Abstract
The age-related loss of anti-oxidant defense reduces recovery from myocardial ischemia/reperfusion injury (MI/R) in aged people. Our previous data showed that inactivation of thioredoxin (Trx) was involved in enhanced aging MI/R injury. Thioredoxin reductase (TrxR), the enzyme known to regulate Trx, is less efficient with age. The aim of the current study was to determine why TrxR activity was reduced and whether reduced TrxR activity contributed to enhanced aging MI/R injury. Both Trx and TrxR activity were decreased in the aging heart, and this difference was further amplified after MI/R. However, MI/R injury did not change TrxR expression between young and aging rats. Increased nitrogen oxide (NOx) but decreased nitric oxide (NO) bioavailability (decreased phosphorylated vasodilator-stimulated phosphoprotein) was observed in aging hearts. Peroxynitrite (ONOO−) was increased in aging hearts and was further amplified after MI/R. TrxR nitration in young and aging hearts was detected by immunoprecipitation (anti-nitrotyrosine) followed by immunoblotting (anti-TrxR). Compared with young hearts, TrxR nitration was increased in the aging hearts, and this was further intensified after MI/R. The ONOO− decomposition catalyst (FeTMPyp) reduced TrxR nitration and increased TrxR and Trx activity. More importantly, FeTMPyp attenuated the MI/R injury in aging hearts as evidenced by decreased caspase-3 and malondialdehyde (MDA) concentration and increased cardiac function. Increased ONOO− nitrated TrxR in the aging heart as a post-translational modification, which may be related to the enhanced MI/R injury of aging rats. Interventions that inhibit nitration and restore TrxR activity might be a therapy for attenuating enhanced MI/R injury in aging heart.
Introduction
The incidence and prevalence of ischemic heart disease increases progressively with age, and both developed and developing countries are faced with an increasingly aging population.1 Not only is there an increased ischemia/reperfusion injury related risk factor with aging, but there is also the possibility of a loss of endogenous protection against myocardial ischemia/reperfusion (MI/R) injury.2 However, the mechanisms responsible for this age-related loss of endogenous protection remain elusive.
Reactive oxygen species (ROS) have long been recognized to act as the major mediator of the aging process and to play critical roles in increased susceptibility of post-ischemic myocardial apoptosis in aging.3,4 However, the outcome of clinical trials with anti-oxidant treatment during myocardial ischemia and reperfusion has been rather disappointing,5 suggesting that other factors in addition to ROS exist. In aging hearts, high levels of nitric oxide (NO) are converted to a number of more reactive derivatives, known collectively as reactive nitrogen species (RNS).6 Peroxynitrite (ONOO−), an extremely important RNS, is a highly reactive species and oxidizes or nitrates (e.g., tyrosine nitration) a variety of molecules, and this has been shown to induce cardiomyocyte death.7 The glutathione (GSH) anti-oxidant system has been demonstrated to be a defense against ONOO−. In addition to the GSH system, most recent studies suggest that the thioredoxin (Trx) system works with the GSH system in a parallel fashion in anti-oxidant defense mechanisms.8 Our previous work reported that Trx activity is decreased in the aging heart by post-transcriptional nitrated modification, which is involved in aging-enhanced MI/R injury.9 Besides the nitrated modification, there were many reasons contributing to inactivation of Trx. Growing evidence has shown that the Trx reductase (TrxR) was found as the key enzyme known to maintain the activity of Trx.10 Mutations in TrxR genes have been identified as causing dilated cardiomyopathy, and new reports showed that TrxR exerts a crucial function during post-ischemic reperfusion.11,12 In 2006, Rohrbach et al. reported that the activity of TrxR was decreased in the aging heart. Meanwhile, decreased TrxR expression enhanced the susceptibility to apoptotic stimuli in in vitro experiments.13 However, why TrxR was inactivated and whether this alteration may involve in aging MI/R injury requires further investigation.
In vitro studies showed that the hexavalent chromium [Cr(VI)]-mediated inhibition of TrxR continues after it is removed, suggesting the modification of TrxR is not easily reversed. Cr(VI) causes increased ONOO− generation, which results in nitrotyrosine modifications to proteins.14,15 High levels of ONOO− exist in the aging heart and might nitrate some proteins, such as TrxR. However, there is a lack of direct evidence demonstrating that TrxR was nitrated and that the nitrated TrxR contributed to its inactivation.
Therefore, the aims of the present study were to: (1) Determine whether TrxR was inactivated in the aging heart subjected to MI/R injury; (2) study whether the inactivation of TrxR was related to its nitration with the high concentration of ONOO− in the aging heart subjected to MI/R injury; and (3) investigate whether treatment with FeTMPyp (ONOO− decomposition catalyst) reduced the nitrated TrxR and attenuated the MI/R injury in aging rats.
Materials and Methods
Animals
All of the investigations conformed to the “Guiding Principles in the Use and Care of Animals” published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996) and approved by the Institutional Animal Care and Use Committee of Capital Medical University. We obtained 6- to 8-month-old (young) and 20- to 24-month old (aging) male Sprague–Dawley rats for the experiment.
Experimental protocol
Sprague–Dawley rats were anesthetized with chloral hydrate (400 mg/kg). Myocardial ischemia was produced by exteriorizing the heart via a left thoracic incision and occluding the left coronary artery (LCA) with a 6-0 silk slipknot. After 30 min of ischemia, the slipknot was released and the myocardium was reperfused for 3 hr. The sham-operated control rats (sham) underwent the same surgical procedures except that the LCA was not occluded. Animals were randomized to treat with vehicle (saline) or FeTMPyp (Cayman Chemical, CAS 133314-07-5) via intraperitoneal injection 10 min prior to reperfusion.
Determination of myocardial ischemia/reperfusion-related injury
Determination of ventricular function
Left ventricular (LV) function was continuously monitored via a MillarMikro-Tip catheter pressure transducer inserted into the LV via the left carotid artery (Powerlab Hardware; AD Instruments, Charlotte, NC). Left ventricular end diastolic pressure (LVEDP), left ventricular systolic pressure (LVSP), and maximal rate of rise/decrease of left ventricular pressure (±dp/dtmax) were derived by computer algorithms.
Determination of caspase-3 protease activity
The substrate Ac-DEVD-AFC (Enzo Life Sciences) was used to determine caspase-3 activity according to the manufacturer's instructions. In brief, 30 mg of myocardial tissue blocks were homogenized in ice-cold lysis buffer and centrifuged at 12,000 rpm for 10 min at 4°C. A 50-μL amount of supernatant was then incubated with buffer containing 10 mM dithiothreitol (DTT) and 5 μL Ac-DEVD-AFC (the final concentration was 200 μM) at 37°C for 1.5 hr. Activity of caspase-3 was determined using a fluorescent microplate reader (BIOTEK, FL-600) at Ex 400 nm, Em 508 nm, and the results were expressed as -fold of the Sham/Vehicle group.
Measurement of malondialdehyde concentration
Malondialdehyde (MDA) concentration is a widely used method to analyze lipid peroxidation in biological material. Cardiac homogenate (10%, wt/vol) was prepared with 0.1 M phosphate-buffered saline (PBS) and centrifuged at 1200×g for 10 min. The supernatant was used to determine MDA concentration with commercially available kits (Nanjing Jiancheng Bioengineering Institute, China).
Detection of total NO and nitrotyrosine content in cardiac tissue
NO has a short half-life and is oxidized to form NO2 and NO3 in vivo. Thus, the detection of NOx (NO·+NO2+NO3) concentration has been demonstrated to reflect total NO formation in vivo. Cardiac tissue samples from risk areas were rinsed and homogenized in deionized water (10%, wt/vol) and centrifuged at 12000 rpm for 10 min. The tissue NO and its in vivo metabolic products (NO2 and NO3) in the supernatant, collectively known as NOx, were determined using a chemiluminescence NO detector (SIEVER 280i NO Analyzer; Ionics Instruments, Boulder, CO), as described in our previous study.9 Nitrotyrosine content has been extensively used by many investigators as a marker for ONOO−, which was determined using an enzyme-linked immunosorbent assay (ELISA) method described in our previous publication.9
Trx and TrxR activity assay
The insulin disulfide reduction assay was used to detect the Trx activity. In brief, 50 μg of cellular protein extracts were pre-incubated at 37°C for 15 min with 2 μL activation buffer (100 mM HEPES, 2 mM EDTA, 1 mg/mL bovine serum albumin [BSA], and 2 mM DTT) to reduce Trx. After addition of 20 μL reaction buffer (100 mM HEPES, 2.0 mM EDTA, 0.2 mM nicotinamide adenine dinucleotide phosphate [NADPH], and 140 μM insulin), the reaction was started by the addition of mammalian Trx reductase (1 μL, 15 mU, Sigma) or water to controls and samples incubated for 30 min at 37°C. The reaction was terminated by adding 125 μL of stopping solution (0.2 M Tris-Cl, 10 M guanidine-HCl, and 1.7 mM 3-carboxy-4-nitrophenyl disulfide, 1mM dinitrothiocyanobenzene (DTNB) followed by absorption measurement at 412 nm. Myocardium supernatants were normalized for total protein content via Bradford assay before determination of TrxR activity by a TrxR activity assay kit (ab83463, Abcam) according to the manufacturer's instructions.
Detection of TrxR protein expression and TrxR nitration
Western blotting was used for detecting TrxR1 and TrxR2 proteins, as described previously.2 Briefly, 80 μg of protein was loaded, and the primary antibodies used were mouse anti-TXNRD1 (ab16847, Abcam) and rabbit anti-TXNRD2 (ab58445, Abcam). The density of the scanned protein bands was measured by image analysis software, and the results were presented as percentage change of the loading control. Immunoprecipitation and immunoblotting were performed by using a procedure described by Ischiropoulos et al.16 Nitrotyrosine was immunoprecipitated with a monoclonal anti-murine 3-nitrotyrosine antibody (ab61392, Abcam). After sample separation, TrxR nitration was detected with a monoclonal antibody against TrxR1 and TrxR2. The blot was developed with Supersignal-Western reagent (Pierce) and visualized with a Kodak Image Station 400.
Statistical analysis
All data were described as mean±standard error of the mean (SEM). Statistical analysis was performed with SPSS 15.0 programs. The t-test was used to compare two independent sample means, and one-way analysis of variance (ANOVA) was used for comparing means of more than two samples. All aging and young rats were randomly assigned to different experiments. A p value of<0.05 was considered to be statistically significant.
Results
Aging rat hearts were more susceptible to myocardial ischemia/reperfusion injury
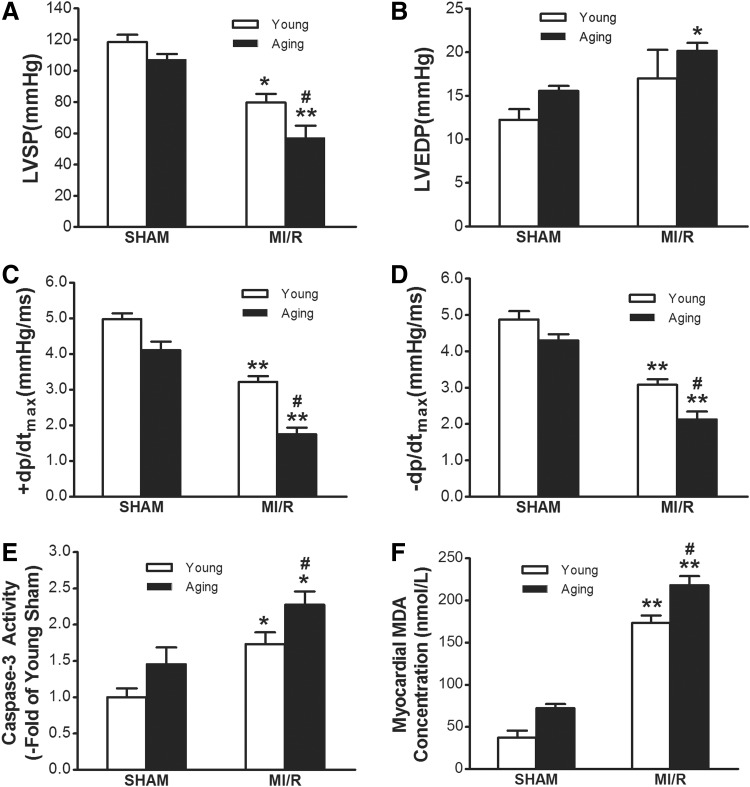
Consistent with previously published results by us and other investigators, aging rat hearts showed more susceptibility to myocardial ischemia/reperfusion injury as evidenced by exaggerated cardiac dysfunction, increased apoptosis and MDA concentration9,17 (Fig. 1).
FIG. 1.
Aging rat hearts were more susceptible to myocardial ischemia/reperfusion injury. (A) Comparison of left ventricular systolic pressure (LVSP) in both the young rats and aging rats after myocardial ischemia/reperfusion (MI/R). (B) Left ventricular end diastolic pressure (LVDEP). (C and D) Maximal rate of rise/decrease of left ventricular pressure (±dp/dtmax). (E) MI/R-induced cardiomyocytes apoptosis with aging. Activity of caspase-3 was determined at the end of re-perfusion. (F) Malondialdehyde (MDA) concentration. MI/R indicates myocardial ischemia for 30 min and reperfusion for 3 hr. The Sham group underwent the same surgical procedures except that the left coronary artery was not occluded. Data are expressed as mean±standard error of the mean (SEM) (n=6–8 each). (*) p<0.05, (**) p<0.01 vs. Sham; (#) p<0.05 vs. Young.
Age-related TrxR activity was decreased in rat hearts subjected to MI/R
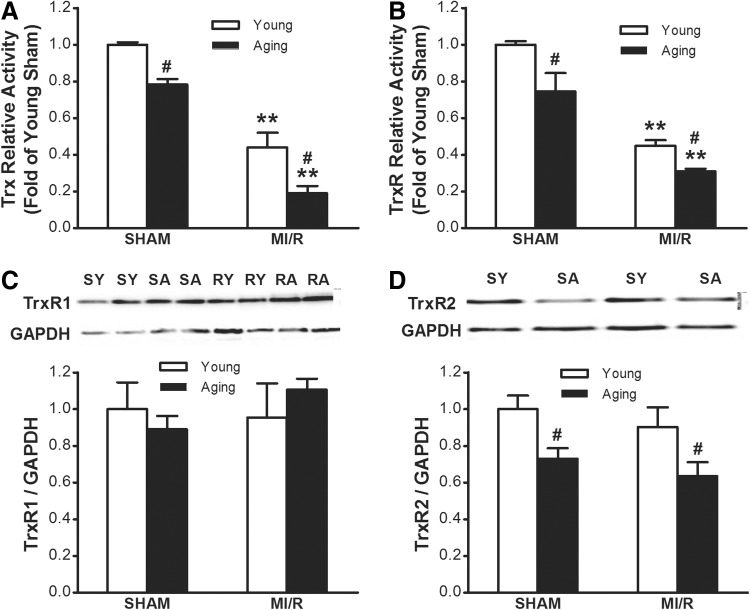
Accumulating evidence indicates that the Trx/TrxR system participates in limiting MI/R, apoptosis, and infarct size.11,18 As summarized in Fig. 2A, compared with young rats, cardiac Trx activity showed a gradual decrease during aging, and this difference was further amplified after MI/R. TrxR was the only enzyme known to catalyze the reduction of Trx and hence is a central component in the Trx system. Thus, TrxR activity was determined. It was shown that cardiac TrxR activity in aging rats was lower than that in the young rats. Moreover, the TrxR activity decreased in aging hearts after MI/R and was lower than that in young adult hearts subjected to MI/R (Fig. 2B). Two major kinds of TrxR protein expression, including TrxR1 and TrxR2, were detected. As shown in Fig. 2C, there was no statistical significance of cardiac TrxR1 protein expression between young and aging rats, whereas TrxR2 protein expression was decreased in Fig. 2D. Moreover, MI/R injury did not change both TrxR1 and TrxR2 expression between the young and aging groups.
FIG. 2.
Age reduced efficiency of thioredoxin (Trx) system in the myocardium. (A and B) Both Trx activity and thioredoxin reductase (TrxR) activity were significantly reduced in the aging rat heart either before or after myocardial ischemia/reperfusion (MI/R). (C) Age-dependent differences in TrxR1 protein expression. (D) Age-dependent differences in TrxR2 protein expression. Densitometry analysis for expression of TrxR1 and TrxR2 protein by western blot (n=8 each). Bar heights represent mean values and brackets indicate standard error of the mean (SEM). (*) p<0.05, (**) p<0.01 vs. Sham; (#) p<0.05 vs. Young. SY, Sham young; SA, Sham aged; RY, reperfused young; RA, reperfused aged.
Production of ONOO− was increased in aging hearts subjected to MI/R
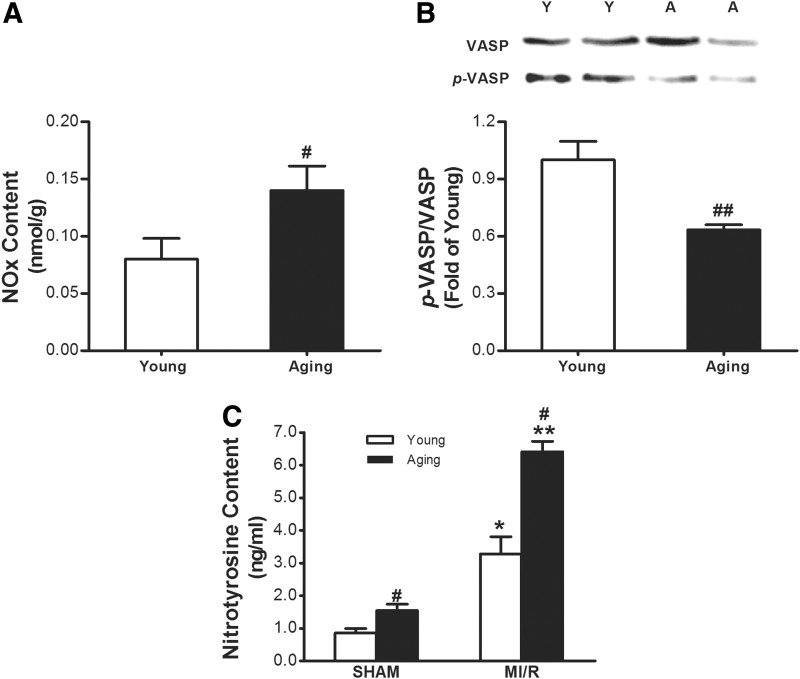
A large number of studies suggest that markedly increased NO and decreased bioavailability are considered to be one of the most important mechanisms that results in age-related cardiac dysfunction.19 Concentrations of NOx (NOx=NO·+NO2+NO3) were measured by using the previously reported vanadium reduction method.9 Our results showed that young adult hearts had a low basal NOx, and aged hearts had a markedly increased basal NOx compared to young adult hearts (Fig. 3A). In vivo vasodilator-stimulated phosphoprotein (VASP) phosphorylation reflected NO bioavailability.20 As illustrated in Fig. 3B, our data demonstrated that although total VASP levels are comparable in both groups, phosphorylated VASP (p-VASP) is markedly reduced in heart tissues isolated from aged rats. Therefore, we believed increased NO contributed to the production of ONOO− with higher superoxide generation in aging. The detection of tissue nitrotyrosine content has been extensively used by many investigators as a marker for ONOO−. As illustrated in Fig. 3C, more nitrotyrosine was detected in myocardial tissue from aging rats compared with young adult rats. Moreover, ischemia/reperfusion-induced overproduction of ONOO− was further amplified in the aging heart.
FIG. 3.
Aging enhances nitrative stress in the heart subjected to myocardial ischemia/reperfusion (MI/R). (A) Total NOx production was increased in the aging heart. (B) Phosphorylated vasodilator-stimulated phosphoprotein (p-VASP) was reduced in heart tissues isolated from aged rat. (C) Nitrotyrosine content, as a marker for ONOO−, was increased in aging rat heart either before or after MI/R. (*) p<0.05, (**) p<0.01 vs. Sham; (#) p<0.05, (##) p<0.01 vs. Young.
Cardiac TrxR was nitrated in aging rats, which was involved in its inactivation
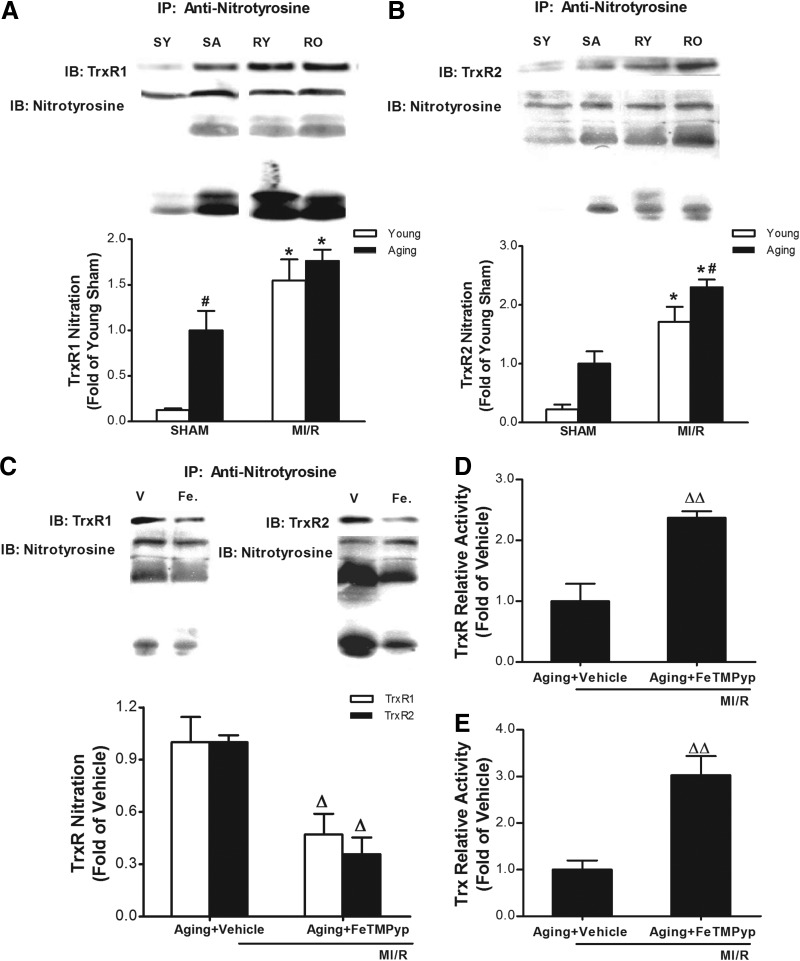
Protein nitration has been shown to be a post-translational protein modification process. We wanted to determine whether reduced TrxR activity observed in the aging heart is caused by increased nitrative stress and subsequent TrxR nitration. First, the TrxR nitration in young and aging hearts was detected by immunoprecipitation (anti-nitrotyrosine) followed by immunoblotting (anti-TrxR). As illustrated in Fig. 4, A and B, nitrated TrxR was not detected in myocardial tissue from a young adult heart, but a clear nitrated band was detected in the hearts of aged rats. Moreover, aging-induced TrxR nitration was further intensified after ischemia/reperfusion. We then determined whether the nitrative modification resulted in TrxR inactivation. Treatment with the peroxynitrite decomposition catalyst FeTMPyp blocked TrxR nitration (Fig. 4C) and attenuated its activity (Fig. 4D). Meanwhile, FeTMPyp restored Trx activity in the aging heart subjected to MI/R (Fig. 4E).
FIG. 4.
The inactivation of thioredoxin reductase (TrxR) was related to its nitration. TrxR nitration in young and aging hearts was detected by immunoprecipitation (anti-nitrotyrosine) followed by immunoblotting (anti-TrxR). Nitration of TrxR1 (A) and TrxR2 (B) in the aging heart subjected to myocardial ischemia/reperfusion (MI/R). The peroxynitrite decomposition catalyst FeTMPyp decreased the nitration of TrxR1 (C) and attenuated its activity (D). (E) Treatment with FeTMPyp attenuated Trx activity in the aging heart subjected to MI/R. Ten minutes before reperfusion, aging rats were randomized to receive vehicle (saline) or FeTMPyp via intra-peritoneal injection (IP). (*) p<0.05 vs. Sham; (#) p<0.05 vs. Young; (Δ) p<0.05, (ΔΔ) p<0.01 vs. Vehicle. IB, immunoblot; SY, Sham young; SA, Sham aging; RY, reperfused young; RO, reperfused old; V, vehicle; Fe, FeTMPyp.
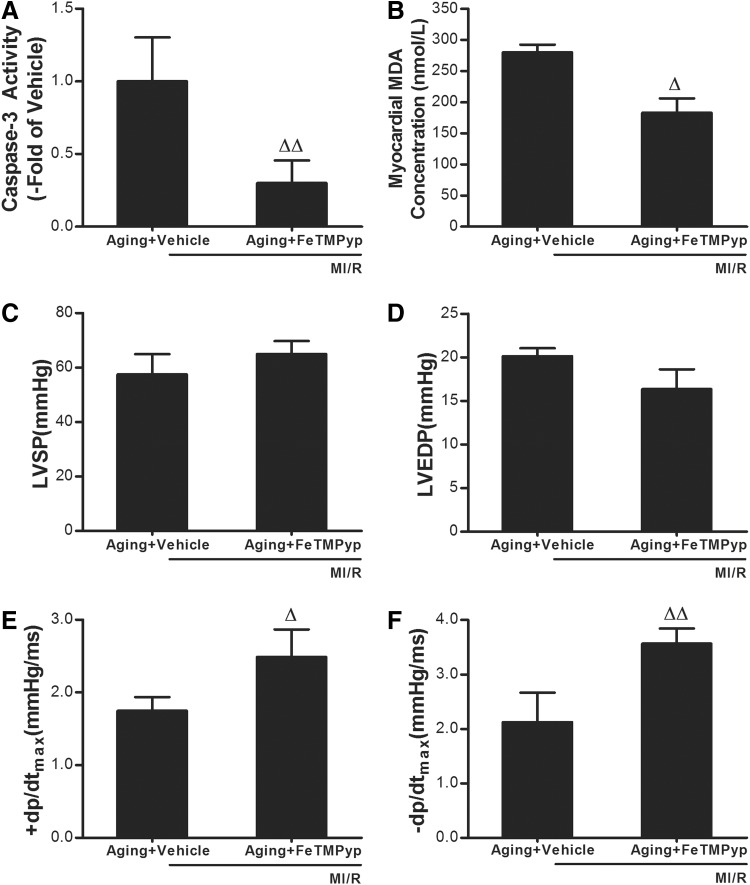
Treatment with the peroxynitrite decomposition catalyst FeTMPyp exerted significant cardiac protective effects against MI/R injury in the aging heart
The data above show that MI/R caused more TrxR nitration in the aging heart, so we then sought to determine whether the TrxR nitration induced more serious aging post-ischemic myocardial injury. As shown in Fig. 5, A and B, FeTMPyp treatment significantly attenuated caspase-3 activity and MDA concentration in the aging heart subjected to MI/R. To determine whether the cardioprotective effects of FeTMPyp were sustained, aging rats were subjected to 30 min of ischemia and 3 h of reperfusion, and the effect of FeTMPyp on cardiac function was determined. As summarized in Fig. 5, C and D, treatment with FeTMPyp restored±dp/dtmax, but not LVSP and LVEDP.
FIG. 5.
FeTMPyp attenuated the myocardial ischemia/reperfusion (MI/R) injury in the aging rats. Effect of FeTMPyp on caspase-3 activity (A) and malondialdehyde (MDA) concentration (B) in the aging heart subjected to MI/R. (C) Effects of FeTMPyp on left ventricular systolic pressure (LVSP) in the aging heart subjected to MI/R. (D) Left ventricular end diastolic pressure (LVDEP). (E and F) Maximal rate of rise/decrease of left ventricular pressure (±dp/dtmax). Ten minutes before reperfusion, rats were randomized to receive vehicle (saline) or FeTMPyp via intraperitoneal injection (IP). Data expressed as mean±standard error of the mean (SEM) (n=8 each). (Δ) p<0.05, (ΔΔ) p<0.01 vs. Vehicle.
Discussion
Baseline cardiac function declines with aging. When the aged heart is exposed to various forms of stress, an amplification of MI/R injury is observed. Higher oxidative stress and lower oxidation defense capabilities are involved in amplified aging MI/R injury. In the present study, we defined for the first time that the enzyme responsible for protein thiol oxidation repair, TrxR, becomes less efficient in the aging heart and even much less efficient after MI/R. Moreover, we have demonstrated that it was the post-translational nitrative modification of TrxR that is responsible for this reduced TrxR activity in the aging heart subjected to MI/R. Finally, we have demonstrated that treatment with a peroxynitrite decomposition catalyst shortly before reperfusion blocked nitrative TrxR inactivation–attenuated aging MI/R injury. Taken together, these results provide strong evidence that nitrative TrxR inactivation might be involved in enhanced aging MI/R injury.
Excessive formation of ROS during post-ischemic reperfusion significantly contributes to tissue injury and functional deterioration of the heart. MI/R injury has been shown to be attenuated by endogenous ROS scavenging systems, such as mitochondrial manganese superoxide dismutase, detoxifying O2, and catalase or GSH peroxidase.20 Growing evidence showed that besides the GSH system, the Trx system, including Trx, TrxR, Trx peroxidase, and NADPH, was a ubiquitous thiol oxidoreductase system that regulates cellular reduction/oxidation (redox) status and cell proliferation/cell survival.21
In 2007, our previous study reported that aging-induced Trx inactivation was one of the causes of enhanced aging MI/R injury.9 However, Trx is directly dependent on TrxRs, which reduce the active site of Trx from the disulfide form to the biologically active dithiol form. TrxR inhibition enhances oxidant susceptibility and favors apoptosis. TrxR was markedly up-regulated in cancer tissues, and molecules that inhibit TrxR promote apoptosis and reduce cancer development.22
Our current results illustrate that the activity of TrxR was decreased in the aging heart, which might contribute to Trx inactivation. In mammals, there are three TrxR isoforms. Thioredoxin reductase-1 (TrxR1) is localized primarily in the cytosol, whereas thioredoxin reductase-2 (TrxR2) is expressed in the mitochondria.11 A third form, thioredoxin reductase-3, is mainly expressed in the testis, and thus, was not included in the current study. We found TrxR1 protein expression was not significantly different in the hearts of the aging rat compared with the young adults, whereas TrxR2 protein expression was significantly decreased. These results were consistent with the study of Rohrbach et al., which suggested that aging is characterized by a specific reduction of TrxR2 and results in the sensitization of cells to oxidative challenge and increased susceptibility of the mitochondrial pathway of apoptosis.13,23 However, our results showed that ischemia/reperfusion injury did not result in these age-related protein expression changes of TrxR1 and TrxR2. Recent data has suggested that there are no alterations in TrxR1 mRNA or protein expression, but caloric restriction recovers TrxR1 activity in senescent cardiac muscles.23 Both our results and those of others have suggested that the regulation of age-related TrxR activity may not be dependent on TrxR protein expression.
Recent studies have demonstrated that besides up-regulation or down-regulation of TrxR expression, TrxR activity was regulated by post-translational modification. In the 1990s, it was found that selenocysteine incorporation increased TrxR activity without an increase in protein synthesis.21 However, there have been few reports of TrxR modification in the past 10 years. There were multiple redox active sites within TrxR, including the flavin adenine dinucleotide (FAD), the carboxy-terminal active site, and the amino-terminal domain dithiol. All of these sites are necessary for electron flow, and therefore TrxR activity and disruption of any one of these could theoretically inhibit its activity.24 Meanwhile, there were many tyrosine residues in TrxR, such as Tyr-114, Tyr-116, and Tyr-200. It is noteworthy that Tyr-200 shields FAD from the solvent and hinders proper binding of the nicotinamide ring of NADP+.25 Growing evidence has shown that Trx can also be modified at the tyrosine residue (protein nitration) in a ONOO−-dependent fashion.26,27 Thus, it was reasonable to speculate the tyrosine residue in TrxR could be nitrated with high levels of ONOO− in the aging heart.
Our study reveals the first evidence of the TrxR nitration in the heart of aging rats subjected to MI/R. However, in this paper the exact tyrosine residues were not tested; they will be investigated in future studies. Moreover, our results suggested that high levels of NO are converted to the more toxic nitrating and oxidant agent ONOO−. As for why NO was increased, it was reported that the vessel wall of aging rats showed an enhanced expression of iNOS isoform.28 Most importantly, our previous work reported that cardiac iNOS expression was significantly up-regulated in aging hearts compared with young hearts. Meanwhile, the increased protein expression of iNOS was paralleled by enhanced iNOS activity.29 Because the iNOS isoform is capable of producing large amounts of NO at a rate that is 100 times greater than normal,30 up-regulation of iNOS contributes to excessive production of NO and ONOO− in the aging heart.
Having demonstrated that TrxR is susceptible to nitration, we further determined whether the activity of TrxR is altered by this post-translational modification. It is worth noting that treatment with the peroxynitrite decomposition catalyst FeTMPyp not only decreased the nitrated TrxR protein but also enhanced its activity, which suggested that the nitration of TrxR may be related to its inactivity. At last we found that FeTMPyp ameliorated the MI/R-induced myocardial apoptosis, lipid peroxidation, and cardiac dysfunction, suggesting that the nitration of TrxR as a post-translational modification may be related to its decreased activity in the aging heart, which was involved in enhanced aging MI/R injury. Moreover, FeTMPyp preserved Trx activity as well as TrxR activity in the aging heart subject to MI/R, which was consistent with our previously published results that another peroxynitrite decomposition catalyst FP-15 increased Trx activity in aging heart.9 TrxR was named for its ability to reduce oxidized Trxs and is necessary for the biological activity of Trx. Therefore, both TrxR and Trx disturbed expression and/or activity play critical roles in aging, and the Trx system may be a novel therapeutic target for age-associated diseases, such as cardiovascular disease.
Our results demonstrate that TrxR activity is decreased in the aging heart subjected to MI/R, which might be related to its nitrative modification. Blocking ONOO− production and inhibiting TrxR inactivation significantly prevented the aging heart from MI/R injury, which suggested that inhibiting nitration and restoring TrxR activity might be a therapy to attenuate enhanced MI/R injury in aging patient.
Acknowledgments
This study was supported by the Natural Sciences Foundation of China (NSFC) grants (30973163 and 81270283, to Huirong Liu; 81170144, to Xiaoliang Wang) and the Funding Project for Academic Human Resources Development in Institutions of Higher Learning Under the Jurisdiction of Beijing Municipality (PHR201106112, to Huirong Liu).
Author Disclosure Statement
No competing interests exist.
References
- 1.Lakatta EG. Levy D. Arterial and cardiac aging: Major hareholders in cardiovascular disease enterprises: Part I: Aging arteries: A “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 2.Boengler K. Schulz R. Heusch G. Loss of cardioprotection with aging. Cardiovasc Res. 2009;83:247–261. doi: 10.1093/cvr/cvp033. [DOI] [PubMed] [Google Scholar]
- 3.Lesnefsky EJ. Hoppel CL. Cardiolipin as an oxidative target in cardiac mitochondria in the aged rat. Biochim Biophys Acta. 2008;1777:1020–1027. doi: 10.1016/j.bbabio.2008.05.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ugarte N. Petropoulos I. Friguet B. Oxidized mitochondrial protein degradation and repair in aging and oxidative stress. Antioxid Redox Signal. 2010;13:539–549. doi: 10.1089/ars.2009.2998. [DOI] [PubMed] [Google Scholar]
- 5.Steinhubl SR. Why have antioxidants failed in clinical trials? Am J Cardiol. 2008;101:14D–19D. doi: 10.1016/j.amjcard.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Fan Q. Chen M. Fang X. Lau WB. Xue L. Zhao L. Zhang H. Liang YH. Bai X. Niu HY. Ye J. Chen Q. Yang X. Liu M. Aging might augment reactive oxygen species (ROS) formation and affect reactive nitrogen species (RNS) level after myocardial ischemia/reperfusion in both humans and rats. Age (Dordr) 2012 doi: 10.1007/s11357-012-9421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji L. Fu F. Zhang L. Liu W. Cai X. Zhang L. Zheng Q. Zhang H. Gao F. Insulin attenuates myocardial ischemia/reperfusion injury via reducing oxidative/nitrative stress. Am J Physiol Endocrinol Metab. 2010;298:E871–E880. doi: 10.1152/ajpendo.00623.2009. [DOI] [PubMed] [Google Scholar]
- 8.Berndt C. Lillig CH. Holmgren A. Thiol-based mechanisms of the thioredoxin and glutaredoxin systems: Implications for diseases in the cardiovascular system. Am J Physiol Heart Circ Physiol. 2007;292:H1227–H1236. doi: 10.1152/ajpheart.01162.2006. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H. Tao L. Jiao X. Gao E. Lopez BL. Christopher TA. Koch W. Ma XL. Nitrative thioredoxin inactivation as a cause of enhanced myocardial ischemia/reperfusion injury in the aging heart. Free Radic Biol Med. 2007;43:39–47. doi: 10.1016/j.freeradbiomed.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monteiro HP. Arai RJ. Travassos LR. Protein tyrosine phosphorylation and protein tyrosine nitration in redox signaling. Antioxid Redox Signal. 2008;10:843–889. doi: 10.1089/ars.2007.1853. [DOI] [PubMed] [Google Scholar]
- 11.Horstkotte J. Perisic T. Schneider M. Lange P. Schroeder M. Kiermayer C. Hinkel R. Ziegler T. Mandal PK. David R. Schulz S. Schmitt S. Widder J. Sinowatz F. Becker BF. Bauersachs J. Naebauer M. Franz WM. Jeremias I. Brielmeier M. Zischka H. Conrad M. Kupatt C. Mitochondrial thioredoxin reductase is essential for early postischemic myocardial protection. Circulation. 2011;124:2892–2902. doi: 10.1161/CIRCULATIONAHA.111.059253. [DOI] [PubMed] [Google Scholar]
- 12.Sibbing D. Pfeufer A. Perisic T. Mannes AM. Fritz-Wolf K. Unwin S. Sinner MF. Gieger C. Gloeckner CJ. Wichmann HE. Kremmer E. Schäfer Z. Walch A. Hinterseer M. Näbauer M. Kääb S. Kastrati A. Schömig A. Meitinger T. Bornkamm GW. Conrad M. von Beckerath N. Mutations in the mitochondrial thioredoxin reductase gene TXNRD2 cause dilated cardiomyopathy. Eur Heart J. 2011;32:1121–1133. doi: 10.1093/eurheartj/ehq507. [DOI] [PubMed] [Google Scholar]
- 13.Rohrbach S. Gruenler S. Teschner M. Holtz J. The thioredoxin system in aging muscle: key role of mitochondrial thioredoxin reductase in the protective effects of caloric restriction? Am J Physiol Regul Integr Comp Physiol. 2006;291:R927–R935. doi: 10.1152/ajpregu.00890.2005. [DOI] [PubMed] [Google Scholar]
- 14.Myers JM. Antholine WE. Myers CR. The intracellular redox stress caused by hexavalent chromium is selective for proteins that have key roles in cell survival and thiol redox control. Toxicology. 2011;281:37–47. doi: 10.1016/j.tox.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myers JM. Myers CR. The effects of hexavalent chromium on thioredoxin reductase and peroxiredoxins in human bronchial epithelial cells. Free Radic Biol Med. 2009;47:1477–1485. doi: 10.1016/j.freeradbiomed.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vadseth C. Souza JM. Thomson L. Seagraves A. Nagaswami C. Scheiner T. Torbet J. Vilaire G. Bennett JS. Murciano JC. Muzykantov V. Penn MS. Hazen SL. Weisel JW. Ischiropoulos H. Prothrombotic state induced by post-translational modification of fibrinogen by reactive nitrogen species. J Biol Chem. 2004;279:8820–8826. doi: 10.1074/jbc.M306101200. [DOI] [PubMed] [Google Scholar]
- 17.Wang K. Zhang J. Liu J. Tian J. Wu Y. Wang X. Quan L. Xu H. Wang W. Liu H. Variations in the protein level of Omi/HtrA2 in the heart of aged rats may contribute to the increased susceptibility of cardiomyocytes to ischemia/reperfusion injury and cell death: Omi/HtrA2 and aged heart injury. Age (Dordr) 2013;35:733–746. doi: 10.1007/s11357-012-9406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lillig CH. Holmgren A. Thioredoxin and related molecules—from biology to health and disease. Antioxid Redox Signal. 2007;9:25–47. doi: 10.1089/ars.2007.9.25. [DOI] [PubMed] [Google Scholar]
- 19.Sartoretto JL. Jin BY. Bauer M. Gertler FB. Liao R. Michel T. Regulation of VASP phosphorylation in cardiac myocytes: Differential regulation by cyclic nucleotides and modulation of protein expression in diabetic and hypertrophic heart. Am J Physiol Heart Circ Physiol. 2009;297:H1697–H1710. doi: 10.1152/ajpheart.00595.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen H. Yu M. Li M. Zhao R. Zhu Q. Zhou W. Lu M. Lu Y. Zheng T. Jiang J. Zhao W. Xiang K. Jia W. Liu L. Polymorphic variations in manganese superoxide dismutase (MnSOD), glutathione peroxidase-1 (GPX1), and catalase (CAT) contribute to elevated plasma triglyceride levels in Chinese patients with type 2 diabetes or diabetic cardiovascular disease. Mol Cell Biochem. 2012;363:85–91. doi: 10.1007/s11010-011-1160-3. [DOI] [PubMed] [Google Scholar]
- 21.Holmgren A. Lu J. Thioredoxin and thioredoxin reductase: Current research with special reference to human disease. Biochem Biophys Res Commun. 2010;396:120–124. doi: 10.1016/j.bbrc.2010.03.083. [DOI] [PubMed] [Google Scholar]
- 22.Witte AB. Anestål K. Jerremalm E. Ehrsson H. Arnér ES. Inhibition of thioredoxin reductase but not of glutathione reductase by the major classes of alkylating and platinum-containing anticancer compounds. Free Radic Biol Med. 2005;239:696–703. doi: 10.1016/j.freeradbiomed.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 23.Xing KY. Lou MF. Effect of age on the thioltransferase (glutaredoxin) and thioredoxin systems in the human lens. Invest Ophthalmol Vis Sci. 2010;51:6598–6604. doi: 10.1167/iovs.10-5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia L. Nordman T. Olsson JM. Damdimopoulos A. Björkhem-Bergman L. Nalvarte I. Eriksson LC. Arnér ES. Spyrou G. Björnstedt M. The mammalian cytosolic selenoenzyme thioredoxin reductase reduces ubiquinone. A novel mechanism for defense against oxidative stress. J Biol Chem. 2003;278:2141–2146. doi: 10.1074/jbc.M210456200. [DOI] [PubMed] [Google Scholar]
- 25.Sandalova T. Zhong L. Lindqvist Y. Holmgren A. Schneider G. Three-dimensional structure of a mammalian thioredoxin reductase: implications for mechanism and evolution of a selenocysteine-dependent enzyme. Proc Natl Acad Sci USA. 2001;98:9533–9538. doi: 10.1073/pnas.171178698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haendeler J. Thioredoxin-1 and posttranslational modifications. Antioxid Redox Signal. 2006;8:1723–1728. doi: 10.1089/ars.2006.8.1723. [DOI] [PubMed] [Google Scholar]
- 27.Tao L. Jiao X. Gao E. Lau WB. Yuan Y. Lopez B. Christopher T. Ramachandrarao SP. Williams W. Southan G. Sharma K. Koch W. Ma XL. Nitrative inactivation of thioredoxin-1 and its role in postischemic myocardial apoptosis. Circulation. 2006;114:1395–1402. doi: 10.1161/CIRCULATIONAHA.106.625061. [DOI] [PubMed] [Google Scholar]
- 28.Cernadas MR. Sánchez de Miguel L. García-Durán M. González-Fernández F. Millás I. Montón M. Rodrigo J. Rico L. Fernández P. de Frutos T. Rodríguez-Feo JA. Guerra J. Caramelo C. Casado S. López-Farré Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res. 1998;83:279–286. doi: 10.1161/01.res.83.3.279. [DOI] [PubMed] [Google Scholar]
- 29.Ferdinandy P. Schulz R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia-reperfusion injury and preconditioning. Br J Pharmacol. 2003;138:532–543. doi: 10.1038/sj.bjp.0705080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li D. Qu Y. Tao L. Liu H. Hu A. Gao F. Sharifi-Azad S. Grunwald Z. Ma XL. Sun JZ. Inhibition of iNOS protects the aging heart against beta-adrenergic receptor stimulation-induced cardiac dysfunction and myocardial ischemic injury. J Surg Res. 2006;131:64–72. doi: 10.1016/j.jss.2005.06.038. [DOI] [PubMed] [Google Scholar]