Summary
Early Drosophila embryogenesis is characterized by shifting from astral microtubule-based to central spindle-based positioning of cleavage furrows. Before cellularization, astral microtubules determine metaphase furrow position by producing Rappaport-like furrows, which encompass rather than bisect the spindle. Their positioning is explained by our finding that the conserved central spindle components centralspindlin (mKLP1 and RacGAP50C), Polo, and Fascetto (Prc1) localize to the astral microtubule overlap region. These components and the chromosomal passenger complex localize to the central spindle, though no furrow forms there. We identify the maternally supplied RhoGEF2 as a key factor in metaphase furrow positioning. Unlike the zygotic, central spindle-localized RhoGEF (Pebble), RhoGEF2 localizes to metaphase furrows, a function distinct from RhoGEF/Pebble and likely due to the absence of a RacGAP50C binding domain. Accordingly, we find that ectopic activation of Rho GTPase generates furrows perpendicular to the central spindle during syncytial divisions. Whereas metaphase furrow formation is myosin independent, these ectopic furrows, like conventional furrows, require myosin as well as microtubules. These studies demonstrate that early Drosophila embryogenesis is primed to form furrows at either overlapping astral microtubules or the central spindle. We propose that the shift to the latter is driven by a corresponding shift from RhoGEF2 to Pebble in controlling furrow formation.
Results and Discussion
Normal and Ectopic Localization of Central Spindle Proteins in the Early Drosophila Embryo
Using both live and fixed fluorescent analysis of cycle 12 Drosophila embryos, we localized conserved central spindle components (Figure 1). We found that Fascetto (Feo, Prc1 homolog), Tumbleweed (Tum, RacGAP50C homolog), and Polo (Plk1 homolog) all localized to the site of metaphase furrow formation (Figure 1A). In addition, the binding partner of Tum in the centralspindlin complex, Pavarotti (Pav, mKLP1 homolog), was previously shown to localize to the metaphase furrows from interphase to metaphase [1]. The chromosomal passenger complex, Aurora B (AurB) and inner centromere protein (INCENP), has no significant localization at sites of metaphase furrow formation (Figure 1A). Localization of the centralspindlin complex and other conserved furrow components to the site of metaphase furrow formation was unexpected. Work from Rappaport and others has shown that, depending on the cell type, cleavage furrow positioning relies on astral microtubules, the central spindle microtubules, or both [2–4]. It is unclear, however, whether furrow induction in both cases relies on the same key furrow signaling molecules. Taken together, our results indicate that the majority of key central spindle-associated furrow components localize at regions of astral-microtubule overlap, the site of metaphase furrow formation in the syncytial Drosophila embryo [5, 6]. This suggests that, at least in Drosophila, astral-microtubule and central spindle-based furrow induction rely on the same set of conserved furrowing components.
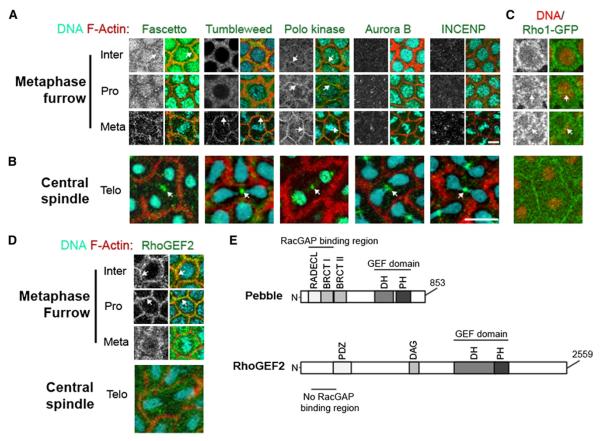
Figure 1.
Central Spindle Proteins Localize to the Metaphase Furrows and the Spindle Midzone
(A) Localization of central spindle proteins (grayscale and green) during metaphase furrow formation in both live and fixed cycle 12 embryos. Arrows indicate colocalization with actin furrows. DNA is cyan and F-actin is red in all panels. Scale bar represents 5 μm.
(B) Telophase localization of central spindle proteins. Arrows indicate accumulations of these proteins at the spindle midzone between two recently divided nuclei. Scale bar represents 10 μm.
(C) Rho1-GFP (grayscale and green) localization 0.5-1 μm below embryo cortex. Arrows in left and right panels highlight a concentrated stripe of RhoA forming directly above each nucleus (red). Nuclei in right panels have been superimposed from a lower z plane in order to highlight nuclear morphology and orientation with respect to the Rho1 stripe. Actin is not labeled in these images.
(D) RhoGEF2 (grayscale and green) localization during metaphase furrow formation and midzone formation at telophase. Arrows indicate colocalization of RhoGEF2 and actin furrows. See also Figure S1.
(E) Schematic representations of conserved protein domains in zygotically expressed RhoGEF/Pebble and the maternally supplied RhoGEF isoform, RhoGEF2.
During the cortical syncytial divisions, astral-based metaphase furrows, but not central spindle-based conventional furrows, are observed. Therefore, we were interested in whether the core furrow signaling components described above also localized to the central spindle during anaphase/telophase. We found that all of the proteins (Feo, Tum, AurB, Polo, and INCENP) showed nearly identical localization to a small region between recently divided nuclei where the central spindle is formed (Figure 1B). This result indicates that despite the lack of conventionally positioned cleavage furrows, the central spindle proteins are still regulated and localized as in somatic cells. These findings were unexpected and left unresolved the explanation for the lack of a cleavage furrow at the central spindle during the cortical syncytial divisions. Therefore, we examined the localization of components downstream of the central spindle components.
Central spindle proteins are thought to position the furrow through localized activation of Rho1 at the cortex [7–9]. Previous studies in fixed embryos have shown that Rho1 is tightly localized at the metaphase furrows [10, 11]. Figure 1C shows live imaging of GFP-Rho1, which, during interphase, is found in an apical ring that extends basally at sites of metaphase furrow ingression, supporting earlier observations. Our live imaging revealed a previously undescribed subcortical stripe of Rho1 that forms during prophase (Figure 1C, arrow). By metaphase, this stripe is well defined, paralleling the metaphase plate. Although this localization does not indicate active zones of Rho1, it is similar to cortical stripes of Rho1 that form just prior to contractile ring formation in mammalian and C. elegans cells [9, 12]. However, by telophase, no localization to the equatorial cortex is observed. Thus, the position of this cortical stripe of Rho1 is equivalent to that found in cells undergoing conventional cytokinesis; however, the timing is altered such that it coincides with metaphase furrow formation.
It is likely that localization of the centralspindlin complex to the sites of metaphase and conventional furrow formation rely on distinct mechanisms. Vesicle trafficking may potentially play a role in their localization, as it does for other metaphase furrow components such as RhoGEF2 and Rho1 [10]. Alternatively, the motor protein Pav may directly drive localization of several of these components to the metaphase furrows relying on overlapping astral microtubules. Taken together, we conclude that all of the necessary central spindle proteins localize to the central spindle of syncytial embryos properly and are therefore potentially competent to accumulate RhoGEF at the midzone region and induce a furrow. Furthermore, the metaphase furrow localization of the central spindle components may indicate potentially novel roles for these proteins outside of the central spindle.
RhoGEF2 Localization Is Specific for Metaphase Furrows Rather Than the Central Spindle
Because all of the necessary proteins were localized to the central spindle, we investigated whether RhoGEF was the missing component preventing formation of conventional cytokinesis furrows. Drosophila expresses both zygotic and maternal forms of RhoGEF, known as Pebble and RhoGEF2, respectively [11, 13]. During the zygotically controlled postcellularization divisions, Pebble is responsible for activating Rho1 at the site of furrow formation and is localized to the midzone region [14, 15]. The role of Pebble in the maternally controlled precellularization divisions is less clear, because loss of Pebble does not disrupt metaphase furrow formation [16]. In addition, although Pebble is present in these syncytial divisions, it is nuclear localized during interphase through prophase and diffusely localized in the cytoplasm throughout the remainder of the cell cycle, with no specific accumulation at the central spindle or metaphase furrows (see Figure S1 available online) [17]. It is unclear why Pebble fails to localize to the central spindle, but previous studies have demonstrated that phosphoregulation of Pebble or an upstream interactor dramatically influences its localization [9]. In contrast, maternal RhoGEF2 localizes to the site of metaphase furrows, and loss of RhoGEF2 produces profound disruptions in their formation [11]. In accordance with previous studies, we observed a clear concentration of RhoGEF2 at the site of metaphase furrow formation from interphase through metaphase (Figure 1D). However, we found that RhoGEF2 does not localize to the central spindle or the equatorial cortex during anaphase and telophase. Therefore, despite the proper localization of central spindle proteins, a conventionally positioned cleavage furrow is missing due to the lack of RhoGEF2 localization during the syncytial divisions.
Sequence analysis of these two RhoGEFs provides insight into the failure of RhoGEF2 to localize to the central spindle. Two protein domains (RADECL and BRCT1) in the N-terminal region of Pebble are required for RacGAP50C binding [8]. Although Pebble and RhoGEF2 both possess functional guanine nucleotide exchange factor (GEF) domains (DH and PH) in their C terminus, RhoGEF2 does not contain the RacGAP binding domains in its N terminus (Figure 1E). This readily explains the lack of RhoGEF2 localization at the central spindle and is in accord with previous work demonstrating that RhoGEF2 relies on an alternative vesicle-based mechanism for localization at the metaphase furrows [10]. RhoGEF2 mutants do not have postcellularization cytokinesis phenotypes, indicating that its primary role is in metaphase furrow formation [11]. Conversely, the conventional RhoGEF, Pebble, is zygotically required immediately in the divisions following cellularization [14, 16].
Ectopic RhoA Activation Induces Furrows down the Central Spindle
Our findings identified RhoGEF2 as the only component absent from the central spindle in syncytial embryos. This raised the possibility of inducing furrow formation at the central spindle if the requirement for RhoGEF were bypassed. We accomplished this by injecting an in vitro purified form of mammalian RhoA (the mammalian homolog of Rho1) that is constitutively active due to a point mutation in the guanosine triphosphate (GTP) binding region (RhoA*) [10]. Embryos bearing a moesin-GFP (actin binding protein) transgene [18] were injected at the beginning of interphase of cycle 12 with Cy5-labeled histones, followed by RhoA* (1 mg/ml) injection. Within the area of the RhoA* injection site (30–35 nuclei), 20% ± 1.3% of the nuclei (n = 8 embryos) formed ectopic furrows at the same position as conventional furrows: in the center and perpendicular to the central spindle (Figures 2A, 2B, and 3; Movie S2). Buffer-injected embryos produced ectopic furrows in 1% ± 0.7% (n = 11 embryos) of the dividing nuclei (Figures 2A, 2B, and 3; Movie S1). Unlike conventional furrows, these ectopic furrows form during prophase and metaphase. These furrows ingress to depths of 3–4 μm, and nuclei below these furrows are displaced basally. These displaced nuclei progress through mitosis and remain connected to the cortex. In addition, we also injected a purified wild-type form of RhoA that could presumably be activated by endogenous GEFs (RhoAwt). We observed ectopic furrows in 6% ± 2.1% of the nuclei (n = 5 embryos), which was significantly lower than in the RhoA*-injected embryos (Figure 3).
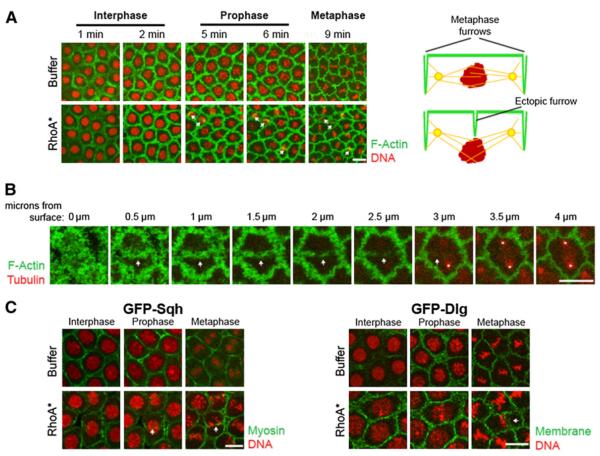
Figure 2.
Ectopic Furrows Induced by Activated RhoA* Injection Are Similar to Cytokinetic Furrows
(A) Living embryos injected during interphase of cycle 12 with either buffer or activated RhoA (RhoA*), the mammalian homolog of Rho1. GFP-Moe (green) labels F-actin and injected Cy5 histone (red) labels DNA (see also Movies S1 and S2). Time following injection is indicated above each panel. Actin is shown at a depth of 3–4 μm below the cortex. Note that arrows indicate the formation of ectopic furrows above nuclei, which have been superimposed from a lower z plane in order to show nuclear orientation and morphology. To the right of each treatment is a schematic of a cross-section through one nucleus at prophase. Note that the ectopic furrows in RhoA*-injected embryos basally displace nuclei from the cortex (see schematic).
(B) Actin-labeled Moe-GFP (green) embryos were injected with RhoA* and rhodamine-labeled tubulin (red) at the beginning of interphase. Images are of one prophase nucleus from the actin cortex (0 μm) to the bipolar spindle (4 μm). Arrow indicates the ectopic furrow forming above the nucleus. Asterisks label the tubulin-rich centrosomes of the spindle.
(C) Embryos with labeled nonmuscle myosin, GFP-Sqh (green), and Cy5-labeled histones (red) were injected with buffer or RhoA*. Embryos with labeled membrane, Dlg-GFP (green), were treated similarly. Both membrane and myosin are observed in the ectopic furrows. Scale bars represent 10 μm.
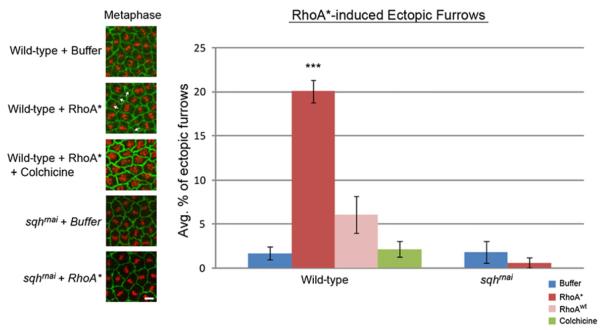
Figure 3.
Ectopic Furrows Require Microtubules and Myosin
Pharmacological and genetic methods were used to disrupt spindle microtubules (colchicine) and myosin (sqhrnai) in embryos injected with buffer, RhoA* (*constitutively active isoform), or RhoAwt (normal isoform) at the beginning of interphase of nuclear cycle 12. The embryos were imaged throughout the following metaphase, and the percent of ectopic furrows was quantified in a region of 5,000 μm2 (graph). Colchicine embryos were labeled with GFP-moesin and RFP-histone. Colchicine was injected immediately after RhoA*. Note the lack of organization of the condensed chromosomes at metaphase indicative of spindle defects. sqhrnai embryos were injected with rhodamine actin (green) and Cy5 histones (red) prior to cycle 12 (see also Figure S2). Data in graph represent the averages of at least five embryos. ***p < 0.001 versus associated buffer control. Error bars indicate SE. Scale bar represents 5 μm.
To determine the orientation of the ectopic furrows in relation to the mitotic spindle, we injected moesin-GFP-expressing embryos during cycle 11 with rhodamine-conjugated tubulin. This was followed by an injection of active RhoA* at the beginning of cycle 12 interphase. All ectopic furrows formed perpendicular to the spindle and bisected the region between the centrosome pairs (Figure 2B). Furthermore, these furrows contained actin, myosin, and membrane, all core components of conventional cytokinetic furrows (Figure 2C).
Both Ectopic and Conventional Cleavage Furrows Require Overlapping Microtubules
Antiparallel overlapping microtubules play a key role in positioning and initiation of the cleavage furrow in many cell types [19]. Therefore, we addressed the role of microtubules in the formation of these ectopic furrows. Embryos were injected with RhoA* at the beginning of interphase of cycle 12 and then immediately injected with colchicine, a microtubule depolymerizer. Previous studies demonstrated that microtubules from interphase through metaphase are not required for metaphase furrow formation [20]. However, ectopic furrows formed at a rate of 2.2% ± 0.9%, compared to 20% ± 1.3% in RhoA*-only injected embryos (Figure 3). Thus, unlike metaphase furrows, ectopic furrows are sensitive to microtubule depolymerization during interphase and prophase. Given the position of ectopic furrows, it is likely that, like conventional furrows, overlapping antiparallel microtubules play an important role in positioning these ectopic furrows. Thus, although RhoA*-induced ectopic furrows form earlier in the cell cycle (prophase/metaphase) than conventional cleavage furrows (anaphase/telophase), both appear to depend on overlap anti-parallel microtubules for furrow establishment and position.
Despite the incorporation of myosin in metaphase furrows, its role in furrow formation is not clear, because metaphase furrows form properly in the absence of myosin [21]. Therefore, we tested whether formation of RhoA-induced ectopic furrows requires myosin. We expressed UAS-sqhrnai during oogenesis using the VP16 α-tubulin Gal4 driver and observed no effect on metaphase furrow formation in buffer-injected embryos, nor did it result in a significant amount of ectopic furrows (1.8% ± 1.2% of nuclei in five embryos; Figure 3). Upon injection of these embryos with RhoA*, we found no significant increase in ectopic furrow formation (0.6% ± 0.6% of nuclei in five embryos). This indicates that myosin is a functional component of these ectopic furrows and, like in conventional furrows, is required for furrow formation. These results indicate that ectopic furrows are functionally equivalent to conventional furrows and distinct from metaphase furrows.
Conclusions
A key event in Drosophila development, the midblastula transition, involves a rapid shift from astral microtubule-based to central spindle-based furrow formation. Here we demonstrate that this is achieved by localizing key furrow determinants to both the astral microtubules and central spindle during the syncytial divisions. Our findings suggest that the switch from astral to central spindle-based furrow formation is mediated by a corresponding switch from maternally supplied RhoGEF2 to zygotically expressed Pebble (RhoGEF). RhoGEF2 is localized to the metaphase furrows during syncytial divisions; then, upon cellularization, Pebble localizes to the central spindle, and traditional furrows form ([14]; Figure 4). Thus, the early Drosophila embryo is poised to form either astral-based or central spindle-based furrows, with RhoGEF-based activation of Rho being the rate-limiting factor driving furrow position (Figure 4). Rappaport's classic experiments as well as studies on experimentally induced monopolar spindles demonstrate that astral microtubules have the potential to induce furrows [3, 22, 23]. In accord with our findings, one of these central spindle components (Prc1) has recently been shown to localize to monopolar astral arrays [24]. We suspect that the same central spindle components localize at astral microtubule plus ends in Rappaport's embryos. The mechanisms guiding this ectopic localization are not known. In syncytial Drosophila embryos, studies have demonstrated that RhoGEF2 is transported to the metaphase furrows via recycling endosome-derived vesicles [10, 25]. Whether other furrow components rely on similar vesicle-based transport mechanisms is unclear.
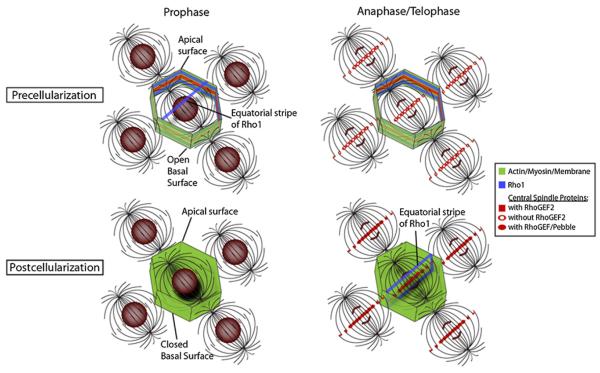
Figure 4.
Schematic of Spatiotemporal Differences of Furrow Determinants in Syncytial and Cellularized Embryos
Apical surfaces have been removed. During the syncytial divisions, central spindle proteins are localized to the metaphase furrows as early as interphase and have midzone localization at telophase, with exception of RhoGEF2. Note the premature equatorial stripe of Rho1 and the absence of a stripe during anaphase/telophase. The cellularized epithelium has a basal cortex and shows localization of the central spindle components, including RhoGEF at the mid-zone during anaphase/telophase, which coincides with an equatorial stripe of Rho1.
Experimental Procedures
Constitutively Active RhoA Injections
RhoA* was injected at the beginning of cycle 12 in embryos also injected with Cy5 histones and rhodamine actin (Figures 2 and 3). Alternatively, embryos derived from stocks bearing the moesin-GFP transgene were used to label actin. A digital zoom was used to capture an ~15,000 μm2 area including and surrounding the injection site. After injection of either RhoA* or buffer, images from the surface to a depth of 6 μm at 1 μm intervals were taken every 30 s. In order to focus on the areas with the highest concentration of RhoA*, we quantified the ectopic furrows in a 5,000 μm2 area centered on the injection site in order to account for dilution of injected protein away from injection site. Ectopic furrows were counted in each embryo and then divided by the number of nuclei in the 5,000 μm2 area to obtain an ectopic furrow percentage. Measurements were pooled from multiple embryos in each treatment and averages were graphed along with standard error measurements.
Supplementary Material
Acknowledgments
We thank the TRiP at Harvard Medical School (NIH/NIGMS grant R01-GM084947) for providing transgenic RNAi fly stocks used in this study. This work was supported by a grant to W.S. from the National Institutes of Health (GM046409).
Footnotes
Supplemental Information
Supplemental Information includes two figures, two movies, and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2012.08.046.
References
- 1.Minestrini G, Harley AS, Glover DM. Localization of Pavarotti-KLP in living Drosophila embryos suggests roles in reorganizing the cortical cytoskeleton during the mitotic cycle. Mol. Biol. Cell. 2003;14:4028–4038. doi: 10.1091/mbc.E03-04-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bringmann H, Hyman AA. A cytokinesis furrow is positioned by two consecutive signals. Nature. 2005;436:731–734. doi: 10.1038/nature03823. [DOI] [PubMed] [Google Scholar]
- 3.Rappaport R. Experiments concerning the cleavage stimulus in sand dollar eggs. J. Exp. Zool. 1961;148:81–89. doi: 10.1002/jez.1401480107. [DOI] [PubMed] [Google Scholar]
- 4.Wheatley SP, Wang Y. Midzone microtubule bundles are continuously required for cytokinesis in cultured epithelial cells. J. Cell Biol. 1996;135:981–989. doi: 10.1083/jcb.135.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazumdar A, Mazumdar M. How one becomes many: blastoderm cellularization in Drosophila melanogaster. Bioessays. 2002;24:1012–1022. doi: 10.1002/bies.10184. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan W, Theurkauf WE. The cytoskeleton and morphogenesis of the early Drosophila embryo. Curr. Opin. Cell Biol. 1995;7:18–22. doi: 10.1016/0955-0674(95)80040-9. [DOI] [PubMed] [Google Scholar]
- 7.Piekny A, Werner M, Glotzer M. Cytokinesis: welcome to the Rho zone. Trends Cell Biol. 2005;15:651–658. doi: 10.1016/j.tcb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Somers WG, Saint R. A RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev. Cell. 2003;4:29–39. doi: 10.1016/s1534-5807(02)00402-1. [DOI] [PubMed] [Google Scholar]
- 9.Yüce O, Piekny A, Glotzer M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J. Cell Biol. 2005;170:571–582. doi: 10.1083/jcb.200501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao J, Albertson R, Riggs B, Field CM, Sullivan W. Nuf, a Rab11 effector, maintains cytokinetic furrow integrity by promoting local actin polymerization. J. Cell Biol. 2008;182:301–313. doi: 10.1083/jcb.200712036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padash Barmchi M, Rogers S, Häcker U. DRhoGEF2 regulates actin organization and contractility in the Drosophila blastoderm embryo. J. Cell Biol. 2005;168:575–585. doi: 10.1083/jcb.200407124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yonemura S, Hirao-Minakuchi K, Nishimura Y. Rho localization in cells and tissues. Exp. Cell Res. 2004;295:300–314. doi: 10.1016/j.yexcr.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Hime G, Saint R. Zygotic expression of the pebble locus is required for cytokinesis during the postblastoderm mitoses of Drosophila. Development. 1992;114:165–171. doi: 10.1242/dev.114.1.165. [DOI] [PubMed] [Google Scholar]
- 14.Albertson R, Cao J, Hsieh TS, Sullivan W. Vesicles and actin are targeted to the cleavage furrow via furrow microtubules and the central spindle. J. Cell Biol. 2008;181:777–790. doi: 10.1083/jcb.200803096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Keefe L, Somers WG, Harley A, Saint R. The pebble GTP exchange factor and the control of cytokinesis. Cell Struct. Funct. 2001;26:619–626. doi: 10.1247/csf.26.619. [DOI] [PubMed] [Google Scholar]
- 16.Lehner CF. The pebble gene is required for cytokinesis in Drosophila. J. Cell Sci. 1992;103:1021–1030. doi: 10.1242/jcs.103.4.1021. [DOI] [PubMed] [Google Scholar]
- 17.Prokopenko SN, Brumby A, O'Keefe L, Prior L, He Y, Saint R, Bellen HJ. A putative exchange factor for Rho1 GTPase is required for initiation of cytokinesis in Drosophila. Genes Dev. 1999;13:2301–2314. doi: 10.1101/gad.13.17.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards KA, Demsky M, Montague RA, Weymouth N, Kiehart DP. GFP-moesin illuminates actin cytoskeleton dynamics in living tissue and demonstrates cell shape changes during morphogenesis in Drosophila. Dev. Biol. 1997;191:103–117. doi: 10.1006/dbio.1997.8707. [DOI] [PubMed] [Google Scholar]
- 19.Glotzer M. The 3Ms of central spindle assembly: microtubules, motors and MAPs. Nat. Rev. Mol. Cell Biol. 2009;10:9–20. doi: 10.1038/nrm2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riggs B, Fasulo B, Royou A, Mische S, Cao J, Hays TS, Sullivan W. The concentration of Nuf, a Rab11 effector, at the microtubule-organizing center is cell cycle regulated, dynein-dependent, and coincides with furrow formation. Mol. Biol. Cell. 2007;18:3313–3322. doi: 10.1091/mbc.E07-02-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Royou A, Field C, Sisson JC, Sullivan W, Karess R. Reassessing the role and dynamics of nonmuscle myosin II during furrow formation in early Drosophila embryos. Mol. Biol. Cell. 2004;15:838–850. doi: 10.1091/mbc.E03-06-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canman JC, Cameron LA, Maddox PS, Straight A, Tirnauer JS, Mitchison TJ, Fang G, Kapoor TM, Salmon ED. Determining the position of the cell division plane. Nature. 2003;424:1074–1078. doi: 10.1038/nature01860. [DOI] [PubMed] [Google Scholar]
- 23.Hu CK, Coughlin M, Field CM, Mitchison TJ. Cell polarization during monopolar cytokinesis. J. Cell Biol. 2008;181:195–202. doi: 10.1083/jcb.200711105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shrestha S, Wilmeth LJ, Eyer J, Shuster CB. PRC1 controls spindle polarization and recruitment of cytokinetic factors during monopolar cytokinesis. Mol. Biol. Cell. 2012;23:1196–1207. doi: 10.1091/mbc.E11-12-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rothwell WF, Zhang CX, Zelano C, Hsieh TS, Sullivan W. The Drosophila centrosomal protein Nuf is required for recruiting Dah, a membrane associated protein, to furrows in the early embryo. J. Cell Sci. 1999;112:2885–2893. doi: 10.1242/jcs.112.17.2885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.