ubiquitin-associated domains of the protein kinase MARK/Par-1. Structure. 2006;14:173–183. doi: 10.1016/j.str.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Pelletier RM. The blood-testis barrier: the junctional permeability, the proteins and the lipids. Prog Histochem Cytochem. 2011;46:49–127. doi: 10.1016/j.proghi.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Redenbach DM, Vogl AW. Microtubule polarity in Sertoli cells: a model for microtubule-based spermatid transport. Eur J Cell Biol. 1991;54:277–290. [PubMed] [Google Scholar]
- Russell LD. Form, dimensions, and cytology of mammalian Sertoli cells. In: Russell LD, Griswold MD, editors. The Sertoli Cell. Clearwater: Cache River Press; 1993. pp. 1–37. [Google Scholar]
- Schmitt-Ulms G, Matenia D, Drewes G, Mandelkow EM. Interactions of MAP/microtubule affinity regulating kinases with the adaptor complex AP-2 of clathrin-coated vesicles. Cell motility and the cytoskeleton. 2009;66:661–672. doi: 10.1002/cm.20394. [DOI] [PubMed] [Google Scholar]
- Smith LB, Milne L, Nelson N, Eddie S, Brown P, Atanassova N, O’Bryan MK, O’Donnell L, Rhodes D, Wells S, Napper D, Nolan P, Lalanne Z, Cheeseman M, Peters J. KATNAL1 regulation of sertoli cell microtubule dynamics is essential for spermiogenesis and male fertility. PLoS Genet. 2012;8:e1002697. doi: 10.1371/journal.pgen.1002697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehbens S, Wittmann T. Targeting and transport: how microtubules control adhesion dynamics. J Cell Biol. 2012;198:481–489. doi: 10.1083/jcb.201206050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su WH, Mruk DD, Cheng CY. Regulation of actin dynamics and protein trafficking during spermatogenesis - insights into a complex process. Crit Rev Biochem Mol Biol. 2013 doi: 10.3109/10409238.2012.758084. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Ohi R, Pellman D. Move in for the kill: motile microtubule regulators. Trends Cell Biol. 2012;22:567–575. doi: 10.1016/j.tcb.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang EI, Xiao X, Mruk DD, Qian XJ, Mok KW, Jenardhanan P, Lee WM, Mathur PP, Cheng CY. Microtubule affinity-regulated kinase 4 (MARK4) is a component of the ectoplasmic specialization in the rat testis. Spermatogenesis. 2012;2:117–126. doi: 10.4161/spmg.20724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanoue T, Nishida E. Molecular recognitions in the MAP kinase cascades. Cellular signalling. 2003;15:455–462. doi: 10.1016/s0898-6568(02)00112-2. [DOI] [PubMed] [Google Scholar]
- Tanwar PS, Kaneko-Tarui T, Zhang L, Teixeira JM. Altered LKB1/AMPK/TSC1/TSC2/mTOR signaling causes disruption of Sertoli cell polarity and spermatogenesis. Hum Mol Genet. 2012;21:4394–4405. doi: 10.1093/hmg/dds272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassan JP, Le Goff X. An overview of the KIN1/PAR-1/MARK kinase family. Biology of the cell/under the auspices of the European Cell Biology Organization. 2004;96:193–199. doi: 10.1016/j.biolcel.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Tilney LG, Bryan J, Bush DJ, Fujiwara K, Mooseker MS, Murphy DB, Snyder DH. Microtubules: evidence for 13 protofilaments. The Journal of cell biology. 1973;59:267–275. doi: 10.1083/jcb.59.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timm T, Balusamy K, Li X, Biernat J, Mandelkow E, Mandelkow EM. Glycogen synthase kinase (GSK) 3beta directly phosphorylates Serine 212 in the regulatory loop and inhibits microtubule affinity-regulating kinase (MARK) 2. The Journal of biological chemistry. 2008a;283:18873–18882. doi: 10.1074/jbc.M706596200. [DOI] [PubMed] [Google Scholar]
- Timm T, Marx A, Panneerselvam S, Mandelkow E, Mandelkow EM. Structure and regulation of MARK, a kinase involved in abnormal phosphorylation of Tau protein. BMC Neuroscience. 2008b;9(Suppl 2):S9. doi: 10.1186/1471-2202-9-S2-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timm T, Matenia D, Li XY, Griesshaber B, Mandelkow EM. Signaling from MARK to tau: regulation, cytoskeletal crosstalk, and pathological phosphorylation. Neuro-degenerative diseases. 2006;3:207–217. doi: 10.1159/000095258. [DOI] [PubMed] [Google Scholar]
- Trinczek B, Brajenovic M, Ebneth A, Drewes G. MARK4 is a novel microtubule-associated proteins/microtubule affinity-regulating kinase that binds to the cellular microtubule network and to centrosomes. The Journal of biological chemistry. 2004;279:5915–5923. doi: 10.1074/jbc.M304528200. [DOI] [PubMed] [Google Scholar]
- Uboha NV, Flajolet M, Nairn AC, Picciotto MR. A calcium- and calmodulin-dependent kinase Ialpha/microtubule affinity regulating kinase 2 signaling cascade mediates calcium-dependent neurite outgrowth. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:4413–4423. doi: 10.1523/JNEUROSCI.0725-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay RD, Kumar AV, Ganeshan M, Balasinor NH. Tubulobulbar complex: cytoskeletal remodeling to release spermatozoa. Reprod Biol Endocrinol. 2012;10:27. doi: 10.1186/1477-7827-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaid KS, Guttman JA, Babyak N, Deng W, McNiven MA, Mochizuki N, Finlay BB, Vogl AW. The role of dynamin 3 in the testis. J Cell Physiol. 2007a;210:644–654. doi: 10.1002/jcp.20855. [DOI] [PubMed] [Google Scholar]
- Vaid KS, Guttman JA, Singaraja RR, Vogl AW. A kinesin is present at unique Sertoli/spermatid adherens junctions in rat and mouse testes. Biol Reprod. 2007b;77:1037–1048. doi: 10.1095/biolreprod.107.063735. [DOI] [PubMed] [Google Scholar]
- Vignaud T, Blanchoin L, Thery M. Directed cytoskeleton self-organization. Trends Cell Biol. 2012;22:671–682. doi: 10.1016/j.tcb.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Vogl AW, Pfeiffer DC, Mulholland D, Kimel G, Guttman J. Unique and multifunctional adhesion junctions in the testis: ectoplasmic specializations. Arch Histol Cytol. 2000;63:1–15. doi: 10.1679/aohc.63.1. [DOI] [PubMed] [Google Scholar]
- Vogl AW, KS Vaid, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol. 2008;636:186–211. doi: 10.1007/978-0-387-09597-4_11. [DOI] [PubMed] [Google Scholar]
- Vogl AW, Weis M, Pfeiffer DC. The perinuclear centriole-containing centrosome is not the major microtubule organizing center in Sertoli cells. Eur J Cell Biol. 1995;66:165–179. [PubMed] [Google Scholar]
- Wade RH. On and around microtubules: an overview. Mol Biotechnol. 2009;43:177–191. doi: 10.1007/s12033-009-9193-5. [DOI] [PubMed] [Google Scholar]
- Watkins JL, Lewandowski KT, Meek SE, Storz P, Toker A, Piwnica-Worms H. Phosphorylation of the Par-1 polarity kinase by protein kinase D regulates 14-3-3 binding and membrane association. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18378–18383. doi: 10.1073/pnas.0809661105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CH, Cheng CY. The blood-testis barrier: its biology, regulation, and physiological role in spermatogenesis. Curr Top Dev Biol. 2005;71:263–296. doi: 10.1016/S0070-2153(05)71008-5. [DOI] [PubMed] [Google Scholar]
- Wong EW, Cheng CY. Polarity proteins and cell-cell interactions in the testis. Int Rev Cell Mol Biol. 2009a;278:309–353. doi: 10.1016/S1937-6448(09)78007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EW, Sun S, Li MW, Lee WM, Cheng CY. 14-3-3 Protein regulates cell adhesion in the seminiferous epithelium of rat testes. Endocrinology. 2009;150:4713–4723. doi: 10.1210/en.2009-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EWP, Cheng CY. Polarity proteins and cell-cell interactions in the testis. Int Rev Cell Mol Biol. 2009b;278:309–353. doi: 10.1016/S1937-6448(09)78007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EWP, Mruk DD, Lee WM, Cheng CY. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:9657–9662. doi: 10.1073/pnas.0801527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Xiang X, Hammer JAr. Motor proteins at the microtubule plus-end. Trends Cell Biol. 2006;16:135–143. doi: 10.1016/j.tcb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Yan HHN, Mruk DD, Wong EWP, Lee WM, Cheng CY. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:8950–8955. doi: 10.1073/pnas.0711264105. [DOI] [PMC free article] [PubMed] [Google Scholar]


