Abstract
Chronic exposure to ultraviolet (UV) irradiation is believed to be the major cause of skin damage that results in premature aging of the skin, so called photoaging, characterized by increases in skin thickness, formation of wrinkles, and loss of skin elasticity. UV induces damage to skin mainly by oxidative stress and collagen degradation. In this study, we examined the photo-protective effect of hydroxysafflor yellow A (HSYA), a major active chemical component isolated from Carthamus tinctorius L., by topical application on the skin of mice. Exposure of the dorsal depilated skin of mice to UV radiation four times a week for 10 weeks induced epidermal hyperplasia, elastin accumulation, collagen degradation, etc. HSYA at the doses of 50, 100, and 200 μg/mouse was topically applied immediately following each UV exposure. The effects of HSYA were evaluated by a series of tests, including macroscopic and histopathological evaluation of skin, pinch test, and redox homeostasis of skin homogenates. Results showed that the UV-induced skin damage was significantly improved after HSYA treatment, especially at doses of 100 and 200 μg/mouse. This protective effect is possibly related to the anti-oxidative property of HSYA and mediated by promoting endogenous collagen synthesis. This is the first study providing preclinical evidence for the protective effect of HSYA against photoaging.
Introduction
Chronic exposure of human skin to ultraviolet (UV) radiation, including UVA (400–320 nm) and UVB (320–290 nm), is known to damage the structure and function of the skin. This damage is referred to collectively as photo-aging, which is characterized by wrinkles, laxity, roughness, and irregular pigmentation1–3 and is associated with many histopathologic changes, such as an accumulation of disorganized elastin fibers (elastosis) and a loss of interstitial collagens.4–6 Previous studies have shown that UV causes photo-oxidative damage to skin, mainly by inducing high levels of reactive oxygen species (ROS), which change gene expression and result in collagen degradation and elastin accumulation.7,8 Under physiological conditions, ROS are part of normal regulatory circuits. The ROS levels are tightly regulated by the anti-oxidants such as superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH). But these defenses will be overwhelmed if the dose of UV light is too high. Once ROS production exceeds the capacity of anti-oxidant defenses, oxidative stress occurs in the deeper layers of the skin.9,10 Oxidative stress causes oxidative damage to cellular components and changes the pattern of gene expression, and thus finally leads to skin pathologies such as non-melanoma and melanoma skin cancers, photo-toxicity, and photoaging.11–13 Given that anti-oxidants may prevent ROS formation or detoxify oxidative stress, regular intake of anti-oxidants and anti-oxidant nutrients as well as topical treatment with anti-oxidants are suggested to be beneficial strategies for preventing UV-mediated cutaneous damage.14–16
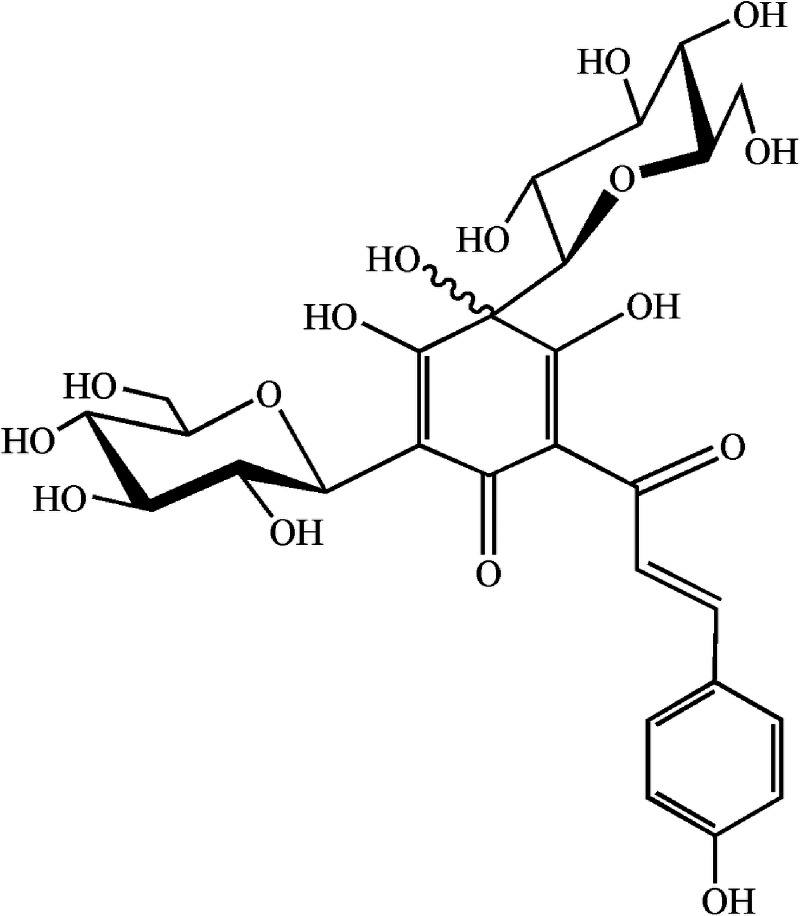
Hydroxysafflor yellow A (HSYA; chemical structure shown in Fig. 1), a major active chemical ingredient isolated from Carthamus tinctorius L., is the principal effective ingredient of Safflor Yellow Injection, which was approved as a new drug in 2005 by the State Food and Drug Administration of China for treating patients with ischemic cardio-cerebrovascular disease.17,18 Studies have demonstrated that HSYA exhibits multiple biomedical activities, such as scavenging oxygen-free radicals,19,20 reducing blood pressure and heart rate,21 and producing anti-inflammatory activity.22 Moreover, numerous research studies have also shown that HSYA exhibits neuroprotective effects against cerebral ischemia/reperfusion injuries, which are closely associated with the formation of ROS.19,23,24 Although the protective effect of HSYA against ischemia is associated with its anti-oxidative activity, little experimental data extend to other disease conditions resulting from oxidative stress, for example, UV-induced photoaging. In this study, to test whether HSYA has a photo-protective activity, we investigated the effect of topical HSYA application on a UV-induced photoaging mouse model. The effectiveness was tested by macroscopic and histopathological evaluation of the skin, the pinch test, and redox homeostasis assay of skin homogenates. Our results demonstrated for the first time that HSYA could protect mouse skin from photoaging induced by UV irradiation and revealed that this effect might be attributable to its free radical scavenging property.
FIG. 1.
Chemical structure of hydroxysafflor yellow A (HSYA) isolated from Carthamus tinctorius.
Materials and Methods
Materials
HSYA was generously provided by Shanxi Huahui Cade Pharmaceutical Co., Ltd. (Shanxi, China). The HYSA was obtained from C. tinctorius L, and its purity (>98%) was determined by high-performance liquid chromatography (HPLC). The HSYA powder was dissolved in ethanol-propylene glycol (7:3, vol/vol), at concentrations of 0.5, 1, and 2 mg/mL. Sample solutions (100 μL/mouse) were applied topically in the dorsal region. HSYA was applied at doses of 50, 100, and 200 μg/mouse.
Commercial kits used for determination of SOD, glutathione peroxidase (GSH-Px), CAT, and malondialdehyde (MDA), as well as the mouse hydroxyproline (Hyp) enzyme-linked immunosorbent assay (ELISA) kit were purchased from Jiancheng institution of Biotechnology (Nanjing, China). All other chemicals used in this study were analytical grade.
Experimental animals and grouping
Female KM mice (20–22 grams), aged approximately 8 weeks, were obtained from the animal center of Guangzhou University of Chinese Medicine (Guangzhou, China). All experimental protocols were approved by the Committee for Animal Care and Use at Guangzhou University of Chinese Medicine (approval number SCXK (Guangzhou)-2008–0020). The mice were housed under conventional conditions at controlled temperature (23±2°C), humidity (55±10%), and light (12 hr light/12 hr darkness, without any UV emission) and given food and water ad libitum throughout the study period. After 1 week of acclimatization to the home cage, animals were randomly divided into seven groups of nine mice each (Table 1). At the start of the experiment, the dorsal skin surface of the mice was shaved with ladies razors (Philips). Briefly, the mice were anesthetized using ether inhalation, and the long hair was sheared using surgical scissors; then the dorsal skin area (2.5×3 cm) was shaved clean. Thereafter, the shaving was performed as required (usually every day).
Table 1.
Treatment Schedule of the Study
| |
|
|
|
HSYA (μg/mouse) |
||
|---|---|---|---|---|---|---|
| Group | Shave | UV radiation | Vehicle 100 μL/mouse | 50 | 100 | 200 |
| NC | − | − | − | − | − | − |
| SC | + | − | − | − | − | − |
| MC | + | + | − | − | − | − |
| VC | + | + | + | − | − | − |
| HSYA-1 | + | + | + | + | − | − |
| HSYA-2 | + | + | + | − | + | − |
| HSYA-3 | + | + | + | − | − | + |
UV, ultraviolet; HSYA, hydroxysafflor yellow A; NC, untreated control; SC, shaved control; MC, model control; VC, solvent control; HSYA-1, treated with HYSA at a dose of 50 μg/mouse; HSYA-2, treated with HYSA at a dose of 100 μg/mouse; HSYA-3, treated with HYSA at a dose of 200 μg/mouse.
Preparation of photoaged mouse model
The mice were irradiated as described previously with slight modifications.25 Simulated solar irradiation was provided by an array of seven UVB lamps, with an emission spectrum between 285 and 350 nm (peak at 310–315 nm), surrounding three UVA lamps (Waldmann UV800, Germany) emitting exclusively UVA in the range of 320–400 nm (peak at 365 nm). The mice were exposed to UV radiation four times a week (except Monday, Wednesday, and Friday) starting with 70 mJ/cm2 for the first week, which was subsequently followed by 140 mJ/cm2 (the second week), then 210 mJ/cm2 (the third week), and 280 mJ/cm2 (the fourth week). Exposure was maintained at 280 mJ/cm2 for the remaining weeks to deliver a total dose of 9.52 J/cm2 over the 10 weeks. During the period of exposure, the mice were group-housed in a stainless steel irradiation chamber in which the animals could move around freely. The integrated UV irradiance was measured with a Waldmann UV meter (Waldmann Lichttechnik GmbH, Germany), and the minimal erythemal dose (MED) ranged between 70 and 80 mJ/cm2 for the mouse skin in our research. The animals were killed 2 days after the final irradiation to allow the recovery from the acute UV effects.
Each UV exposure was followed by treatment with the sample solutions (three different doses of HSYA) or the vehicle (100 μL per mouse). The formulation was spread evenly on the shaved dorsal area of each mouse until no residue was observed, with caution against applying outside the demarcated (shaved) area.
Macroscopic evaluation of dorsal skin
Skin was examined for photo-damage every week for 10 weeks. The UV-exposed dorsal skin of each mouse was photographed while the mouse was under anesthesia. The grade of photo-damage was determined according to evaluation criteria shown in Table 2, modified from Bissett et al.26,27 The grading score ranged from 0 for normal skin to 6 for severely photo-damaged skin.
Table 2.
Grading Scale for Evaluation of Photoaging
| Grade | Evaluation criteria |
|---|---|
| 0 | No wrinkles or laxity; fine striations running the length of the body |
| 1 | Fine striations |
| 2 | Disappearance of all fine striations |
| 3 | Shallow wrinkles |
| 4 | A few deep wrinkles and laxity |
| 5 | Increased deep wrinkles |
| 6 | Severe wrinkles; development of tumors/lesions |
Evaluation of recovery from stretching (pinch test)
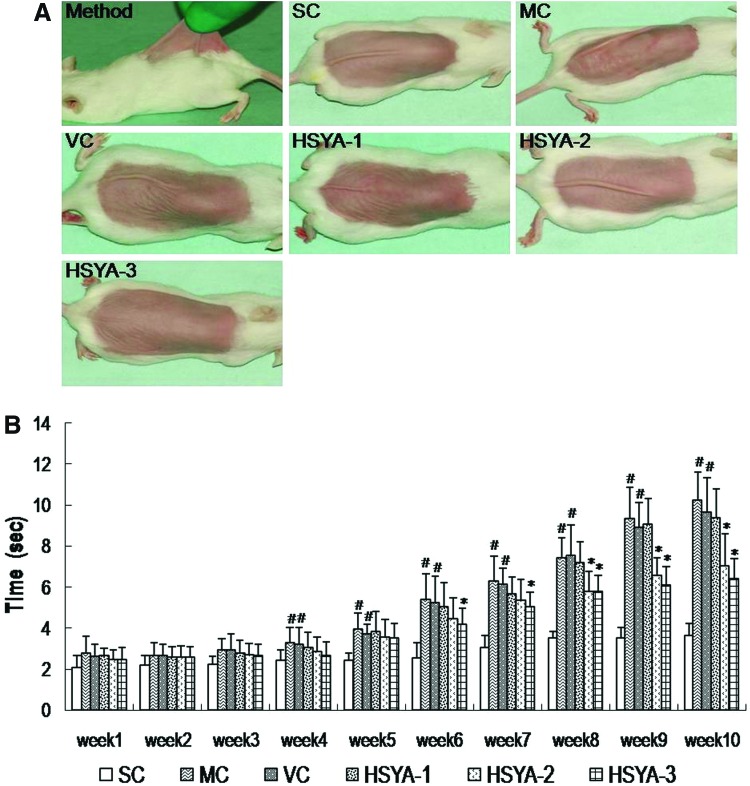
Pinch testing was carried out on mice according to the method of Tsukahara under ether anesthesia.26,28 The dorsal skin at the midline of mice was picked up with the fingers as much as possible (to a degree that does not lift the animal into the air), and the pinch was subsequently released (see Fig. 3A, below). The time until the skin recovered to the original state was measured.
FIG. 3.
Evaluation of sagging by pinch testing. (A) Photographs of pinch testing carried out according to the method of Bryce and Bogdan. (B) Recovery time in the pinch test, performed at the end of week 10, in groups with or without UV exposure. Data represent means±standard deviation (SD) (n=9). (#) p<0.05 compared with the SC group; (*) p<0.05 compared with the VC group. SC, shaved control; MC, model control; VC, solvent control; HSYA-1, treated with HYSA at a dose of 50 μg/mouse; HSYA-2, treated with HYSA at a dose of 100 μg/mouse; HSYA-3, treated with HYSA at a dose of 200 μg/mouse. (Color image available online at www.liebertpub.com/rej).
Skin anti-oxidant indicators analysis
At the end of 10 weeks, the mice were sacrificed by cervical dislocation, and the dorsal skin surface was quickly removed. The removed skin tissue (usually 0.4 gram) was carefully washed two times with cold normal saline (0.9%, wt/vol, NaCl), and then was homogenized (10,000 rpm, 20 sec) with Ultra Turrax (T18 Basic, IKA) in 9 volumes of cold normal saline. The homogenate was centrifuged at 3000×g for 20 min at 4°C, and total supernatant was saved for subsequent assays. Skin total SOD activity, GSH-Px activity, CAT activity, and MDA levels were determined with the corresponding diagnostic kits (Nanjing Jiancheng Bioengineering Inst., Nanjing, China). The principles of these kits were briefly as follows.
SOD activity was determined using the xanthine oxidase method, on the basis of its ability to inhibit the oxidation of hydroxylamine by the xanthine–xanthine oxidase system. GSH-Px activity was measured by quantifying the rate of hydrogen peroxide (H2O2)-induced oxidation of GSH to oxidized GSH (GSSG), catalyzed by GPH-Px. CAT activity was measured according to the ammonium molybdate spectrophotometric method, based on the fact that ammonium molybdate can rapidly terminate the H2O2 degradation reaction catalyzed by CAT and react with the residual H2O2 to generate a yellow complex that is monitored by the absorbance at 405 nm. MDA was determined by the thiobarbituric acid (TBA) method, based on its reaction with TBA to form TBA reactive substances (TBARS).
Protein concentration was measured using the method of Lowry and others,29 using bovine serum albumin as standard.
Determination of collagen content
Collagen content could be converted from Hyp content by multiplying with a conversion factor 7.46.30 Hyp, a characteristic amino acid of collagen, in the skin samples was measured by an Hyp ELISA kit according to the manufacturer's instructions (Jiancheng Inst. of Biotechnology, Nanjing, China) after the samples were hydrolyzed in 6 M HCl at 110°C for 24 hr.
Histological examination
The dorsal skin samples (1×0.4 cm) removed at week 10 were fixed in 10% buffered formalin for at least 24 hr, progressively dehydrated in solutions containing an increasing percentage of ethanol (70%, 80%, 95%, and 100%, vol/vol), cleared in Histoclear (ASONE, Tokyo, Japan), embedded in paraffin under vacuum, and sectioned at 5-μm thickness. Hematoxylin & Eosin (H&E) staining was used for routine examination of the tissue and quantification of epidermal hyperplasia. Elastin fibers were stained with the Gomori aldehyde fuchsin method31 to evaluate the skin elasticity of irradiated and non-irradiated mice.
To quantify epidermal hyperproliferation following UV exposure, the thickness of the epidermis was measured at 10 randomly selected locations per slide using an optical microscope (Leica DMLB) with 200×magnification. Each slide was photographed digitally using a Leica DC 300 camera, and the thickness was obtained through the image analysis program Image J 1.36 (Wayne Rasband, National Institutes of Health, Bethesda, MD).32,33
Statistical analysis
All quantitative data were presented as mean±standard deviation (SD). The changes in variable parameters between treated groups and control group were analyzed by one-way analysis of variance (ANOVA) followed by the Dunnett test as a post hoc comparison. A value of p<0.05 was considered to be significant in all cases.
Results
HSYA prevented UV-induced macroscopic skin lesions
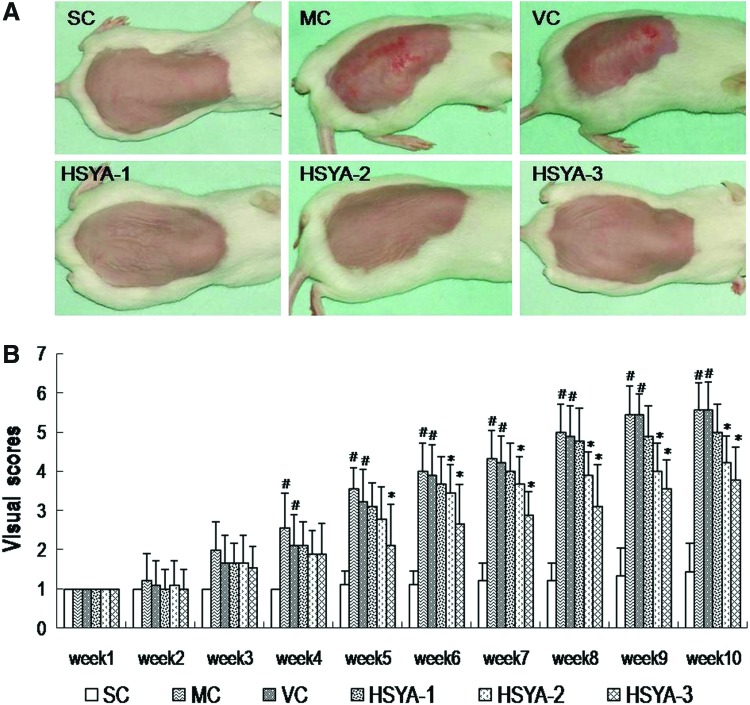
Macroscopic effects of UV irradiation on mouse skin are shown in Fig. 2. Because the mice in the untreated control (NC) group were not shaved until they were sacrificed, they were not examined weekly for macroscopic changes in the skin. Thus, the NC control is omitted in Fig. 2.
FIG. 2.
Hydroxysafflor yellow A (HSYA) prevents ultraviolet (UV)-induced macroscopic skin lesions in mice in vivo. (A) Macroscopic changes in the skin of mice upon various treatments at the end of experimental period of 10 weeks. (B) Results of the visual score of different experimental groups during the 10-week study period. Data represent means±standard deviation (SD) (n=9). (#) p<0.05 compared with the SC group; (*) p<0.05 compared with the VC group. SC, shaved control; MC, model control; VC, solvent control; HSYA-1, treated with HYSA at a dose of 50 μg/mouse; HSYA-2, treated with HYSA at a dose of 100 μg/mouse; HSYA-3, treated with HYSA at a dose of 200 μg/mouse. (Color image available online at www.liebertpub.com/rej).
Starting from week 4, skin lesions were observed in MC group mice. However, the UV-induced skin lesions were partially restored by HSYA from the 6th week, especially at high doses (100–200 μg/mouse). As shown in Fig. 2A, in the last week of this experiment, the mice in the SC group showed fine striations running the length of the body (head-to-tail direction) on their dorsal skin. This minute change is visible to the naked eye, but could not be observed in both the macroscopic and histological photographs. We consider this perhaps a result from shaving, an effect that might be masked in other groups by damage caused by the UV radiation.
At the end of the 10 weeks, seven out of nine (77.78%) MC mice and six out of nine (66.67%) VC mice exhibited deep-wrinkled, leathery skin with some flesh-colored lesions, whereas no lesions but a few shallow wrinkles were observed in the mouse skin of the HSYA-3 group (Fig. 2A). In the HSYA-2 group, three mice (33.33%) showed localized lesions, and the remaining mice displayed a few shallow wrinkles (Fig. 2A). In the HSYA-1 group, four mice (44.44%) showed extensive lesions, and the other five exhibited several deep coarse wrinkles (Fig. 2A).
Statistically, as shown in Fig. 2B, at the end of the experiment, the visual scores of the MC group and the VC group showed no significant differences, but both were markedly higher than that in the SC group. However, the scores of the HSYA-2 group and HSYA-3 group were significantly decreased compared with the VC group. These results indicated that 10 weeks of UV exposure caused mouse skin damage mainly involving wrinkles and extensive lesions in appearance, but HSYA (especially 100–200 μg/mouse) effectively prevented these macroscopic damages.
HSYA promoted recovery from stretching in the pinch test
At the end of 10 weeks of treatment, the period that mouse skin recovered from stretch was examined by the pinch test. Figure 3A shows photographs of mouse dorsal skin after being stretched for 1 sec. Compared with the SC group, the mice in the VC and MC groups needed a significantly longer time to recover from the pinch (Fig. 3B); there was no obvious difference between the VC and MC groups. Although the HSYA-1 group showed no statistical difference from the VC group, the HSYA-2 group and HSYA-3 group did have significant reductions in recovery time compared with the VC group. These data indicated that HSYA (especially 100–200 μg/mouse) could promote the ability of the skin to regain its initial shape after deformation.
HSYA elevated the activities of skin anti-oxidant enzymes
It has been widely recognized that SOD, GSH-Px, and CAT play important roles in protecting skin from oxidative damage.14–16 As shown in Table 3, the activities of SOD, GSH-Px, and CAT showed no significant differences either between the NC and SC groups or between the MC and VC groups, indicating that the hair removal treatment and the vehicle had no effect on these indicators. Moreover, compared to the SC group, the activities of SOD, GSH-Px, and CAT in the MC group were significantly decreased by 23.0%, 50.4%, and 60.3%, respectively (all p<0.05). However, when compared with the VC group, GSH-Px and CAT activities in the HSYA-3 group and SOD activity in the HSYA-2 group showed remarkable increases (all p<0.05). These data showed that HSYA (100–200 μg/mouse) could effectively up-regulate the decreased anti-oxidative enzyme activities caused by UV exposure.
Table 3.
Effects of HSYA on SOD, GSH-Px, and CAT Activities, As Well As MDA and Collagen Contents on Photoaging Mouse Skin
| Group | SOD UA/mgprot | GSH-Px UB/mgprot | CAT UC/mgprot | MDA nmol/mgprot | Collagen (μg/mg) |
|---|---|---|---|---|---|
| NC | 34.45±6.12 | 670.63±91.85 | 14.22±1.17 | 6.63±1.17 | 23.27±5.22 |
| SC | 31.76±6.87 | 669.29±81.58 | 14.87±1.42 | 7.50±1.61 | 22.31±2.96 |
| MC | 24.46±4.69# | 332.29±44.82# | 5.90±0.95# | 20.38±2.08# | 17.06±4.02# |
| VC | 24.65±4.62# | 358.11±77.66# | 5.48±1.02# | 19.55±2.09# | 17.57±4.43# |
| HSYA-1 | 27.25±4.46# | 340.85±72.02# | 5.97±1.04# | 19.88±1.48# | 22.08±2.16* |
| HSYA-2 | 31.02±5.22* | 323.19±60.41# | 6.53±0.88# | 8.02±1.11** | 16.79±3.73# |
| HSYA-3 | 26.36±4.49# | 565.75±100.01**# | 9.58±1.40*# | 11.16±1.19*# | 23.08±5.28** |
Each value represents the mean±standard devation (SD) (n=9).
p<0.05 compared with the SC group; *p<0.05 and **p<0.01 compared with the VC group.
One unit of SOD activity was defined as the amount of the enzyme inhibiting the oxidation by 50%.
One unit of glutathione peroxidase was defined as the amount of the enzyme leading 1 μmol GSH oxidized per min.
One unit of catalase activity was defined as the amount of enzyme that reduces 1 μmol of H2O2 per second under defined conditions.
HSYA, hydroxysafflor yellow A; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; CAT, catalase; MDA, malondialdehyde; NC, untreated control; SC, shaved control; MC, model control; VC, solvent control.
HSYA decreased skin MDA levels
Increased MDA level is a well-accepted marker of oxidative stress. As shown in Table 3, no significant difference was found in MDA values either between the NC and SC groups, or between the MC and VC groups. When compared to the SC group, an obvious increase in MDA content was found in the MC group (p<0.05). However, the contents of MDA were significantly decreased in the HSYA-2 and HSYA-3 groups as compared with the VC group (p<0.01 and p<0.05, respectively). These data showed that the hair removal treatment and the vehicle did not influence MDA content, but HSYA (100–200 μg/mouse) markedly inhibited the increase of MDA concentration induced by repeated UV exposures.
HSYA increased skin collagen content
Collagen content was converted from hydroxyproline content by multiplying with a conversion factor 7.46.30 As seen in Table 3, our results showed that no significant difference was observed in collagen content either between the NC and SC groups or between the MC and VC groups. They also showed that repeated UV irradiation could significantly decrease the skin collagen content of MC mice and VC mice, which was 76.5% and 78.6% of SC mice, respectively (all p<0.05 vs. SC group). Although the HSYA-2 group (100 μg/mouse) showed no statistical difference from the VC group, HSYA treatment at the doses of 50 and 200 μg/mouse could markedly increase the collagen content (p<0.05 and p<0.01 vs. VC group, respectively). These data demonstrated UV radiation reduced collagen levels in the skin, whereas HSYA (especially 200 μg/mouse) reversed this UV radiation-caused collagen damage.
HSYA maintained the structural integrity of the skin
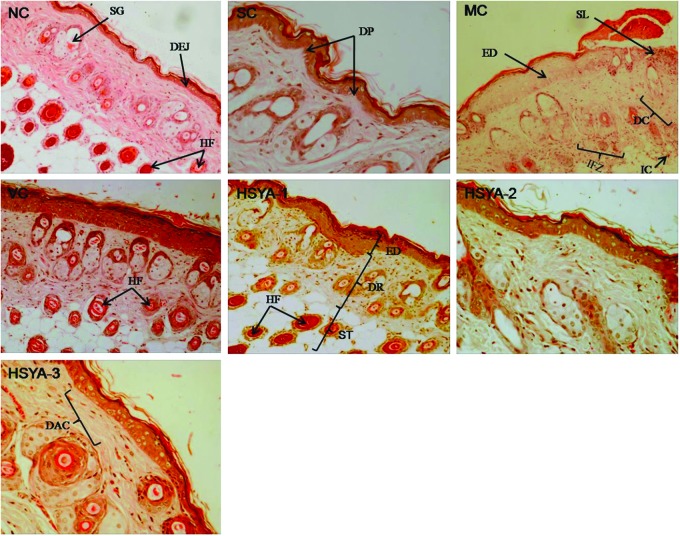
At the end of the 10-week period, mouse skin in groups NC and SC (Fig. 4) showed almost similar features, which displayed a complete and clear structure. The epidermis had multiple layers of squamous cells, covered by a thin layer of keratin. The dermal–epidermal junction (DEJ), a wavy thin line of cells between the dermis and epidermis, could be observed clearly along with the dermal papilla (DP). Moreover, the dermis showed an ordered arrangement of collagen fibers, the thickness and distribution of which were uniform. As shown in Fig. 5, NC and SC, the dermis also contained large amounts of elastic fibers (bundles of elastin), which were stained deep purple, branched, and woven into the net. In addition, clusters of sebaceous glands were attached to the numerous hair follicles. Deeper dermis showed abundant fat with regularly distributed hair follicles; vascular channels were also seen in regular distribution. Inflammatory infiltrations were not observed in or underneath the dermis (Fig. 4NC and SC).
FIG. 4.
Hematoxylin & Eosin (H&E) staining of mouse skin in: NC group (100×); SC group, having the same or similar characteristics to the skin in NC group (200×); MC group, showing destroyed structure and inflammation (100×; note the inflammatory cells); VC group, having the similar features to that in MC group (100×); HSYA-1 group (100×)); HSYA-2 group (200×)); HSYA-3 group (200×)). ED, epidermis; DEJ, dermal–epidermal junction; DR, dermis; SP, dermal papilla; ST, subcutaneous tissue; HF, hair follicle; SG, sebaceous glands; IC, inflammatory cells; IFZ, inflammation zone; DAC, densely arranged collagen fibers which were stacked on top of each other in an orderly arrangement; SL, skin lesion; DC, degraded collagen fibers which were messily arranged. (Color image available online at www.liebertpub.com/rej).
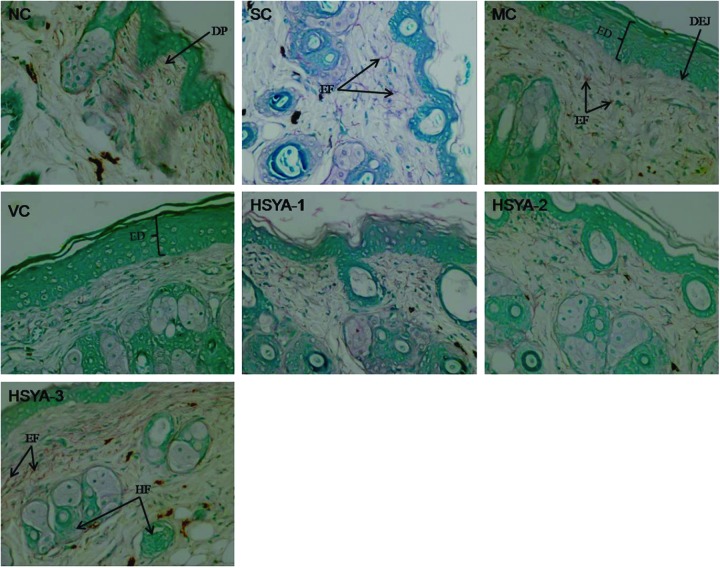
FIG. 5.
Gomori aldehyde fuchsin staining of mouse skin in: NC group (200×; note that the arrangement of the elastic fibers is web-like and has a random pattern. SC group, having the same or similar characteristics to the skin in NC group (200×)); MC group, showing large quantities of tangled, fractured thickened, degraded fibers (200×)); VC group, having the similar features to that in MC group (200×); HSYA-1 group (200×); HSYA-2 group (200×); HSYA-3 group (200×). DP, dermal papillae; EF, elastic fibers; ED, epidermis; DEJ, dermal–epidermal junction; HF, hair follicle. NC, untreated control; SC, shaved control; MC, model control; VC, solvent control; HSYA-1, treated with HYSA at a dose of 50 μg/mouse; HSYA-2, treated with HYSA at a dose of 100 μg/mouse; HSYA-3, treated with HYSA at a dose of 200 μg/mouse. (Color image available online at www.liebertpub.com/rej).
After a 10-week treatment period, UV-irradiated mouse skin (MC group) displayed the following features (Figs. 4MC, 5MC). First, the epidermis showed hyperkeratosis and hyperplasia with thickening of the stratum corneum. Second, the flattening of the DEJ with disappearance of dermal papillae, which resulted in a decrease in surface contact area by approximately 35%, could be seen clearly. Third, beneath the DEJ, collagen fibers had coagulated into homogeneous eosinophilic material, and there was obvious fracture phenomenon (Fig. 4). Fourth, dermal elastic fibers significantly decreased in number, thickened, and became tangled and broken (Fig. 5). Moreover, hair follicles showed crowding and necrosis, with a thick eosinophilic outline, and sebaceous glands disappeared. In addition, inflammatory infiltrations were clear in the entire dermis and between fat cells and hair follicles.
In addition, the histopathological features of the VC group were quite similar to the MC group. The most striking features also included an obviously thickened stratum corneum, a clear flattening of the DEJ, decreased collagen fibers, and disorganized elastic fibers (Figs. 4VC, 5VC). Furthermore, similar inflammatory infiltrates were also observed in and underneath the dermis.
The skin of mice in the HSYA-1 group showed severe epidermal hyperplasia with a markedly thickened stratum corneum and there was a flattened DEJ with loss of the dermal papillae. The dermal collagen fibers were fragmented, sparse, and disorganized (Fig. 4HSYA-1). Meanwhile, the elastic fibers were reduced, broken, and tangled (Fig. 5HSYA-1). Inflammatory infiltrates were still present in the entire dermis. However, the histopathology of the mouse skin in the HSYA-2 group showed mild hyperplasia of the epidermis with a slightly thickened stratum corneum. The structure of the DEJ returned to normal and showed some small dermal papillae. Dermis also tended to be normal. The superficial dermis showed an orderly arrangement of collagen fibers and elastic fibers, although some of these were incomplete. Diffuse inflammation was not observed in the HSYA-2 group (Figs. 4 and 5).
The mice treated with HSYA at a dose of 200 μg/mouse (HSYA-3 group) showed a smooth surface with no wrinkling. The epidermis had multiple layers of squamous cells, covered by thin layer of stratum corneum and a small number of stratum spinosum cells. The wavy DEJ and dermal papillae could be clearly seen. The number of collagen and elastin fibers was significantly increased and they both showed an ordered arrangement (Figs. 4HSYA-3 and 5HSYA-3). The regenerating epidermis and hair follicles were also present. In addition, the upper portion of the skin showed a decreased number of hair follicles and slight inflammation.
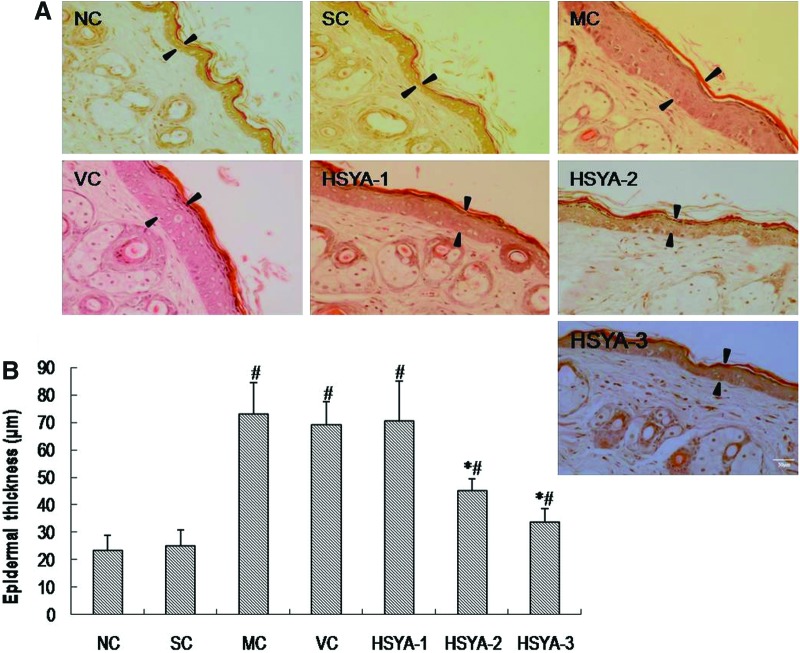
HSYA inhibited UV-induced epidermal hyperproliferation
It is well known that the irregular thickening of the epidermal layer is a characteristic feature of epidermal hyperproliferation caused by UV.3,34,35 The inhibitory effect of HSYA on UV-induced epidermal hyperproliferation was evaluated by directly measuring the thickness of epidermis. The results are shown in Fig. 6. With regard to epidermal thickness, no statistically significant difference was found either between the NC and SC groups or between the MC and VC groups. For the MC group, there was an obvious increase in epidermal thickness (p<0.05, vs. SC group). However, the thickness was significantly decreased in the HSYA-2 and HSYA-3 groups as compared with the VC group (p<0.05 and p<0.05, respectively). These data indicated that topical treatment with HSYA (100 and 200 μg/mouse) significantly inhibited UV-induced epidermal hyperproliferation.
FIG. 6.
Induction of epidermal hyperproliferation by ultraviolet (UV) and inhibition by hydroxysafflor yellow A (HSYA). (A) Photographs (200×) of epidermal hyperproliferation in the skin of mice upon various treatments by Hematoxylin & Eosin (H&E) stain. Scale bar, 50 μm. Arrowheads indicate the width of epidermis. (B) The average thickness of the epidermis in the skin of mice upon various treatments. Data represent means±standard deviation (SD) (n=6). (#) p<0.01 compared with the SC group; (*) p<0.05 compared with the VC group. NC, untreated control; SC, shaved control; MC, model control; VC, solvent control; HSYA-1, treated with HYSA at a dose of 50 μg/mouse; HSYA-2, treated with HYSA at a dose of 100 μg/mouse; HSYA-3, treated with HYSA at a dose of 200 μg/mouse. (Color image available online at www.liebertpub.com/rej).
Discussion
In recent decades, a growing body of evidence has demonstrated that UV-induced oxidative stress causes further oxidative damage to cellular components (i.e., lipids, proteins, and/or DNA) and changes the pattern of gene expression, finally leading to skin pathologies (such as photoaging).11,36,37 In the present study, we evaluated the anti-oxidative damage effects of HSYA by measuring the levels of SOD, GSH-Px, and CAT, which are widely regarded as indicators of oxidative stress, in the skin of mice.9,12,36 Our results showed that after UV irradiation for 10 weeks, mouse skin displayed a notable decrease in activities of SOD, GPH-Px, and CAT. However, topical HSYA (especially 100–200 μg/mouse) treatment significantly elevated activities of these enzymes, indicating that HSYA could exert favorable effects against skin oxidative injury induced by UV irradiation. Previous studies have demonstrated that HSYA can scavenge hydroxyl radicals, inhibit lipid peroxidation, and protect the tissues (especially brain tissue) from oxidative damage.19,38 Consistently, this study provides direct in vivo evidence that HSYA confers protection against UV-induced skin damage through its anti-oxidative action. Because HSYA has more than one phenolic hydroxyl group in its molecule, its anti-oxidant efficacy may be attributed to its multiple phenolic hydroxyl groups.38,39
It is well known that UV-induced oxidative stress leads to lipid peroxidation (LPO), which results in the accumulation of MDA (a stable end-product of LPO),40,41 and the level of MDA is considered to be indirect evidence of photoaging.40–42 To further elucidate whether the skin-protective effect of HSYA was related to its anti-oxidative efficiency, we also performed biochemical analysis to determine the changes in skin MDA levels. In accordance with previous resports,26,40,42 exposure of the skin to UV radiation for 10 weeks caused an obvious increase in MDA levels, indicating that UV irradiation exhausted the endogenous reducing power and catalyzed the lipid peroxidation. However, topical HSYA (especially 100–200 μg/mouse) treatment could significantly down-regulate the skin MDA level. Thus, the protective effect of HSYA in UV-induced skin injury may be at least partially related to its anti-lipid peroxidative properties.
Furthermore, recent advances in the field of aesthetic and anti-aging medicine have also demonstrated that UV-induced photoaging is manifested primarily as reduction in skin elasticity,2,4,6,37 and that the loss of integrity of elastic fibers directly leads to the marked reduction of skin elasticity and the formation of sagging skin.2,4,5,43–45 In this study, it was found that when mouse skin was exposed under UV for 10 weeks, it exhibited an obviously extended recovery time in the pinch test (indicating visible reduction in skin elasticity), and the normal fibrillary pattern of skin elastic fibers was replaced by large quantities of thickened, tangled, degraded, and non-functional fibers. However, HSYA treatment (100–200 μg/mouse) markedly shortened the recovery time and reversed the disrupted elastic fibers caused by UV, strongly revealing that HSYA had the potential to maintain the integrity of dermal elastic fibers, and could further attenuate chronic UV-induced skin sagging and reduction of elasticity.
Because the reduction of collagen is responsible for the old and wrinkled appearance,37,43,46,47 we further examined the effect of continuous UV irradiation on collagen content, as well as arrangement and structure of collagen fibers in the mouse skin. On the basis of histological observation and measurement of Hyp content, collagen fibers were fragmented and the arrangement of these fibers was severely disturbed in mouse skin after chronic UV exposure, which was consistent with the markedly reduced hydroxyproline content. HSYA treatment (especially at the dose of 200 μg/mouse) could significantly prevent UV-induced reduction of collagen content and maintain the integrity of collagen structure in irradiated mouse skin. These results indicated that HSYA might help prevent skin from wrinkles and sagging, mainly caused by the degradation of skin collagen.
Additionally, it is also well recognized that photo-aged skin is characterized by an exaggerated epidermal hyperplasia, which accelerates the formation of wrinkles and results in rough dry skin.2,6,11 In accordance with previous results,26,27,42 we also found that the thickness of the epidermis was significantly increased by UV irradiation. The increase in epidermal thickness was markedly inhibited by topical treatment of HSYA (100–200 μg/mouse). These data suggested that HSYA was capable of inhibiting the increase in skin thickness induced by repeated UV irradiation and maintaining skin smooth.
In conclusion, our results showed that topical HSYA treatment increased collagen content, inhibited epidermal hyperproliferation, and maintained the integrity of dermal collagen and elastic fibers. This may be related to its ability to inhibit photo-oxidative stress-mediated skin injury by increasing the activities of anti-oxidant enzymes (SOD, GSH-Px, CAT) and reducing MDA level. Taken together, these results clearly demonstrate that topical HSYA application is able to protect skin from UV-induced oxidative damage and suggest it may help prevent photoaging.
Acknowledgments
This work was supported by grants from the Guangdong International Cooperation Projects in 2012, Guangdong Province, P.R. China (Project No. 2012B050300002), and the Innovative Experimental Projects of College Students of Guangdong (No.AAE112121A07)
Author Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Diffey BL. Solar ultraviolet radiation effects on biological system. PhysMed Biol. 1991;36:299–328. doi: 10.1088/0031-9155/36/3/001. [DOI] [PubMed] [Google Scholar]
- 2.Fisher GJ. Wang ZQ. Fatta SC. Varani J. Kang S. Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl Med. 1997;337:1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- 3.Chung JH. Photoaging in Asians. Photodermatol Photo. 2003;19:109–121. doi: 10.1034/j.1600-0781.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- 4.Sams WM. Smith J. The histochemistry of chronically sun damaged skin. J Invest Dermatol. 1961;37:447–452. doi: 10.1038/jid.1961.141. [DOI] [PubMed] [Google Scholar]
- 5.Uitto J. Fazio MJ. Olsen DR. Molecular mechanisms of cutaneous aging. Age-associated connective tissue alterations in the dermis. J Am Acad Dermatol. 1989;21:614–622. [PubMed] [Google Scholar]
- 6.Scharffetter-Kochanek K. Brenneisen P. Wenk J. Herrmann G. Ma W. Kuhr L. Meewes C. Wlaschek M. Photoaging of the skin from phenotype to mechanisms. Exp Gerontol. 2000;35:307–316. doi: 10.1016/s0531-5565(00)00098-x. [DOI] [PubMed] [Google Scholar]
- 7.Herrling TH. Jung K. Fuchs J. Measurements of UV-generated free radicals/reactive oxygen species (ROS) in skin. Spectrochim Acta Part A. 2006;63:840–845. doi: 10.1016/j.saa.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Oikarinen A. Karvonen J. Uitto J. Hannuksela M. Connective tissue alterations in skin exposed to natural and therapeutic UV-radiation. Photodermatology. 1985;2:15–26. [PubMed] [Google Scholar]
- 9.Sies H. Biochemistry of oxidative stress. Angew Chem. 1986;25:1058–1071. [Google Scholar]
- 10.Jurkiewicz BA. Buettner GR. Ultraviolet-light-induced free radical formation in skin: An electron paramagnetic resonance study. Photochem Photobiol. 1994;59:1–4. doi: 10.1111/j.1751-1097.1994.tb04993.x. [DOI] [PubMed] [Google Scholar]
- 11.Lober CW. Fenske NA. Photoaging and the skin: Differentiation and clinical response. Geriatrics. 1990;45:36–42. [PubMed] [Google Scholar]
- 12.Kasapoglu M. Ozben T. Alterations of antioxidant enzymes and oxidative stress markers in aging. Exp Gerontol. 2001;36:209–220. doi: 10.1016/s0531-5565(00)00198-4. [DOI] [PubMed] [Google Scholar]
- 13.Matsumura Y. Ananthaswamy HN. Toxic effect of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195:298–308. doi: 10.1016/j.taap.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 14.Gilchrest BA. A review of skin ageing and its medical therapy. Br J Dermatol. 1996;135:867–875. doi: 10.1046/j.1365-2133.1996.d01-1088.x. [DOI] [PubMed] [Google Scholar]
- 15.Masaki H. Role of antioxidants in the skin: Anti-aging effects. J Dermatol Sci. 2010;58:85–90. doi: 10.1016/j.jdermsci.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Nichols JA. Katiyar SK. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Z. Yang J. Jia Y. Tian Y. Wen A. Pharmacokinetic properties of hydroxysafflor yellow A in healthy Chinese female volunteers. J Ethnopharmacol. 2009;124:635–638. doi: 10.1016/j.jep.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 18.Lin XT. The clinical application of Hydroxysafflor yellow. Chin J Health Care Med. 2012;14:332–333. [Google Scholar]
- 19.Tian J. Li G. Liu Z. Fu F. Hydroxysafflor yellow A inhibits rat brain mitochondrial permeability transition pores by a free radical scavenging action. Pharmacology. 2008;82:121–126. doi: 10.1159/000141653. [DOI] [PubMed] [Google Scholar]
- 20.Ji DB. Zhang LY. Li CL. Ye J. Zhu HB. Effect of hydroxysafflor yellow A on human umbilical vein endothelial cells under hypoxia. Vascul Pharmacol. 2009;50:137–145. doi: 10.1016/j.vph.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Nie PH. Zhang L. Zhang WH. Rong WF. Zhi JM. The effects of hydroxysafflor yellow A on blood pressure and cardiac function. J Ethnopharmacol. 2012;139:746–750. doi: 10.1016/j.jep.2011.11.054. [DOI] [PubMed] [Google Scholar]
- 22.Wu Y. Wang L. Jin M. Zang BX. Hydroxysafflor yellow A alleviates early inflammatory response of bleomycin-induced mice lung injury. Biol Pharm Bull. 2012;35:515–522. doi: 10.1248/bpb.35.515. [DOI] [PubMed] [Google Scholar]
- 23.Zhu H. Wang Z. Ma C. Tian J. Fu F. Li C. Guo D. Roeder E. Liu K. Neuroprotective effects of hydroxysafflor yellow A: In vivo and in vitro studies. Planta Med. 2003;69:429–433. doi: 10.1055/s-2003-39714. [DOI] [PubMed] [Google Scholar]
- 24.Kong SZ. Xian YF. Ip SP. Lai XP. Shi XG. Lin ZX. Su ZR. Protective effects of hydroxysafflor yellow A on β-amyloid-induced neurotoxicity in PC12 cells. Neurochem Res. 2013;38:951–960. doi: 10.1007/s11064-013-1002-7. [DOI] [PubMed] [Google Scholar]
- 25.Kim SY. Kim SJ. Lee JY. Kim WG. Park WS. Sim YC. Lee SJ. Protective effects of dietary soy isoflavones against UV-induced skin-aging in hairless mouse model. J Am Coll Nutr. 2004;23:157–162. doi: 10.1080/07315724.2004.10719356. [DOI] [PubMed] [Google Scholar]
- 26.Agrawal R. Kaur IP. Inhibitory effect of encapsulated curcumin on ultraviolet-induced photoaging in mice. Rejuvenation Res. 2010;13:397–410. doi: 10.1089/rej.2009.0906. [DOI] [PubMed] [Google Scholar]
- 27.Bissett DL. Chatterjee R. Hannon DP. Photoprotective effect of topical anti-inflammatory agents against ultraviolet radiation-induced chronic skin damage in the hairless mouse. Photodermatol Photo. 1990;7:153–158. [PubMed] [Google Scholar]
- 28.Tsukahara K. Moriwaki S. Hotta M. Fujimura T. Sugiyama-Nakagiri Y. Sugawara S. Kitahara T. Takema Y. The effect of sunscreen on skin elastase activity induced by ultraviolet-A irradiation. Biol Pharm Bull. 2005;28:2302–2307. doi: 10.1248/bpb.28.2302. [DOI] [PubMed] [Google Scholar]
- 29.Lowry OH. Rosebrough NJ. Farr AL. Randall RJ. Protein measurement with the Folinphenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 30.Neuman R. Logan M. The determination of collagen and elastin in tissues. J Biol Chem. 1950;186:549–556. [PubMed] [Google Scholar]
- 31.Proctor GB. Horobin RW. The aging of Gomori's aldehyde-fuchsin: The nature of the chemical changes and the chemical structures of the coloured components. Histochemistry. 1983;77:255–267. doi: 10.1007/BF00506568. [DOI] [PubMed] [Google Scholar]
- 32.Gaspar LR. Maia Campos PM. Rheological behavior and the SPF of sunscreens. Int J Pharm. 2003;250:35–44. doi: 10.1016/s0378-5173(02)00462-3. [DOI] [PubMed] [Google Scholar]
- 33.Ouhtit A. Muller HK. Davis DW. Ullrich SE. McConkey D. Ananthaswamy HN. Temporal events in skin injury and the early adaptive responses in ultraviolet-irradiated mouse skin. Am J Pathol. 2000;156:201–207. doi: 10.1016/S0002-9440(10)64720-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brenneisen P. Sies H. Scharffetter-Kochanek K. Ultraviolet-B irradiation and matrix methalloproteinases: From induction via signaling to initial events. Ann NY Acad Sci. 2002;973:31–43. doi: 10.1111/j.1749-6632.2002.tb04602.x. [DOI] [PubMed] [Google Scholar]
- 35.Hirobe T. Furuya R. Akiu S. Ifuku O. Fukuda M. Keratinocytes control the proliferation and differentiation of cultured epidermal melanocytes from ultraviolet radiation B-induced pigmented spots in the dorsal skin of hairless mice. Pigment Cell Res. 2003;15:391–399. doi: 10.1034/j.1600-0749.2002.02052.x. [DOI] [PubMed] [Google Scholar]
- 36.Wenk J. Brenneisen P. Meewes C. Wlaschek M. Peters T. Blaudschun R. Ma W. Kuhr L. Schneider L. Scharffetter-Kochanek K. UV-induced oxidative stress and photoaging. Curr Probl Dermatol. 2001;29:83–94. doi: 10.1159/000060656. [DOI] [PubMed] [Google Scholar]
- 37.Yaar M. Gilchrest BA. Photoageing: Mechanism, prevention and therapy. Br J Dermatol. 2007;157:874–887. doi: 10.1111/j.1365-2133.2007.08108.x. [DOI] [PubMed] [Google Scholar]
- 38.Jin M. Li JR. Wu W. Study on the antioxidative effect of Safflor Yellow. Zhongguo Zhong Yao Za Zhi. 2004;29:447–449. [PubMed] [Google Scholar]
- 39.Shan LQ. Ma S. Qiu XC. Zhou Y. Zhang Y. Zheng LH. Ren PC. Wang YC. Fan QY. Ma BA. Hydroxysafflor Yellow A protects spinal cords from ischemia/reperfusion injury in rabbits. BMC Neuroscience. 2010;11:98. doi: 10.1186/1471-2202-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spiteller G. Lipid peroxidation in aging and age-dependent diseases. Exp Gerontol. 2001;36:1425–1457. doi: 10.1016/s0531-5565(01)00131-0. [DOI] [PubMed] [Google Scholar]
- 41.Stadtman ER. Berlett BS. Reactive oxygen-mediated protein oxidation in aging and disease. Chem Res Toxicol. 1997;10:485–494. doi: 10.1021/tx960133r. [DOI] [PubMed] [Google Scholar]
- 42.Zhuang Y. Hou H. Zhao X. Zhang Z. Li B. Effects of collagen and collagen hydrolysate from jellyfish (Rhopilema esculentum) on mice skin photoaging induced by UV irradiation. J Food Sci. 2009;74:H183–H188. doi: 10.1111/j.1750-3841.2009.01236.x. [DOI] [PubMed] [Google Scholar]
- 43.Johnston KJ. Oikarinen AI. Lowe NJ. Clark JG. Uitto J. Ultraviolet radiation-induced connective tissue changes in the skin of hairless mice. J Invest Dermatol. 1984;82:587–590. doi: 10.1111/1523-1747.ep12261342. [DOI] [PubMed] [Google Scholar]
- 44.Fujimura T. Haketa K. Hotta M. Kitahara T. Loss of skin elasticity precedes to rapid increase of wrinkle levels. J Dermatol Sci. 2007;47:233–239. doi: 10.1016/j.jdermsci.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Kligman LH. Akin FJ. Kligman AM. Sunscreens promote repair of ultraviolet radiation-induced dermal damage. J Invest Dermatol. 1983;81:98–102. doi: 10.1111/1523-1747.ep12542169. [DOI] [PubMed] [Google Scholar]
- 46.Fisher GJ. The pathophysiology of photoaging of the skin. Cutis. 2005;75:5–8. [PubMed] [Google Scholar]
- 47.Murakami H. Shimbo K. Inoue Y. Takino Y. Kobayashi H. Importance of amino acid composition to improve skin collagen protein synthesis rates in UV-irradiated mice. Amino Acids. 2012;42:2481–2489. doi: 10.1007/s00726-011-1059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]