Abstract
Objective
The purpose of this study was to evaluate the short- and long-term efficacy, safety, and tolerability of ziprasidone in adolescents with schizophrenia.
Methods
Subjects ages 13–17 years with schizophrenia (American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th ed. [DSM-IV]) were enrolled in a 6 week, randomized, double-blind, placebo-controlled multicenter trial (RCT) followed by a 26 week open-label extension study (OLE). Subjects were randomized in a 2:1 ratio to flexible-dose oral ziprasidone (40–160 mg/day, based on weight) or placebo. Primary end-point was change from baseline in Brief Psychiatric Rating Scale–Anchored (BPRS-A) total score. Safety assessments included adverse events, vital signs, laboratory measures, electrocardiograms, weight and body mass index, and movement disorder ratings.
Results
Planned interim analysis for the primary end-point in the RCT resulted in early termination of both studies because of futility. In the RCT, 283 subjects received ziprasidone (n=193) or placebo (n=90). In the intent-to-treat analysis population, the least squares mean (SE) BPRS–A score decrease from baseline at week 6 was not significantly different (p=0.15; −14.16 [0.78] for ziprasidone and −12.35 [1.05] for placebo). Per-protocol analysis was significant (p=0.02). In the OLE, 221 subjects entered the OLE and received ziprasidone for a median of 99 days. The mean (SD) change in BPRS-A score from end of RCT to end of OLE (last observation carried forward) was −6.9 (8.9). The most common treatment-emergent adverse events (≥10%) for all causalities during the RCT were somnolence and extrapyramidal disorders, and during OLE was somnolence only. No subjects had Fridericia's corrected QT (QTcF) ≥500 ms in the RCT or OLE phases. One completed suicide occurred during the OLE phase. For RCT and OLE, no clinically significant changes were reported in metabolic indices and laboratory measures.
Conclusions
Ziprasidone failed to separate from placebo in treatment of schizophrenia in adolescents. Ziprasidone was generally well tolerated with an overall neutral weight and metabolic profile.
Clinical Trials Registry
NCT00257192 and NCT00265382 at ClinicalTrials.gov.
Introduction
Schizophrenia is rare in children up to the age of 12 years (Burd and Kerbeshian 1987); however, up to one third of people with schizophrenia have illness onset during adolescence (Hafner et al. 1993; Beratis et al. 1994; American Psychiatric Association 2004). Schizophrenia, when it does develop in childhood or adolescence, appears to be associated with greater functional impairments than when onset occurs during adulthood (Young and Findling 2004). The chronic course, severe functional impairments, and poor prognosis create a great need to identify effective and safe treatments for adolescents with schizophrenia.
The advent of atypical antipsychotics has led to an increase in their use for the management of schizophrenia and other disorders in children and adolescents, and there is a growing body of evidence to support the use of these agents in young patients with schizophrenia and bipolar disorder (Masi and Liboni 2011). Nevertheless, there are fewer treatment studies in adolescents with schizophrenia than in adult patients. Robust data to guide clinical practice were scarce until relatively recently. Several studies of atypical antipsychotics for schizophrenia in the adolescent population have resulted in United States Food and Drug Administration (FDA) approved use of aripiprazole, olanzapine, risperidone, quetiapine, and paliperidone in adolescents (Findling et al. 2008; Haas et al. 2009 a,b; Kryzhanovskaya et al. 2009; Singh et al. 2011; Findling et al. 2012).
Recent studies have shown that although typical and atypical antipsychotics appear to have similar efficacy for schizophrenia, atypical antipsychotics have a reduced propensity for neurologic side effects, including extrapyramidal symptoms (Findling et al. 2010). However, among the atypical antipsychotics, quetiapine, risperidone, and olanzapine can have significant weight gain, endocrine, and metabolic side effects (De Hert et al. 2011; Maayan and Correll 2011).
Ziprasidone is a second-generation atypical antipsychotic agent used in the management of schizophrenia and acute bipolar mania in adults (Keck et al. 1998; Daniel et al. 1999; Keck et al. 2003; Potkin et al. 2005; Keck et al. 2009). The efficacy and safety of ziprasidone in schizophrenia and bipolar I disorder have been studied mainly in subjects >18 years of age; there is limited information on its use in children and adolescents in rigorous randomized clinical trials (DelBello et al. 2008). Here, we report the findings of a 6 week randomized, double-blind, placebo-controlled trial (RCT) of the efficacy, safety, and tolerability of flexibly dosed ziprasidone compared with placebo for the treatment of schizophrenia in adolescent subjects, followed by an open-label 26 week extension (OLE) study.
Methods
The studies were planned to be conducted in accordance with the principles of the Declaration of Helsinki, all International Conference on Harmonisation Good Clinical Practice (GCP) guidelines, and with all local regulatory requirements. Written informed consent from the subject's legal guardian and informed assent from the subject were obtained prior to study entry. Ethics review boards from each participating center approved the study protocol prior to any subject recruitment.
The study was conducted at 70 international sites with 25 in the United States, 25 in Europe (Russia, Ukraine), 15 in Asia (India, Malaysia, and Singapore), and 5 in Central and South America (Peru, Columbia, and Costa Rica). Four sites were terminated for GCP violations. The RCT (ClinicalTrials.gov: NCT00257192) took place between April 2006 and March 2009, and the 26 week OLE study (ClinicalTrials.gov: NCT00265382) took place from June 2006 to June 2009. The safety data for the studies were monitored by an independent data safety monitoring board (DSMB). A planned interim analysis was performed when approximately two thirds of the planned number of enrolled subjects had completed the study. The DSMB reviewed the interim analysis results and recommended termination of the study for futility; no safety concerns were identified. Both the RCT and OLE study were terminated on March 23, 2009.
Study participants
RCT phase
The study was a 6 week, randomized, double-blind, placebo-controlled investigation of the efficacy, safety, and tolerability of flexibly dosed ziprasidone compared with placebo for the treatment of schizophrenia in adolescents ages 13–17 years (inclusive). Inpatient and outpatient boys and girls, who met the Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) criteria for schizophrenia (American Psychiatric Association 1994), confirmed by the Structured Clinical Interview for DSM-IV – Child Edition (KID-SCID) (Matzner et al. 1997) were recruited. Eligible subjects had symptoms for 7 days prior to screening, had a screening and baseline (randomization) Brief Psychiatric Rating Scale – Anchored (BPRS-A) score of ≥35, and a score of ≥4 on at least one of the four items (unusual thought content, hallucinations, suspiciousness, or conceptual disorganization) (Woerner et al. 1988).
Subjects were excluded if they had a substance-induced psychotic disorder or behavioral disturbance, a DSM-IV–defined psychoactive substance or alcohol abuse/dependence in the preceding month, a rating of 7 on the single suicidal ideation item on the Child Depression Rating Scale-Revised (CDRS-R) (Poznanski et al. 1985), significant mental retardation, or autism or pervasive developmental disorder, or if they were judged by investigator to be at imminent risk of suicide or homicide. Other general criteria for exclusion included serious/unstable medical conditions, history of significant cardiovascular disease, cardiac arrhythmias, conduction abnormalities, QT prolongation, clinically significant electrocardiographic (ECG) abnormalities, and Fridericia's corrected QT (QTcF) interval ≥460 ms at screening or baseline.
Subjects were not permitted to take any other antipsychotic agents, mood stabilizers, stimulants, antidepressants (including monoamine oxidase inhibitors), anti-emetics, several antihypertensives (propranolol, reserpine, clonidine, methyldopa), or any medication that is known to prolong the QT interval. Subjects were included if they had been on stable doses of select medications (some hormones, antihypertensive agents, diuretics, and oral hypoglycemic agents) to treat a stable clinical condition for at least 2 months before study entry.
Lorazepam (up to 2 mg/day), or, if not available, diazepam (up to 5 mg/day) could be used as needed for anxiety or agitation, except within 6 hours before assessments. Permissible medications for insomnia included lorazepam, diphenhydramine, or zolpidem; for extrapyramidal symptoms, they included benztropine, other anticholinergics, or propranolol, and were dosed per investigator's discretion.
Subjects were assessed at baseline, then weekly until week 6 (Fig. 1). Subjects could withdraw from the study at any time, or at the discretion of the investigator or study sponsor for safety, symptomatology, or administrative reasons. Subjects were to be discontinued from the RCT and not allowed to enter the OLE phase if they had syncopal episodes suggestive of cardiac arrhythmia, QT prolongation (QTcF ≥460 ms, or increase from baseline ≥60 ms), ventricular arrhythmia, were at imminent risk of suicide, or were pregnant.
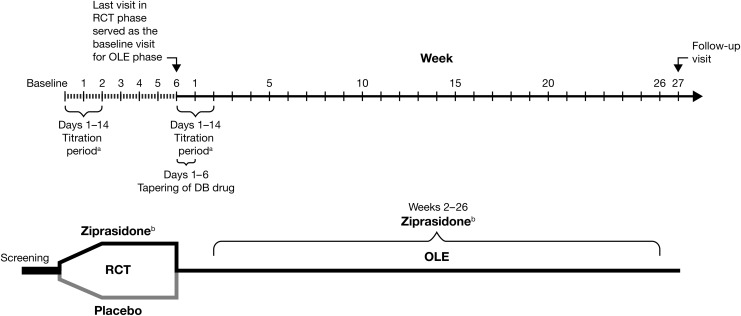
FIG. 1.
Study design for randomized controlled and open-label extension trials. DB, double blind; OLE, open-label extension; RCT, randomized controlled trial. aDose titration: 20 mg/day start (night), increased by 20 mg every 2 days to target dose. bFlexible dose: Ziprasidone 40–80 mg/day (<45 kg), ziprasidone 120–160 mg/day (≥45 kg).
OLE phase
The RCT phase was followed by a 26 week OLE and enrolled subjects who had participated in the earlier 6 week trial, met the required eligibility criteria, and wished to receive treatment with open-label ziprasidone. The final visit of the RCT phase (week 6 or early termination) served as the baseline visit for the OLE study. Subjects were tapered off their study double-blind medication during the first 6 days of the OLE study, while the open-label active medication was titrated up over 2 weeks (Fig. 1).
Subjects were discontinued from the RCT phase but allowed to enter the OLE phase under the following circumstances: insufficient clinical response after end of titration, requiring rescue medication (mood stabilizer, antidepressants, stimulants), increased suicidality (≥3 points higher on CDRS-R suicidality item 13 than at baseline and maintaining that increase for two consecutive visits), or not reaching the minimum threshold total daily dose of ziprasidone (80 mg/day for subjects with ≥45 kg body weight and 40 mg/day for subjects with <45 kg body weight).
Subjects were assessed at baseline, week 1, and week 2, then every 4 weeks (weeks 6, 10, 14, 18, 22, 26) during treatment, with a follow-up visit at week 27.
Dosing
RCT phase
Subjects were randomized in a 2:1 ratio to receive either ziprasidone or placebo in a double-blind fashion. Twice-daily ziprasidone capsules were given with meals. Medication was supplied in childproof blister cards. Ziprasidone was initiated at 20 mg/day then titrated over the first 1–2 weeks to a target dose of 120–160 mg/day for subjects weighing ≥45 kg and 60–80 mg/day for subjects weighing<45 kg). After reaching target dose, ziprasidone could be flexibly dosed at 80–160 mg/day (40–80 mg/day for subjects weighing<45 kg).
OLE phase
Subjects were tapered off the double-blind treatment during the first 6 days of the OLE phase. Ziprasidone was titrated up from a 20 mg b.i.d. starting dose during the first 2 weeks with the goal of achieving the target dose by day 14. For subjects with a body weight ≥45 kg, the target dose range was 80–160 mg/day (80 mg/day maximum for subjects weighing<45 kg). After the week 2 visit, dosing was flexible within the target range at investigator discretion, with a minimum dose of 40 mg/day for all subjects.
Outcome and safety assessments
RCT phase
The prespecified primary efficacy end-point was change from baseline to week 6 in BPRS-A total score, administered at baseline and at the weekly visits or early termination (Woerner et al. 1988). The secondary efficacy end-points were the change from baseline to week 6 in the Positive and Negative Syndrome Scale (PANSS) total score (Kay et al. 1987), administered at baseline, week 2, and week 6 (or early termination) and the Clinical Global Impressions-Severity (CGI-S) score (Guy 1976), administered on all weekly visits. Other assessments included the CGI-Improvement (CGI-I) and Children's Global Assessment Scale (CGAS), a clinician-rated global assessment item for children based on symptoms and social functioning in home, school, and community settings (Shaffer et al. 1983).
Safety assessments included the following: Adverse event (AE) reporting; clinical laboratory testing; physical examination; blood pressure and pulse rate; body weight, height, and body mass index (BMI); 12-lead ECG, and QTcF measurements.
All observed or volunteered AEs (in response to open-ended investigator queries), the severity (mild, moderate, severe) of the events, and the investigator's assessment of their relationship to the study treatment were recorded. Independent of severity, a serious AE (SAE) was defined as any AE that either resulted in death, was life threatening, required inpatient hospitalization or prolongation of existing hospitalization, resulted in a persistent or significant disability/incapacity, or resulted in a congenital anomaly/birth defect.
The AE database was searched programmatically at the end of the study to identify possibly suicide-related AEs (PSRAEs) using text strings as per FDA recommendations. Events were reviewed and classified by an independent panel of experts, who categorized these PSRAEs according to the Columbia Classification Algorithm of Suicide Assessment (C-CASA) (Posner et al. 2007). Additionally, subjects were evaluated for depression with the CDRS-R scale (Poznanski et al. 1985) and for extrapyramidal symptoms with movement disorder scales (Simpson-Angus Rating Scale [SARS], Barnes Akathisia Rating Scale [BARS], and Abnormal Involuntary Movement Scale [AIMS]) (Simpson and Angus 1970; Guy 1976; Barnes 1989).
OLE phase
Subjects were assessed using the BPRS-A total score, CGI-S, and CGAS for efficacy. All the safety assessments conducted during the RCT phase were also performed during the OLE phase. New-onset AEs and AEs that began in the preceding RCT period but were ongoing in the OLE period were reported in the OLE phase.
Statistical analyses
RCT phase
The 6 week study was designed to have 85% statistical power to show a difference between ziprasidone and placebo, equivalent to the median drug–placebo difference seen in the adult trials (i.e., 5% level, two sided) of statistical significance. The projected sample size needed was 276 randomized subjects (2:1 enrollment with 184 receiving ziprasidone and 92 receiving placebo). A modified intent-to-treat (mITT) analysis set was defined as all subjects who were randomized, had baseline measurements, had taken at least one dose of study treatment, and had had at least one postbaseline visit. The per-protocol (PP) analysis set (prespecified in the protocol) included all subjects in the mITT analysis set who did not violate any major inclusion/exclusion criteria and who did not have any prespecified major protocol violations that could impact the interpretation of the primary end-point. Subjects (total 51 ziprasidone treated and 22 placebo treated) were excluded from the PP analysis set when they had major prespecified protocol deviations, such as dosing deviations (failure to reach or remain within target dose range, missed>20% of study doses), took prohibited concomitant medications, had two or more positive urine drug screens at any two visits, had BPRS-A total score<35 (or score<4 on predefined items at screening or baseline), or were rated by noncertified raters. The safety analysis set included all subjects who were randomized and had taken at least one dose of the study drug.
Analyses of change from baseline at week 6 in BPRS-A total score, PANSS total score, and CGI-S score were conducted using mixed-model repeated measures analysis of covariance (MMRM) with treatment, region, visit, and visit-by-treatment interaction as fixed effects and baseline score as a covariate. Subject effect was included in the mixed model as a random effect. The unstructured covariance matrix option was used. The Hochberg procedure was applied to preserve type I error in the analyses of the key secondary end-points, PANSS and CGI-S.
A planned interim analysis occurred with the first 184 randomized subjects. The study could stop early for efficacy (p≤0.0124, two sided) or for futility (p≥0.4772, two sided) or continue as planned. The interim analysis resulted in a recommendation to terminate the study for futility. Among the 184 subjects (two thirds of the planned sample size of 276) included in the interim analysis, the date of randomization ranged from May 2006 to November 2008. A subsequent 100 subjects were randomized from November 2008 to February 2009. Because of the speed of enrollment during the interim analysis process, all but one subject had completed the RCT when it was stopped. The α significance level was adjusted for the multiple analyses, and final analysis employed a two-sided p value<0.0462, based on the probability of stopping early for futility estimated at 3.65%.
OLE phase
As this was an open-label study with no comparator, no inferential statistics were performed. All quantitative variables were described by standard descriptive statistics. Only the safety analysis set was defined for this phase, and included all subjects who took at least one dose of study drug.
Post-hoc assessments—RCT phase
Post-hoc analyses for efficacy (BPRS-A) were explored by geographic region (United States, Europe [Russia, Ukraine], Asia [India, Malaysia, and Singapore], and Central/South America [Peru, Columbia, and Costa Rica]) using the MMRM model to test for treatment difference.
Independent audit
A companion study to this trial (for pediatric acute bipolar mania) was undertaken to address an FDA pediatric written request and Pediatric Research Equity Act (PREA) commitment. In that study, three study sites were cited for GCP deficiencies. The FDA asked the study sponsor to conduct an audit of all sites in the pediatric acute bipolar mania study. In the bipolar study, the independent auditor found that 81% (193 of 237) of the subjects had reliable data, and 19% had unreliable data, and a sensitivity analysis of the “reliable” subset demonstrated efficacy versus placebo (p=0.0006). Nevertheless, after review of the audit results, the FDA concluded that the pediatric bipolar mania study was unreliable. As many sites in the pediatric bipolar mania study overlapped with this adolescent schizophrenia study, the study sponsor elected to conduct an additional independent quality assurance audit of all study sites for all subjects in the adolescent schizophrenia study as well. The independent audit group developed a set of severity evaluation criteria, categorizing all the audit findings as “essential” or “minor,” based on their potential for impact on the reliability of the data. For reliability of the safety data, the criteria were: The data from the site had to be trusted, all SAEs had to have been reported, and a subject had to have received at least one dose of the study drug or placebo. For reliability of the efficacy data, the essential factors were: Presence of signed informed consent, the subject having the condition (i.e., was the diagnosis performed appropriately and were key inclusion/exclusion criteria met), documentation that the subject received the drug, and the subject having been appropriately evaluated for the primary efficacy end-point (BPRS-A).
Results
Subjects
RCT phase
A total of 342 subjects were screened, 284 subjects were randomized, and 283 subjects were treated (193 ziprasidone and 90 placebo). Of the treated subjects, 187 (66.1%) subjects completed and 96 (33.9%) subjects discontinued the study (Table 1). Most discontinuations were for reasons not related to the study drug and occurred more frequently in the placebo group (38.9%) than in the ziprasidone group (22.3%). A higher percentage of subjects in the placebo group discontinued because of insufficient clinical response (Table 1).
Table 1.
Subject Disposition
| |
RCT |
OLE |
|
|---|---|---|---|
| Ziprasidone | Placebo | Ziprasidone | |
| Screened | 342 | N/A | |
| Assigned to treatment | 284a | 221 | |
| Treated, n | 193 | 90 | 221 |
| Completed, n (%) | 135 (69.9) | 52 (57.8) | 76 (34.4) |
| Discontinued, n (%) | 58 (30.1) | 38 (42.2) | 145 (65.6) |
| Reason for discontinuation, n (%) | |||
| Subject died | N/A | N/A | 1 (0.5)d |
| Related to study drug | 15 (7.8) | 3 (3.3) | 97 (43.9) |
| Adverse event | 13 (6.7) | 3 (3.3) | 5 (2.3) |
| Laboratory abnormality | 1 (0.5) | - | N/A |
| Study terminated by sponsor | 1 (0.5) | - | 92 (41.6) |
| Not related to study drug | 43 (22.3) | 35 (38.9) | 47 (21.3) |
| Adverse event | 8 (4.1) | 7 (7.8) | 16 (7.2) |
| Laboratory abnormality | 0 | 1 (1.1) | 1 (0.5) |
| Lost to follow-up | 3 (1.6) | 3 (3.3) | 3 (1.4) |
| Other | 18 (9.3) | 22 (24.4) | 14 (6.3) |
| Insufficient clinical response | 18 (9.3)b | 18 (20.0)b | 6 (2.7) |
| Miscellaneous | 0 | 4 (4.4)c | 8 (3.6)e |
| Withdrawal of consent | 14 (7.3) | 2 (2.2) | 13 (5.9) |
One subject was randomized but untreated.
Includes withdrawal of subject because of lack of efficacy, insufficient clinical response, and worsening of disease under study.
Includes one subject who needed to travel, one subject who was enrolled into OLE phase by investigator, one subject who had a protocol violation, and one subject who terminated RCT phase to roll over to OLE phase.
Subject committed suicide.
Includes grandparents who terminated their rights to the participant, subject who needed to reside in residential facility that did not permit research subjects, subject whose guardian withdrew consent, and withdrawal because of the sponsor's decision (subject did not follow protocol).
N/A, not applicable; OLE, open-label extension; RCT, randomized controlled trial.
Overall, subjects in both treatment groups had comparable demographic and baseline disease characteristics (Table 2). Subjects ranged in age from 12 to 17 years with an overall mean age of 15.3 years in the ziprasidone group (four subjects were 12 years old at study start) and 15.4 years in the placebo group. The mean modal dose for ziprasidone-treated subjects who completed the titration phase, from week 3 to week 6 or early termination, was 129.3 mg/day (n=138, subjects weighing ≥45 kg) and 67.8 mg/day (n=18, subjects weighing<45 kg) in the mITT analysis set and, 135.8 mg/day (n=99, subjects weighting ≥45 kg) and 65.3 mg/day (n=15, subjects weighing<45kg) in the PP analysis set.
Table 2.
Subject Demographics and Schizophrenia Characteristics
| |
RCT |
|
|||
|---|---|---|---|---|---|
| |
Ziprasidone |
Placebo |
OLE |
||
| Male | Female | Male | Female | Total | |
| n | 109 | 84 | 62 | 28 | 221 |
| Age (years) | |||||
| Mean±SD | 15.2±1.4 | 15.3±1.3 | 15.4±1.5 | 15.5±1.3 | 15.3±1.4 |
| Race, n (%) | |||||
| White | 62 (56.9) | 54 (64.3) | 43 (69.4) | 17 (60.7) | 140 (63.3) |
| Black | 10 (9.2) | 7 (8.3) | 2 (3.2) | 0 | 11 (5.0) |
| Asian | 21 (19.3) | 17 (20.2) | 12 (19.4) | 5 (17.9) | 44 (19.9) |
| Hispanic | 6 (5.5) | 3 (3.6) | 1 (1.6) | 2 (7.1) | 9 (4.1) |
| Other | 10 (9.2) | 3 (3.6) | 4 (6.5) | 4 (14.3) | 17 (7.7) |
| Ethnicity, n (%) | |||||
| Hispanic/Latino | 15 (13.8) | 6 (7.1) | 3 (4.8) | 6 (21.4) | 25 (11.3) |
| Not Hispanic | 94 (86.2) | 78 (92.9) | 59 (95.2) | 22 (78.6) | 196 (88.7) |
| Weight (kg) | |||||
| Mean±SD | 61.4±15.1 | 61.0±16.1 | 66.1±15.8 | 60.1±15.1 | 61.7±14.5 |
| Range | 30.0–106.0 | 38.5–126.8 | 37.0–106.4 | 31.5–105.0 | 31.5–105.0 |
| Height (cm) | |||||
| Mean±SD | 167.5±10.8 | 161.5±7.9 | 170.5±9.2 | 161.8±9.1 | 165.7±10.0 |
| Range | 123.0–190.0 | 143.0–180.3 | 148.0–186.7 | 139.0–178.0 | 123.0–190.0 |
| Schizophrenia, paranoid type (n) | 127 | 57 | 139 | ||
| Duration of current episode (months) | |||||
| Mean (range) | 9.6 (0–96) | 9.2 (0–84) | 9.4 (0–84) | ||
| Number of prior episodes | |||||
| Mean (range) | 1.1 (0–6) | 1.2 (0–8) | 1.1 (0–8) | ||
OLE, open-label extension; RCT, randomized controlled trial.
The proportion of subjects taking ≥1 concomitant medications was higher in the ziprasidone group (50.8%) than in the placebo group (38.9%). The most commonly used concomitant drug was lorazepam, which was taken at comparable frequency in the ziprasidone (22.3%) and placebo groups (23.3%). Subjects in the ziprasidone and placebo groups, respectively, may have taken other permissible drugs, including diazepam (5.2% vs. 5.7%), diphenhydramine or diphenhydramine hydrocholoride (8.8% vs. 5.5%), propranolol (2.6% vs. 0), and zolpidem or zolpidem tartrate (4.1% vs. 5.5%).
OLE phase
All 221 subjects who entered the OLE study were treated with ziprasidone and of these, 151 subjects received ziprasidone and 70 subjects received placebo in the preceding RCT. The RCT study was terminated for futility early by the study sponsor. By the time of study termination, all but one subject in the RCT had completed the RCT study visits. Because of the early termination of the RCT study for futility, participation of 92 of the 221 treated subjects in the OLE phase was ended by the study sponsor (Table 1). The mean length of the OLE study was 108.1 (range: 2–209, median: 99) days. The mean modal daily dose during weeks 2–26 and early termination was 125.0 mg/day for all subjects weighing ≥45 kg (n=175) and 64.8 mg/day for all subjects weighing <45 kg (n=21).
Efficacy end-points
RCT phase
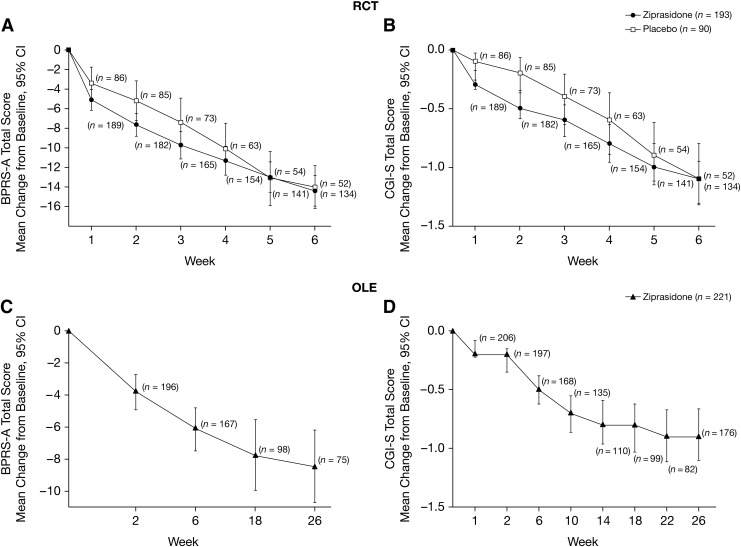
In the mITT analysis set, mean (SD) baseline BPRS-A scores were comparable in ziprasidone (n=189) and placebo (n=87) groups: 50.9 (10.1) and 50.3 (9.8), respectively (Fig. 2). The least squares (LS) mean (SE) scores in BPRS-A total score decreased from baseline to week 6 in both the ziprasidone and placebo groups with no significant difference (Table 3).
FIG. 2.
Primary efficacy end-points (modified intent-to-treat [mITT] analysis set). Mean change from baseline in BPRS-A total score and CGI-S total score in RCT (A, B) and OLE (C, D). BPRS-A, Brief Psychiatric Rating Scale – Anchored; CGI-S, Clinical Global Impressions-Severity; CI, Confidence interval.
Table 3.
Summary of Primary and Secondary End-Points at Week 6 of RCT
| |
Ziprasidone |
Placebo |
Difference from placebo |
p value |
|||
|---|---|---|---|---|---|---|---|
| End-point | n | LS mean (SE) | n | LS mean (SE) | LS mean (SE) | 95% CI | |
| mITT analysis set | |||||||
| BPRS-A Total Score | 189 | −14.2 (0.8) | 87 | −12.4 (1.1) | −1.8 (1.3) | −4.3 to 0.7 | 0.15 |
| PANSS Total Score | 183 | −23.6 (1.4) | 86 | −21.0 (1.7) | −2.6 (2.0) | −6.5 to 1.4 | 0.20 |
| PANSS Positive Subscale | 183 | −7.2 (0.4) | 86 | −5.9 (0.6) | −1.3 (0.7) | −2.6 to 0.1 | 0.04 |
| PANSS Negative Subscale | 183 | −5.5 (0.4) | 86 | −5.1 (0.5) | −0.4 (0.6) | −1.6 to 0.7 | 0.47 |
| CGI-S Score | 190 | −1.1 (0.1) | 87 | −0.8 (0.1) | −0.2 (0.1) | −0.5 to 0.1 | 0.13 |
| CGI-I Score | 190 | 2.7 (0.1) | 87 | 2.9 (0.1) | −0.2 (0.1) | −0.5 to −0.1 | 0.18 |
| PP analysis set | |||||||
| BPRS-A Total Score | 141 | −15.0 (0.9) | 67 | −11.7 (1.2) | −3.3 (1.5) | −6.2 to −0.4 | 0.03 |
| PANSS Total Score | 136 | −24.1 (1.7) | 67 | −19.6 (2.1) | −4.5 (2.4) | −9.3 to 0.2 | 0.06 |
| PANSS Positive Subscale | 136 | −7.3 (0.5) | 67 | −5.3 (0.7) | −2.0 (0.8) | −3.5 to −0.5 | 0.01 |
| PANSS Negative Subscale | 136 | −5.8 (0.6) | 67 | −4.6 (0.6) | −1.2 (0.7) | −2.6 to 0.2 | 0.10 |
| CGI-S Score | 142 | −1.2 (0.1) | 68 | −0.8 (0.1) | −0.3 (0.2) | −0.65 to 0.0 | 0.05 |
| CGI-I Score | 142 | 2.6 (0.1) | 68 | 2.9 (0.1) | −0.3 (0.2) | −0.6 to 0.1 | 0.12 |
BPRS-A, Brief Psychiatric Rating Scale–Anchored; CGI-S, Clinical Global Impressions-Severity; CGI-I, Clinical Global Impressions-Improvement; CI, confidence interval; LS, least squares; mITT, modified intent-to-treat; PANSS, Positive and Negative Symptom Scale; PP, per protocol; RCT, randomized controlled trial; SE, standard error.
Mean (SD) baseline PANSS total scores for ziprasidone (n=190) and placebo (n=88) were 88.9 (18.5) and 87.4 (17.9), respectively. Mean (SD) baseline PANSS negative subscale scores for ziprasidone (n=190) and placebo (n=88) were 23.4 (6.5) and 22.6 (6.5), whereas the PANSS positive subscale scores for ziprasidone (n=190) and placebo (n=88) were 21.9 (5.6) and 21.7 (5.5), respectively. There was no statistically significant difference for the PANSS total score but there was significantly different improvement on the PANSS positive subscale for the ziprasidone group (p<0.05, Table 3). Mean (SD) baseline CGI-S score for the ziprasidone group (n=190) was 4.7 (0.7) and for the placebo group (n=88) was 4.6 (0.7) and decreased from baseline until week 6 (Fig. 2). For the ziprasidone group (n=190), the mean (SD) baseline CGI-I score was 4.7 (0.7) and for the placebo group (n=88) it was 4.6 (0.7). The LS mean (SE) difference compared with placebo in change from baseline to week 6 for the CGI-S score was −0.21 (0.1) and for the CGI-I score was −0.19 (0.1); neither was statistically different (p=0.13 and p=0.18, respectively).
For the PP analysis set, the mean baseline (SD) BPRS-A scores for ziprasidone (n=141) and placebo (n=67) were 50.8 (9.7) and 50.6 (10.4), respectively. For ziprasidone (n=142) and placebo (n=68) respectively, the mean baseline (SD) scores were: PANSS total score (88.1 [17.6] and 88.7 [18.7]), PANSS negative subscale (23.2 [6.4] and 23.3 [6.9]), and PANSS positive subscale (21.8 [5.6] and 21.9 [5.8]). Mean (SD) baseline CGI-S score for the ziprasidone group (n=142) was 4.7 (0.8) and for the placebo group (n=68) was 4.7 (0.7). The mean (SD) baseline CGI-I score for the ziprasidone group (n=142) was 3.5 (0.8) and for the placebo group (n=68) was 3.8 (0.9). The results for the PP analysis set (n=142 ziprasidone and n=68 placebo) for BPRS-A (p=0.03) and for the PANSS positive subscale (p=0.04) showed statistically significant difference in favor of ziprasidone.
Mean CGAS scores increased throughout the study (higher scores indicate improvement in functioning), but CGAS scores for the ziprasidone and placebo groups were similar at each time point.
Independent audit findings for RCT
The independent audit report found that this study had similar audit findings to those of the companion study conducted on pediatric bipolar mania, which the FDA assessed as unreliable. The efficacy data for 241 of the total 287 subjects across 70 study sites were considered reliable (84.0%), whereas data for 43 of the subjects were not considered reliable. Sensitivity analysis based on the reliable mITT analysis set (n=235) confirmed that ziprasidone did not separate from placebo in the treatment of pediatric schizophrenia (p=0.25).The independent auditor determined that all but two subjects included in the RCT had reliable safety data.
Post-hoc analyses for RCT
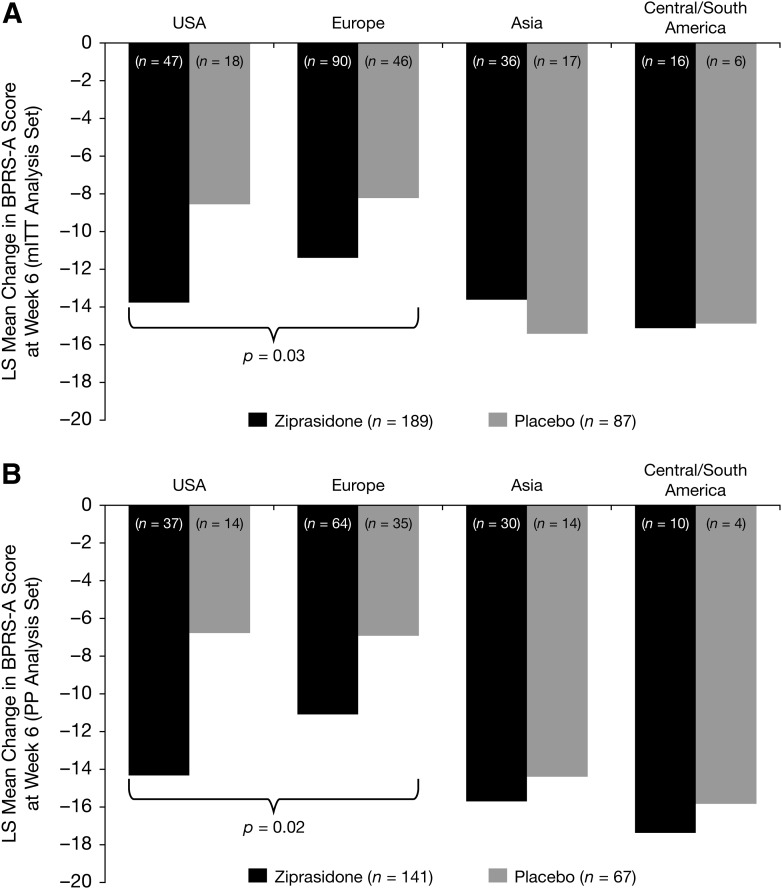
Post-hoc analysis explored regional differences. The mean (SD) baseline BPRS-A scores were consistent across ziprasidone and placebo treatment groups in North America (53.0 [11.7], n=47 versus 53.9 [13.0], n=18), in Europe (50.4 [9.0], n=90 versus 50.3 [8.7], n=46), in South/Central America (53.2 [12.7], n=16 versus 48.7 [10.7], n=6), and in Asia (48.6 [8.9], n=36 versus 47.0 [8.1], n=17). The results indicated a significant treatment effect at week 6 for the United States and European/Eastern European regions for the mITT analysis and PP analysis sets (p=0.03 and p=0.02). The test was based on a contrast that averaged the effect across the two regions. There was no significant treatment effect for the Asia or South/Central America regions for either the mITT or PP analysis sets [p>0.5 for each comparison]). Whereas the response to ziprasidone was consistent among regions, the placebo response observed in Asia and Central/South America was higher than in other regions (Fig. 3).
FIG. 3.
Primary end-point by geographical region among the mITT analysis set (A) and the PP analysis set (B). BPRS-A, Brief Psychiatric Rating Scale – Anchored; m ITT, intent to treat; LS, least square; PP, per protocol. p value reported is based on contrast averaged across United States and European/Eastern European regions.
OLE phase
Mean (SD) baseline (last observation in RCT phase) BPRS-A scores were 39.8 (12.8) (n=220, one subject had missing data at baseline), and continued to decrease throughout the study (Fig. 2). Mean (SD) baseline CGI-S scores for the group (n=221) were 3.9 (1.1) and decreased slightly from baseline to the end of the OLE phase (Fig. 2). Mean (SD) CGAS scores increased from baseline throughout the OLE phase, suggesting an improvement in overall functioning (at baseline: 53.2 [13.8], n=221; at week 26: 65.6 [12.4], n=76; and at week 26 last observation carried forward: 60.5 [14.2], n=197).
Safety assessments
RCT phase
Of 193 subjects in the ziprasidone group, 147 reported 458 AEs, whereas 49 of 90 subjects in the placebo group reported 110 AEs for any reason. Of all treatment-emergent AEs from all causalities (≥5% incidence), somnolence and extrapyramidal disorders were the most frequent (≥10%) in the ziprasidone group compared with placebo (Table 4). More subjects in the ziprasidone group (n=14) discontinued for treatment-related AEs (7.3% vs. 3.3%) than in the placebo group (n=3). Similar proportions of ziprasidone-treated subjects (n=22, 11.4%) and placebo-treated subjects (n=11, 12.2%) discontinued treatment for AEs from all causes. One subject each from the ziprasidone and placebo groups discontinued treatment because of increased alanine aminotransferase levels (ALT). In the ziprasidone group, two subjects discontinued because of QT prolongation. One subject (male, age 16 years, 80 mg/day ziprasidone) had a mild QT prolongation on day 27 (QTcF 468 ms) that resolved, after treatment discontinued, by day 33. Another subject (female, age 17 years, 120 mg/day ziprasidone) had a QTcF reading of 394–402 ms on day 1 and a QTcF reading of 456–470 ms on day 23 (multiple recordings were done); the prolongation resolved on day 31 after discontinuation of treatment. Three subjects discontinued treatment because of sedation or somnolence; none were reported in the placebo group.
Table 4.
Incidence of Treatment-Emergent Adverse Events (All Causalities) Occurring in ≥5% of Subjects
| |
RCT |
OLE |
|
|---|---|---|---|
| n | Ziprasidone 193 | Placebo 90 | Ziprasidone 221 |
| Adverse event, n (%) | |||
| Somnolence | 38 (19.7) | 6 (6.7) | 33 (14.9) |
| Extrapyramidal disorder | 22 (11.4) | 1 (1.1) | (<5) |
| Nausea | 19 (9.8) | 2 (2.2) | (<5) |
| Dizziness | 18 (9.3) | 1 (1.1) | 8 (3.6) |
| Insomnia | 18 (9.3) | 13 (14.4) | (<5) |
| Fatigue | 17 (8.8) | 4 (4.4) | 11 (5.0) |
| Headache | 15 (7.8) | 2 (2.2) | 20 (9.0) |
| Tremor | 15 (7.8) | 1 (1.1) | 14 (6.3) |
| Akathisia | 13 (6.7) | 3 (3.3) | (<5) |
| Vomiting | 12 (6.2) | 3 (3.3) | (<5) |
| Overdose | 12 (6.2) | 4 (4.4) | (<5) |
OLE, open-label extension; RCT, randomized controlled trial.
Among treatment-emergent SAEs, 13 were reported in 10 ziprasidone subjects, and 2 were reported in 1 placebo subject. None were judged to be drug-related by the investigator. Six subjects on ziprasidone and one subject on placebo had SAEs that led to permanent discontinuation. One SAE was an overdose of sertraline (not the study drug), which was considered a suicidal gesture leading to permanent discontinuation from the study on day 7; all other SAEs were related to the disease under study. One subject was hospitalized for suicidal ideation and completed the study. Other SAEs in the ziprasidone group were laceration to wrist (accidental), hostility, worsening of anxiety, depression, hallucinations, and other symptoms related to schizophrenia. In the placebo group, one subject had two SAEs (aggression, increase in psychosis) during the RCT phase.
There were 21 subjects with PSRAEs, 7.8% (n=15) and 6.7% (n=6) of subjects in the ziprasidone and placebo groups, respectively, according to the Columbia Classification system (Posner et al. 2007). Of these, four events in the ziprasidone group were considered SAEs. Fifteen subjects (11 subjects receiving ziprasidone and 4 subjects receiving placebo) had PSRAE that were adjudicated as non-suicide related, and classified as “Other: accident, psychiatric, or medical events.” Two subjects receiving ziprasidone and one subject receiving placebo displayed self-injurious behavior; two subjects receiving ziprasidone and one subject receiving placebo had suicidal ideation; and one subject receiving ziprasidone attempted suicide. The mean (SD) CDRS-R total score decreased from baseline at all visits, and decreases from baseline to week 6 were numerically similar in the ziprasidone group (−7.9 [7.9]) relative to the placebo group (−6.5 [5.5]).
Overdoses were reported as AEs for 11 subjects on ziprasidone and 4 subjects receiving placebo; all were the result of dosing errors (11 subjects [9 ziprasidone, 2 placebo] caused by a prescriber/instruction error, 3 caused by a subject or parent error, and 1 subject was noted as “patient overdosed” with insufficient further information). Overdosing ranged from 1 to 30 days at 100–200 mg/day for subjects weighing<45 kg, and from 1 to 19 days, at 180–400 mg/day for subjects weighing ≥45 kg. Ten of the 15 subjects reported no AE during the overdose. The majority of the AEs that occurred simultaneously with incorrect dosing were mild or moderate in severity. One subject (58 kg) had AEs of severe tremor and overdose (180–320 mg/day for 13 days), and was discontinued because of the overdose. Some of the overdoses appeared to be related to study staff at the site misunderstanding the drug dispensing instructions for the study drug blister packs. Partway through the study, the study team placed a temporary hold on new enrollments until the study drug in new packaging was available and shipped to the sites. The number of overdosings of the study drug caused by investigator error decreased after this change was instituted. One subject was discontinued because of overdose with sertraline (not the study drug) in a suicide attempt. Other clinically relevant AEs reported during the period of overdosing were akathisia (n=3), tremor (n=2), dyskinesia (n=2), extrapyramidal symptoms (n=2), and one each of fatigue, lethargy, sedation, insomnia, somnolence, blood prolactin increase, blood bilirubin increase, blood creatinine increase, blood phosphorous decrease, neutrophil count increase, and petechiae.
Among the treatment-emergent AEs (all causalities), the majority were nervous system disorder AEs and included somnolence, extrapyramidal disorders, akathisia, tremor, and musculoskeletal stiffness (Table 4). However, no statistically significant differences between the treatment groups were observed in these symptoms based on any movement disorder scale (SARS, BARS, and AIMS) administered (Table 5).
Table 5.
Movement Disorder Scales
| |
RCT |
OLE |
||||
|---|---|---|---|---|---|---|
| Mean (SD) | n | Ziprasidone | n | Placebo | n | Ziprasidone |
| SARS total score | ||||||
| Baseline | 189 | 0.7 (2.1) | 87 | 0.6 (1.7) | 221 | 0.9 (2.2) |
| Change from baseline to week 6− or week 26−LOCF | 189 | 0.3 (2.5) | 86 | −0.1 (0.6) | 206 | −0.2 (1.5) |
| BARS | ||||||
| Baseline | 190 | 0.1 (0.5) | 88 | 0.1 (0.3) | 221 | 0.1 (0.4) |
| Change from baseline to week 6− or week 26−LOCF | 190 | 0.0 (0.6) | 87 | 0.0 (0.2) | 206 | 0.1 (0.5) |
| AIMS | ||||||
| Baseline | 190 | 0.2 (0.8) | 88 | 0.3 (0.9) | 221 | 0.3 (1.2) |
| Change from baseline to week 6− or week 26−LOCF | 190 | 0.0 (0.8) | 87 | 0.0 (0.7) | 206 | 0.0 (1.0) |
RCT, randomized controlled trial; OLE, open-label extension; LOCF, last observation carried forward; BARS, Barnes Akathisia Rating Scale; AIMS, Abnormal Involuntary Movement Scale; SARS, Simpson-Angus Rating Scale.
The height, weight, waist circumference, and BMI z-score derived from Centers for Disease Control and Prevention (CDC) growth charts were comparable between the ziprasidone and placebo groups at baseline and throughout the study (Tables 6 and 7). The mean changes from baseline in supine hemodynamic measures and pulse rate were small for both treatment groups, and were clinically insignificant (Table 6).
Table 6.
Vital Signs and Metabolic Measures
| |
RCT |
OLE |
|||||
|---|---|---|---|---|---|---|---|
| |
Ziprasidone |
Placebo |
Ziprasidone |
||||
| Baseline | Change from baseline to week 6 | Baseline | Change from baseline to week 6 | Baseline | Change from baseline to week 26 | Change from baseline to week 26/EOT | |
| Vital signs | |||||||
| Height (cm) | |||||||
| n | 193 | 135 | 90 | 52 | 221 | 75 | 203 |
| Mean±SD | 164.9±10.1 | 0.2±0.7 | 167.8±10.0 | 0.0±0.6 | 165.8±10.0 | 166.3±10.3 | 166.7±9.7 |
| Weight (kg) | |||||||
| n | 193 | 134 | 90 | 52 | 221 | 76 | 204 |
| Mean±SD | 61.2±15.5 | −0.1±2.4 | 64.3±15.7 | 0.0±2.0 | 61.7±14.5 | 1.7±5.6 | 0.7±4.6 |
| BMI z (kg/m2) | |||||||
| n | 193 | 134 | 90 | 52 | 221 | 75 | 203 |
| Mean±SD | 0.3±1.1 | 0.0±0.2 | 0.4±1.1 | 0.0±0.2 | 0.3±1.1 | 0.5±1.0 | 0.3±1.1 |
| Absolute BMI percentile | |||||||
| n | 193 | 134 | 90 | 52 | 221 | 75 | 203 |
| Mean±SD | 57.5±31.1 | 57.3±31.2 | 60.2±30.8 | 55.0±32.3 | 58.6±31.2 | 67.4±28.4 | 59.7±31.4 |
| Waist (cm) | |||||||
| n | 188 | 129 | 85 | 47 | 212 | 74 | 194 |
| Mean±SD | 76.9±12.9 | −0.2±2.7 | 78.0±13.1 | 0.1±2.8 | 77.2±11.8 | 1.1±6.5 | 0.3±5.1 |
| Supine hemodynamic measures | |||||||
| SBP (mm Hg) | |||||||
| n | 193 | 135 | 90 | 52 | 221 | 76 | 205 |
| Mean±SD | 113.6±9.2 | −0.6±9.7 | 113.7±8.5 | 2.8±8.6 | 114.6±9.9 | −0.9±11.4 | −1.2±10.4 |
| DBP (mm Hg) | |||||||
| n | 193 | 135 | 90 | 52 | 221 | 76 | 205 |
| Mean±SD | 71.0±8.5 | −0.9±7.7 | 72.1±7.6 | 1.0±6.8 | 71.6±8.9 | −0.1±7.7 | −1.1±9.0 |
| Pulse (bpm) | |||||||
| n | 193 | 135 | 90 | 52 | 221 | 76 | 205 |
| Mean±SD | 76.0±10.5 | −1.3±11.3 | 77.1±10.7 | −1.1 (11.4) | 75.7±10.4 | −1.6±11.5 | −0.7±11.8 |
| QTcF (ms) | |||||||
| n | 193 | 134 | 89 | 52 | 221 | 73 | 179 |
| Mean±SD | 392.0±17.3 | 5.1±15.9 | 389.9±20.1 | 0.3±17.0 | 392.0±16.7 | 4.3±20.3 | 3.8±18.9 |
| Metabolic measures | |||||||
| Fasting glucose (mg/dL) | |||||||
| n | 159 | 115 | 78 | 48 | 182 | 63 | 146 |
| Mean±SD | 90.0±16.1 | −2.8±18.8 | 91.3±14.0 | 3.2±17.0 | 90.8±16.3 | 1.1±12.9 | 1.2±18.8 |
| Insulin (μU/dL) | |||||||
| n | 145 | 112 | 68 | 38 | 36 | 11 | 24 |
| Mean±SD | 17.7±47.6 | −1.2±30.8 | 13.1±17.9 | 7.3±22.3 | 15.0±17.1 | −3.9±24.1 | 3.8±25.9 |
| Total fasting cholesterol (mg/dL) | |||||||
| n | 164 | 125 | 75 | 50 | 185 | 69 | 156 |
| Mean±SD | 158.9±31.3 | −8.3±25.4 | 158.9±30.7 | −5.3±20.2 | 157.0±30.8 | −4.0±30.0 | −5.8±27.6 |
| Fasting HDL-C (mg/dL) | |||||||
| n | 150 | 115 | 74 | 49 | 179 | 63 | 146 |
| Mean±SD | 48.1±12.1 | −0.4±9.4 | 48.2±12.2 | −1.3±10.2 | 47.6±11.8 | 2.2±12.1 | 0.3±11.2 |
| Fasting LDL-C (mg/dL) | |||||||
| n | 150 | 115 | 74 | 49 | 179 | 63 | 146 |
| Mean±SD | 91.7±25.7 | −7.5±19.2 | 89.6±25.0 | −2.6±16.9 | 89.5±25.1 | −5.3±24.5 | −5.0±22.8 |
| Fasting triglycerides (mg/dL) | |||||||
| n | 150 | 115 | 74 | 49 | 179 | 63 | 146 |
| Mean±SD | 102.7±50.1 | −3.9±63.6 | 114.1±73.2 | −13.2±85.9 | 107.0±63.8 | −4.1±55.2 | −7.1±61.8 |
Normal values for fasting glucose: 60–<100 mg/dL, insulin: 6–27 μmol/dL, total cholesterol: 85–169 mg/dL, HDL-C: 40–75 mg/dL, LDL-C: 62–129 mg/dL, triglycerides: 26–109 mg/dL.
BMI, body mass index; bpm, beats/min; DBP, diastolic blood pressure; EOT, end of treatment; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; OLE, open-label extension; QTcF, Fridericia-corrected QT interval; RCT, randomized controlled trial; SBP, systolic blood pressure.
Table 7.
BMI-z Score Change from Baseline (A) and Categoric Weight Change from Baseline (B)
|
A. BMI-z score change from baseline | ||||
|---|---|---|---|---|
| |
RCT |
OLE |
|
|
| Baseline | Ziprasidone | Placebo | Ziprasidone | |
| n | 193 | 90 | 221 | |
| n (%) | n (%) | n (%) | ||
| < −2 | 0 | 0 | 0 | |
| ≥−2 to<−1.5 | 8 (4.2) | 5 (5.6) | 12 (5.4) | |
| ≥−1.5 to<−1 | 23 (11.9) | 7 (7.8) | 21 (9.5) | |
| ≥−1 to<−0.5 | 21 (10.9) | 7 (7.8) | 26 (11.8) | |
| ≥−0.5 to<0 | 24 (12.4) | 14 (15.6) | 28 (12.7) | |
| ≥0 to<0.5 | 34 (17.6) | 14 (15.6) | 33 (14.9) | |
| ≥0.5 to<1.0 | 30 (15.5) | 11 (12.2) | 34 (15.4) | |
| ≥1.0 to<1.5 | 24 (12.4) | 19 (21.1) | 35 (15.8) | |
| ≥1.5 to<2.0 | 20 (10.4) | 8 (8.9) | 23 (10.4) | |
| ≥2 to<2.5 | 7 (3.6) | 5 (5.6) | 9 (4.1) | |
| ≥2.5 to<3 | 2 (1.0) | 0 | 0 | |
| ≥3 | 0 | 0 | 0 | |
| Week 6 or week 26/early termination change from baseline | ||||
| n | 134 | 52 | 221 | |
| n (%) | n (%) | n (%) | ||
| < −2 | 0 | 0 | 0 | |
| ≥−2 to<−1.5 | 0 | 0 | 1 (0.5) | |
| ≥−1.5 to<−1 | 0 | 0 | 6 (3.0) | |
| ≥−1 to<−0.5 | 6 (4.5) | 1 (1.9) | 10 (4.9) | |
| ≥−0.5 to<0 | 83 (61.9) | 29 (55.8) | 105 (51.7) | |
| ≥ 0 to<0.5 | 42 (31.3) | 22 (42.3) | 69 (34.0) | |
| ≥ 0.5 to<1.0 | 3 (2.2) | 0 | 6 (3.0) | |
| ≥ 1.0 to<1.5 | 0 | 0 | 3 (1.5) | |
| ≥ 1.5 to<2.0 | 0 | 0 | 3 (1.5) | |
| ≥ 2 to<2.5 | 0 | 0 | 0 | |
| ≥2.5 to<3 | 0 | 0 | 0 | |
| ≥3 | 0 | 0 | 0 | |
| B. Weight categories at baseline and shift from baselinea | ||||
| n | 193 | 90 | 221 | |
| Baseline underweight, n(%) | 1 (0.5) | 1 (1.1) | 1 (0.5) | |
| Baseline normal weight, n(%) | 140 (72.5) | 58 (64.4) | 155 (70.1) | |
| Baseline overweight, n(%) | 31 (16.1) | 22 (24.4) | 45 (20.4) | |
| Baseline Obese, n(%) | 21 (10.9) | 9 (10.0) | 20 (9.1) | |
|
RCT: Shift from baseline to week 6/ET | ||||
|---|---|---|---|---|
| Ziprasidone | Underweight | Normal weight | Overweight | Obese |
| Baseline underweight | 1 | 0 | 0 | 0 |
| Baseline normal weight | 1 | 130 | 1 | 0 |
| Baseline overweight | 0 | 4 | 22 | 2 |
| Baseline obese | 0 | 0 | 1 | 17 |
|
RCT: Shift from baseline to week 6/ET | ||||
|---|---|---|---|---|
| Placebo | Underweight | Normal weight | Overweight | Obese |
| Baseline underweight | 0 | 1 | 0 | 0 |
| Baseline normal weight | 0 | 54 | 1 | 0 |
| Baseline overweight | 0 | 4 | 18 | 0 |
| Baseline obese | 0 | 0 | 0 | 9 |
|
OLE: shift from baseline to week 26/ET | ||||
|---|---|---|---|---|
| Ziprasidone | Underweight | Normal weight | Overweight | Obese |
| Baseline underweight | 1 | 0 | 0 | 0 |
| Baseline normal weight | 1 | 136 | 6 | 0 |
| Baseline overweight | 0 | 5 | 31 | 5 |
| Baseline obese | 0 | 0 | 5 | 13 |
Based on Centers for Disease Control and Prevention (CDC) growth charts:
BMI percentile<5th was defined as underweight; BMI percentile ≥5th and<85th was defined as normal weight; BMI percentile ≥85th and <95th was defined as overweight; and BMI percentile ≥95th was defined as obese.
BMI, body mass index; ET, end of treatment; OLE, open-label extension; RCT, randomized controlled trial.
The mean baseline and mean changes from baseline in the metabolic indices did not show a clinically significant difference between treatment groups (Table 6). Overall, for fasting glucose and lipid values, most of the shifts in both treatment groups were from abnormal to normal laboratory values (Table 8).
Table 8.
Categoric Change in Metabolic Measures
| |
RCT |
OLE |
||||
|---|---|---|---|---|---|---|
| |
Ziprasidone |
Placebo |
Ziprasidone |
|||
| Category change from baseline | n | n (%) | n | n (%) | n | n (%) |
| Glucose | ||||||
| Normal to abnormal | 136 | 1 (0.7) | 60 | 2 (3.3) | 159 | 2 (1.3) |
| Borderline to abnormal | 19 | 0 | 16 | 0 | 23 | 2 (8.7) |
| Abnormal to normal | 4 | 2 (50.0) | 2 | 2 (100) | 5 | 3 (60.0) |
| Triglycerides | ||||||
| Normal to abnormal | 73 | 6 (8.2) | 34 | 6 (17.7) | 91 | 7 (7.8) |
| Borderline to abnormal | 45 | 16 (35.6) | 19 | 0 | 47 | 10 (21.3) |
| Abnormal to normal | 32 | 12 (37.5) | 21 | 4 (19.1) | 46 | 12 (26.1) |
| Cholesterol | ||||||
| Normal to abnormal | 110 | 1 (0.9) | 49 | 0 | 131 | 2 (1.5) |
| Borderline to abnormal | 39 | 7 (18.0) | 17 | 2 (11.8) | 45 | 8 (17.8) |
| Abnormal to normal | 15 | 5 (33.3) | 9 | 2 (22.2) | 14 | 6 (42.9) |
| LDL-C | ||||||
| Normal to abnormal | 116 | 1 (0.9) | 59 | 0 | 147 | 2 (1.4) |
| Borderline to abnormal | 23 | 4 (17.4) | 8 | 2 (25.0) | 28 | 3 (10.7) |
| Abnormal to normal | 11 | 4 (36.4) | 7 | 3 (42.9) | 9 | 2 (22.2) |
| HDL-C | ||||||
| Normal to abnormal | 126 | 12 (9.5) | 64 | 7 (10.9) | 155 | 6 (3.9) |
| Abnormal to normal | 24 | 16 (66.7) | 10 | 5 (50.0) | 29 | 14 (48.3) |
Glucose:<100 mg/dL - normal, 100–125 mg/dL - borderline,>125 mg/dL - abnormal.
Triglycerides:<90 mg/dL- normal, 90–130 mg/dL - borderline,>130 mg/dL - abnormal.
Cholesterol:<170 mg/dL - normal, 170–199 mg/dL - borderline,>199 mg/dL - abnormal.
LDL-C:<110 mg/dL - normal, 110–129 mg/dL - borderline,>129 - abnormal.
HDL-C:>= 35 mg/dL - normal,<35 - abnormal.
OLE, OLE, open-label extension; RCT, randomized controlled trial; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
In the ziprasidone group, there was a small increase in the mean QTcF intervals compared with the placebo group at all time points (mean changes from baseline at week 1 through week 6 ranged from 3.9 to 10.8 ms in the ziprasidone group vs. −3.6 to 3.6 ms in the placebo group). QTcF prolongation (≥460 ms) was reported in five ziprasidone-treated subjects and in none in the placebo group; QTcF increase of ≥60 ms from baseline occurred in four subjects on ziprasidone (maximum change from baseline of 70 ms [n=1], 63 ms [n=2], and 66 ms [n=1]) and none on placebo. No subjects reported a QTcF of ≥500 ms.
OLE phase
Among 221 subjects in the safety analysis set, 139 subjects reported 393 AEs, and 94 AEs were considered to be treatment related by the investigator. Among treatment-emergent AEs that occurred more frequently with ziprasidone than with placebo at a rate of ≥5% in either treatment group were: Fatigue, somnolence, headache, dizziness, and tremor (Table 4).
Out of total 145 subjects (65.6%) who discontinued the study, 92 (41.6%) were discontinued by the study sponsor because of early termination of the study and 5 (2.3%) discontinued because of AEs considered related to study drug. These AEs were sedation (n=1), somnolence (n=1), weight increase (n=1), weight decrease (n=1), and muscle rigidity (n=1). A total of 22 subjects (10.0%) discontinued the study because of treatment-emergent AEs. The causality of most discontinuations for AEs was the disease under study. Nine subjects were reported as discontinued because of SAEs: Schizophrenia (n=4), auditory hallucination (n=1), suicidal ideation (n=1), suicidal behavior (n=1), drug ineffective (n=1), and sexual activity increased (n=1). For one of these subjects, the SAE (schizophrenia) occurred before dosing and was, therefore, not considered treatment emergent. One subject had an AE of viral infection (hepatitis B), unrelated to the study drug, that led to permanent discontinuation.
Ten subjects (4.5%) had 19 PSRAEs, and most were reported as SAEs. One subject (17-year-old Asian female, weighing 52 kg), who was in the placebo group in the preceding RCT phase and was on 160 mg/day ziprasidone during the OLE phase, committed suicide during the study on day 23. The investigator considered the causality of the event to be unknown or related to study drug (poor control of symptoms of schizophrenia).
A total of 35 SAEs in 18 subjects were reported. Two subjects (0.9%) had SAEs that were judged to be treatment related (completed suicide [described previously] and acute dystonic reaction).
Three AEs of overdose were reported for two subjects; all were the result of investigator or subject dosing error. One subject (17-year-old male weighing 78.5 kg) was reported as having had a “possible overdose” during the titration period (days 1–7), although the maximal allowed daily dose was not exceeded, and then had an AE of overdose on day 8 (220 mg/day). One subject (18-year-old male weighing 67.8 kg) took, by error, 180 mg/day ziprasidone instead of the prescribed 160 mg/day on day 23.
The majority of treatment-emergent AEs (all causalities) were related to nervous system disorders and included somnolence, headache, and tremor (Table 4). Nonetheless, movement disorders assessments (SARS, BARS, and AIMS) showed little or no change from baseline values for the majority of subjects (Table 5).
Changes in BMI z-score from baseline were generally small; the majority of subjects had BMI z-scores between −1 and<1 at weeks 6 and 26 (Tables 6 and 7). Mean changes in supine hemodynamic measures were small and not clinically significant (Table 6). For fasting glucose and lipids, changes from normal to abnormal values were seen only in a small number of subjects. A higher proportion of subjects with abnormal baseline values experienced a change to normal values, compared with the proportion of subjects whose values changed from normal to abnormal (Table 8).
In the mean QTcF intervals, there were increases ranging from 2.5 to 8.7 ms at all time points, but no particular pattern over time was apparent. No subjects reported QTcF ≥500 ms, and only one subject had QTcF ≥460 ms. Two subjects had increases of ≥60 ms from baseline measurements (69 ms and 60 ms). One subject had an AE of ECG ST segment elevation, and one subject had an AE of ECG T-wave inversion; both AEs subsequently resolved without any intervention.
Discussion
Ziprasidone therapy failed to show superiority compared with placebo in the treatment of schizophrenia in adolescent subjects using the BPRS-A measure based on the ITT analysis set during the 6 week RCT phase. However, in the PP analysis set, there was a significant difference in the ziprasidone group compared with placebo. All other secondary end-points (except PANSS positive subscale) were not significantly different between the treatment groups in both the mITT and PP analysis sets. No new or unexpected safety concerns arose in this pediatric population in the RCT phase or in the extended follow-up during the OLE phase when compared with other pediatric studies (Toren et al. 2004; DelBello et al. 2008).
Ziprasidone is an approved treatment for schizophrenia in adults (Keck et al. 1998; Daniel et al. 1999) and the results of this study in the adolescent population were surprising. We conducted post-hoc analyses to examine treatment differences for BPRS-A by geographic location in both the mITT and PP analysis sets. The difference in efficacy between ziprasidone and placebo was found to be significant across the United States and European/Eastern European regions. Whereas the placebo response was similar across United States and European/Eastern European regions, the placebo response was markedly higher in Asia and South/Central America. The failure of the overall study to demonstrate ziprasidone's efficacy over placebo could relate to the much higher placebo response observed in Asia and Central/South America, which accounted for 27% of the study population. Whereas a differential country response has been observed in trials of ziprasidone in adults (Vieta et al. 2010), this is the first observation of a differential regional response to ziprasidone in adolescents. Another study of olanzapine in adolescent subjects with schizophrenia also reported geographic differences (Kryzhanovskaya et al. 2009).
Ziprasidone was generally well tolerated in adolescents with schizophrenia, with no new safety findings in the RCT or OLE phase compared with the adult studies ( Keck et al. 1998; Daniel et al. 1999). Whereas in the ziprasidone group the incidence of treatment-related SAEs was higher than in the placebo group, none were considered treatment related during the RCT, and there was a>1% occurrence during the OLE phase. The most commonly occurring AEs (somnolence and extrapyramidal disorders) overall were attributable to known pharmacologic effects of ziprasidone. However, movement disorders assessments did not show any clinically significant change from baseline in the majority of subjects in either phase of the study. No deaths were reported in the RCT phase of the study, and only one death occurred during the OLE phase, a suicide that the investigator attributed to poor control of symptoms of schizophrenia. An important limitation of this study was that ziprasidone serum levels were not measured during the study to ensure appropriate dosing, although the selected doses were determined based on previous studies (DelBello et al. 2008). Ziprasidone treatment was not associated with any clinically significant change from baseline in various hemodynamic measures, weight (BMI z-score), fasting glucose, lipids, and other metabolic indices.
Conclusions
Ziprasidone failed to separate from placebo in treatment of schizophrenia in adolescents. Factors that may have contributed to lack of statistical significance (based on mITT analysis set) include geographic region, and require further investigation. Ziprasidone was generally well tolerated, with an overall neutral weight and metabolic profile.
Clinical Significance
We report the results of a short-term, double-blind, randomized controlled trial and of a long-term open-label extension trial of ziprasidone monotherapy in adolescents with schizophrenia. When compared with the proven efficacy of ziprasidone monotherapy in adults with schizophrenia, these data indicate that ziprasidone was not efficacious in adolescents. Geographic variability in response may be a contributing factor that needs further exploration. The long-term data suggest that ziprasidone was well tolerated with a neutral metabolic profile, consistent with all prior studies.
Acknowledgments
Editorial support was provided by H. Koeller, PhD, of PAREXEL, and funded by Pfizer Inc.
Disclosures
Dr. Findling receives or has received research support from, acted as a consultant for, received royalties from, and/or served on a speaker's bureau for Abbott, Addrenex, Alexza, American Psychiatric Press, AstraZeneca, Biovail, Bristol-Myers Squibb, Dainippon Sumitomo Pharma, Forest, GlaxoSmithKline, Guilford Press, Johns Hopkins University Press, Johnson & Johnson, KemPharm Lilly, Lundbeck, Merck, National Institutes of Health, Neuropharm, Novartis, Noven, Organon, Otsuka, Pfizer, Physicians' Post-Graduate Press, Rhodes Pharmaceuticals, Roche, Sage, Sanofi-Aventis, Schering-Plough, Seaside Therapeutics, Sepracore, Shionogi, Shire, Solvay, Stanley Medical Research Institute, Sunovion, Supernus Pharmaceuticals, Transcept Pharmaceuticals, Validus, WebMD, and Wyeth. Dr. Çavuş was an employee of Pfizer during the study conduct and manuscript preparation and is now an employee of Bristol-Myers Squib. This study was supported by funding from Pfizer Inc. Drs. Pappadopulos, Vanderburg and Schwartz are employees of Pfizer, and Mr. Gundapaneni is a contracted employee of Pfizer. Dr. DelBello has received research support from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen,Johnson & Johnson, Martek, Merck, Novartis, Pfizer, Repligen, Schering-Plough, Shire, and Somerset. She is on the lecture bureau of or has received consulting fees from Bristol-Myers Squibb, Pfizer, and Merck.
Drs. Findling, Çavuş, Pappadopulos, Vanderburg, and DelBello contributed to the conceptualization, drafting, and revision of the manuscript; statistical support was provided by Dr. Schwartz and Mr. Gundapaneni. All co-authors have reviewed and approved the manuscript.
References
- American Psychiatric Association. 4th. Washington, DC: American Psychiatric Association; 1994. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- American Psychiatric Association. 2nd. Arlington, VA: American Psychiatric Association; 2004. Practice Guideline for the Treatment of Patients with Schizophrenia. [Google Scholar]
- Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- Beratis S. Gabriel J. Hoidas S. Age at onset in subtypes of schizophrenic disorders. Schizophr Bull. 1994;20:287–296. doi: 10.1093/schbul/20.2.287. [DOI] [PubMed] [Google Scholar]
- Burd L. Kerbeshian J. A North Dakota prevalence study of schizophrenia presenting in childhood. J Am Acad Child Adolesc Psychiatry. 1987;26:347–350. doi: 10.1097/00004583-198705000-00012. [DOI] [PubMed] [Google Scholar]
- Daniel DG. Zimbroff DL. Potkin SG. Reeves KR. Harrigan EP. Lakshminarayanan M. Ziprasidone 80 mg/day and 160 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: A 6-week placebo-controlled trial. Ziprasidone Study Group. Neuropsychopharmacology. 1999;20:491–505. doi: 10.1016/S0893-133X(98)00090-6. [DOI] [PubMed] [Google Scholar]
- De Hert M. Dobbelaere M. Sheridan EM. Cohen D. Correll CU. Metabolic and endocrine adverse effects of second-generation antipsychotics in children and adolescents: A systematic review of randomized, placebo controlled trials and guidelines for clinical practice. Eur Psychiatry. 2011;26:144–158. doi: 10.1016/j.eurpsy.2010.09.011. [DOI] [PubMed] [Google Scholar]
- DelBello MP. Versavel M. Ice K. Keller D. Miceli J. Tolerability of oral ziprasidone in children and adolescents with bipolar mania, schizophrenia, or schizoaffective disorder. J Child Adolesc Psychopharmacol. 2008;18:491–499. doi: 10.1089/cap.2008.008. [DOI] [PubMed] [Google Scholar]
- Findling RL. Johnson JL. McClellan J. Frazier JA. Vitiello B. Hamer RM. Lieberman JA. Ritz L. McNamara NK. Lingler J. Hlastala S. Pierson L. Puglia M. Maloney AE. Kaufman EM. Noyes N. Sikich L. Double-blind maintenance safety and effectiveness findings from the Treatment of Early-Onset Schizophrenia Spectrum (TEOSS) study. J Am Acad Child Adolesc Psychiatry. 2010;49:583–594. doi: 10.1016/j.jaac.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling RL. McKenna K. Earley WR. Stankowski J. Pathak S. Efficacy and safety of quetiapine in adolescents with schizophrenia investigated in a 6–week, double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol. 2012;22:327–342. doi: 10.1089/cap.2011.0092. [DOI] [PubMed] [Google Scholar]
- Findling RL. Robb A. Nyilas M. Forbes RA. Jin N. Ivanova S. Marcus R. McQuade RD. Iwamoto T. Carson WH. A multiple-center, randomized, double-blind, placebo-controlled study of oral aripiprazole for treatment of adolescents with schizophrenia. Am J Psychiatry. 2008;165:1432–1441. doi: 10.1176/appi.ajp.2008.07061035. [DOI] [PubMed] [Google Scholar]
- Guy W. Rockville, MD: National Institutes of Mental Health; 1976. ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education and Welfare Publication (ADM) 76–338. [Google Scholar]
- Haas M. Eerdekens M. Kushner S. Singer J. Augustyns I. Quiroz J. Pandina G. Kusumakar V. Efficacy, safety and tolerability of two dosing regimens in adolescent schizophrenia: Double-blind study. Br J Psychiatry. 2009a;194:158–164. doi: 10.1192/bjp.bp.107.046177. [DOI] [PubMed] [Google Scholar]
- Haas M. Unis AS. Armenteros J. Copenhaver MD. Quiroz JA. Kushner SF. A 6-week, randomized, double-blind, placebo-controlled study of the efficacy and safety of risperidone in adolescents with schizophrenia. J Child Adolesc Psychopharmacol. 2009b;19:611–621. doi: 10.1089/cap.2008.0144. [DOI] [PubMed] [Google Scholar]
- Hafner H. Maurer K. Loffler W. Riecher–Rossler A. The influence of age and sex on the onset and early course of schizophrenia. Br J Psychiatry. 1993;162:80–86. doi: 10.1192/bjp.162.1.80. [DOI] [PubMed] [Google Scholar]
- Kay SR. Fiszbein A. Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keck P., Jr. Buffenstein A. Ferguson J. Feighner J. Jaffe W. Harrigan EP. Morrissey MR. Ziprasidone 40 and 120 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 4-week placebo-controlled trial. Psychopharmacology (Berl) 1998;140:173–184. doi: 10.1007/s002130050755. [DOI] [PubMed] [Google Scholar]
- Keck PE., Jr. Versiani M. Potkin S. West SA. Giller E. Ice K. Ziprasidone in the treatment of acute bipolar mania: A three-week, placebo-controlled, double-blind, randomized trial. Am J Psychiatry. 2003;160:741–748. doi: 10.1176/appi.ajp.160.4.741. [DOI] [PubMed] [Google Scholar]
- Keck PE., Jr. Versiani M. Warrington L. Loebel AD. Horne RL. Long-term safety and efficacy of ziprasidone in subpopulations of patients with bipolar mania. J Clin Psychiatry. 2009;70:844–851. doi: 10.4088/jcp.08m04045. [DOI] [PubMed] [Google Scholar]
- Kryzhanovskaya L. Schulz SC. McDougle C. Frazier J. Dittmann R. Robertson–Plouch C. Bauer T. Xu W. Wang W. Carlson J. Tohen M. Olanzapine versus placebo in adolescents with schizophrenia: A 6-week, randomized, double-blind, placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48:60–70. doi: 10.1097/CHI.0b013e3181900404. [DOI] [PubMed] [Google Scholar]
- Maayan L. Correll CU. Weight gain and metabolic risks associated with antipsychotic medications in children and adolescents. J Child Adolesc Psychopharmacol. 2011;21:517–535. doi: 10.1089/cap.2011.0015. [DOI] [PubMed] [Google Scholar]
- Masi G. Liboni F. Management of schizophrenia in children and adolescents: Focus on pharmacotherapy. Drugs. 2011;71:179–208. doi: 10.2165/11585350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Matzner F. Silva R. Silvan M. Chowdhury M. Nastasi L. Preliminary test-retest reliability of the KID-SCID, in 1997 Annual Meeting New Research Program and Abstracts. Washington, DC. American Psychiatric Association; 1997. pp. 172–173. [Google Scholar]
- Posner K. Oquendo MA. Gould M. Stanley B. Davies M. Columbia Classification Algorithm of Suicide Assessment (C-CASA): Classification of suicidal events in the FDA's pediatric suicidal risk analysis of antidepressants. Am J Psychiatry. 2007;164:1035–1043. doi: 10.1176/appi.ajp.164.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potkin SG. Keck PE., Jr. Segal S. Ice K. English P. Ziprasidone in acute bipolar mania: A 21–day randomized, double-blind, placebo-controlled replication trial. J Clin Psychopharmacol. 2005;25:301–310. doi: 10.1097/01.jcp.0000169068.34322.70. [DOI] [PubMed] [Google Scholar]
- Poznanski EO. Freeman LN. Mokros HB. Children's Depression Rating Scale–Revised. Psychopharmacol Bull. 1985;21:979–989. [Google Scholar]
- Shaffer D. Gould MS. Brasic J. Ambrosini P. Fisher P. Bird H. Aluwahlia S. A children's global assessment scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- Simpson GM. Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Singh J. Robb A. Vijapurkar U. Nuamah I. Hough D. A randomized, double-blind study of paliperidone extended-release in treatment of acute schizophrenia in adolescents. Biol Psychiatry. 2011;70:1179–1187. doi: 10.1016/j.biopsych.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Toren P. Ratner S. Laor N. Weizman A. Benefit-risk assessment of atypical antipsychotics in the treatment of schizophrenia and comorbid disorders in children and adolescents. Drug Saf. 2004;27:1135–1156. doi: 10.2165/00002018-200427140-00005. [DOI] [PubMed] [Google Scholar]
- Vieta E. Ramey T. Keller D. English PA. Loebel AD. Miceli J. Ziprasidone in the treatment of acute mania: A 12-week, placebo-controlled, haloperidol-referenced study. J Psychopharmacol. 2010;24:547–558. doi: 10.1177/0269881108099418. [DOI] [PubMed] [Google Scholar]
- Woerner MG. Mannuzza S. Kane JM. Anchoring the BPRS: An aid to improved reliability. Psychopharmacol Bull. 1988;24:112–117. [PubMed] [Google Scholar]
- Young CM. Findling RL. Pharmacologic treatment of adolescent and child schizophrenia. Expert Rev Neurother. 2004;4:53–60. doi: 10.1586/14737175.4.1.53. [DOI] [PubMed] [Google Scholar]