Abstract
Phosphate is an indispensable nutrient for the formation of nucleic acids and the cell membrane. Adequate phosphate balance is a prerequisite for basic cellular functions ranging from energy metabolism to cell signaling. More than 85% of body phosphate is present in the bones and teeth. The remaining phosphate is distributed in various soft tissues, including skeletal muscle. A tiny amount, around 1% of total body phosphate, is distributed both in the extracellular fluids and within the cells. Impaired phosphate balance can affect the functionality of almost all human systems, including muscular, skeletal, and vascular systems, leading to an increase in morbidity and mortality of the involved patients. Currently, measuring serum phosphate level is the gold standard to estimate the overall phosphate status of the body. Despite the biological and clinical significance of maintaining delicate phosphate balance, serum levels do not always reflect the amount of phosphate uptake and its distribution. This article briefly discusses the potential that some of the early consequences of phosphate toxicity might not be evident from serum phosphate levels.
Keywords: Klotho, FGF23, Vitamin D, Calcium
Introduction
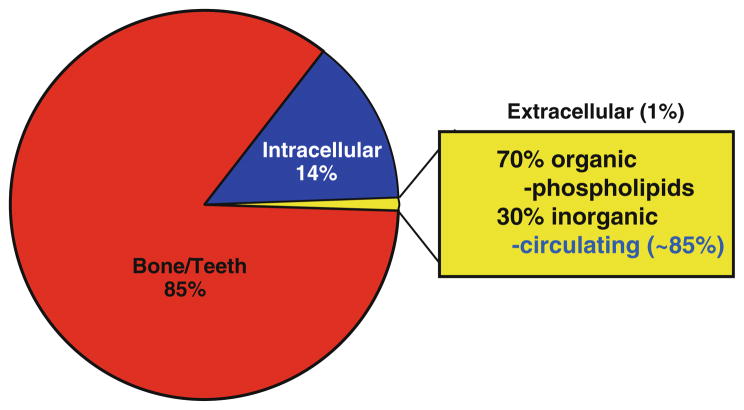
Phosphorus is regularly consumed through food and, once absorbed, phosphorus binds with oxygen and is distributed in the various parts of the body as phosphate. A number of important cellular and systemic organ functions, including energy metabolism, maintenance of mineral ion equilibrium, and adequate cell signaling require physiological phosphate balance (serum levels around 2.5–4.5 mg/dl in humans). Of relevance, infants have higher serum phosphate levels than adults [1]. Most of the body phosphate is present in the bone as hydroxyapatite, and only 1% of the total phosphate is available in the extracellular compartment (Fig. 1) [2–5]. Serum measurements of extracellular phosphate therefore reveal only a tiny fraction of total body phosphate and might not always reflect the amount of phosphate uptake and its distribution. Of relevance, in a healthy individual, roughly two-thirds of consumed phosphate is excreted in urine and one-third is excreted through the stool to maintain homeostatic balance.
Fig. 1.
Phosphate distribution in various compartments of the body. Please note that circulating serum phosphate measurement estimates only a tiny pool of total body phosphate
The physiological phosphate balance is mostly accomplished by cross-organ talk among kidney, intestine, bone, and parathyroid gland [3, 6–10]. Phosphate absorption, following food ingestion, takes place mostly in the small intestine [11]. Intestinal phosphate absorption is a complex process, partly assisted by sodium-dependent phosphate transporter 2b (NaPi-2b), present in the luminal side of the intestinal cells. The levels of 1,25-dihydroxyvitamin D and dietary phosphate could influence the intestinal transport activity of NaPi-2b [12]. For example, 1,25-dihydroxyvitamin D can induce intestinal NaPi-2b protein to increase phosphate absorption in the gut. In a similar line of observation, following acute phosphate administration, the absorption ability of phosphate was reduced in mice with genetically suppressed NaPi-2b activity when compared with control mice [13]. It must be noted that the human relevance of NaPi-2b knockout mice is not yet clear, as loss-of-function mutations of human NaPi-2b gene do not significantly alter phosphate balance in the affected individual [14].
Phosphate is freely filtered through the glomeruli; more than 80% of the filtered phosphate is reabsorbed in the proximal tubular epithelial cells and a tiny fraction is reabsorbed in the distal tubule. Comparable to intestinal phosphate absorption, renal phosphate reabsorption in the proximal part of the tubules is also partly accomplished by sodium-dependent phosphate uptake through NaPi-2a and NaPi-2c transporters; parathyroid hormone (PTH) has a major influence on the activity of NaPi-2a and NaPi-2c that is present in the luminal side of the proximal tubular epithelial cells [15, 16]. PTH can inhibit NaPi-dependent phosphate reabsorption and thereby increase urinary phosphate excretion [15, 16]. The physiological phosphate balance is uniquely maintained by the ability of the kidney to adjust phosphate handling, as phosphate restriction could increase reabsorption, whereas phosphate intake could reduce the reabsorption process; such adaptation to a low or high phosphate-containing diet is partly accomplished by the insertion or retrieval of renal NaPi transporters from the brush border membrane. Recent studies have shown that fibroblast growth factor 23 (FGF23) and klotho can also suppress renal NaPi activity [17–20].
Our understanding of the critical role of the kidney in the regulation of systemic phosphate metabolism is significantly enhanced by the identification of the FGF23–klotho system. In the accompanying section, we briefly discuss the role for the FGF23–klotho system in phosphate metabolism that is relevant to this article. For further details on the molecular and clinical aspects of FGF23–klotho functions, readers are recommended to see several recently published review articles [3, 7, 21–30].
FGF23–klotho system
Bone-derived FGF23 is a protein of around 30 kDa; the NH2-terminal contains the FGF receptor (FGFR)-binding domain, and the COOH-terminal contains the klotho-binding site [31]. In vitro studies have shown the binding ability of FGF23 to FGFR1c, FGFR3c, and FGFR4 [17, 32–35], although in vivo studies could not detect noteworthy responses of FGF23 through FGFR3 and FGFR4 [36]. In contrast to other autocrine and paracrine members of FGFs, the interaction between endocrine FGF23 with its receptors and subsequent activation of signaling network requires klotho as a cofactor; the FGF23–klotho–FGFR complex can activate downstream signaling phosphoproteins, including FGF receptor substrate-2a (FRS2a) and extracellular signal-regulated kinase 1/2 (Erk1/2) [37, 38].
Klotho is a 130-kDa membrane protein [28] that can be cleaved from the plasma membrane by disintegrin and metalloproteinases (ADAM-10 and ADAM-17) [39]. The expression of klotho is mostly detected in the kidney (distal convoluted tubules), in the brain (choroid plexus epithelium), and in the parathyroid gland [40]. Although FGF23 is a circulatory protein, the restricted expression of klotho provides the tissue specificity for FGF23 functions. The essential role of klotho in FGF23-mediated phosphate regulation is convincingly shown in various experimental models. For example, wild-type mice, challenged with bioactive FGF23 protein, had significantly reduced serum phosphate levels, whereas such bioactive protein lost its phosphate-lowering ability in mice without klotho activity (either klotho knockout mice or Fgf23/klotho double-knockout mice) [41]. Similarly, hypophosphatemic phex mutant mice became hyperphosphatemic when klotho function was eliminated, even though the phex/klotho double-mutant mice have higher serum levels of FGF23 than phex mutant mice [42, 43]. A similar line of observation was also noted in humans; for instance, loss-of-function mutation in the human klotho gene resulted in severe hyperphosphatemia, although the affected patient with tumoral calcinosis had high serum levels of FGF23 [44].
The ability to reduce serum phosphate levels by the activated FGF23–klotho system is observed in both animal and human studies [41, 43–52]. For example, in autosomal dominant hypophosphatemic rickets (ADHR) patients, increased activity of FGF23 caused by gain-of-function mutations of the human FGF23 gene is associated with hypophosphatemia as a result of excessive urinary phosphate loss [46]. Such a phosphate-wasting phenotype is also observed in transgenic mice with forced expression of human FGF23 [53], and genetically eliminating the functions of Fgf23 from mice leads to hyperphosphatemia [54]. More importantly, restoring the functions of human FGF23, either by genetic manipulation, or by therapeutic inoculation in the Fgf23 knockout mice, can reverse hyperphosphatemia to hypophosphatemia, clearly showing the in vivo phosphate-regulating abilities of FGF23 [55].
The interaction of bone-derived FGF23 and mostly kidney-derived klotho can increase urinary phosphate excretion by reducing the reabsorption ability of NaPi-2a and NaPi-2c co-transporters and by inhibiting synthesis of the active vitamin D metabolite, 1,25-dihydroxyvitamin D [17–19]. It is important to mention that whether the suppression of renal NaPi co-transporter activity by the FGF23–klotho pathway is a direct effect [20] or is mediated through other FGF23 target molecules is not yet clear and requires additional experimental clarification.
Because physiological phosphate homeostasis is maintained by delicate cross-organ interactions among the kidney, intestine, bone, and parathyroid gland (Fig. 2) [3, 6–10], pathology involving any of these organs can lead to altered phosphate balance in the form of either hypophosphatemia or hyperphosphatemia. Particularly, the renal transport system is essential for the physiological regulation of organic and inorganic ion balance, and most of the chronic renal diseases without therapeutic intervention usually progress to irreversible renal fibrosis that affects survival [56–59]. The cause and consequences of such obvious dysregulation of phosphate is detailed in numerous publications [60–64], and instead of reproducing such information, we have listed a few disorders related to hypo-and hyperphosphatemic conditions as Table 1.
Fig. 2.
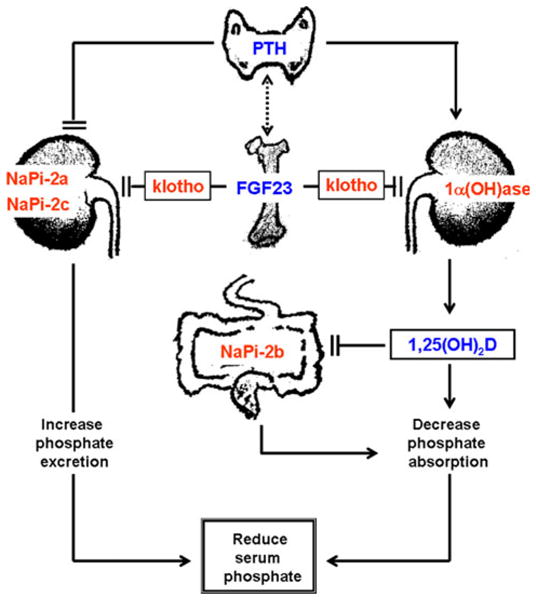
Simplified diagram showing multi-organ interactions in regulation of phosphate homeostasis. Fibroblast growth receptor (FGF)23 produced in the bone cells can suppress renal NaPi-2a and NaPi-2c co-transporter activities to increase the urinary excretion of phosphate. Similarly, FGF23 can also suppress renal expression of 1α (OH)ase to reduce production of 1,25-dihydroxyvitamin D [1,25(OH)2D], which can suppress intestinal NaPi-2b activities to reduce phosphate absorption, resulting in decreased serum phosphate levels [3]. Of relevance, parathyroid hormone (PTH) can induce the expression of the 1α(OH)ase, and thereby can increase the production of 1,25(OH)2D, which in turn can inhibit PTH and 1α(OH)ase expression. Such transcriptional repression feedback maintains vitamin D homeostasis. The figure is adopted with modification from our earlier publication [29]
Table 1.
Partial list of pathological events or diseases that can induce hypophosphatemia and hyperphosphatemia [3, 27, 93]
| Hypophosphatemia |
| Respiratory alkalosis |
| Alcoholism |
| Bone marrow transplant |
| Chronic diarrhea |
| Chronic liver disease |
| Diabetic ketoacidosis |
| Diuretics |
| Extensive burns |
| FGF23-induced hypophosphatemic rickets |
| FGF23-mediated tumor-induced osteomalacia |
| Fibrous dysplasia |
| Hormones (insulin, glucagon, cortisol) |
| Hyperparathyroidisms |
| Intestinal malabsorption |
| MacCune–Albright syndrome |
| Renal transplant |
| Renal tubular defects (Fanconi syndrome) |
| Salicylate poisoning |
| Severe dietary deficiency |
| Severe sepsis |
| Vitamin D deficiency |
| Hyperphosphatemia |
| Acromegaly |
| Bisphosphonate therapy |
| Hemolysis |
| Hypoparathyroidism |
| Intravenous/oral phosphate therapy |
| Magnesium deficiency |
| Metabolic acidosis |
| Phosphate enema |
| Renal failure |
| Respiratory acidosis |
| Rhabdomyolysis |
| Tumor calcinosis |
| Tumor lysis syndrome |
| Vitamin D intoxication |
Human phosphate toxicity
Phosphate toxicity resulting from excessive accumulation of phosphate can provoke a wide range of local and systemic manifestations in the body. A higher occurrence of vascular calcification in patients with chronic kidney disease (CKD) is one of the commonly encountered consequences of phosphate toxicity [65–68]. More importantly, phosphate toxicity and low serum vitamin D levels have been implicated as independent risk factors for patients with CKD. In the 1960s, administration of inorganic phosphate, by intravenous or oral routes, was used as a therapy to treat hypercalcemia and provided direct evidence of the consequences of human phosphate toxicity. For instance, inorganic phosphate treatment, either orally or intravenously, induced various features of phosphate toxicity, not only in organ damage, but also in their survival; disodium or dipotassium salt (Na2HPO4 or K2HPO4) was administrated orally (the cumulative dose of phosphate varied from 1.2 to 21.6 g; average, 9.6 g) or administered intravenously (the cumulative dose of phosphate varied from 2.3 to 6.2 g; average, 3.33 g). Despite similar serum phosphate levels in pre- and postphosphate treatment, two patients developed diarrhea during oral phosphate administration with a cumulative dose of 8.8 and 6.5 g, respectively. Pulmonary edema was encountered during phosphate infusion in a patient receiving a cumulative dose of 3.1 g phosphate that raised the serum phosphate level to 5.6 g/dl. In addition, one of the intravenously phosphate-treated patients, who received a cumulative dose of 3.1 g phosphate, died of an acute myocardial infarction. Finally, autopsies were performed on six cases of phosphate-treated patients, and five of those showed features of ectopic calcification, detected mostly in the lungs, heart, and kidney [69].
Phosphate solution was also widely used as an enema, especially for the treatment of constipation and preoperative bowel preparation in children. Phosphate toxicity induced by such phosphate enemas is reported to cause tetany and respiratory and intestinal disorders [70]. For example, a 28-month-old boy who received phosphate enemas for preoperative bowel preparation developed hyperthermia, tachycardia, tachypnea, cyanosis, and tetany immediately after the administration of the phosphate-containing enema; the patient also developed respiratory failure and severe metabolic acidosis and was treated with artificial ventilation [70]. Some of these abnormal symptoms following a phosphate-containing enema can be attributed to abnormal mineral ion and electrolyte balance induced by phosphate toxicity. In a similar line of observation, when hypertonic phosphate solution was rectally administered to a 4-year-old chronically constipated girl with normal renal function, she developed phosphate toxicity (23 mg/dl) with breathing difficulties and a depressed level of consciousness [71]. In accord with the pediatric group of patients, 14 elderly patients who received a phosphate-containing enema showed significantly elevated serum phosphate levels within an hour, followed by a marked reduction of serum calcium levels by 12 h [72].
Recently, complications associated with sodium–phosphate (Na–P) treatment were compiled [73]. The 109 patients treated with Na–P compounds showed numerous complications, including electrolyte disturbances, renal failure, and colonic ulceration [73]. Furthermore, 171 cases of renal failure were reported to the U.S. Food and Drug Administration (FDA) following use of an Na–P compound [73]. In a separate retrospective study using the U.S. Food and Drug Administration Adverse Event Reporting System, analyzing 2,097,223 files from 2004 to 2009 (first 9 months), 178 patients treated with Na–P tablet preparations were reported for higher occurrence of adverse drug reactions in the kidneys [74], again suggesting the potential harmful effects of uncontrolled phosphate ingestion. Dose-dependent time-course animal studies have provided further insights in the pathomechanisms of phosphate toxicity.
Experimental phosphate toxicity
The damage inflected by phosphate toxicity can range from biochemical alterations to tumor growth [75] to mammalian aging, with the resultant effect being compromised survival [45]. Genetically engineered klotho knockout mice develop phosphate toxicity by 3 weeks of age that leads to body weight loss, kyphosis, infertility, generalized organ atrophy, and significantly reduced lifespan [43, 45, 48, 76–78]. Some of these changes that were noted in klotho knockout mice bear similarities to human aging. Extensive molecular and biochemical analysis, performed on klotho knockout mice, suggests that increased renal activity of NaPi-2a leads to increase serum retention of phosphate; importantly, genetically reducing phosphate toxicity in klotho knockout mice, by generating NaPi2a/klotho double-knockout mice, could suppress aging phenotypes [45]. Notably, the klotho knockout mice regained fertility and extended their survival when serum phosphate levels were genetically reduced, as noted in the NaPi2a/klotho double-knockout mice [45]. However, when NaPi2a/klotho double-knockout mice were fed with a high-phosphate diet, these mice lost their reproductive abilities and other aging features reappeared, clearly suggesting that diet-induced phosphate toxicity can promote the aging process. Again, when phosphate toxicity was induced in NaPi2a/klotho double-knockout mice by providing a high-phosphate diet (1.2%), features resembling premature aging, including generalized tissue atrophy and cardiovascular calcification, reappeared, and all the mice died by 15 weeks of age. Of particular significance, none of the NaPi2a/klotho double-knockout mice fed with a normal phosphate diet (0.6%) died until 20 weeks of the observational period [45]. These in vivo genetic and dietary manipulation studies clearly demonstrated that the mammalian aging process could be adversely influenced by phosphate toxicity to compromise survival. In a separate animal study, when acute phosphate toxicity (7- to 20-fold increase over control by 4 h) was induced by using a commercially available phosphate-containing enema (30–50 ml/kg), 100% mortality was noted in the experimental animals [79]. Moreover, genetically inducing phosphate toxicity could significantly impair survival of leptin-deficient obese mice [80]. Furthermore, experimental studies have shown that excessive dietary phosphate intake could increase the growth and size of lung tumors [75].
Although the pathological consequences of phosphate toxicity on organ damage and survival are obvious, the mechanism by which phosphate toxicity accelerates tissue injuries is not clear. Phosphate toxicity can exert cytotoxic effects on various organs to compromise their functionality. For instance, an increased rate of apoptosis, induced by phosphate toxicity, could be suppressed by reducing phosphate burden [45]. In fact, patients exposed to a phosphate-containing enema showed features of necrotic changes of the abdominal tissues, including loss of internal and external sphincters as a consequence of extensive tissue necrosis [81]. In a similar line of observation, when hypertonic phosphate solution was intradermally injected to rabbits, a pronounced erythema and indurations were noted by 24 h, which eventually progressed to central necrosis and full-thickness tissue loss by 5–7 days [81], implicating local erosive effects of phosphate toxicity induced by cytotoxic effects. Moreover, dietary phosphate could stimulate the AKT-mediated signaling network and can provoke an increase in lung tumorigenesis [75]. Similarly, studies have found that extracellular phosphate can induce MAPK signaling networks, and particularly can activate Erk1/2 phospho-protein to exert yet to be defined cellular functions [82]. Detailed understanding of phosphate-mediated cell signaling will help to explain the molecular mechanisms of phosphate toxicity.
Can features of phosphate toxicity appear in normophosphatemia?
Evidence from human studies
Recent studies have linked serum phosphate levels with cardiovascular disorders, including calcification. In fact, studies have found increased risk of cardiovascular diseases even when serum phosphate levels were within the upper limit of normal range [83]. In the Cholesterol and Recurrent Events (CARE) study, a relationship was established between serum phosphate levels and the rate of cardiovascular disorders [83]; in this study, the baseline serum phosphate levels were measured in 4,127 fasting participants before the incidence of myocardial infarction and followed up for a median period of 59.7 months. Participants with serum phosphate >3.5 mg/dl had a higher adjusted hazard ratio for death compared with those with serum phosphate <3.5 mg/dl. In a similar line of observation, higher serum levels of phosphate were found to be associated with increased risk of cardiac dysfunction, although the serum phosphate levels of most of the individuals were within the upper limit of normal range [83].
The Framingham Offspring Study was the first community-based examination of the association between normal-range phosphate levels and cardiovascular disorders in the general population. In this cohort, 3,368 individuals (mean age, 44 ± 10 years) without any cardiovascular or renal disorders were followed up for about 16 years [84]. Higher incidence of cardiovascular diseases was found to be associated with serum phosphate levels; individuals with 3.5 mg/dl serum phosphate levels had 1.55 times higher hazard ratios than those with levels below 2.8 mg/dl [84]. Again, it is important to note that a serum level of 3.5 mg/dl is within the accepted normal range.
In a similar line of study, an association between serum phosphate levels and the Coronary Artery Risk Development in Young Adults (CARDIA) was evaluated for 3,015 individuals (mean age, 25.2 years), with mean serum phosphate and calcium levels of 3.6 (1.3–5.7) and 9.5 (7.1–13.2) mg/dl, respectively [85]. The presence of coronary artery calcium, assessed by computed tomography 15 years later, was found to be significantly associated with serum phosphate levels. There was a clear difference between the groups with serum phosphate values of first quartile (<3.3 mg/dl) versus the fourth quartile (>3.9 mg/dl) [85]. Similarly, in a community-dwelling follow-up study of 12.6 years, higher serum levels of phosphate were shown to be associated with death among 15,732 adult participants with a mean phosphate level of 3.4 mg/dl (1–9.1) [86]. These human studies with a significant number of participants clearly suggest the risk of cardiovascular events and death even within the upper limit of normal serum phosphate levels in the general population. One important question that is not yet clearly understood is why serum phosphate levels differ in the general population, and recent genome-wide association studies have shed some light on this aspect.
Using 16,264 participants of four large cohorts with normal phosphate metabolism, a genome-wide association study found polymorphisms in seven loci with minor allele frequencies of 0.08–0.49 associated with serum phosphate levels [87]. Three loci were identified near genes encoding the NaPi-2a co-transporter, the calcium-sensing receptor (CASR), and FGF23, and the investigators concluded that these genetic variants might determine the serum phosphate levels in the general population [87]. However, additional follow-up studies are needed to validate such observations. It is important to note that in accord with the human studies, experimental studies have also shown various phosphate-induced physical, biochemical and morphological changes, despite the lack of significant changes in the serum levels.
Evidence from experimental studies
The consequences of phosphate toxicity are easier to identify when serum phosphate levels are abnormally high; however, whether certain features of phosphate toxicity might appear even in normophosphatemic conditions are not yet clear. At present, determining serum phosphate level is the gold standard to estimate the overall phosphate status of the body. In this section, we provide experimental evidence suggesting that some of the early consequences of phosphate toxicity might not be evident from serum levels of phosphate because this level does not always reflect the amount of phosphate uptake and its distribution.
The high phosphate-fed experimental animal model helped us to understand whether features of phosphate toxicity might appear even in a normophosphatemic microenvironment. In an experimental study using 8-week-old Wistar male rats fed with various amounts of phosphate (0.3%, 0.6%, 0.9%, 1.2%, or 1.5%) with a constant amount of calcium (0.6%) for 4 weeks, significantly increased urinary and fecal excretion of phosphate followed the high-phosphate diet consumption [88]. Interestingly, despite the dietary phosphate load for 4 weeks, there was no statistically significant increase in serum phosphate or calcium levels in animals fed 1.5% phosphate compared with 0.3% phosphate-fed control animals. Even though no marked changes in serum phosphate levels were noted between high and normal phosphate-fed animals, the high (1.5%) phosphate-fed rats had lesser body weight gain (58 ± 6 g/4 weeks) as compared with the control (0.3%) phosphate-fed rats (95 ± 5 g/4 weeks), clearly suggesting an adverse impact of the high-phosphate diet without marked changes in serum phosphate levels [88].
In a separate study, 1-month-old male rats (n = 30) were fed with a control diet (Ca:P = 1:1) or experimental diets of either Ca:P = 1:2 or Ca:P = 1:3 for 8 weeks. In accordance with the earlier study, a high-phosphate diet reduced growth; the rat that received Ca:P = 1:2 was 11% lighter whereas the rat that received Ca:P = 1:3 was 29% lighter than control rats fed with the Ca:P = 1:1 diet. Moreover, rats that received Ca:P = 1:3 had significantly shorter femurs, compared with rats fed Ca:P = 1:1 and Ca:P = 1:2 (P = 0.001 and 0.019, respectively) [89]. Both groups of rates with high phosphate consumption had significantly (P <0.001) lower bone mineral content (BMC) and areal bone mineral density (BMD) than control rats [89]. It is important to note that such extensive changes in the body weight and skeletal anomalies were present in spite of normal serum calcium and phosphate levels in the various groups [89], reinforcing the idea that serum phosphate levels might not always reflect phosphate burden.
It is important to note that high serum phosphate can induce compensatory hyperparathyroidism in both human and animal models [90, 91]. Experimental studies have shown that feeding animals with a high-phosphate diet can induce hyperparathyroidism, even when the serum phosphate level stays within the normal range during the first several months [92]. In an study conducted on rabbits fed with a high-phosphate diet (Ca:P = 1:7) for 1–6 months, compared with a control group that received a normal phosphate diet (Ca:P = 1:0.7), a gradual increase of serum PTH levels was noted in animals that consumed the high-phosphate diet, although serum phosphate levels in high phosphate diet-fed animals remained unchanged over the course of the first 3 months [92]. Summarizing the aforementioned experimental evidence, it is clear that the features of phosphate toxicity can appear in a normophosphatemic microenvironment.
Conclusion
Although measuring serum phosphate level is the gold standard to estimate the overall phosphate status of the body, the amount of intracellular phosphate or phosphate storage is not taken into consideration in such traditional methods of phosphate measurement. In this brief review article, we have provided both human and experimental evidence that clearly suggests that certain features of phosphate toxicity might appear even in normophosphatemic conditions, thereby exposing the limitation of serum phosphate measurements to detect early events of phosphate toxicity. We believe that tissue injuries inflicted by chronic phosphate toxicity caused by extremely high serum retention of phosphate are mostly irreversible, whereas the effects of phosphate toxicity that appear in normophosphatemic microenvironments are more likely to be reversible, or even preventable. Based on the results of human and experimental studies, it is obvious that reducing phosphate burden and maintaining phosphate balance by adequate uptake are crucial for a healthy life because phosphate toxicity can inflict irreversible organ damage that compromises the quality of life.
Acknowledgments
Some of the original research that formed the basis of this review article was performed by Drs. Mutsuko Ohnishi (MD, PhD), Shigeko Kato, (PhD), Junko Akiyoshi, (MD), Kazuyoshi Uchihashi, (MD, PhD), Khadijah Turkistani (BDS), and Yonggeun Hong (PhD) of the Department of Oral Medicine, Infection and Immunity at the Harvard School of Dental Medicine, Boston, MA, USA, and supported by a grant (R01-DK077276 to M.S. Razzaque) from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Conflict of interest None.
Contributor Information
Satoko Osuka, Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Room: 304, 188 Longwood Avenue, Boston, MA 02115, USA.
Mohammed S. Razzaque, Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Room: 304, 188 Longwood Avenue, Boston, MA 02115, USA. Department of Pathology, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan
References
- 1.Slatopolsky E, Rutherford WE, Rosenbaum R, Martin K, Hruska K. Hyperphosphatemia. Clin Nephrol. 1977;7:138–146. [PubMed] [Google Scholar]
- 2.Gaasbeek A, Meinders AE. Hypophosphatemia: an update on its etiology and treatment. Am J Med. 2005;118:1094–1101. doi: 10.1016/j.amjmed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Razzaque MS. The FGF23–klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol. 2009;5:611–619. doi: 10.1038/nrendo.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iotti S, Lodi R, Gottardi G, Zaniol P, Barbiroli B. Inorganic phosphate is transported into mitochondria in the absence of ATP biosynthesis: an in vivo 31P NMR study in the human skeletal muscle. Biochem Biophys Res Commun. 1996;225:191–194. doi: 10.1006/bbrc.1996.1152. [DOI] [PubMed] [Google Scholar]
- 5.Hutson SM, Williams GD, Berkich DA, LaNoue KF, Briggs RW. A 31P NMR study of mitochondrial inorganic phosphate visibility: effects of Ca2+, Mn2+, and the pH gradient. Biochemistry. 1992;31:1322–1330. doi: 10.1021/bi00120a007. [DOI] [PubMed] [Google Scholar]
- 6.Drezner M. Phosphorus homeostasis and related disorders. In: Bilezikian J, Raisz L, Rodan G, editors. Principles in bone biology. 2. Academic Press; New York: 2002. pp. 321–338. [Google Scholar]
- 7.Razzaque MS. FGF23-mediated regulation of systemic phosphate homeostasis: is klotho an essential player? Am J Physiol Renal Physiol. 2009;296:F470–F476. doi: 10.1152/ajprenal.90538.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Econs MJ. New insights into the pathogenesis of inherited phosphate wasting disorders. Bone (NY) 1999;25:131–135. doi: 10.1016/s8756-3282(99)00108-8. [DOI] [PubMed] [Google Scholar]
- 9.Miyamoto K, Ito M, Segawa H, Kuwahata M. Molecular targets of hyperphosphataemia in chronic renal failure. Nephrol Dial Transplant. 2003;18(suppl 3):iii79–iii80. doi: 10.1093/ndt/gfg1020. [DOI] [PubMed] [Google Scholar]
- 10.Quarles LD. FGF23, PHEX, and MEPE regulation of phosphate homeostasis and skeletal mineralization. Am J Physiol Endocrinol Metab. 2003;285:E1–E9. doi: 10.1152/ajpendo.00016.2003. [DOI] [PubMed] [Google Scholar]
- 11.Marks J, Debnam ES, Unwin RJ. Phosphate homeostasis and the renal–gastrointestinal axis. Am J Physiol Renal Physiol. 2010;299:F285–F296. doi: 10.1152/ajprenal.00508.2009. [DOI] [PubMed] [Google Scholar]
- 12.Hattenhauer O, Traebert M, Murer H, Biber J. Regulation of small intestinal Na-Pi type IIb cotransporter by dietary phosphate intake. Am J Physiol Gastrointest Liver Physiol. 1999;277:G756–G762. doi: 10.1152/ajpgi.1999.277.4.G756. [DOI] [PubMed] [Google Scholar]
- 13.Sabbagh Y, O’Brien SP, Song W, Boulanger JH, Stockmann A, Arbeeny C, et al. Intestinal Npt2b plays a major role in phosphate absorption and homeostasis. J Am Soc Nephrol. 2009;20:2348–2358. doi: 10.1681/ASN.2009050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corut A, Senyigit A, Ugur SA, Altin S, Ozcelik U, Calisir H, et al. Mutations in SLC34A2 cause pulmonary alveolar microlithiasis and are possibly associated with testicular microlithiasis. Am J Hum Genet. 2006;79:650–656. doi: 10.1086/508263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tenenhouse HS. Regulation of phosphorus homeostasis by the type IIa Na/phosphate cotransporter. Annu Rev Nutr. 2005;25:197–214. doi: 10.1146/annurev.nutr.25.050304.092642. [DOI] [PubMed] [Google Scholar]
- 16.Murer H, Forster I, Hilfiker H, Pfister M, Kaissling B, Lotscher M, et al. Cellular/molecular control of renal Na/Pi-cotransport. Kidney Int Suppl. 1998;65:S2–S10. [PubMed] [Google Scholar]
- 17.Gattineni J, Bates C, Twombley K, Dwarakanath V, Robinson ML, Goetz R, et al. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Renal Physiol. 2009;297:F282–F291. doi: 10.1152/ajprenal.90742.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohnishi M, Nakatani T, Lanske B, Razzaque MS. In vivo genetic evidence for suppressing vascular and soft-tissue calcification through the reduction of serum phosphate levels, even in the presence of high serum calcium and 1,25-dihydroxyvitamin D levels. Circ Cardiovasc Genet. 2009;2:583–590. doi: 10.1161/CIRCGENETICS.108.847814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyamoto K, Ito M, Kuwahata M, Kato S, Segawa H. Inhibition of intestinal sodium-dependent inorganic phosphate transport by fibroblast growth factor 23. Ther Apher Dial. 2005;9:331–335. doi: 10.1111/j.1744-9987.2005.00292.x. [DOI] [PubMed] [Google Scholar]
- 20.Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, et al. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010;24:3438–3450. doi: 10.1096/fj.10-154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imel EA, Econs MJ. Fibroblast growth factor 23: roles in health and disease. J Am Soc Nephrol. 2005;16:2565–2575. doi: 10.1681/ASN.2005050573. [DOI] [PubMed] [Google Scholar]
- 22.Razzaque MS, Lanske B. The emerging role of the fibroblast growth factor-23-klotho axis in renal regulation of phosphate homeostasis. J Endocrinol. 2007;194:1–10. doi: 10.1677/JOE-07-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest. 2008;118:3820–3828. doi: 10.1172/JCI36479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Razzaque MS. Does FGF23 toxicity influence the outcome of chronic kidney disease? Nephrol Dial Transplant. 2009;24:4–7. doi: 10.1093/ndt/gfn620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukagawa M, Hamada Y, Nakanishi S, Tanaka M. The kidney and bone metabolism: nephrologists’ point of view. J Bone Miner Metab. 2006;24:434–438. doi: 10.1007/s00774-006-0719-7. [DOI] [PubMed] [Google Scholar]
- 26.Kuro-o M. Overview of the FGF23–klotho axis. Pediatr Nephrol. 2010;25:583–590. doi: 10.1007/s00467-009-1260-4. [DOI] [PubMed] [Google Scholar]
- 27.Razzaque MS. Osteo-renal regulation of systemic phosphate metabolism. IUBMB Life. 2011;63:240–247. doi: 10.1002/iub.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nabeshima Y. The discovery of alpha-klotho and FGF23 unveiled new insight into calcium and phosphate homeostasis. Cell Mol Life Sci. 2008;65:3218–3230. doi: 10.1007/s00018-008-8177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Razzaque MS. Phosphate toxicity: new insights into an old problem. Clin Sci (Lond) 2011;120:91–97. doi: 10.1042/CS20100377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juppner H. Phosphate and FGF-23. Kidney Int. 2011;79(suppl 121):S24–S27. doi: 10.1038/ki.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamashita T. Structural and biochemical properties of fibroblast growth factor 23. Ther Apher Dial. 2005;9:313–318. doi: 10.1111/j.1744-9987.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 32.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005;16:107–137. doi: 10.1016/j.cytogfr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature (Lond) 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 36.Liu S, Vierthaler L, Tang W, Zhou J, Quarles LD. FGFR3 and FGFR4 do not mediate renal effects of FGF23. J Am Soc Nephrol. 2008 doi: 10.1681/ASN.2007121301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuro-o M. Klotho as a regulator of fibroblast growth factor signaling and phosphate/calcium metabolism. Curr Opin Nephrol Hypertens. 2006;15:437–441. doi: 10.1097/01.mnh.0000232885.81142.83. [DOI] [PubMed] [Google Scholar]
- 38.Medici D, Razzaque MS, Deluca S, Rector TL, Hou B, Kang K, et al. FGF-23-klotho signaling stimulates proliferation and prevents vitamin D-induced apoptosis. J Cell Biol. 2008;182:459–465. doi: 10.1083/jcb.200803024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extra-cellular domain of klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA. 2007;104:19796–19801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242:626–630. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 41.Nakatani T, Bara S, Ohnishi M, Densmore MJ, Taguchi T, Goetz R, et al. In vivo genetic evidence of klotho-dependent functions of FGF23 in regulation of systemic phosphate homeostasis. FASEB J. 2009;23:433–441. doi: 10.1096/fj.08-114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Razzaque MS. Therapeutic potential of klotho–FGF23 fusion polypeptides: WO2009095372. Expert Opin Ther Pat. 2010;20:981–985. doi: 10.1517/13543771003774100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakatani T, Ohnishi M, Razzaque MS. Inactivation of klotho function induces hyperphosphatemia even in presence of high serum fibroblast growth factor 23 levels in a genetically engineered hypophosphatemic (Hyp) mouse model. FASEB J. 2009;23:3702–3711. doi: 10.1096/fj.08-123992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, Sorenson AH, et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest. 2007;117:2684–2691. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohnishi M, Razzaque MS. Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. FASEB J. 2010;24:3562–3571. doi: 10.1096/fj.09-152488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. The ADHR Consortium. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 47.Gribaa M, Younes M, Bouyacoub Y, Korbaa W, Ben Charfeddine I, Touzi M, et al. An autosomal dominant hypophosphatemic rickets phenotype in a Tunisian family caused by a new FGF23 missense mutation. J Bone Miner Metab. 2010;28:111–115. doi: 10.1007/s00774-009-0111-5. [DOI] [PubMed] [Google Scholar]
- 48.Ohnishi M, Nakatani T, Lanske B, Razzaque MS. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D1 alpha-hydroxylase. Kidney Int. 2009;75:1166–1172. doi: 10.1038/ki.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Imanishi Y, Hashimoto J, Ando W, Kobayashi K, Ueda T, Nagata Y, et al. Matrix extracellular phosphoglycoprotein is expressed in causative tumors of oncogenic osteomalacia. J Bone Miner Metab. 2011 doi: 10.1007/s00774-011-0290-8. [DOI] [PubMed] [Google Scholar]
- 50.Kitaoka T, Namba N, Miura K, Kubota T, Ohata Y, Fujiwara M, et al. Decrease in serum FGF23 levels after intravenous infusion of pamidronate in patients with osteogenesis imperfecta. J Bone Miner Metab. 2011;29:598–605. doi: 10.1007/s00774-011-0262-z. [DOI] [PubMed] [Google Scholar]
- 51.Benet-Pages A, Orlik P, Strom TM, Lorenz-Depiereux B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet. 2005;14:385–390. doi: 10.1093/hmg/ddi034. [DOI] [PubMed] [Google Scholar]
- 52.Sun Y, Wang O, Xia W, Jiang Y, Li M, Xing X, et al. FGF23 analysis of a Chinese family with autosomal dominant hypophosphatemic rickets. J Bone Miner Metab. 2011 doi: 10.1007/s00774-011-0285-5. [DOI] [PubMed] [Google Scholar]
- 53.Bai X, Miao D, Li J, Goltzman D, Karaplis AC. Transgenic mice overexpressing human fibroblast growth factor 23(R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology. 2004;145:5269–5279. doi: 10.1210/en.2004-0233. [DOI] [PubMed] [Google Scholar]
- 54.Sitara D, Razzaque MS, Hesse M, Yoganathan S, Taguchi T, Erben RG, et al. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 2004;23:421–432. doi: 10.1016/j.matbio.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DeLuca S, Sitara D, Kang K, Marsell R, Jonsson K, Taguchi T, et al. Amelioration of the premature aging-like features of Fgf-23 knockout mice by genetically restoring the systemic actions of FGF-23. J Pathol. 2008;216:345–355. doi: 10.1002/path.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Razzaque MS. Does renal ageing affect survival? Ageing Res Rev. 2007;6:211–222. doi: 10.1016/j.arr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 57.Taguchi T, Nazneen A, Al-Shihri AA, Turkistani KA, Razzaque MS. Heat shock protein 47: a novel biomarker of phenotypically altered collagen-producing cells. Acta Histochem Cytochem. 2011;44:35–41. doi: 10.1267/ahc.11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Razzaque MS, Taguchi T. Cellular and molecular events leading to renal tubulointerstitial fibrosis. Med Electron Microsc. 2002;35:68–80. doi: 10.1007/s007950200009. [DOI] [PubMed] [Google Scholar]
- 59.Taguchi T, Razzaque MS. The collagen-specific molecular chaperone HSP47: is there a role in fibrosis? Trends Mol Med. 2007;13:45–53. doi: 10.1016/j.molmed.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 60.Orrego JJ, Sheehan M. Hyperphosphatemia. Endocr Pract. 2010;16:524–525. doi: 10.4158/EP.16.3.524. [DOI] [PubMed] [Google Scholar]
- 61.Blokker M. Hyperphosphatemia and its treatment. 1983. CANNT J. 2008;18:26–27. [PubMed] [Google Scholar]
- 62.Baker WL. Hypophosphatemia. Am J Nurs. 1985;85:998–1003. [PubMed] [Google Scholar]
- 63.Malluche HH, Monier-Faugere MC. Hyperphosphatemia: pharmacologic intervention yesterday, today and tomorrow. Clin Nephrol. 2000;54:309–317. [PubMed] [Google Scholar]
- 64.Knochel JP. Hypophosphatemia. West J Med. 1981;134:15–26. [PMC free article] [PubMed] [Google Scholar]
- 65.Huybers S, Bindels RJ. Vascular calcification in chronic kidney disease: new developments in drug therapy. Kidney Int. 2007;72:663–665. doi: 10.1038/sj.ki.5002477. [DOI] [PubMed] [Google Scholar]
- 66.Razzaque MS. The dualistic role of vitamin D in vascular calcifications. Kidney Int. 2011;79:708–714. doi: 10.1038/ki.2010.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goldsmith DJ, Covic A, Sambrook PA, Ackrill P. Vascular calcification in long-term haemodialysis patients in a single unit: a retrospective analysis. Nephron. 1997;77:37–43. doi: 10.1159/000190244. [DOI] [PubMed] [Google Scholar]
- 68.Razzaque MS, St-Arnaud R, Taguchi T, Lanske B. FGF-23, vitamin D and calcification: the unholy triad. Nephrol Dial Transplant. 2005;20:2032–2035. doi: 10.1093/ndt/gfh991. [DOI] [PubMed] [Google Scholar]
- 69.Goldsmith RS, Ingbar SH. Inorganic phosphate treatment of hypercalcemia of diverse etiologies. N Engl J Med. 1966;274:1–7. doi: 10.1056/NEJM196601062740101. [DOI] [PubMed] [Google Scholar]
- 70.Everman DB, Nitu ME, Jacobs BR. Respiratory failure requiring extracorporeal membrane oxygenation after sodium phosphate enema intoxication. Eur J Pediatr. 2003;162:517–519. doi: 10.1007/s00431-002-0987-8. [DOI] [PubMed] [Google Scholar]
- 71.Marraffa JM, Hui A, Stork CM. Severe hyperphosphatemia and hypocalcemia following the rectal administration of a phosphate-containing Fleet pediatric enema. Pediatr Emerg Care. 2004;20:453–456. doi: 10.1097/01.pec.0000132217.65600.52. [DOI] [PubMed] [Google Scholar]
- 72.Grosskopf I, Graff E, Charach G, Binyamin G, Spinrad S, Blum I. Hyperphosphataemia and hypocalcaemia induced by hypertonic phosphate enema–an experimental study and review of the literature. Hum Exp Toxicol. 1991;10:351–355. doi: 10.1177/096032719101000509. [DOI] [PubMed] [Google Scholar]
- 73.Belsey J, Epstein O, Heresbach D. Systematic review: adverse event reports for oral sodium phosphate and polyethylene glycol. Aliment Pharmacol Ther. 2009;29:15–28. doi: 10.1111/j.1365-2036.2008.03837.x. [DOI] [PubMed] [Google Scholar]
- 74.Ehrenpreis ED, Parakkal D, Semer R, Du H. Renal risks of sodium phosphate tablets for colonoscopy preparation: a review of adverse drug reactions reported to the US Food and Drug Administration. Colorectal Dis. 2011;13:e270–e275. doi: 10.1111/j.1463-1318.2011.02679.x. [DOI] [PubMed] [Google Scholar]
- 75.Jin H, Xu C-X, Lim H-T, Park S-J, Shin J-Y, Chung Y-S, et al. High dietary inorganic phosphate increases lung tumorigenesis and alters Akt signaling. Am J Respir Crit Care Med. 2009;179:59–68. doi: 10.1164/rccm.200802-306OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nabeshima Y. Klotho: a fundamental regulator of aging. Ageing Res Rev. 2002;1:627–638. doi: 10.1016/s1568-1637(02)00027-2. [DOI] [PubMed] [Google Scholar]
- 77.Kuro-o M. Disease model: human aging. Trends Mol Med. 2001;7:179–181. doi: 10.1016/s1471-4914(01)01921-9. [DOI] [PubMed] [Google Scholar]
- 78.Nabeshima Y. Toward a better understanding of Klotho. Sci Aging Knowledge Environ. 2006;2006:pe11. doi: 10.1126/sageke.2006.8.pe11. [DOI] [PubMed] [Google Scholar]
- 79.Martin RR, Lisehora GR, Braxton M, Jr, Barcia PJ. Fatal poisoning from sodium phosphate enema. Case report and experimental study. JAMA. 1987;257:2190–2192. [PubMed] [Google Scholar]
- 80.Ohnishi M, Kato S, Razzaque MS. Genetic induction of phosphate toxicity significantly reduces the survival of hyper-cholesterolemic obese mice. Biochem Biophys Res Commun. 2011;415:434–438. doi: 10.1016/j.bbrc.2011.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pietsch JB, Shizgal HM, Meakins JL. Injury by hypertonic phosphate enema. Can Med Assoc J. 1977;116:1169–1170. [PMC free article] [PubMed] [Google Scholar]
- 82.Yamazaki M, Ozono K, Okada T, Tachikawa K, Kondou H, Ohata Y, et al. Both FGF23 and extracellular phosphate activate Raf/MEK/ERK pathway via FGF receptors in HEK293 cells. J Cell Biochem. 2010;111:1210–1221. doi: 10.1002/jcb.22842. [DOI] [PubMed] [Google Scholar]
- 83.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 84.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D’Agostino RB, Sr, Gaziano JM, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879–885. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 85.Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA. Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol. 2009;20:397–404. doi: 10.1681/ASN.2008020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Foley RN, Collins AJ, Ishani A, Kalra PA. Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2008;156:556–563. doi: 10.1016/j.ahj.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 87.Kestenbaum B, Glazer NL, Kottgen A, Felix JF, Hwang SJ, Liu Y, et al. Common genetic variants associate with serum phosphorus concentration. J Am Soc Nephrol. 2010;21:1223–1232. doi: 10.1681/ASN.2009111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tani Y, Sato T, Yamanaka-Okumura H, Yamamoto H, Arai H, Sawada N, et al. Effects of prolonged high phosphorus diet on phosphorus and calcium balance in rats. J Clin Biochem Nutr. 2007;40:221–228. doi: 10.3164/jcbn.40.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huttunen MM, Tillman I, Viljakainen HT, Tuukkanen J, Peng Z, Pekkinen M, et al. High dietary phosphate intake reduces bone strength in the growing rat skeleton. J Bone Miner Res. 2007;22:83–92. doi: 10.1359/jbmr.061009. [DOI] [PubMed] [Google Scholar]
- 90.Calvo MS. Dietary phosphorus, calcium metabolism and bone. J Nutr. 1993;123:1627–1633. doi: 10.1093/jn/123.9.1627. [DOI] [PubMed] [Google Scholar]
- 91.Kemi VE, Karkkainen MU, Rita HJ, Laaksonen MM, Outila TA, Lamberg-Allardt CJ. Low calcium:phosphorus ratio in habitual diets affects serum parathyroid hormone concentration and calcium metabolism in healthy women with adequate calcium intake. Br J Nutr. 2010;103:561–568. doi: 10.1017/S0007114509992121. [DOI] [PubMed] [Google Scholar]
- 92.Bai RJ, Cheng XG, Yan D, Qian ZH, Li XM, Qu H, et al. Rabbit model of primary hyperparathyroidism induced by high-phosphate diet. Domest Anim Endocrinol. 2011 doi: 10.1016/j.domaniend.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 93.Bacchetta J, Salusky IB. Evaluation of hypophosphatemia: lessons from patients with genetic disorders. Am J Kidney Dis. 2011 doi: 10.1053/j.ajkd.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]