Abstract
Mustard gas, used in chemical warfare since 1917, is a mutagenic and carcinogenic agent that produces severe dermal lesions for which there are no effective therapeutics; it is currently seen as a potential terrorist threat to civilian populations. Sulforaphane, found in cruciferous vegetables, is known to induce enzymes that detoxify compounds such as the sulfur mustards that react through electrophilic intermediates. Here, we observe that a single topical treatment with sulforaphane induces mouse epidermal levels of the regulatory subunit of glutamate-cysteine ligase, the rate-limiting enzyme in glutathione biosynthesis, and also increases epidermal levels of reduced glutathione. Furthermore, a glutathione S-transferase, GSTA4, is also induced in mouse skin by sulforaphane. In an in vivo model in which mice are given a single mutagenic application of the sulfur mustard analog 2-(chloroethyl) ethyl sulfide (CEES), we now show that therapeutic treatment with sulforaphane abolishes the CEES-induced increase in mutation frequency in the skin, measured four days after exposure. Sulforaphane, a natural product currently in clinical trials, shows promise as an effective therapeutic against mustard gas.
Keywords: sulfur mustard, electrophile, carcinogen, natural product, 4-hydroxy-nonenal
Introduction
Mustard gas (bis-[2-chloroethyl]sulfide, SM) was first used in chemical warfare in 1917 (Papirmeister et al., 1991), and its potential use by terrorists against civilian populations is currently considered a plausible threat by the Department of Homeland Security (http://www.dhs.gov/xlibrary/assets/prep_chemical_fact_sheet.pdf). Dermal exposure results in severe chemical burns affecting all layers of the skin (Papirmeister et al., 1991), and patients require lengthy hospitalization. Despite recent progress in identifying agents that ameliorate some of the effects of mustards (Anumolu et al., 2010; Gordon et al., 2010; O'Neill et al., 2010; Anumolu et al., 2011), there are currently no effective therapies for the acute effects of dermal exposure. Mustard gas and a less toxic analog, 2-(chloroethyl) ethyl sulfide (CEES), induce DNA damage through structurally analogous electrophilic intermediates and are mutagenic (Ogston et al., 1946; Fox and Scott, 1980; Liu et al., 2010; Boulware et al., 2012). Epidemiological evidence suggests that chronic SM exposure leads to increased risk for respiratory malignancies (Wada et al., 1968; Easton et al., 1988), and there have been reports of carcinogenesis in mice following a single exposure (Heston, 1953).
Evidence has been presented (Drasch et al., 1987; Noort et al., 2002; Hattersley et al., 2008) suggesting that sulfur mustard has a relatively long biological half-life in skin, allowing ongoing damage to accumulate over a period of days. We have therefore been interested in identifying potential therapeutic agents that might act to detoxify the active electrophiles in situ and thereby decrease the overall level of macromolecular alkylation. We have recently shown (Boulware et al., 2012) that 2,6-dithiopurine (DTP), a nucleophilic scavenger of many electrophilic DNA-damaging agents (MacLeod et al., 1993; Qing et al., 1996), including CEES (Liu et al., 2010), can block CEES-induced mutagenesis in mouse skin. In this model, DTP was applied therapeutically beginning one h after CEES treatment. This suggests that biologically significant DNA damage continues to occur long after the initial treatment, providing a therapeutic window of hours to days. We therefore hypothesized that treatments that increase the organism’s endogenous capacity for detoxifying electrophiles, for example through the glutathione (GSH) conjugation pathway (Lu, 2009), might also provide therapeutic benefit.
Sulforaphane (SFN), a natural isothiocyanate found in cruciferous vegetables, blocks DNA damage, mutagenesis and carcinogenesis caused by electrophilic chemicals (Cheung and Kong, 2010; Kwak and Kensler, 2010), by inducing several Phase II detoxication enzymes that collectively enhance the conjugation of electrophiles to cellular reduced glutathione (GSH). Effects on both the biosynthesis of GSH, and the expression of GSH S-transferases (GSTs) have been observed (Marrot et al., 2008; Lu, 2009; Higgins and Hayes, 2011). In cultures of normal human epidermal keratinocytes, SFN has shown marginal protective effects against SM toxicity (Gross et al., 2006). Therapeutic and/or protective effects of SFN are currently under study in clinical trials for cancer and other chronic diseases (http://clinicaltrials.gov/ct2/results?term=sulforaphane). We now report the abolition of CEES-induced mutagenesis in an animal model by therapeutic treatment with SFN.
Materials and Methods
Husbandry and treatment of C57BL/6 and Big Blue mice with CEES were as described previously (Boulware et al., 2012). Briefly, our standard mutagenesis protocol utilized a single topical treatment of shaved dorsal epidermis of Big Blue mice with 20 µL of 200 mM CEES dissolved in ethanol. This resulted in a total dose of 4 µmol CEES, and a dose density of ~ 2.3 µmol/cm2. Control mice received ethanol only. As described previously, this treatment resulted in a 4–5-fold increase in mutation frequency with no apparent changes in skin morphology detectable by histopathology (Boulware et al., 2012). All animal treatments were approved by the Institutional Animal Care and Use Committee. R-Sulforaphane (SFN) was obtained from LKT Laboratories (St. Paul, MN) and dissolved in acetone before use. The indicated doses of SFN were applied to the shaved dorsum of mice in 200 µL acetone. At the indicated time points, mice were sacrificed and the dorsal skin excised. Epidermal samples were prepared as previously described by scraping the epidermal layer of the epidermis into radioimmunoprecipitation assay buffer (50 mM Tris-HCl [pH7.4], 150 mM NaCl, 1mM EDTA, 1% Triton X-100, protease cocktail [P8340; Sigma Aldrich], phosphatase inhibitor cocktails 1 and 2 [P2850, P5726, Sigma-Aldrich] (Abel et al., 2010). Approximately one-third of the epidermal scraping was immediately frozen in liquid nitrogen and reserved for analysis of GSH content. The remaining epidermis was homogenized in RIPA buffer for immunoblot analysis as previously described (Abel et al., 2010; Abel et al., 2011). Quantitative analysis of reduced GSH content was performed using a fluorescent assay as described (Abel et al., 2011). Antibodies for GST, GSTA4, and tubulin were as described (Abel et al., 2010; Abel et al., 2011). Antisera specific for mouse GCLC and GCLM, the catalytic and regulatory subunits of glutamate-cysteine ligase, respectively, were kindly provided by Dr. Terry Kavanagh.
Analyses of mutation frequencies in genomic DNA prepared from treated skins of female Big Blue mice were performed as described (Boulware et al., 2012). Data were analyzed by two-tailed Student’s t-test, and a p value of 0.05 or less is indicated by asterisks in the Figures.
Results
Sulforaphane induces phase II detoxification enzymes in mouse skin
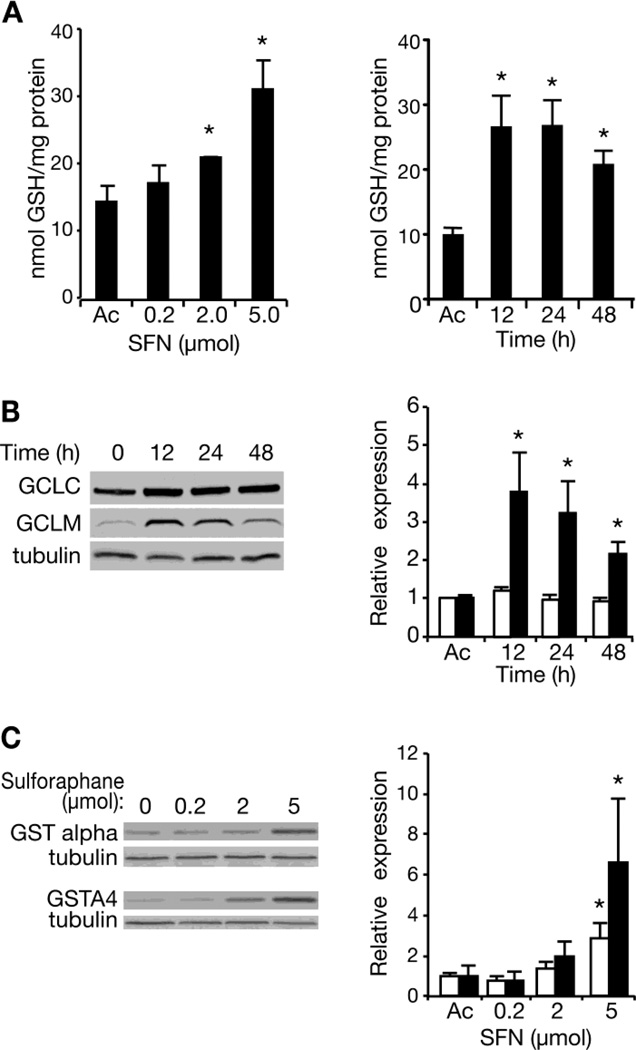
SFN is known to increase levels of cellular reduced glutathione (GSH) and of glutathione-S-transferases (GSTs) (Cheung and Kong, 2010). We applied SFN to the skin of C57BL/6 mice and measured levels of reduced GSH in epidermal extracts 24 h later. Increasing single doses of SFN produced increases in epidermal GSH (Figure 1A, left panel); the increase was statistically significant with doses of 2 or 5 µmol. Significant increases were seen at the 5 µmol dose as early as 12 h post-treatment, and GSH levels remained significantly elevated for at least 48 h (Figure 1A, right panel).
Fig. 1. Induction of Phase II detoxication genes in mouse skin by SFN.
A. Epidermal extracts were assayed for reduced GSH content 24 h after the mice received various doses of SFN (left panel), applied topically in acetone, or at various times after receiving 5 µmol SFN (right panel). Each graph represents the mean and standard deviation of data from three mice per experimental group in three independent experiments. In all panels, asterisks indicate statistically significant differences from the acetone control, p<0.05. B. Epidermal extracts were assayed for GCLC and GCLM by immunoblotting 0, 12, 24 or 48 h after the mice received 5 µmol SFN; a representative immunoblot is shown. Both proteins were quantitated in the same gels. Data were normalized in each experiment to the level of tubulin seen in a duplicate gel, and data from three independent experiments are plotted in the right-hand panel. Open bars, GCLC; solid bars, GCLM. C. Epidermal extracts were assayed for GST-alpha and GSTA4 by immunoblotting 24 h after the mice received various doses of SFN (left panel); representative gels are shown. Data from three independent experiments were normalized to tubulin controls in the same gel and are presented in the right-hand panel. Open bars, GST-alpha; solid bars, GSTA4.
The rate limiting enzyme in GSH biosynthesis, glutamate-cysteine ligase, consists of a catalytic subunit (GCLC) and a regulatory subunit (GCLM). Expression of the genes for both of these subunits has been shown to be induced by SFN treatment in other systems through a Nrf2-dependent process (Marrot et al., 2008; Lu, 2009; Kwak and Kensler, 2010; Higgins and Hayes, 2011), and we have previously observed SFN-induction of nuclear NRF2 and GCLM in vitro in a human keratinocyte tumor cell line (Abel et al., 2011). We hypothesized that SFN-induction of one or both subunits might underlie the observed increase in epidermal GSH levels. Epidermal extracts were prepared at various times following a treatment with 5 µmol SFN, proteins in the extracts were separated by gel electrophoresis, and the levels of GCLC and GCLM (relative to tubulin as a control) were determined by immunoblotting with antibodies to the two subunits. Levels of GCLC increased slightly after SFN treatment, but the increase was not statistically significant (Figure 1B). On the other hand, GCLM levels increased significantly within 12 h of SFN treatment, and remained significantly elevated for at least 48 h.
We also assayed several GSTs 24h after treatment with various doses of SFN. No significant changes were seen in mu- or pi-class GSTs (data not shown), but the alpha-class GSTs were significantly increased by 5 µmol SFN (Figure 1C). Indeed, analyses with antibodies specific for GSTA4 revealed a significant 6-fold elevation of this enzyme by SFN. Thus, it seemed possible that enhancement of endogenous detoxication by SFN might block CEES-induced mutagenesis.
Sulforaphane abolishes CEES-induced mutagenesis in vivo
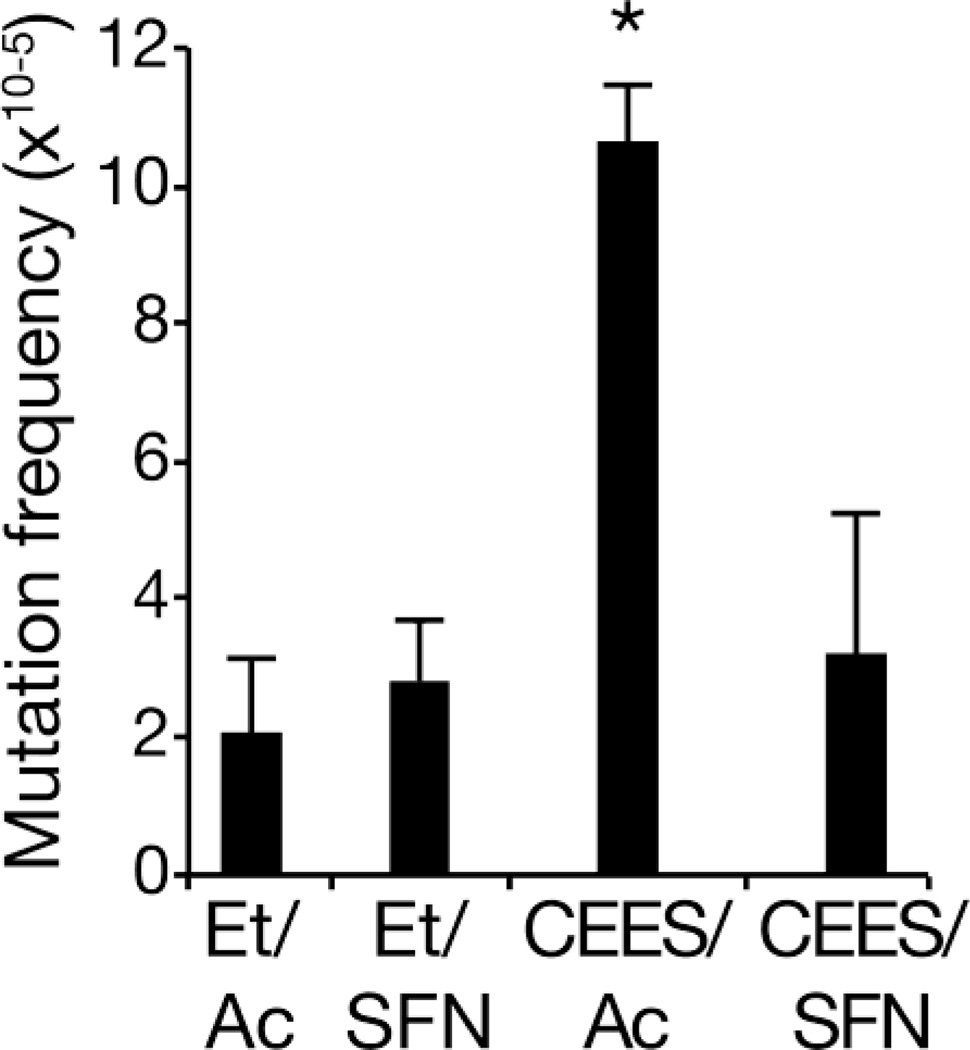
We used the Big Blue strain of C57BL/6 mice to test this hypothesis. In these mice, multiple copies of a bacteriophage genome, containing a reporter gene, are incorporated into the mouse genome (Kohler et al., 1991). After in vivo treatment with a DNA-damaging agent, phage DNA can be recovered and mutations in the reporter can be enumerated by a blue/white plaque assay (Kohler et al., 1991). We have previously shown that topical treatment with CEES induces a robust increase in mutation frequency in mice, using DNA recovered from the skin 4 days following treatment (Boulware et al., 2012). In the current experiments, we measured a background mutation frequency (vehicle only controls) of ~2 × 10−5 . Treatment of mice with 5 µmol SFN only did not significantly alter the mutation frequency (Figure 2; p>0.05). As expected from previous work (Boulware et al., 2012), we found that in vivo treatment with 200 mM CEES increased the mutation frequency by ~5-fold over the background mutation frequency (Figure 2; p<0.0002). However, when CEES treatment was followed one h later by treatment with 5 µmol SFN, with a total of three repeated treatments at 24 h intervals, the mutation frequency decreased to 3.2 × 10−5, not significantly different than the control value (p>0.05). Thus, therapeutic SFN completely blocked CEES-induced mutation in this model.
Fig. 2.
Abolition of CEES-induced mutagenesis by sulforaphane. Big Blue mice (3 mice per group) were treated once with either ethanol (Et) or 200 mM CEES in ethanol (6). Beginning one h post-CEES, 4 daily treatments with acetone (Ac) or 5 µmol SFN dissolved in acetone were given. Genomic DNA was isolated from treated skin 4 days post-CEES, and mutation frequency determined; mean and standard deviation are plotted for each group. *, significantly different than Et/Ac control, p<0.0002.
Discussion
SFN has been widely studied as a cancer chemopreventive agent due to its ability to induce phase II detoxication activities, particularly those involved with conjugation of cellular GSH to electrophilic intermediates of chemical carcinogens (Kwak and Kensler, 2010). Many such phase II genes induced by SFN, including various GSH S-transferases and GSH biosynthetic enzymes, are under the control of the Nrf2 signaling pathway, and the ability of SFN to prevent chemical carcinogenesis is abrogated in the absence of Nrf2 (Kwak and Kensler, 2010). We previously showed in vitro that SFN can induce GCLM in keratinocytes, and we have now extended this to mouse epidermis (Figure 1B). Correlated with this, epidermal GSH levels (Figure 1A) and GSTA4 levels (Figure 1C) are also increased. SM and CEES produce macromolecular damage through an electrophilic intermediate, generally thought to be an episulfonium ion (Ogston et al., 1946; Liu et al., 2010). These findings suggest that GSH-conjugation of this intermediate may account in part for the observed ability of topical SFN to abolish CEES-induced mutagenesis in the treated skin (Figure 2). We believe that this represents the first report of a direct effect of SFN on mutagenesis in vivo.
In addition to direct production of macromolecular damage through chemical adduct formation, CEES treatment has been reported to induce oxidative damage in mouse skin (Black et al., 2010; Inturi et al., 2011). One component of the oxidative damage induced by CEES in mouse skin is thought to be derived from 4-hydroxynonenal, a lipid peroxidation product (Pal et al., 2009). 4-hydroxynonenal is known to be a mutagen (Feng et al., 2003) and may therefore contribute to the increased mutation frequency found after CEES-treatment. GST4A specifically detoxifies 4-hydroxynonenal (Abel et al., 2010; Balogh and Atkins, 2011). Thus, part of the effectiveness of SFN in abolishing CEES-induced mutagenesis may derive from a reduction in epidermal 4-hydroxynonenal levels by GST4A-mediated conjugation. Further experiments would be necessary to determine the relative importance of effects of SFN treatment on the direct mutagenesis pathway and the indirect pathway through induction of oxidative DNA damage.
Large numbers of civilians could be exposed to high levels of mustard gas in a terrorist incident (e.g., http://publicintelligence.net/national-planning-scenarios-version-21-3-2006-final-draft/, Scenario 5), inducing acute skin lesions that require hospitalization (Papirmeister et al., 1991). However, it is likely that a higher number of people would be exposed to lower, mutagenic doses without overt toxicity. Given the known mutagenicity and carcinogenicity (Heston, 1953; Wada et al., 1968; Fox and Scott, 1980; Easton et al., 1988) of mustard gas, this population might be at higher risk for mustard-related cancers later in life. We previously demonstrated abolition of CEES-induced mutagenesis in this model (Boulware et al., 2012) using DTP, a non-toxic, nucleophilic scavenger (MacLeod et al., 1993; Qing et al., 1996). As noted above, the fact that both compounds are effective when given post-exposure is likely due to the existence of a skin reservoir of long-lived mustard that damages DNA over an extended time-course (Drasch et al., 1987; Noort et al., 2002; Hattersley et al., 2008). For SFN, the induction of the ability to conjugate lipid peroxides such as 4-hydroxynonenal may also contribute to the therapeutic response. The present results suggest that SFN is as effective as DTP in blocking mutagenesis. Since their modes of action are complementary, it is expected that a therapeutic strategy incorporating both agents might be effective against mustard gas exposure.
Highlights.
Sulforaphane induces increased levels of glutathione in mouse skin
Sulforaphane induces increased levels of GSTA4 in mouse skin
Sulforaphane, applied after CEES-treatment, completely abolishes CEES-mutagenesis
The therapeutic effect may suggest a long biological half-life for CEES in this system
Acknowledgements
We thank Terry Kavanagh, Ted Mills and Rick Wood for thoughtful comments on the manuscript, Terry Kavanagh for providing antibodies, Jennifer Bubel and Steve Carbajal for technical support, and Rebecca Deen and Joi Holcomb for manuscript preparation. This work was supported by grant U01 NS058191 from NINDS through the CounterACT Program, and by Center Grants from the NCI (P30 CA016672) and the NIEHS (P30 ES007784).
Abbreviations
- SM
Sulfur mustard [bis(2-chloroethyl)sulfide]
- CEES
2-(chloroethyl) ethyl sulfide
- DTP
2,6-dithiopurine
- SFN
Sulforaphane
- GSH
Reduced glutathione
- GST
Glutathione S-transferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel EL, Angel JM, Riggs PK, Langfield L, Lo HH, Person MD, Awasthi YC, Wang LE, Strom SS, Wei Q, DiGiovanni J. Evidence that Gsta4 modifies susceptibility to skin tumor development in mice and humans. J Natl Cancer Inst. 2010;102:1663–1675. doi: 10.1093/jnci/djq392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel EL, Bubel JD, Simper MS, Powell L, McClellan SA, Andreeff M, Macleod MC, DiGiovanni J. Protection against 2-chloroethyl ethyl sulfide (CEES) - induced cytotoxicity in human keratinocytes by an inducer of the glutathione detoxification pathway. Toxicol Appl Pharmacol. 2011;255:176–183. doi: 10.1016/j.taap.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Anumolu SS, DeSantis AS, Menjoge AR, Hahn RA, Beloni JA, Gordon MK, Sinko PJ. Doxycycline loaded poly(ethylene glycol) hydrogels for healing vesicant-induced ocular wounds. Biomaterials. 2010;31:964–974. doi: 10.1016/j.biomaterials.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anumolu SS, Menjoge AR, Deshmukh M, Gerecke D, Stein S, Laskin J, Sinko PJ. Doxycycline hydrogels with reversible disulfide crosslinks for dermal wound healing of mustard injuries. Biomaterials. 2011;32:1204–1217. doi: 10.1016/j.biomaterials.2010.08.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogh LM, Atkins WM. Interactions of glutathione transferases with 4-hydroxynonenal. Drug Metab Rev. 2011;43:165–178. doi: 10.3109/03602532.2011.558092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black AT, Hayden PJ, Casillas RP, Heck DE, Gerecke DR, Sinko PJ, Laskin DL, Laskin JD. Expression of proliferative and inflammatory markers in a full-thickness human skin equivalent following exposure to the model sulfur mustard vesicant, 2-chloroethyl ethyl sulfide. Toxicol Appl Pharmacol. 2010;249:178–187. doi: 10.1016/j.taap.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware S, Fields T, McIvor E, Powell KL, Abel EL, Vasquez KM, Macleod MC. 2,6-Dithiopurine, a nucleophilic scavenger, protects against mutagenesis in mouse skin treated in vivo with 2-(chloroethyl) ethyl sulfide, a mustard gas analog. Toxicol Appl Pharmacol. 2012;263:203–209. doi: 10.1016/j.taap.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KL, Kong AN. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010;12:87–97. doi: 10.1208/s12248-009-9162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drasch G, Kretschmer E, Kauert G, von Meyer L. Concentrations of mustard gas [bis(2-chloroethyl)sulfide] in the tissues of a victim of a vesicant exposure. J Forensic Sci. 1987;32:1788–1793. [PubMed] [Google Scholar]
- Easton DF, Peto J, Doll R. Cancers of the respiratory tract in mustard gas workers. Br J Ind Med. 1988;45:652–659. doi: 10.1136/oem.45.10.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Hu W, Amin S, Tang MS. Mutational spectrum and genotoxicity of the major lipid peroxidation product, trans-4-hydroxy-2-nonenal, induced DNA adducts in nucleotide excision repair-proficient and -deficient human cells. Biochemistry. 2003;42:7848–7854. doi: 10.1021/bi034431g. [DOI] [PubMed] [Google Scholar]
- Fox M, Scott D. The genetic toxicology of nitrogen and sulphur mustard. Mutat Res. 1980;75:131–168. doi: 10.1016/0165-1110(80)90012-3. [DOI] [PubMed] [Google Scholar]
- Gordon MK, Desantis A, Deshmukh M, Lacey CJ, Hahn RA, Beloni J, Anumolu SS, Schlager JJ, Gallo MA, Gerecke DR, Heindel ND, Svoboda KK, Babin MC, Sinko PJ. Doxycycline hydrogels as a potential therapy for ocular vesicant injury. J Ocul Pharmacol Ther. 2010;26:407–419. doi: 10.1089/jop.2010.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross CL, Nealley EW, Nipwoda MT, Smith WJ. Pretreatment of human epidermal keratinocytes with D,L-sulforaphane protects against sulfur mustard cytotoxicity. Cutan Ocul Toxicol. 2006;25:155–163. doi: 10.1080/15569520600859985. [DOI] [PubMed] [Google Scholar]
- Hattersley IJ, Jenner J, Dalton C, Chilcott RP, Graham JS. The skin reservoir of sulphur mustard. Toxicol In Vitro. 2008;22:1539–1546. doi: 10.1016/j.tiv.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Heston WE. Occurrence of tumors in mice injected subcutaneously with sulfur mustard and nitrogen mustard. J Natl Cancer Inst. 1953;14:131–140. [PubMed] [Google Scholar]
- Higgins LG, Hayes JD. Mechanisms of induction of cytosolic and microsomal glutathione transferase (GST) genes by xenobiotics and pro-inflammatory agents. Drug Metab Rev. 2011;43:92–137. doi: 10.3109/03602532.2011.567391. [DOI] [PubMed] [Google Scholar]
- Inturi S, Tewari-Singh N, Gu M, Shrotriya S, Gomez J, Agarwal C, White CW, Agarwal R. Mechanisms of sulfur mustard analog 2-chloroethyl ethyl sulfide-induced DNA damage in skin epidermal cells and fibroblasts. Free Radic Biol Med. 2011;51:2272–2280. doi: 10.1016/j.freeradbiomed.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler SW, Provost GS, Fieck A, Kretz PL, Bullock WO, Sorge JA, Putman DL, Short JM. Spectra of spontaneous and mutagen-induced mutations in the lacI gene in transgenic mice. Proc Natl Acad Sci U S A. 1991;88:7958–7962. doi: 10.1073/pnas.88.18.7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak MK, Kensler TW. Targeting NRF2 signaling for cancer chemoprevention. Toxicol Appl Pharmacol. 2010;244:66–76. doi: 10.1016/j.taap.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Powell KL, Thames HD, MacLeod MC. Detoxication of sulfur half-mustards by nucleophilic scavengers: robust activity of thiopurines. Chem Res Toxicol. 2010;23:488–496. doi: 10.1021/tx900190j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod MC, Qing WG, Powell KL, Daylong A, Evans FE. Reaction of nontoxic, potentially chemopreventive purinethiols with a direct-acting, electrophilic carcinogen, benzo[a]pyrene-7,8-diol 9,10-epoxide. Chem Res Toxicol. 1993;6:159–167. doi: 10.1021/tx00032a004. [DOI] [PubMed] [Google Scholar]
- Marrot L, Jones C, Perez P, Meunier JR. The significance of Nrf2 pathway in (photo)-oxidative stress response in melanocytes and keratinocytes of the human epidermis. Pigment Cell Melanoma Res. 2008;21:79–88. doi: 10.1111/j.1755-148X.2007.00424.x. [DOI] [PubMed] [Google Scholar]
- Noort D, Benschop HP, Black RM. Biomonitoring of exposure to chemical warfare agents: a review. Toxicol Appl Pharmacol. 2002;184:116–126. [PubMed] [Google Scholar]
- O'Neill HC, White CW, Veress LA, Hendry-Hofer TB, Loader JE, Min E, Huang J, Rancourt RC, Day BJ. Treatment with the catalytic metalloporphyrin AEOL 10150 reduces inflammation and oxidative stress due to inhalation of the sulfur mustard analog 2-chloroethyl ethyl sulfide. Free Radic Biol Med. 2010;48:1188–1196. doi: 10.1016/j.freeradbiomed.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogston AG, Holiday ER, Philpot JSL, Stocken LA. The replacement reactions of β-β'-dichlorodiethyl sulphide and of some analogues in aqueous solution: The isolation of β-chloro-β'-hydroxydiethyl sulphide. Trans Faraday Soc. 1946;44:45–52. [Google Scholar]
- Pal A, Tewari-Singh N, Gu M, Agarwal C, Huang J, Day BJ, White CW, Agarwal R. Sulfur mustard analog induces oxidative stress and activates signaling cascades in the skin of SKH-1 hairless mice. Free Radic Biol Med. 2009;47:1640–1651. doi: 10.1016/j.freeradbiomed.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papirmeister B, Feister AJ, Robinson SI, Ford RD. Medical Defense Against Mustard Gas: Toxic Mechanisms and Pharmacological Implications. Boca Raton, FL: CRC Press, Inc; 1991. [Google Scholar]
- Qing WG, Powell KL, MacLeod MC. Kinetics of the reaction of a potential chemopreventive agent, 2,6-dithiopurine, and its major metabolite, 2,6-dithiouric acid, with multiple classes of electrophilic toxicants. Chem Res Toxicol. 1996;9:1298–1304. doi: 10.1021/tx960088n. [DOI] [PubMed] [Google Scholar]
- Wada S, Miyanishi M, Nishimoto Y, Kambe S, Miller RW. Mustard gas as a cause of respiratory neoplasia in man. Lancet. 1968;1:1161–1163. doi: 10.1016/s0140-6736(68)91863-1. [DOI] [PubMed] [Google Scholar]