Abstract
A Chinese herbal preparation, SiMiaoFang (SMF), has been used clinically for treating arthralgia by virtue of its anti-inflammatory and pain-relieving activities. However, no evidence base links SMF to anti-osteoarthritis (OA), particularly its link to inhibiting cartilage matrix degradation. In this study, we undertook a characterization of anti-OA activity of SMF using an in vivo rat model induced by anterior cruciate ligament transection and medial meniscus resection (ACLT+MMx) together with in vitro studies with chondrocytes for further molecular characterization. ACLT+MMx rats were treated with SMF at doses of 0.63, 1.25, and 2.5 grams/kg per day for 6 weeks. SMF treatments significantly inhibited cartilage matrix degradation, as indicated by increasing proteoglycan and collagen content, particularly type II collagen expression in articular cartilage, decreasing CTX-II (collagen type II degradation marker), and increasing CPII (collagen type II synthesis marker) in circulation. Moreover, SMF suppressed synovial inflammation and inhibited release of interleukin-1β (IL-1β) and tumor necrosis factor-α in serum. The levels of serum prostaglandin E2 and nitric oxide productions were decreased via suppression of the production of cyclooxygenase-2 and inducible nitric oxide synthase, respectively. Importantly, SMF interfered with OA-augmented expression of matrix metalloproteinases (MMPs) -3 and -13 and aggrecanases (ADAMTS) -4 and -5, which are considered to be key enzymes in cartilage matrix degradation, and simultaneously augmented OA-reduced tissue inhibitors of metalloproteinases (TIMPs) -1 and -3 expression in the joints. The largest changes in these parameters were found at the highest dose. Meanwhile, SMF significantly decreased MMP-3 and -13 and increased TIMP-1 and -3 at mRNA and protein levels in IL-1β–induced chondrocytes. These findings provide the first evidence that SMF effectively treats OA by inhibiting cartilage matrix degradation.
Introduction
Osteoarthritis (OA), a musculoskeletal disorder of the bone and joint,1 is the most common form of arthritis, affecting millions of people worldwide, and the major cause of disability in the elderly, affecting about 80% of individuals over the age of 75.2 OA is characterized by progressive destruction of articular cartilage. An imbalance between the biosynthesis and degradation of matrix components leads to a progressive destruction of the tissue and finally causes complete damage of the articular surface. Cartilage matrix consists largely of proteoglycan and collagen; the loss of proteoglycan occurs early in the process of cartilage degeneration3 and is followed by the catabolism of collagen fibrils, leading to the loss of cartilage structural integrity.4 Interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), the predominant cytokines involved in the initiation and processes of cartilage degradation, are potent inhibitors of cartilage matrix synthesis.5 In addition, a relevant role as mediators of cartilage damage is carried out by nitric oxide (NO) and prostaglandins (PGE2),6,7 and the contribution of these molecules to cartilage degradation stems from their ability to enhance the production and activation of aggrecanases (ADAMTS) and matrix metalloproteinases (MMPs), which inhibit proteoglycan and collagen biosynthesis and induce chondrocyte apoptosis.8,9 ADAMTS and MMPs are up-regulated in osteoarthritic-affected cartilage and act as downstream key players in the inflammatory signal cascade.10,11 Additionally, MMP-induced cartilage degeneration is also regulated by inhibition of endogenous tissue inhibitors of metalloproteinase (TIMPs),12 and the imbalance in the ratio of TIMPs and MMPs results in continued matrix destruction in OA.13
The current treatment strategies for OA are to decrease symptoms, improve function, and delay time to surgery. There are three kinds of therapeutic agents: Disease-modifying OA drugs (DMOADs) such as hyaluronic and glucosamine; nonsteroidal anti-inflammatory drugs (NSAIDs), such as loxoprofen and nabumetone; and steroid and biological response modifiers, which are all clinically used to relieve the severity of OA, slow disease progression, and prevent subsequent joint damage.2 Although these drugs have good effects on OA, there were reports that long-term application of them causes side effects on the gastrointestinal tract.14
SiMiaoFang (SMF) is a compound prescription comprised of four ingredients—Phellodendri Chines Cortex, Atractylodis rhizoma, Coicis Semen, and Achyranthis bidentatae Radix—in a specified ratio.15 SMF has been used in traditional Chinese medicine since the Qing Dynasty (16th century CE) because of its function of clearing heat and removing dampness. Today, SMF is commonly used clinically for treatment of arthralgia due to its anti-inflammatory and pain-relieving activities,16 as in gouty arthritis and rheumatoid arthritis.17–19 But SMF's activity and mechanism of anti-OA remain uncharacterized, in particular its link to inhibiting cartilage matrix degradation. In this study we investigated the effects of SMF on cartilage matrix degradation, pro-inflammatory cytokines, and inflammatory mediators and mainly explored the further mechanism of MMPs/TIMPs using in vivo and in vitro assays, as part of an ongoing effort to identify novel and potent agents for the prevention and treatment of OA.
Materials and Methods
Herbal preparation
SMF was prepared as described in the Chinese Pharmacopeia of 2010. Briefly, the four ingredients—Phellodendri Chinensis Cortex (250 grams, beaked with salt), Coicis seuen (250 grams), Atractylodis Rhizoma (125 grams), and Achyranthis bidentatae radix (125 grams)—were pulverized to a fine powder, suspended in distilled water to a concentration of 0.25 gram/mL, and mixed well before administration. The samples for cell culture assays were prepared by extracting the powder with water (three times, for 1 h each). The combined extracts of SMF were concentrated in vacuo and dissolved in dimethylsulfoxide (DMSO; 1 g/mL). All of the samples were stored at 4°C.
The representative chemical compositions of berberine (0.7347%), glycerol trioleate (0.01%), atractylenolide (0.0028%), and β-ecdysone (0.0118%) in SMF were determined by high-performance liquid chromatography (HPLC) analysis.
OA Animals model and treatment
Ten-week-old male Sprague–Dawley rats (Animal Science Laboratory of Peking University Health Science department, PR China) were anesthetized with halothane. After being shaved and disinfected, the right knee joint was exposed through a medial par patellar approach. The patella was dislocated laterally and the knee placed in full flexion, followed by anterior cruciate ligament transection and medial meniscus resection (ACLT+MMx) with micro-scissors. In sham-operated negative controls, the right knee joint was exposed and incisions were closed after subluxation of the patella and washing the joint surface with saline. The rats were randomly assigned to six groups: ACLT+MMx without treatment (Model, n=10), sham operated (Sham, n=10), ACLT+MMx rats treated with 0.16 gram/kg glucosamine (GS, n=10), and ACLT+MMx rats treated with SMF intra-gastrically at a daily dose of 0.63 gram/kg, 1.25 grams/kg, or 2.50 grams/kg (n=10 in per group) for 6 days per week for 6 weeks. Dose calculations followed guidelines correlating dose equivalents between humans and laboratory animals, on the basis of ratios of body surface area,20 and 1.25 grams/kg is the dose equivalent based on a recommended dose of 0.1 gram/kg in human. Un-treated control rats induced by ACLT+MMx and sham rats received distilled water only.
All animals were maintained on a 12-hr light/dark cycle under constant temperature (24±2°C) and humidity (55%±5%), and were allowed free access to food and water. All procedures for consideration of animal welfare were reviewed and approved by the ethical committee of China Academy of Traditional Chinese Medicine.
Histological analysis
Animals were sacrificed after 6 weeks of treatment. The tibia and femur with synovium were fixed in 4% paraformaldehyde for 24 hr, de-calcified in 10% EDTA, and embedded in paraffin. Tissue sections (4 μm) were mounted on common slides for stained with Hematoxylin & Eosin (H&E), Toluidine Blue, and Masson's Trichrome as described.21,22 Cartilage histopathological features were analyzed using the scoring system modified by Mankin et al. (score range 0–12, from normal to complete disorganization and hypo-cellularity).23 Synovium histopathology was evaluated according to Yoshimi histological grading (score range 0–18, from normal to most severe reaction).24 An Image-Pro Plus 6.0 System (IMS) image analysis system was used for quantitative analysis of Toluidine Blue and Masson staining. Six fields of view were randomly selected from each slice, and an index of positive staining was determined from the area of positive staining and the average optical density. The positive index was calculated as integrated optical density (IOD) (positive area×average OD). All sections were randomized and evaluated by a trained observer who was blinded to the treatment groups.
Serum radioimmunoassay and enzyme-linked immunosorbent assay analysis
Animal blood was collected from the abdominal aorta and serum was analyzed for IL-1β, TNF-α, PGE2, and cyclooxygenase (COX) -1 and -2, by radioimmunoassay assay (RIA). NO and inducible nitric oxide synthase (iNOS) were detected by assay kits. Assays for serum CTX-II (collagen type II degradation marker) and CPII (collagen type II synthesis marker) levels were performed with serum Pre-Clinical CartiLaps ELISA kit (R & D System, USA) and pro-collagen type II C-pro-peptide ELISA kit (R & D System, USA),25 respectively.
Immunoblotting analysis
Paraffin sections (4 μm) of joint tissue were mounted on poly-l-lysine–coated slides. The paraffin sections were de-waxed by routine method and incubated for 10 min with 3% hydrogen peroxide (H2O2). Each section was incubated with blocking serum (Vectastain ABC Kit) at room temperature for 30 min and then with primary rabbit monoclonal antibody against type II collagen (dilution 1/30, Biosynthesis Biotechnology, China), rabbit polyclonal antibody against ADAMTS-4 (dilution 1/100, Abcam Biotechnology, UK), rabbit polyclonal antibody against ADAMTS-5 (dilution 1/20, Abcam Biotechnology, UK), rabbit polyclonal antibody against MMP-3 (dilution 1/50, Abcam Biotechnology, UK), rabbit polyclonal antibody against MMP-13 (dilution 1/50, Abcam Biotechnology, UK), rabbit monoclonal antibody against TIMP-1 (dilution 1/80, Abcam Biotechnology, UK) and rabbit monoclonal antibody against TIMP-3 (dilution 1/50, Abcam Biotechnology, UK), respectively overnight at 4°C, sections incubated in phosphate-buffered saline (PBS) without antibody served as negative controls. After incubation with biotinylated secondary antibody, sections were incubated with avidin–biotin complex reagent containing horseradish peroxidase for 30 min. The sections were then stained with 3,3′-diaminobenzidine (DAB) (Sigma). The Image-Pro Plus 6.0 System image analysis system was used for quantitative analysis.
Chondrocytes culture, cell viability, and samples collecting
Normal human chondrocytes were obtained commercially (ScienCell, USA). The chondrocytes were maintained in the special chondrocyte medium (ScienCell, USA) and 5% heat-inactivated fetal bovine serum (FBS; vol/vol), at 37°C in a humidified atmosphere of 95% air and 5% CO2. At the third passage, cells were seeded at a density of 1×106 cells per dish or 5×103 cells per well in a 96-well plate and cultured to approximately 80% confluence, then made quiescent in serum-free medium for 24 hr. Chondrocytes were treated with SMF at 1.25, 3.125, and 7.8125 μg/mL for 1 hr, and then treated with IL-1β at 10 ng/mL for another 24 hr of incubation; DMSO and glucosamine (100 μg/mL) treatment were negative and positive controls. At the end of the incubation period, cell viability was determined by the MTT assay.26 Chondrocytes in the dish were collected for further study using a western blot assay and real-time PCR assay, respectively.
Western blot
After treatment, all chondrocytes were harvested in cold PBS. The pellet was resuspended in lysis buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 5 mM EDTA, 0.1% sodium dodecyl sulfate [SDS], 0.5% NP-40) containing 10 mM phenylmethylsulfonyl fluoride (PMSF) and 2 mg/mL aprotinin. Protein was obtained to detect the levels of MMPs and TIMPs in chondrocytes by western blotting. The western blot protocol and semi-quantitative analysis were carried out as described.27 The following antibodies were used: Rabbit anti-MMP-3 polyclonal antibody (dilution 1/100, Abcam Biotechnology, UK), rabbit anti-MMP-13 monoclonal antibody (dilution 1/100, Abcam Biotechnology, UK), rabbit anti-TIMP-1 polyclonal antibody (dilution 1/50, Abcam Biotechnology, UK), rabbit anti-TIMP-3 polyclonal antibody (dilution 1/50, Abcam Biotechnology, UK), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (internal control, rabbit polyclonal antibody, dilution 1:200, Santa Cruz Biotechnology, Inc., Santa Cruz, CA). All experiments were done in triplicate. The relative quantity of each antibody was measured by Alpha Ease FC (Fluorchem FC2) software. The density ratio of protein to GAPDH was calculated from the band density.
Real-time quantitative PCR
After treatment, total RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA) from the chondrocytes according to the manufacturer's instructions. The total RNA (2 μg) was reverse transcribed to complementary (c) DNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems Foster City, CA) according to the instruction manual. The specific transcripts were quantified by quantitative real-time PCR using a Quanti Tect SYBR Green PCR Kit (QIAGEN K.K., Tokyo, Japan) and analyzed with an ABI 7500 real-time PCR system (Applied Biosystems, USA). Gene-specific primers were used for MMP-3 (forward, TTTTGGCCATCT CTTCCTTCA; reverse, TGTGGATGCCTCTTGGGTATC), MMP-13 (forward, TGAGGATACA GGCAAGACTC T; reverse, CAATACGGTT ACTCCAGATGC), TIMP-1 (forward, CTTCTGG CATCCTGTTGTT G; reverse, AGAAGGCCGTCTGTGGGT), TIMP-3 (forward, TACCGAG GCTTCACCAAGA TG; reverse, TCCCACCTCTCCACGAAGTTG), and GAPDH (forward, GAAGGT GAAGG TCGGAGTC; reverse, GAAGATGGTGATGGGATTTC). The messenger (m) RNA levels of MMP-3 and -13 and TIMP-1 and -3 were normalized to the GAPDH mRNA level. PCR was performed as 95°C for 10 min, followed by 40 cycles of 95°C for 30 sec, and 60°C for 1 min. The quantification data was analyzed with ABI Prism analysis software. The relative mRNA expression was calculated with the comparative threshold cycle (CT) method.28
Statistical analysis
The software of SPSS version 11.0 for Windows (SPSS Inc, Chicago, IL) and was used for statistical analysis. Continuous variables were expressed as means±standard error of the mean (SEM). Content of collagen and proteoglycans were analyzed with non-parametric statistics (Kruskal–Wallis test). Other data were analyzed by one-way analysis of variance (ANOVA) followed by least significant difference (LSD) or Dunnett T3 test. Differences were considered statistically significant when p was less than 0.05.
Results
Effects of SMF on histopathology of articular cartilage and synovium in ACLT+MMx rats
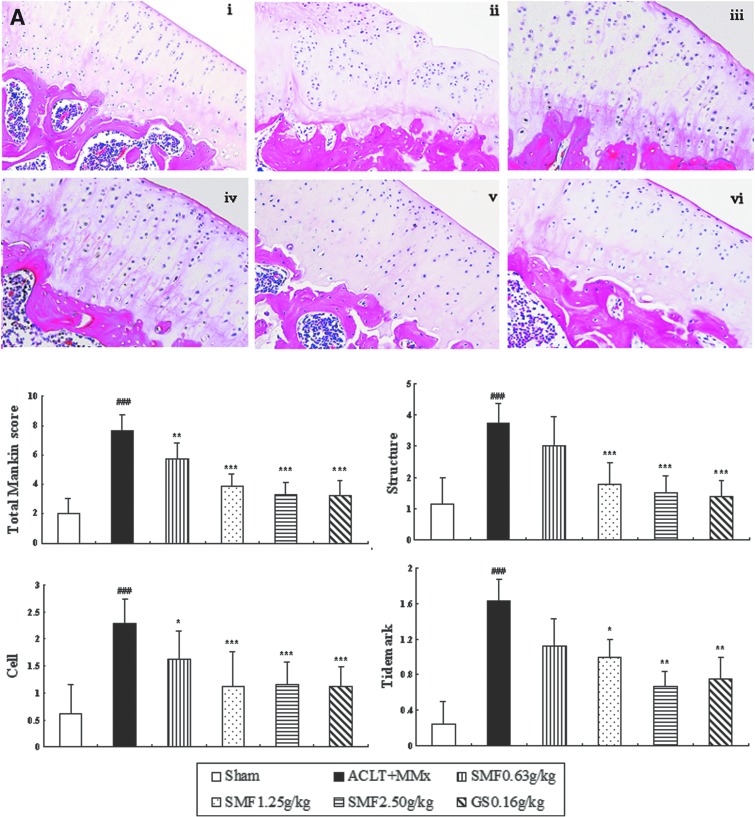
To characterize the anti-OA activity of SMF, we treated ACLT+MMx rats with SMF and compared the activity with glucosamine. Histological analysis of sections revealed significant histopathological changes in the medial tibia plateau of un-treated ACLT+MMx rats (Fig.1A, ii), as indicated by surface irregularity, disorganization of articular cartilage with apparent cloning of chondrocytes in the transitional and radial zones, and disrupted tidemark. As expected, the overall Mankin score was significantly increased by ACLT+MMx. Treatment of ACLT+MMx rats with SMF or glucosamine substantially restored cartilage morphology (Fig. 1A, iii–vi), as indicated by normal cartilage surfaces with normal cellularity in the transitional and radial zones and normal and intact. The overall modified Mankin scores of articular cartilage were significantly lower in SMF- and glucosamine-treated rats than in untreated ACLT+MMx rats (p<0.05, 0.01, or 0.001) (Fig. 1A).
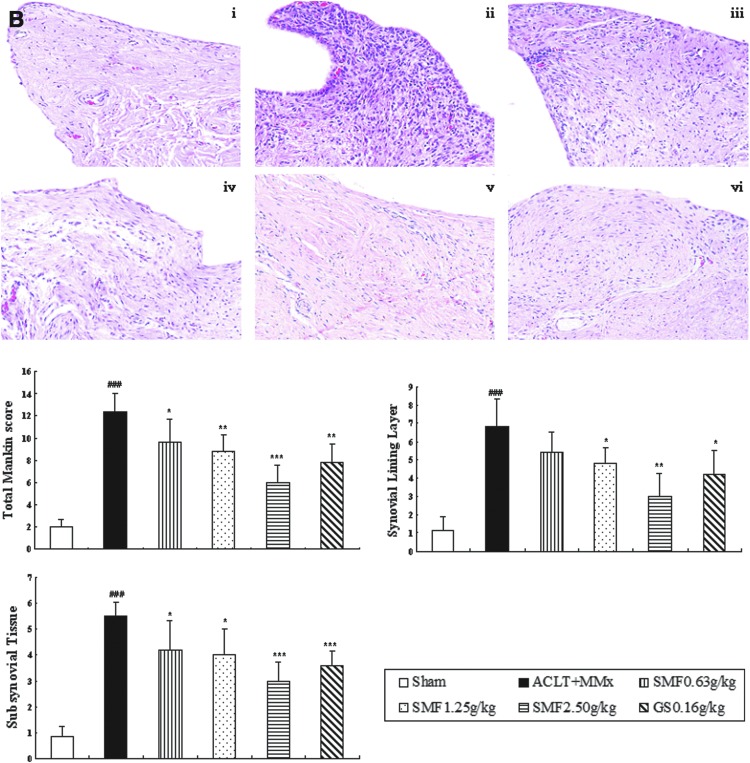
FIG. 1.
Effects of SiMiaoFang (SMF) on histopathology of articular cartilage and synovium in anterior cruciate ligament transection and medial meniscus resection (ACLT+MMx) rats. The effect of SMF on morphology of articular cartilage (A) and synovium (B). Cartilage histology was observed in ACLT+MMx and sham-operated rats at the end of the 6-week treatment period. Photomicrographs taken at 200×magnification of representative cartilage sections from one animal per treatment group are shown: Sham (i), untreated ACLT+MMx rats (ii), ACLT+MMx rats treated with 0.625 gram/kg (iii), 1.25 grams/kg (iv), 2.50 grams/kg (v), ACLT+MMx rats treated with glucosamine 0.16 gram/kg (vi). Data are the mean±standard deviation (SD) of samples from 10 rats. p values are for the one-way analysis of variance (ANOVA) comparing treatment group with untreated ACLT+MMx rats. (***) p<0.001 and (**) p<0.01, compared with ACLT+MMx; (###) p<0.001, compared with sham group. (A) Significant histopathological changes were evident in the untreated ACLT+MMx rats, as indicated by surface irregularity and disorganization of articular cartilage with apparent cloning of chondrocytes in the transitional and radial zones (i). The cartilage histology of ACLT+MMx rats treated with SMF or glucosamine (ii–iv, vi), restored cartilage morphology (Aii–iv), as indicated by surface regularity of cartilage with normal cellularity in the transitional and radial zones. The overall Mankin scores of both the SMF and glucosamine groups were significantly decreased compared to untreated ACLT+MMx rats. (B) Untreated ACLT+MMX rats appeared more hypertrophic with infiltration of inflammatory cells in synovial tissue compared with sham rats (Bi–ii). SMF repressed massive cellular infiltration and dose-dependently inhibited the histological severity scores in ACLT+MMx rats. (Color images available online at www.liebertpub.com/rej)
Un-treated ACLT+MMX rats appeared more hypertrophied, and there was infiltration of inflammatory cells in synovium tissue compared with sham rats (Fig. 1B i–ii). SMF repressed inflammatory cellular infiltration and dose-dependently inhibited the histological severity scores of synovium in ACLT+MMx rats (Fig. 1B).
Effects of SMF on cartilage matrix in the joints of ACLT+MMx rats
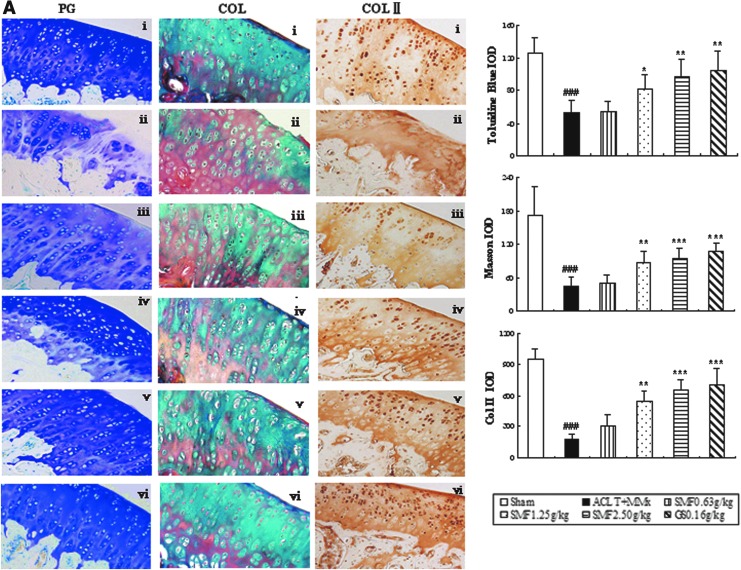
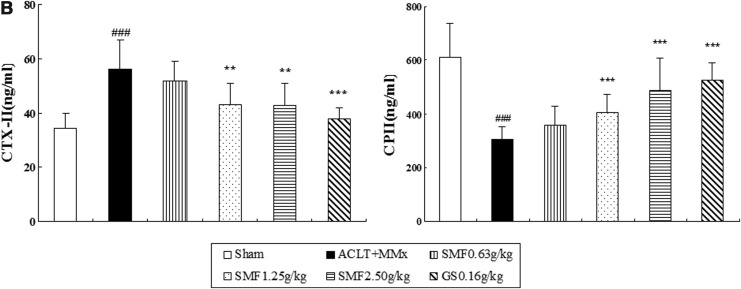
Proteoglycan and collagen are major constituents of articular cartilage matrix and were evaluated by Toluidine Blue and Masson Trichrome staining. In the untreated ACLT+MMx rats, proteoglycan and collagen were significantly reduced, as shown in Fig. 2A ii. Furthermore, immunohistochemistry revealed a significant decrease in typeII collagen, followed with a significant increase in CTX-II and a decrease in CPII in the serum of untreated ACLT+MMx rats compared with the Sham group (Fig. 2B).
FIG. 2.
The effect of SiMiaoFang (SMF) on cartilage matrix components in anterior cruciate ligament transection and medial meniscus resection (ACLT+MMx) rats. (A) The effect of SMF on proteoglycan (PG), collagen (COL), and type II collagen (COLII). Toluidine Blue-O, Masson staining, and immunohistochemistry were used to assess the proteoglycan (PG), collagen (COL), and type II collagen (COLII). Photomicrographs taken at 200×magnification of representative cartilage sections from one animal per group are shown on the left: Sham (i), untreated ACLT+MMx rats (ii), ACLT+MMx rats treated with 0.625 gram/kg (iii), 1.25 grams/kg (iv), 2.50 grams/kg (v), ACLT+MMx rats treated with glucosamine 0.16 gram/kg (vi). Data represents the mean±SD of samples from 10 rats in each group. Comparing treatment group with untreated ACLT+MMx rats. (***) p<0.001, (**) p<0.01, and (*) p<0.05, compared with ACLT+MMx; (###) p<0.001, compared with the sham group. (B) The effects of SMF on CTXII and CPII in ACLT+MMx rats serum. (B) Data represent the mean±SD of samples from 10 rats in each group. Comparing treatment group with untreated ACLT+MMx rats. (***) p<0.001 and (**) p<0.01, compared with ACLT+MMx; (###) p<0.001, compared with the sham group.
Treatment with SMF 1.25 and 2.5 grams/kg or glucosamine significantly inhibited the cartilage matrix degradation, as indicated by restoring the loss of proteoglycan and collagen (Fig. 2A), and up-regulated the expression of type II collage. Particularly at the dose of 2.5 grams/kg, there were around 1.8-fold, 2.1-fold, and 3.7-fold increase in proteoglycan (p<0.01), collagen (p<0.001) and type II collage (p<0.001), respectively, as well as a significant difference with a 60% increase in CPII (p<0.001) and a 24% decrease in CTX-II (p<0.01) compared to those of untreated ACLT+MMx rats (Fig. 2B).
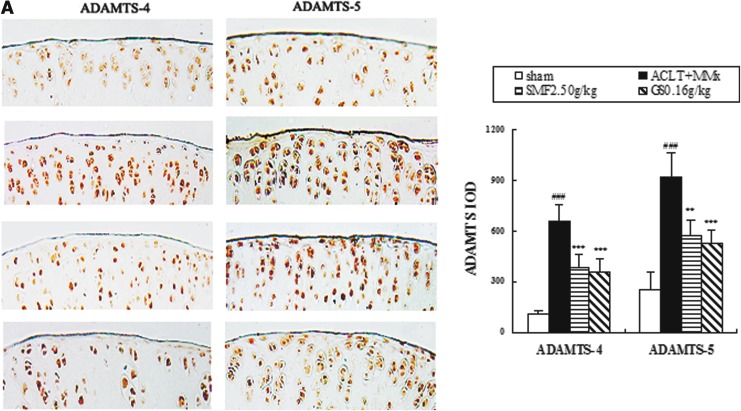
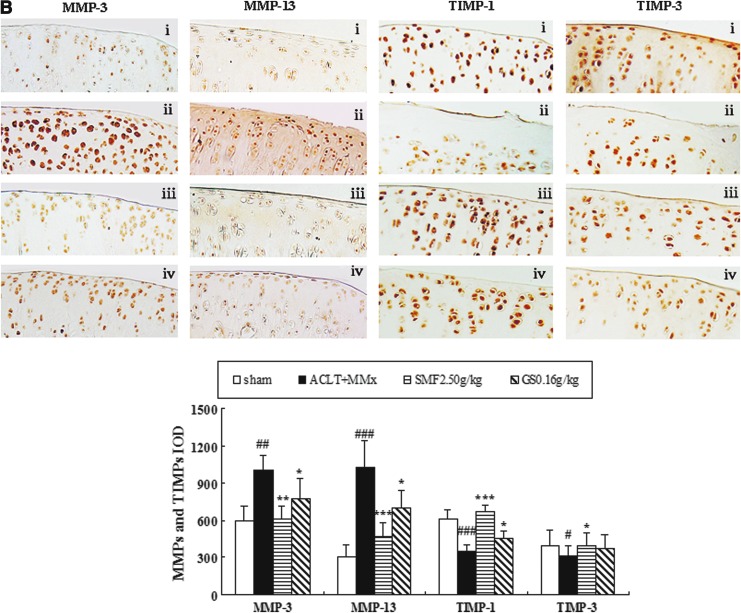
Effects of SMF on ADAMTS-4 and -5, MMP-3 and -13, and TIMP-1 and -3 expression in the joints of ACLT+MMx rats
The effects of SMF treatment on expression of ADAMTS, MMPs, and TIMPs in ACLT+MMx rats were assessed by immunohistochemistry. Representative joint sections from SMF 2.5 gram/kg or glucosamine treatment groups and quantitative analysis are shown in Fig. 3. ACLT+MMx induced increased expression of ADAMT-4 and -5 and MMP-3 and -13 and decreased expression of TIMP-1 and -3 in the joints. Compared with un-treated ACLT+MMx rats, treatment with SMF 2.5 grams/kg increased positive expression by 42% in ADAMT-4 (p<0.001) and 38% in ADAMT-5 (p<0.01) (Fig. 3A). Meanwhile, SMF 2.5 grams/kg also induced a clear down-regulation of MMP-3 and -13 (p<0.001 and p<0.01, respectively) and marked up-regulation of TIMP-1 and -3 (p<0.001 and p<0.05, respectively) compared with untreated ACLT+MMx rats (Fig. 3B). ADAMTS-4 and -5, MMP-3 and -13, and TIMP-1 and -3 were expressed in the chondrocytes of the knee joint of all groups, and the positive staining was predominantly cytoplasmic or cytosolic in the chondrocytes. These results showed that SMF exerted anti-OA activity in vivo through ADAMTS and MMPs/TIMPs system.
FIG. 3.
The effects of SiMaioFang (SMF) on aggrecanases-1 and -2 (ADAMTS-4 and -5), matrix metalloproteinases-3 and -13 (MMP-3 and -13), and tissue inhibitors of metalloproteinases-1 and -3 (TIMP-1 and -3) in the knee joints. (A) The effects of SMF on expression of ADAMTS-4 and -5 in the knee joints. (B) The effects of SMF on expression of MMP-3 and -13 and TIMP-1 and -3 in the knee joints. These enzymes expressions were assessed by quantitative immunohistochemistry. Photomicrographs taken at 200×magnification of representative cartilage sections from one animal per group are shown: Sham (i), anterior cruciate ligament transection+medial meniscus resection ACLT+MMx (ii), ACLT+MMx+SMF at 2.50 grams/kg (iii), ACLT+MMx+glucosamine 0.16 gram/kg (iv). Quantitative immunohistochemistry analyses of the expression of these enzymes in the tibia are shown, and data represent the mean±standard deviation (SD) of samples from 10 rats in each group. Comparing treatment group with untreated ACLT+MMx rats. (***) p<0.001, (**) p<0.01, and (*) p<0.05, compared with ACLT+MMx; (###) p<0.001, (##) p<0.01, and (#) p<0.05, compared with the sham group. (Color images available online at www.liebertpub.com/rej)
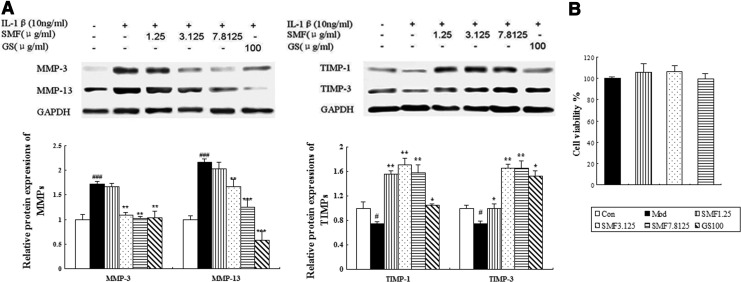
Effects of SMF on the production and gene expression of MMP-3 and -13 and TIMP-1 and -3 in human chondrocytes
It is well known that MMPs/TIMPs play a key role in OA progression according to in vivo results. We carried out in vitro studies to elucidate further effects on MMPs/TIMPs by western blotting. The expression of MMP-3 and -13 was significantly down-regulated when incubated with SMF at 3.125 or 7.8125 μg/mL or after incubation with glucosamine. The down-regulating effects were augmented as the dose of SMF increased, and SMF at 7.8125 μg/mL induced a 40% decrease in MMP-3 (p<0.01) and a 42% decrease in MMP-13 (p<0.001) compared to untreated IL-1β–induced chondrocytes. The down-regulated effect of SMF on MMP-13 expression is weaker than that of glucosamine. Meanwhile, the expression of TIMP-1 and -3 was remarkably up-regulated when incubated with SMF at all three concentrations or with glucosamine. The largest increases in the two parameters were found at SMF 3.125 μg/mL (both p<0.01) and with 2.2-fold up-regulation, respectively. The expression of TIMP-1 and -3 was up-regulated slightly more by glucosamine than by SMF (Fig. 4A). Meanwhile, SMF at doses of 1.25, 3.125, and 7.8125 μg/mL did not affect IL-1β–induced chondrocyte viability (Fig. 4B).
FIG. 4.
The effects of SiMiaoFang (SMF) on matrix metalloproteinases-3 and -13 (MMP-3 and-13) and tissue inhibitors of metalloproteinases-1 and -3 (TIMP-1 and -3) expression in IL-1β–induced chondrocytes. (A) Western blot analysis of MMP-3 and -13 and TIMP-1 and -3 expression in human chondrocytes were carried out as described in Materials and Methods. Representative blots are shown above, and quantitative analysis and cell viability are shown below. Values given are the mean±standard deviation (SD) of three independent experiments. (***) p<0.001, (**) p<0.01, and (*) p<0.05, compared with Model (Mod); (###) p<0.001 and (#) p<0.05, compared with the Control (Con) group. (B) The effect of SMF on viability of IL-1β–induced chondrocytes. GS, glucosamine.
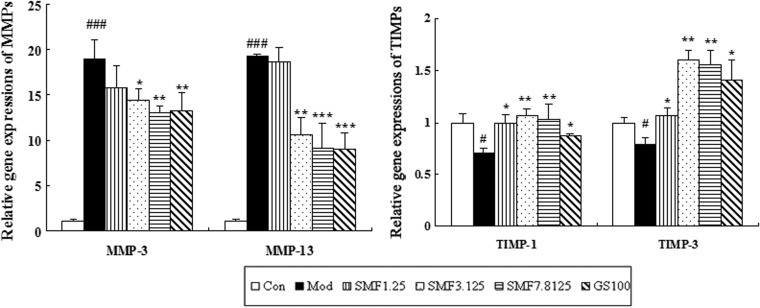
To further validate the above mechanisms, we also assessed the effects of SMF on mRNA expression of MMP-3 and-13 and TIMP-1 and -3 in IL-1β–induced chondrocytes by real-time quantitative PCR. SMF significantly inhibited the IL-1β–induced gene expression of MMP-3 and-13 in a dose-dependent manner and SMF at the highest concentration produced with a 31% decrease in MMP-3 mRNA (p<0.01) and 52% decrease in MMP-13 mRNA (p<0.001) compared with the model group. Interestingly, the effects of SMF and glucosamine on gene expression of TIMP-1 and -3 were similar to those in protein levels. SMF at 3.125 μg/mL induced 1.5-fold and 2.0-fold up-regulation in TIMP-1 and -3 mRNA compared with the model group, respectively (both p<0.01) (Fig. 5).
FIG. 5.
The effects of SiMiaoFang (SMF) on matrix metalloproteinases-3 and -13 (MMP-3 and -13) and tissue inhibitors of metalloproteinases-1 and -3 (TIMP-1 and -3) mRNA expression in IL-1β–induced chondrocytes. Real-time PCR analyses of MMP-3 and -13 and TIMP-1 and -3 gene expression in human chondrocytes were carried out as described in Materials and Methods. Quantitative analysis is shown and values given are the mean±standard deviation (SD) of three independent experiments. (***) p<0.001, (**) p<0.01, and (*) p<0.05, compared with Model (Mod); (###) p<0.001 compared with the Control (Con) group.
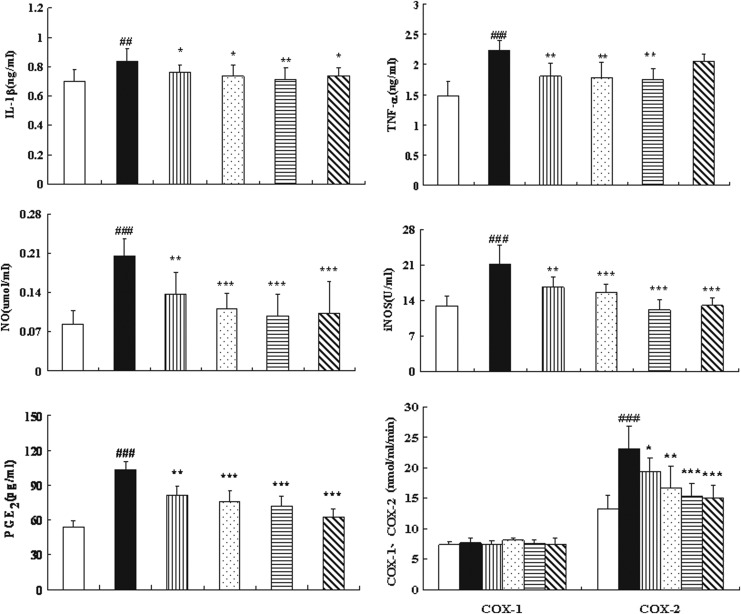
Effects of SMF on pro-inflammatory cytokines and inflammatory mediators in ACLT+MMx rats
Because the high levels of serum IL-β in mono-iodoacetate–induced OA rats and serum TNF-α as well as IL-6 in OA patients were observed, respectively.29,30 We detected pro-inflammatory cytokines and inflammatory mediators in the serum of ACLT+MMx rats, and the results are indicated in Fig. 6. Treatment of ACLT+MMx rats with SMF at 1.25 or 2.5 grams/kg, or with glucosamine, resulted in a significant difference in IL-1β and with a 15% decrease at the highest dose of 2.5 grams/kg compared with untreated ACLT+MMx rats (p<0.01). SMF at any of three doses significantly decreased levels of circulating TNF-α by approximately 20% compared to those of untreated ACLT+MMx rats (all p<0.01).
FIG. 6.
The effects of SiMiaoFang (SMF) on interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), nitric oxide (NO), inducible nitric oxide synthase (iNOS), prostaglandin E (PGE2), cyclooxygenase-1 (Cox-1), and Cox-2 in serum of cruciate ligament transection+medial meniscus resection (ACLT+MMx) rats. Blood was obtained from the abdominal aorta at 6 weeks of treatment after the ACLT+MMx operation and sham-operated, and serum was used for the assays of IL-1β, TNF-α, NO, iNOS, PGE2, COX-1, and COX-2. Data represent the mean±standard deviation (SD) of samples from 10 rats in each group compared the treatment group with untreated ACLT+MMx rats. (***) p<0.001, (**) p<0.01, and (*) p<0.05, compared with ACLT+MMx; (###) p<0.001, (##) p<0.01, compared with the sham group.
There was a trend of decreasing in serum PGE2 with an increasing dose of SMF; SMF at 2.5 grams/kg resulted in a 30% decrease compared with untreated ACLT+MMx rats (p<0.001). SMF did not cause any effect on serum COX-1 levels. However, at any of three doses of SMF, there were significant differences in COX-2 levels compared with untreated ACLT+MMx rats, and the highest dose resulted in a 33% decrease in COX-2 (p<0.001), which was comparable to the decrease induced by glucosamine treatment.
SMF treatment at all three doses significantly decreased levels of NO and iNOS in serum, and SMF at 2.5 grams/kg or glucosamine significantly decreased contents of NO and iNOS by 50% and 42%, respectively, compared to those of untreated ACLT+MMx rats (p<0.001, respectively).
Discussion
OA is a degenerative joint disease with multiple underlying pathogenic mechanisms caused by various risk factors. The balance of degradation and synthesis of cartilage matrix plays a key role in inhibiting the destruction of articular cartilage. Therefore, inhibiting degradation and promoting synthesis of cartilage matrix is an important issue in the treatment of OA. In the present study, we evaluated the anti-OA activity of SMF in vivo using a ACLT+MMx rat model. The results showed SMF could clearly inhibit the degradation of cartilage matrix, as indicated by increasing proteoglycan and collagen content, particularly type II collagen expression in articular cartilage, and decreasing CTX-II and increasing CPII in circulation, showing a significant anti-joint degeneration.
Cartilage matrix is composed of proteoglycan and collagen. Type II collagen is the most important biomarker for OA because of a 90–95% content in total collagen.31 It has been recognized that MMP-3 and -13 play important roles in the degradation of cartilage matrix during the development of OA. MMP-3 can degrade proteoglycan and type II collagen.32 MMP-13 can degrade type II collagen and is considered the primary collagenase in OA.33 The expression and production of MMPs are regulated at the mRNA and protein levels by various factors; the most important effect is from the TIMP family.34 The enzymatic activities of MMP-3 and −13 are inhibited by TIMP-1, and TIMP-3 has been recognized as a potent inhibitor of MMP-3.35,36 Under normal conditions, TIMPs bind to active MMPs in a ratio of 1:1 to make an inactive complex. Therefore, an imbalance in the ratio of TIMPs to MMPs causes continued matrix destruction in OA.13,15
In the present study, we demonstrated that SMF significantly interfered with the OA-induced expression of MMP-13 and -3, while augmenting that of TIMP-1 and -3 in the joints of ACLT+MMx rats. These results are supported by our in vitro study, where IL-1–augmented expression of MMP-3 and -13 was inhibited and expression of TIMP-1 and -3 was up-regulated in protein and gene levels by SMF in IL-1β–induced chondrocytes. A recent study has also showed that the primary component berberine inhibited the induction of MMP-13 and -3 and increased TIMP-1 expression in IL-1β– chondrocytes.37,38 Thus, SMF can block cartilage matrix degradation by mediating the balance of MMPs/TIMPs.
Moreover, ADAMTS-4 and -5 are considered to be the most efficient aggrecanases.39,40 Recent papers have reported that deleting ADAMTS-4 and -5 in mice significantly protected against proteoglycan degradation and decreased the severity of murine OA.41 As the most likely candidates to play a role in the pathogenesis of OA, ADAMTS-4 and -5 are inhibited by TIMP-3.42 Our study shows that SMF markedly decreased ADAMTS-4 and -5 expression and increased TIMP-3 expression in ACLT+MMx rat joints, which may be one of mechanisms of SMF anti-OA activity.
The progression of OA is now believed to be relevant to inflammation in the early stages of the disease. Among the pro-inflammatory cytokines involved in OA, IL-1β and TNF-α are considered the major players. IL-1β and TNF-α produced by activated synoviocytes, mononuclear cells, or chondrocytes can directly induce degradation of cartilage matrix43,44 and promote the production of PGE2 and NO as well as up-regulation of MMP-3 and -13, which together induce the progression of OA.45–48 Among the numerous pro-inflammatory enzymes, inducible COX-2 is a key enzyme for production of PGE2, and iNOS regulates the generation of NO. Our results showed that SMF significantly decreased the secretion of IL-1β and TNF-α in serum. Additionally, SMF markedly inhibited the release of PGE2 and NO in circulation through suppression of COX-2 and iNOS production, respectively. SMF was able to suppress the inflammatory response of arthritis. Wang et al. reported that SMF effectively down-regulated over-expression of IL-1β, TNF-α, and IL-6 mRNA in adjuvant arthritis (AA) rats.49 The dose of SMF used in AA rats is similar to OA rats in our study. Moreover, all SMF four ingredients contain the anti-inflammatory substances, such as berberine and atractylodine, which were confirmed to have anti-inflammatory activity.50–53 Thus, we deduce that SMF inhibits inflammation response in OA by interfering with secretion of pro-inflammatory cytokines and inflammatory mediators.
Because glucosamine is effective not only in prevention of cartilage matrix degradation but also against inflammatory symptoms in OA animal experiments,54–56 we used it as the control drug, and its activity was also proved in our study. Compared with glucosamine at the clinically equivalent dose, SMF at 2.5 grams/kg had a similar ability to suppress cartilage matrix components mass loss and anti-inflammation. Moreover, both SMF and glucosamine were able to regulate the balance of the MMPs/TIMPs system, which has been confirmed by in vitro results. Meanwhile, SMF exerted anti-OA activity with no evidence of adverse effect on viscera (heart, liver, spleen, lung, and kidney), body weight, skin, hair, and activities of ACLT+MMx rats. It is worth mentioning that the cost of SMF is far lower than glucosamine in the marketplace. Thus, SMF could be a potential novel agent that could effectively prevent cartilage matrix degradation in OA.
In conclusion, these results demonstrated that SMF effectively inhibited cartilage matrix degradation and interfered with the secretions of pro-inflammatory cytokines and inflammatory mediators in ACLT+MMx rats. The effects of preventing cartilage matrix were mainly from the direct suppressing MMPs expression, simultaneous up-regulation of TIMPs production, and then maintaining the balance of MMPs to TIMPs in the joints and chondrocytes. Moreover, down-regulation of the expression of ADMATS-4 and -5 was highly effective in ACLT+MMx rats. Accordingly, this study provides the first evidence that SMF can effectively treat OA.
Acknowledgments
This work was supported by the grants from project of National Natural Science Foundation of China (81072900)
Author Disclosure Statement
Ying Xu and Qian Liu contributed equally to this paper and all authors have no conflicts of interest to disclose.
References
- 1.Harris ED., Jr. The bone and joint decade: A catalyst for progress. Arthritis Rheum. 2001;44:1969–1970. doi: 10.1002/1529-0131(200109)44:9<1969::AID-ART342>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 2.Brosseau L. Wells GA. Kenny GP. Reid R. Maetzel A. Tugwell P. Huijbregts M. McCullough C. De Angelis G. Chen L. The implementation of a community-based aerobic walking program for mild to moderate knee osteoarthritis (OA): A knowledge translation (KT) randomized controlled trial (RCT): Part II: Clinical outcomes. BMC Public Health. 2012;12:1073–1088. doi: 10.1186/1471-2458-12-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mankin HJ. Lippiello L. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. J Bone Joint Surg Am. 1970;52:424–434. [PubMed] [Google Scholar]
- 4.Jubb RW. Fell HB. The breakdown of collagen by chondrocytes. J Pathol. 1980;130:159–167. doi: 10.1002/path.1711300304. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes JC. Martel-Pelletier J. Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39:237–246. [PubMed] [Google Scholar]
- 6.Mazzetti I. Grigolo B. Pulsatelli L. Dolzani P. Silvestri T. Roseti L. Meliconi R. Facchini A. Differential roles of nitric oxide and oxygen radicals in chondrocytes affected by osteoarthritis and rheumatoid arthritis. Clin Sci (Lond) 2001;101:593–599. [PubMed] [Google Scholar]
- 7.Henrotin Y. Kurz B. Aigner T. Oxygen and reactive oxygen species in cartilage degradation: Friends or foes? Osteoarthritis Cartilage. 2005;13:643–654. doi: 10.1016/j.joca.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Abramson SB. Osteoarthritis and nitric oxide. Osteoarthritis Cartilage. 2008;16:S15. doi: 10.1016/S1063-4584(08)60008-4. [DOI] [PubMed] [Google Scholar]
- 9.Attur M. Al-Mussawir HE. Patel J. Kitay A. Dave M. Palmer G. Pillinger MH. Abramson SB. Prostaglandin E2 exerts catabolic effects in osteoarthritis cartilage: Evidence for signaling via the EP4 receptor. J Immunol. 2008;181:5082–5088. doi: 10.4049/jimmunol.181.7.5082. [DOI] [PubMed] [Google Scholar]
- 10.Cawston TE. Wilson AJ. Understanding the role of tissue degrading enzymes and their inhibitors in development and disease. Best Pract Res Clin Rheumatol. 2006;20:983–1002. doi: 10.1016/j.berh.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Song RH. Tortorella MD. Malfait AM. Alston JT. Yang Z. Arner EC. Griggs DW. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 2007;56:575–585. doi: 10.1002/art.22334. [DOI] [PubMed] [Google Scholar]
- 12.Burger D. Rezzonico R. Li JM. Modoux C. Pierce RA. Welgus HG. Dayer JM. Imbalance between interstitial collagenase and tissue inhibitor of metalloproteinases 1 in synoviocytes and fibroblasts upon direct contact with stimulated T lymphocytes: Involvement of membrane-associated cytokines. Arthritis Rheum. 2004;41:1748–1759. doi: 10.1002/1529-0131(199810)41:10<1748::AID-ART7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 13.Lin N. Liu C. Xiao C. Jia H. Imada K. Wu H. Ito A. Triptolide, a diterpenoid triepoxide, suppresses inflammation and cartilage destruction in collagen-induced arthritis mice. Biochem Pharmacol. 2007;73:136–146. doi: 10.1016/j.bcp.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 14.Reginster JY. Neuprez A. Lecart MP, et al. Role of glucosamine in the treatment for osteoarthritis. Rheumatol Int. 2012;32:2959–2967. doi: 10.1007/s00296-012-2416-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.China Pharmacopoeia Committee. Chinese Pharmacopoeia. Chemical Industry Press; Beijing, PR China: 2005. [Google Scholar]
- 16.Jing Y. Li WL. Experimental study on anti-inflammatory effect and analgesic effect of Simiao powder. J Henan Univ Chin Med. 2008;23:33–34. [Google Scholar]
- 17.Shi XD. Li GC. Qian ZX. Jin ZQ. Song Y. Randomized and controlled clinical study of modified prescriptions of Simiao Pill in the treatment of acute gouty arthritis. Chin J Integr Med. 2008;14:17–22. doi: 10.1007/s11655-007-9001-7. [DOI] [PubMed] [Google Scholar]
- 18.Wang X. Zhang X. Zhang L. Li Y. Effects and mechanisms of Simiao pill on adjuvant arthritis rats model. Zhongguo Zhong Yao Za Zhi. 2010;35:2889. [PubMed] [Google Scholar]
- 19.Zhao J. Zha Q. Jiang M. Cao H. Lu A. Expert consensus on the treatment of rheumatoid arthritis with Chinese patent medicines. J Altern Complement Med. 2013;19:111–1188. doi: 10.1089/acm.2011.0370. [DOI] [PubMed] [Google Scholar]
- 20.CDER. FDA. Guidance for Industry, Pharmacol Toxicol; Washington, DC: 2005. Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. [Google Scholar]
- 21.Hayami T. Pickarski M. Wesolowski GA. McLane J. Bone A. Destefano J. Rodan GA. Duong le T. The role of subchondral bone remodeling in osteoarthritis: Reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model. Arthritis Rheum. 2004;50:1193–1206. doi: 10.1002/art.20124. [DOI] [PubMed] [Google Scholar]
- 22.Gruber HE. Marshall GJ. Nolasco LM. Kirchen ME. Rimoin DL. Alkaline and acid phosphatase demonstration in human bone and cartilage: Effects of fixation interval and methacrylate embedments. Biotech Histochem. 1988;63:299–306. doi: 10.3109/10520298809107604. [DOI] [PubMed] [Google Scholar]
- 23.Mankin HJ. Dorfman H. Lippiello L. Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53:523–537. [PubMed] [Google Scholar]
- 24.Yoshimi T. Kikuchi T. Obara T. Yamaguchi T. Sakakibara Y. Itoh H. Iwata H. Miura T. Effects of high-molecular-weight sodium hyaluronate on experimental osteoarthrosis induced by the resection of rabbit anterior cruciate ligament. Clin Orthop. 1994;298:296–304. [PubMed] [Google Scholar]
- 25.Nelson F. Dahlberg L. Laverty S. Reiner A. Pidoux I. Ionescu M. Fraser G L. Brooks E. Tanzer M. Rosenberg LC. Dieppe P. Robin Poole A. Evidence for altered synthesis of type II collagen in patients with osteoarthritis. J Clin Invest. 1998;102:2115–2125. doi: 10.1172/JCI4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen WP. Tang JL. Bao JP. Wu LD. Thymoquinone inhibits matrix metalloproteinase expression in rabbit chondrocytes and cartilage in experimental osteoarthritis. Exp Biol Med. 2010;235:1425–1431. doi: 10.1258/ebm.2010.010174. [DOI] [PubMed] [Google Scholar]
- 27.Xu Y. Zhang ZJ. Geng F. Su SB. White KN. Bligh SA. Wang ZT. Treatment with Qing'E, a kidney-invigorating Chinese herbal formula, antagonizes the estrogen decline in ovariectomized mice. Rejuvenation Res. 2010;13:479–488. doi: 10.1089/rej.2009.1000. [DOI] [PubMed] [Google Scholar]
- 28.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45–e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashkavand Z. Malekinejad H. Amniattalab A. Rezaei-Golmisheh A. Vishwanath BS. Silymarin potentiates the anti-inflammatory effects of Celecoxib on chemically induced osteoarthritis in rats. Phytomedicine. 2012;19:1200–1205. doi: 10.1016/j.phymed.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Stannus O. Jones G. Cicuttini F. Parameswaran V. Quinn S. Burgess J. Ding C. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthritis Cartilage. 2010;18:1441–1447. doi: 10.1016/j.joca.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Naito K. Watari T. Muta T. Furuhata A. Iwase H. Igarashi M. Kurosawa H. Nagaoka I. Kaneko K. Low-intensity pulsed ultrasound (LIPUS) increases the articular cartilage type II collagen in a rat osteoarthritis model. J Orthop Res. 2009;28:361–369. doi: 10.1002/jor.20995. [DOI] [PubMed] [Google Scholar]
- 32.Jo H. Park JS. Kim EM. Jung MY. Lee SH. Seong SC. Park SC. Kim HJ. Lee MC. The in vitro effects of dehydroepiandrosterone on human osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2003;11:585–594. doi: 10.1016/s1063-4584(03)00094-3. [DOI] [PubMed] [Google Scholar]
- 33.Goldring MB. Otero M. Plumb DA. Dragomir C. Favero M. El Hachem K. Hashimoto K. Roach HI. Olivotto E. Borzì RM. Marcu KB. Roles of inflammatory and anabolic cytokines in cartilage metabolism: Signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur Cell Mater. 2011;21:202–220. doi: 10.22203/ecm.v021a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brew K. Dinakarpandian D. Nagase H. Tissue inhibitors of metalloproteinases: Evolution, structure and function. Biochim Biophys Acta. 2000;1477:267. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 35.Gomis-Ruth FX. Maskos K. Betz M. Bergner A. Huber R. Suzuki K. Yoshida N. Nagase H. Brew K. Bourenkov GP. Bartunik H. Bode W. Mechanism of inhibition of the human matrix metalloproteinase stromelysin-1 by TIMP-1. Nature. 1997;389:77–80. doi: 10.1038/37995. [DOI] [PubMed] [Google Scholar]
- 36.Wetzel M. Li L. Harms KM. Roitbak T. Ventura PB. Rosenberg GA. Khokha R. Cunningham LA. Tissue inhibitor of metalloproteinases-3 facilitates Fas-mediated neuronal cell death following mild ischemia. Cell Death Differ. 2008;15:143–151. doi: 10.1038/sj.cdd.4402246. [DOI] [PubMed] [Google Scholar]
- 37.Moon PD. Jeong HS. Chun CS. Kim HM. Baekjeolyusin‐tang and its active component berberine block the release of collagen and proteoglycan from IL‐1β‐stimulated rabbit cartilage and down‐regulate matrix metalloproteinases in rabbit chondrocytes. Phytother Res. 2011;25:844–850. doi: 10.1002/ptr.3353. [DOI] [PubMed] [Google Scholar]
- 38.Hu P. Chen W. Tang J L. Bao JP. Wu LD. Protective effects of berberine in an experimental rat osteoarthritis model. Phytother Res. 2011;25:878–885. doi: 10.1002/ptr.3359. [DOI] [PubMed] [Google Scholar]
- 39.Zeng W. Corcoran C. Collins-Racie LA. Lavallie ER. Morris EA. Flannery CR. Glycosaminoglycan-binding properties and aggrecanase activities of truncated ADAMTSs: Comparative analyses with ADAMTS-5,-9,-16 and-18. Biochim Biophys Acta. 2006;1760:517–524. doi: 10.1016/j.bbagen.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Fushimi K. Troeberg L. Nakamura H. Lim NH. Nagase H. Functional differences of the catalytic and non-catalytic domains in human ADAMTS-4 and ADAMTS-5 in aggrecanolytic activity. J Biol Chem. 2008;283:6706–6716. doi: 10.1074/jbc.M708647200. [DOI] [PubMed] [Google Scholar]
- 41.Majumdar MK. Askew R. Schelling S. Stedman N. Blanchet T. Hopkins B. Morris EA. Glasson SS. Double-knockout of ADAMTS-4 and ADAMTS-5 in mice results in physiologically normal animals and prevents the progression of osteoarthritis. Arthritis Rheum. 2007;56:3670–3674. doi: 10.1002/art.23027. [DOI] [PubMed] [Google Scholar]
- 42.Kashiwagi M. Tortorella M. Nagase H. Brew K. TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4) and aggrecanase 2 (ADAM-TS5) J Biol Chem. 2001;276:12501–12504. doi: 10.1074/jbc.C000848200. [DOI] [PubMed] [Google Scholar]
- 43.Aida Y. Maeno M. Suzuki N. Shiratsuchi H. Motohashi M. Matsumura H. The effect of IL-1β on the expression of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in human chondrocytes. Life Sci. 2005;77:3210–3221. doi: 10.1016/j.lfs.2005.05.052. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi M. Squires G R. Mousa A. Tanzer M. Zukor D J. Antoniou J. Feige U. Poole A R. Role of interleukin‐1 and tumor necrosis factor α in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005;52:128–135. doi: 10.1002/art.20776. [DOI] [PubMed] [Google Scholar]
- 45.Loeser RF. Carlson CS. Carlo MD. Cole A. Detection of nitrotyrosine in aging and osteoarthritic cartilage: Correlation of oxidative damage with the presence of interleukin‐1β and with chondrocyte resistance to insulin‐like growth factor 1. Arthritis Rheum. 2002;46:2349–2357. doi: 10.1002/art.10496. [DOI] [PubMed] [Google Scholar]
- 46.Del Carlo M. Loeser RF. Nitric oxide-mediated chondrocyte cell death requires the generation of additional reactive oxygen species. Arthritis Rheum. 2002;46:394–403. doi: 10.1002/art.10056. [DOI] [PubMed] [Google Scholar]
- 47.Tortorella M D. Malfait A M. Deccico C. Arner E. The role of ADAM-TS4 (aggrecanase-1) and ADAM-TS5 (aggrecanase-2) in a model of cartilage degradation. Osteoarthritis and cartilage/OARS, Osteoarthritis Cartilage. 2001;9:539. doi: 10.1053/joca.2001.0427. [DOI] [PubMed] [Google Scholar]
- 48.Xian YF. Mao QQ. Ip SP. Lin ZX. Che CT. Comparison on the anti-inflammatory effect of Cortex Phellodendri Chinensis and Cortex Phellodendri Amurensis in 12-O-tetradecanoyl-phorbol-13-acetate-induced ear edema in mice. J Ethnopharmacol. 2011;137:1425–1430. doi: 10.1016/j.jep.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 49.Wang X. Zhang X. Zhang L. Li Y. Effects and mechanisms of Simiao pill on adjuvant arthritis rats model. Zhongguo Zhong Yao Za Zhi. 2010;35:2889–2892. [PubMed] [Google Scholar]
- 50.Huang DW. Chung CP. Kuo YH. Lin YL. Chiang W. Identification of compounds in adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) seed hull extracts that inhibit lipopolysaccharide-induced inflammation in RAW 264.7 macrophages. J Agric Food Chem. 2009;57:10651–10657. doi: 10.1021/jf9028514. [DOI] [PubMed] [Google Scholar]
- 51.Han SB. Lee CW. Yoon YD. Lee JH. Kang JS. Lee KH. Yoon WK. Lee K. Park S K. Kim HM. Prevention of arthritic inflammation using an oriental herbal combination BDX-1 isolated from Achyranthes Bidentata and Atractylodes Japonica. Arch Pharm Res. 2005;28:902–908. doi: 10.1007/BF02973875. [DOI] [PubMed] [Google Scholar]
- 52.Seo MJ. Kim SJ. Kang TH. Rim HK. Jeong HJ. Um JY. Hong SH. Kim HM. The regulatory mechanism of β-eudesmol is through the suppression of caspase-1 activation in mast cell-mediated inflammatory response. Immunopharmacol Immunotoxicol. 2011;33:178–185. doi: 10.3109/08923973.2010.491082. [DOI] [PubMed] [Google Scholar]
- 53.Liu K. Luo T. Zhang Z. Wang T. Kou J. Liu B. Huang F. Modified Si-Miao-San extract inhibits inflammatory response and modulates insulin sensitivity in hepatocytes through an IKKβ/IRS-1/Akt-dependent pathway. J Ethnopharmacol. 2011;136:473–479. doi: 10.1016/j.jep.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 54.Gouze J N. Bordji K. Gulberti S. Terlain B. Netter P. Magdalou J. Fournel-Gigleux S. Ouzzine M. Interleukin‐1β down‐regulates the expression of glucuronosyltransferase I, a key enzyme priming glycosaminoglycan biosynthesis: Influence of glucosamine on interleukin‐1β–mediated effects in rat chondrocytes. Arthritis Rheum. 2001;44:351–360. doi: 10.1002/1529-0131(200102)44:2<351::AID-ANR53>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 55.Tiraloche G. Girard C. Chouinard L. Sampalis J. Moquin L. Ionescu M. Reiner A. Poole AR. Laverty S. Effect of oral glucosamine on cartilage degradation in a rabbit model of osteoarthritis. Arthritis Rheum. 2005;52:1118–1128. doi: 10.1002/art.20951. [DOI] [PubMed] [Google Scholar]
- 56.Oegema TR. Deloria LB. Sandy JD. Hart DA. Effect of oral glucosamine on cartilage and meniscus in normal and chymopapain‐injected knees of young rabbits. Arthritis Rheum. 2002;46:2495–2503. doi: 10.1002/art.10499. [DOI] [PubMed] [Google Scholar]