Abstract
We have previously demonstrated that cocaine- and amphetamine-regulated transcript (CART) peptide colocalizes with GABA, dynorphin, D1 receptors, and substance P in some neurons in the nucleus accumbens. One of the main nuclei that receive accumbal efferents is the ventral pallidum, and both dynorphin and substance P have been shown to be present in the cell bodies and terminals of this projection. Thus, we investigated whether CART peptide is also present in the ventral pallidum in terminals that originate in the accumbens. The anterograde tracer PHA-L colocalized with CART in neuronal processes in the ventral pallidum when injected into the nucleus accumbens. Also, CART colocalized with the retrograde tracer r-BDA in accumbens cell bodies after the tracer was injected into the ventral pallidum. Using electron microscopic immunocytochemistry, we examined CART terminals in the ventral pallidum and found that CART-immunoreactive terminals formed symmetric synapses consistent with inhibitory GABAergic synapses. These synapses closely resemble GABAergic synapses in the substantia nigra pars reticulata, another nucleus that receives some CART–containing accumbal efferents. Lastly, we found that intra-pallidal injection of CART 55–102 inhibited cocaine-induced locomotion, indicating that CART peptide in the ventral pallidum can have functional effects.
Keywords: drug addiction, psychostimulants, neuropeptides, limbic system, neuroanatomy, behavior
A fragment of a CART peptide was first discovered in an extract from ovine hypothalamus . It was later found that an mRNA was increased after acute administration of cocaine or amphetamine, and the mRNA sequence included the portion coding for the peptide discovered by Speiss, et al. (Spiess et al., 1981, Douglass et al., 1995). The mRNA was named CART for “Cocaine and Amphetamine Regulated Transcript” (Douglass et al., 1995). The increase in the level of the transcript in response to psychostimulant drug administration suggested that the product of this gene was a substance related to the action of psychostimulant drugs, and the increased level further suggested an increased need for or release of the gene product. Recently, it was shown that cocaine administration increased the proportion of CART neurons in the nucleus accumbens (NAcc) that demonstrated Fos immunoreactivity (Hubert and Kuhar, 2008), which was reversible, and saline did not cause this increase. These findings strengthen the idea that CART-containing neurons are activated by injection of cocaine. Also, it was recently shown that intra-accumbal injections of CART 55–102 decrease cocaine self-administration (Jaworski et al., 2008). CART peptides have also been shown to be involved in a variety of other physiologic functions, including food intake, stress, endocrine control and other processes (Hurd et al., 1999, Larsen et al., 2002, Hunter and Kuhar, 2003, Dominguez et al., 2004, Kuhar et al., 2005, Murphy, 2005, Rogge et al., 2008)
In the NAcc, CART peptides are colocalized with GABA, substance P and dynorphin in some of the medium spiny neurons (Dallvechia-Adams et al., 2002, Hubert and Kuhar, 2005, 2006). In addition, CART peptides are found in the same neurons that contain dopamine D1 receptors (Hubert and Kuhar, 2006). Taken together, these data demonstrate that CART neurons are a subset of NAcc medium spiny neurons that are part of the direct pathway of information that flows through the basal ganglia (Wichmann and DeLong, 1998). Previous work on the anatomy of CART neurons has shown a CART-containing projection from the NAcc core to the substantia nigra pars reticulata (SNr) of the rat (Dallvechia-Adams et al., 2002).
The ventral pallidum (VP) is another major recipient of NAcc efferents, and the possibility of this nucleus receiving a projection of CART-containing neurons from the NAcc has not previously been investigated. The VP is a nucleus involved in processes associated with both the basal ganglia and extended amygdala systems (Kretschmer, 2000, Heimer, 2003, Smith et al., 2009). The connectivity of VP afferents and efferents reflects this classification, sending and receiving projections from the NAcc core and shell, amygdala, pedunculopontine tegmentum, lateral hypothalamus and other regions (Zahm, 1989, Groenewegen et al., 1993, Churchill and Kalivas, 1994). It has been shown that injections of opiates into the VP modulate the hedonic value or “liking” of natural rewards such as sucrose, as well as elicit locomotor activity (Churchill and Kalivas, 1999, Smith and Berridge, 2005, 2007). The VP is requisite for reward and motivational processes and encodes hedonic events (Smith et al., 2009). Since psychomotor stimulants affect the reward and reinforcement circuits in mammals, as well as locomotor systems, we examined whether CART neurons in the NAcc project to the VP and have a role in psychomotor stimulant-induced locomotion.
In this study, in order to determine if CART-containing neurons project to the VP, we performed anterograde and retrograde tracing studies of CART-containing neurons from the NAcc to the VP. It has been well documented that the NAcc has a significant projection to the VP, but it is not known whether the subset of NAcc neurons that contain CART contribute to this projection. We also examined the morphology of CART-containing terminals at their synapses in the VP at the electron microscopic level. In addition, studies were conducted to assess the functional effect of injecting CART peptide into the VP on saline- and cocaine-stimulated locomotor activity.
Experimental Procedures
Animals and Tissue Preparation
Sixteen adult, male, Sprague-Dawley rats (Charles River, Wilmington MA) were used in the anatomical experiments. For perfusion, all animals were given an injection of pentobarbital (10mg/kg, i.p.) Within minutes of loss of tail pinch reflex, all rats were transcardially perfused with at least 100 ml of cold, oxygenated Ringer’s solution. This was followed by perfusion with 500 ml of fixative containing 4.0% paraformaldehyde and 0.1% glutaraldehyde, dissolved in phosphate buffer (PB, 0.1M, pH 7.4). Brains were removed from the skull and stored in 30% sucrose solution in PB (for immunofluorescence or light microscopy) or PBS (for electron microscopy) at 4°C until brains were saturated. The brains were then cut into 60 µm-thick coronal sections on a freezing microtome. Sections were put in a 1.0% sodium borohydride solution dissolved in phosphate buffered saline (PBS) for 20 min and rinsed with PBS before being processed for immunocytochemistry. The anesthesia and euthanasia procedures were carried out according to the National Institutes of Health Guidelines, and a protocol describing these experiments has been approved by the Institutional Animal Care and Use Committee of Emory University.
Tract tracing experiments
For anterograde tracing experiments, all rats received either a unilateral or bilateral iontophoretic injection of the anterograde tracer PHA-L in the NAcc (AP +1.6mm; LM +/− 0.8mm; DV −7.3mm (Paxinos and Watson, 1986). After being anesthetized with isoflurane, the rats were fixed in a stereotaxic frame (Knopf). A glass micropipette (20–35 m tip diameter), containing PHA-L (2.5% in 0.1 M, pH 8.0, phosphate buffer; (Vector, Burlingame, CA) was placed in the NAcc shell, and iontophoretic delivery of tracer was performed with a 7-nA positive current for 20 minutes via a 7-seconds-on/7-seconds-off cycle. After the appropriate survival period (10–12 days), the rats were perfusion fixed as described above. The brains were serially cut (60-m-thick sections) and reacted with sodium borohydride. To reveal the injection site, sections of each rat brain was processed for PHA-L using light microscopy as described above. To examine the colocalization of PHA-L and CART, the double immunofluorescence method was used.
The retrograde experiments involved the stereotaxic, iontophoretic injection of rhodamine-conjugated dextran amines (R-DA) using the same parameters as the anterograde tracing experiments. The injections were made into the VP at the coordinates AP −.26mm; LM +/− 2.5mm; DV −8.0mm (Paxinos and Watson, 1986). We were not able to visualize at the injection sites using an antibody for R-DA using LM procedures, presumably because of interference from the attached rhodamine moiety. Colocalization of r-BDA and CART in the NAcc was also studied using fluorescence immunochemistry.
Antisera
Commercially available antibodies for PHA-L raised in mouse (Vector, Burlingame, CA), and custom antibodies for CART peptide raised in rabbit (Koylu et al., 1997) were used. For Immunofluorescence, secondary antibodies raised in donkey coupled to either CY3 or FITC (Jackson Laboratories, West Grove, PA) were used to visualize the localization of the proteins of interest.
Immunofluorescent Localization of CART Peptides and PHA-L
The tissue sections were preincubated in a solution containing 5% normal donkey serum (NDS), 1.0% bovine serum albumin (BSA), and 0.3% Triton X-100 in PBS for one hour. They were then incubated overnight with primary antisera for CART and/or c-Fos diluted to 0.5–1.0 µg/ml in a solution containing 1.0% NDS, 1.0% BSA, 0.3% Triton X-100 in PBS. Next, the sections were rinsed in PBS and transferred for one hour to a secondary antibody solution containing FITC- (for CART) and CY3-(for c-Fos) conjugated secondary antibodies diluted 1:100 in the primary antibody diluent solution. The tissue sections were then washed in PBS and incubated in cupric sulfate solution for 30 minutes to enhance visualization. Sections were then mounted on gelatin-coated slides and dried, and a coverslip was applied with Vectashield mounting agent (Vector, Burlingame, CA) and sealed using clear fingernail polish. Specimens were observed throughout the rostrocaudal length (2.7 to 0.7 mm according to Paxinos and Watson (Paxinos and Watson, 1986) of the nucleus accumbens using a Zeiss LSM 410 confocal microscope. Accumbal neurons that displayed CART-IR were scanned on the confocal microscope for the presence of double labeling. Due to the limitations of darkfield, oil-immersion confocal microscopy, where it is difficult to precisely define the border between core and shell, no distinction is made between the accumbens core and shell.
The antisera for CART peptide is directed against the C-terminal portion and will therefore recognize proCART and its various processed forms. While this manuscript refers to “CART peptide”, what is being localized are several CART peptides (Koylu et al., 1998), including the active peptides, CART 55–102 and CART 62–102.
Light Microscope Procedures
The sections were preincubated in a solution containing 10% normal goat serum (NGS), 1.0% bovine serum albumin (BSA), and 0.3% Triton X-100 in PBS for one hour. They were then incubated overnight with either primary antisera diluted at 0.5–1.0 µg/ml in a solution containing 1.0% NGS, 1.0% BSA and 0.3% Triton X-100 in PBS. Next, the sections were rinsed in PBS and transferred for one hour to a secondary antibody solution containing biotinylated goat-anti-rabbit IgGs (Vector, Burlingame, CA) diluted 1:200 in the primary antibody diluent solution. After rinsing, sections were put in a solution containing 1:100 avidin-biotin-peroxidase complex (ABC, Vector, Burlingame, California). The tissue was then washed in PBS and 0.05M TRIS buffer before being transferred to a solution containing 0.01M imidazole, 0.0005% hydrogen peroxide, and 0.025% 3,3’-diaminobenzidine tetrahydrochloride (DAB, Sigma, St. Louis, MO) in TRIS for 7–10 minutes. Sections were then mounted on gelatin-coated slides and dried, and a coverslip was applied with Permount.
Electron Microscope Procedures
After vibratome cutting, sections were transferred to a cryoprotectant solution (PB, 0.05M, pH 7.4 containing 25% sucrose and 10% glycerol) for 20 min. They were then frozen in a −80°C freezer for 20 min, returned to a decreasing gradient of cryoprotectant solutions and rinsed in PBS. Sections then underwent immunocytochemical procedures for the immunoperoxidase localization of CART in a manner identical to that for the light microscope, except that the incubation in the primary antisera was carried out at 4 C for 48 hours and that Triton-X-100 was omitted from all incubation solutions.
The sections were then transferred to PB (0.1M, pH 7.4) for 10 minutes and exposed to 1% osmium tetroxide for 20 minutes. They were then rinsed with PB and dehydrated in an increasing gradient of ethanol. Uranyl acetate (1%) was added to the 70% alcohol to increase contrast at the electron microscope. The sections were then treated with propylene oxide before being embedded in epoxy resin (Durcupan, ACM, Fluka, Buchs, Switzerland) for 12 hr, mounted on microscope slides and placed in a 60°C oven for 48 hrs.
Pieces of VP tissue were taken out from the slides and glued on the top of resin blocks with cyanoacrylate glue. They were cut into 60 nm ultrathin sections with an ultramicrotome (Ultracut T2, Leica, Nussloch, Germany) and serially collected on single-slot Pioloform-coated copper grids. The sections were stained with lead citrate for 5 min (Reynolds, 1963) and examined with a Zeiss EM-10C electron microscope (Thornwood, NY).
Locomotor Activity Studies
Rats (n = 5) which had injection cannulae implanted were anesthetized with isoflurane (4% in O2 at 1 liter per minute). A 26 ga bilateral guide cannula assembly (Plastics One, Roanoke, VA) was implanted 2mm above the VP (the injection cannula extended 2 mm past the guide cannula to the target region). Stereotaxic coordinates relative to bregma for the guide cannula were: AP −.26mm; LM +/− 2.5mm; DV −6.0mm (Paxinos and Watson, 1986). The guide cannula assembly was secured to the skull with small stainless steel screws and dental cement. Dummy cannula extending 0.5 mm beyond the guide cannula tips were inserted and a dust cap was attached to the top of the cannula assembly. After surgery, the rats were given meloxicam (1 mg/kg, s.c.) once to minimize pain and discomfort and allowed to recover for at least 7 days before experiments.
Stainless steel injection cannula (33 ga) cut to extend 2 mm beyond the guide cannula was used for infusions. PE-10 tubing was used to connect the injection cannula to 25µl syringes (Hamilton; Reno, NV) which were attached to infusion pumps (Harvard Apparatus; Cambridge, MA). For the infusions, the rats were confined to a small polyethylene box. Each infusion was given in a volume of 0.5 µl/side over 30 s. The injector cannula was left in place for an additional 30 s after the infusion, then the injector cannula was removed, and the dummy cannula and dust cap were reinserted. For the rats that received both CART peptide (Peptides International Inc., Louisville, KY) and cocaine (NIDA; National Institute of Health, Bethesda, MD), the i.p. cocaine injection was given 1 min after the CART infusion.
Locomotor activity was measured in photocell cages (Omnitech Electronics; Columbus, OH) measuring 40 × 40 × 30 cm. Each cage had 32 photocells (16 front to back and 16 side to side) positioned 5 cm off the cage floor. Each cage was isolated in a stainless steel box equipped with a ventilated fan, 10W light bulb and a keyhole to observe the rats. The distance traveled (in cm) was calculated by measuring the consecutive breaks of adjacent photocell beams. Operation of the photocell cages and data collection was done by an IBM computer.
For the infusions, each rat was put into the photocell cage for 1 h for habituation, given the infusion, then returned to the cages for an additional hour. Prior to the start of the experiments, each rat was habituated to the testing procedure and environment by receiving daily sham injections for 3 days.
Results
Anterograde projections of CART neurons from the NAcc to the VP
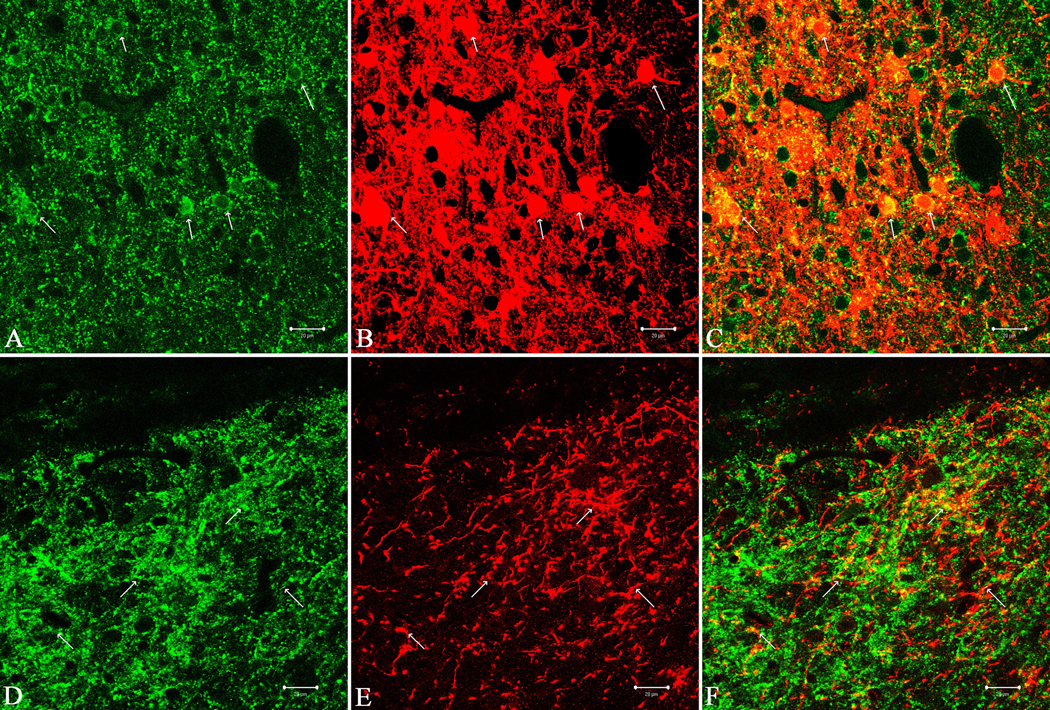
Eight rats were injected intra-accumbally and unilaterally with PHA-L as described in Methods. A typical distribution of the PHA-L in brain is shown in Figure 1. The immunohistochemical localization of both PHA-L and CART peptides were determined in the NAcc and VP, and an example of the results is shown in Figure 2. Fluorescence immunocytochemistry for CART peptide alone (green) demonstrated a dense locus of immunoreactivity in the neuropil of the subcommissural VP (Figure 2D) and in the NAcc (Figure 2A), and the latter is well established. It was of interest that no cell bodies staining for CART were found in the VP. Also, PHA-L (red) was found in the VP (Figure 2E), and colocalization studies revealed that there was a significant colocalization of both PHA-L and CART in the VP (yellow stain, Figure 2F). Also, both CART and PHA-L were found in the NAcc (Figure 2B and C), particularly near the injection site, as expected. The colocalization of CART and PHA-L in the VP indicates that CART peptide-containing neurons project to the VP.
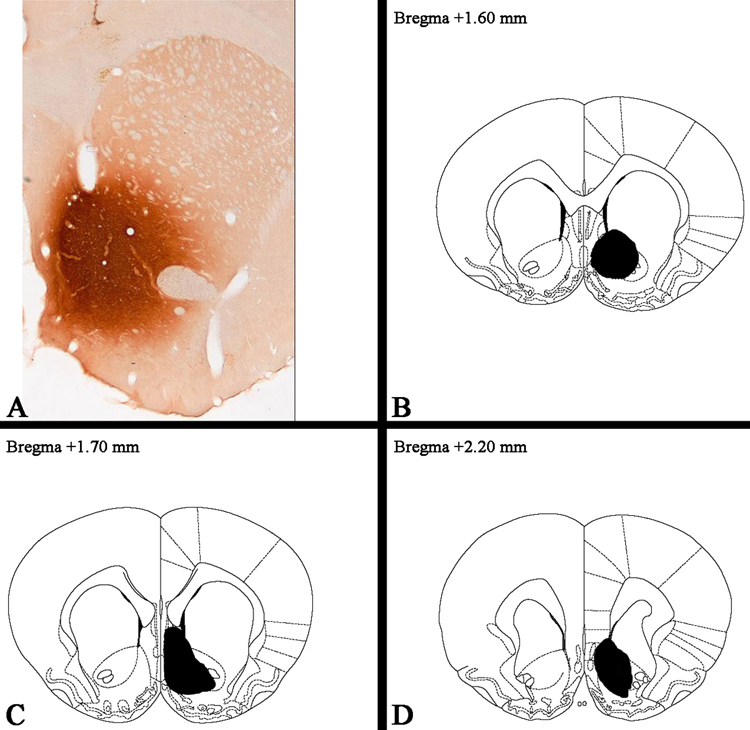
Figure 1.
Anterograde studies and site of injection and distribution of PHA-L in the rat nucleus accumbens (NAcc). The upper left portion of the figure shows an injection site immunoreactive for PHA-L . The three schematics shown are from one representative rat and demonstrate the extent of the spread of the stain, at three different anatomical levels according to Paxinos and Watson (1986).
Figure 2.
Fluorescence immunocytochemistry of CART peptides and PHA-L in the NAc (A–C) and VP (D–F) in anterograde studies. CART peptide is shown in green and PHA-L in red. Colocalization sites are stained yellow and arrows indicate some of the colocalization sites for CART and PHA-L. See text for details. Scale Bars: 20µm
A retrograde tracer injected into the subcommissural VP labeled CART-containing cell bodies in the NAcc
Injection of R-DA into the VP (n=8) ventral to the decussation of the anterior commissure resulted in the labeling of cell bodies in the NAcc and examples of the results are shown in Figure 3. The injections evaluated in this study were localized using immunofluorescence in the VP immediately ventral to the anterior commissure according to the atlas of Paxinos and Watson (Paxinos and Watson, 1986) (Figure 3). The injection sites in the VP displayed strong CART immunoreactivity, in addition to a very strong presence of the fluorescent tracer, as expected, and they colocalized near the injection site, also as expected (Figure 3A, B, C). In the NAcc (Figure 3D, E, F,), cell bodies were clearly labeled by R-DA (Figure 3E). Many of these cell bodies colocalized (Figure 3F) both CART peptides and R-DA, confirming that CART is present in the NAcc to VP projection.
Figure 3.
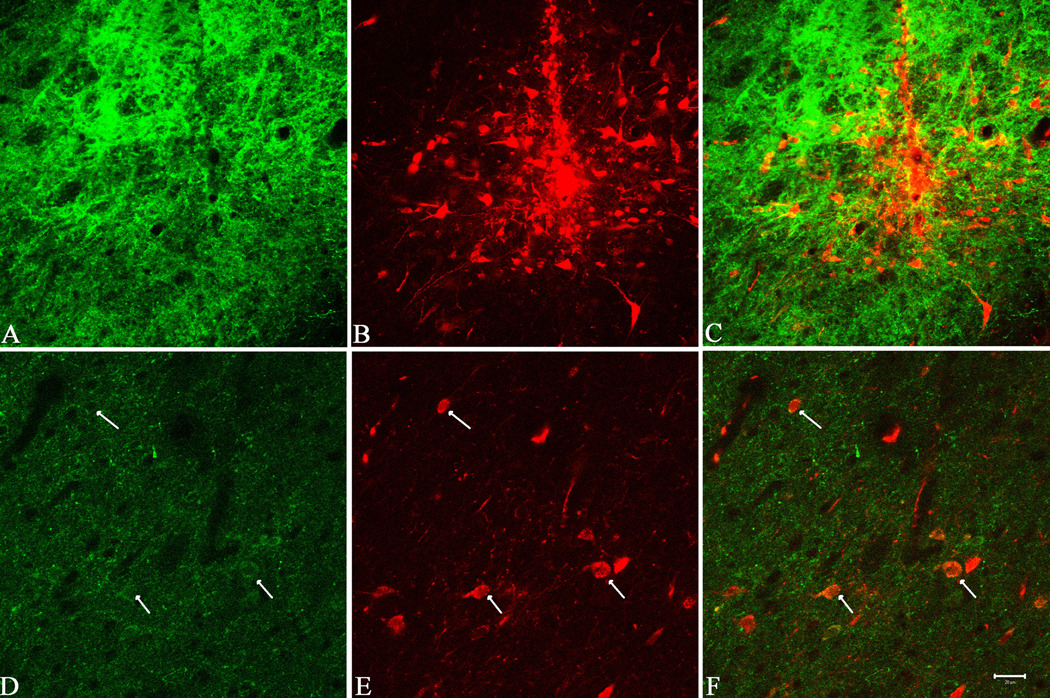
Fluorescence immunocytochemistry for CART peptides and Rhodamine-conjugated dextran amines (R-DA) in the VP (A–C) and in the Nucleus Accumbens (D–F) in the retrograde transport studies. CART peptide immunoreactivity is shown in green and rhodamine is red. Colocalization is identified by the yellow colored elements. See text for details and interpretation.
Electron microscopic analysis of CART-immunoreactive nerve terminals reveals symmetric synapses onto VP dendrites
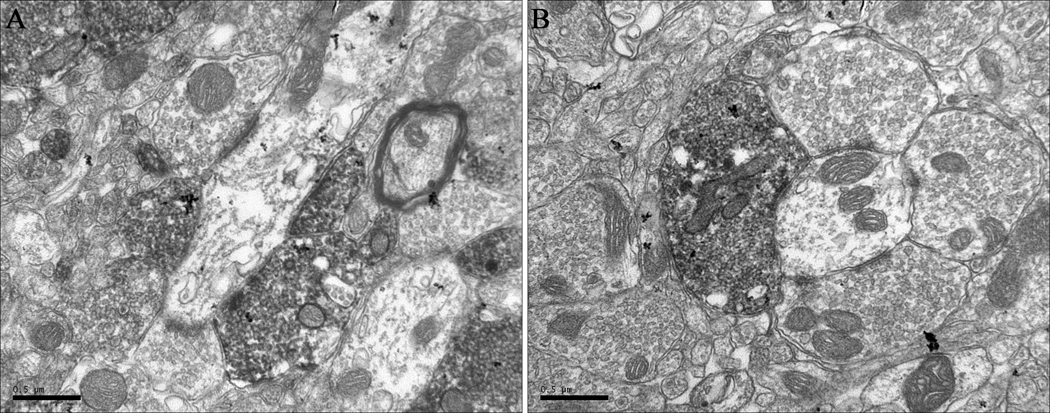
CART immunoreactivity was examined in the VP (n=4 animals) at the electron microscopic level as described in Methods. The majority of elements labeled were axon terminals (n=161 total immunopositive terminals examined), with additional labeling observed in preterminal axons (Figure 4). No cell bodies or dendrites were immunoreactive for CART, confirming the light microscopic observations. For 62 of the CART peptide–positive nerve terminals, the synaptic junctions were either not visible or not easily identifiable. In the remaining 99 nerve terminals, CART peptide immunoreactivity was only present in presynaptic elements and nerve terminals making symmetric synapses on dendritic elements, even though unstained, asymmetric junctions were observed in the same fields. The terminals that displayed CART immunoreactivity were small and densely packed with synaptic vesicles (Figure 4A, B). Since our anterograde tracing experiments suggest that these CART projections are from the NAcc, it is reasonable to assume that the morphology of the terminals is similar to that of NAcc terminals that synapse in other nuclei. Terminals of NAcc neurons in the SN do indeed have similar morphology to the CART-immunoreactive terminals in the VP (Hubert et al., 2001).
Figure 4.
EM immunoperoxidase immunocytochemistry for CART in the VP. The CART antibody labeled presynaptic elements exclusively. A and B are photos from two different animals with basically the same information. The morphology of these CART-staining nerve terminals is similar to that of accumbal-derived terminals found in the substantia nigra, which suggests they are terminals of the same type of neuron. Scale Bar = 500nm. See text for discussion.
Intra-VP injection of CART peptide inhibits cocaine-stimulated locomotor activity but has no effect on basal locomotor activity
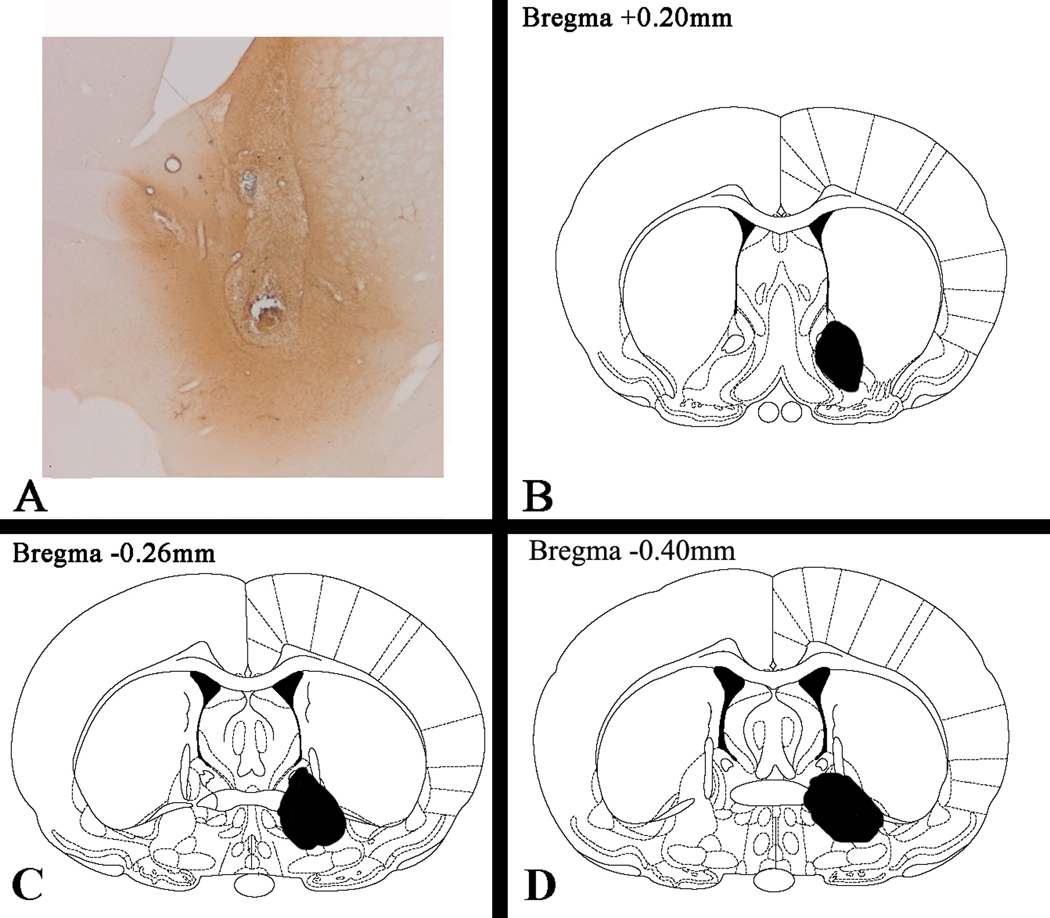
The location and distribution of CART peptide after injection into the VP was first simulated by injecting BDA, a marker that is relatively easy to visualize, through the cannulae in a manner identical to that of the CART injections. The BDA was then visualized by a chromogenic reaction. As expected, the injection site was in the subcommissural VP (Figure 5).
Figure 5.
Confirmation of the site of injection of and the distribution of CART peptide in the VP in a typical animal. BDA, which was easy to visualize, was injected through indwelling cannulae in a manner identical to that of CART injections as described in the text. Coordinates of coronal sections are according to Paxinos and Watson (1986). See text for additional details.
In behavioral studies, injection of CART peptide into the VP had no effect on basal locomotor activity at all time points tested and at both doses (Figure 6). However, at 10 and 20 minutes post-cocaine injection, there was a significant effect of CART peptide on locomotor activity of rats with respect to treatment (F(2,36)=9.536, p<0.05). Post-hoc comparisons using Tukey’s test indicate a significant decrease in locomotor activity in the group receiving 2.5 µg of CART in the VP at the 10- and 20-minute time points. These results parallel those found for intra-accumbal administrations of CART on cocaine- or amphetamine-induced locomotor activity (Jaworski et al., 2003a, Jaworski et al., 2003b, Kim et al., 2003).
Figure 6.
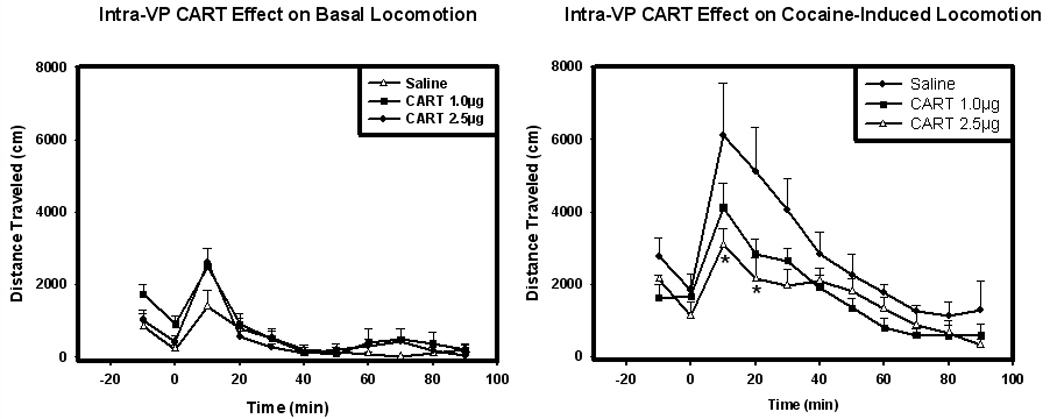
Locomotor activity of rats following injections of Intra-VP CART alone and Intra-VP CART plus i.p. cocaine (10mg/kg). Injection of CART peptide into the VP had no effect on basal locomotor activity (left side) at all times points tested and at all doses infused (Figure 6). However, at 10 and 20 minutes post-cocaine injection (right side), there was a significant effect on locomotor activity of rats with respect to treatment (F(2,36)=9.536, p<0.05; Figure 6). Post-hoc comparisons using Tukey’s test indicate a significant decrease in locomotor activity in the group receiving 2.5 µg of CART in the VP at the 10- and 20-minute time points (asterisks).
Discussion
The VP was first noticed as a nucleus that received a significant projection from the nucleus accumbens (Heimer, 2003). Due to the fact that it, as well as the NAcc, receives projections from both the basal ganglia proper and nuclei that are now recognized as part of the extended amygdala system, it has been postulated that the VP is a site for limbic-motor integration (Mogenson and Yang, 1991). Our results demonstrate that CART peptide is present in the NAcc to VP projection and likely plays a role in the motor functionality of the VP.
The high level of CART immunoreactivity in the VP demonstrated in this study is supported by a previous immunocytochemical study that examined CART immunoreactivity throughout the rat brain (Koylu et al., 1998). No CART immunoreactive cell bodies were observed in the VP, but a dense innervation of neuronal processes was present. An anterograde tracer injected into the NAcc produced labeled neuronal axons and terminals in the VP. These processes were often colocalized with CART peptide immunoreactivity suggesting that CART peptide-containing neurons in the NAcc projected to the VP. These findings were confirmed by additional studies where a retrograde tracer injected into the VP labeled CART-immunoreactive cell bodies in the NAcc. The NAcc to VP projection is known to contain GABA and a number of neuropeptides. The discovery of CART peptide and its localization with substance P and dynorphin in GABAergic accumbal efferents suggest a role for CART in neuronal functions in nuclei that receive projections from the NAcc, including the VP. These results indicate a CART pathway between the NAcc and the VP.
The VP has long been considered part of the basal ganglia in a loop that includes the NAcc. This circuit was proposed to subserve locomotor activity in the context of repetitive and reinforced behavior (Kretschmer, 2000). Formerly, the VP was considered to be simply a relay nucleus for processing this information, but increased anatomical and behavioral studies have demonstrated that the VP integrates information from several limbic sources including the NAcc (Fuller et al., 1987, Klitenick et al., 1992). Injection of a number of drugs, including µ-opiate, δ-opiate, dopaminergic and glutamatergic agonists, into the VP can induce spontaneous locomotor activity (Shreve and Uretsky, 1989, Austin and Kalivas, 1990, Klitenick et al., 1992, Napier and Chrobak, 1992, Hooks and Kalivas, 1994, Gong et al., 1997). Intrapallidal injections of the GABA-A agonist, muscimol, can attenuate locomotor activity induced by pharmacological stimulation of the NAcc (Austin and Kalivas, 1989). Also, injection of a D1 agonist, but not a D2 agonist, into the VP increases locomotor activity (Gong et al., 1999). This is interesting in that it suggests that dopamine receptors in the VP, in addition to the more well-known NAcc dopaminergic system, can be involved in locomotor behavior. Since locomotor activity can be modulated by manipulation of a variety of neurotransmitter systems in the VP, it is not surprising that CART injected into the VP can reduce psychomotor stimulant-induced locomotor activity as shown in this study. Thus, the actions of CART in the VP seem to attenuate dopaminergic function, as is also observed in the NAcc (Jaworski et al., 2003a, Kim et al., 2003, Jaworski et al., 2007, Hubert et al., 2008, Jaworski et al., 2008, Rogge et al., 2008) and the VTA (Jaworski et al., 2008).
Studies on the NAcc and its dopaminergic innervation have often found that agents that produce locomotor activity are generally reinforcing (Mogenson et al., 1980). This seems to hold true in the VP as well. Intracranial self-stimulation (ICSS) is supported throughout the VP in rats (Panagis et al., 1995). Psychomotor stimulants such as cocaine decrease the current threshold for ICSS in the VP (Panagis and Spyraki, 1996). Dopamine antagonists increase the threshold for ICSS, though this might be a secondary effect of general motoric inhibition. Intra-VP administration of amphetamine and cocaine also induce a conditioned place preference (CPP), which supports a role for pallidal DA in reward-related processes (Gong et al., 1996). Interestingly, injections of substance P, which is colocalized with CART in NAcc efferents, into the VP also produce CPP (Nikolaus et al., 1999). If both substance P and CART are released from accumbal terminals in the VP as suggested by our anatomical findings, it will be interesting to examine if they are mutually inhibitory, and under what conditions each transmitter is released. It is also important to discover if CART peptide injections into the VP can attenuate the local effects of dopamine relative to reward and reinforcement, as is seen in the NAcc (Jaworski et al., 2003a, Jaworski et al., 2008).
Recent research has examined the balance between the neural substrates of incentive and reward, i.e., between “wanting” and “liking”, in the VP (Smith and Berridge, 2005, Smith et al., 2009). Opioids and GABA, both contained in NAcc efferents to the VP, are both involved in the wanting of food reward, but only opiates affected the reward value of sucrose. Smith and Berridge (Smith and Berridge, 2005) mapped regions of the VP to determine the key area that enhances reward and incentive properties of food and sucrose and demonstrated that the posterior VP is a “hotspot” for opiate modulation of these processes. CART peptide also has been shown to modulate feeding behavior, although all of the sites mediating this are unknown (Rogge et al., 2008). However, given that lesions of the VP can produce adipsia and aphagia, and that CART significantly innervates the VP as shown here, it is possible that CART actions in the VP can affect feeding behavior by modulation of “wanting” and/or “liking” circuits. The modulation of “wanting” circuits may also be predicted by the ability of CART to modulate locomotion induced by manipulation of dopaminergic systems.
Acknowledgments
The support of NIH grants RR00165, DA15162, DA15040, DA02156, and The Georgia Research Alliance.
Abbreviations
- CART
Cocaine and Amphetamine-Regulated Transcript
- NAcc
Nucleus Accumbens
- VP
Ventral Pallidum
- SNr
Substantia Nigra pars Reticulata
- PB
Phosphate Buffer
- PBS
Phosphate-Buffered Saline
- PHA-L
Phaseolus Vulgaris Leucoagglutinin
- r-DA
Rhodamine-conjugated Dextran Amines
- FITC
Fluorescein Isothiocyanate
- BSA
Bovine Serum Albumin
- NDS
Normal Donkey Serum
- NGS
Normal Goat Serum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Austin MC, Kalivas PW. Blockade of enkephalinergic and GABAergic mediated locomotion in the nucleus accumbens by muscimol in the ventral pallidum. Jpn J Pharmacol. 1989;50:487–490. doi: 10.1254/jjp.50.487. [DOI] [PubMed] [Google Scholar]
- Austin MC, Kalivas PW. Enkephalinergic and GABAergic modulation of motor activity in the ventral pallidum. J Pharmacol Exp Ther. 1990;252:1370–1377. [PubMed] [Google Scholar]
- Churchill L, Kalivas PW. A topographically organized gamma-aminobutyric acid projection from the ventral pallidum to the nucleus accumbens in the rat. J Comp Neurol. 1994;345:579–595. doi: 10.1002/cne.903450408. [DOI] [PubMed] [Google Scholar]
- Churchill L, Kalivas PW. The involvement of the mediodorsal nucleus of the thalamus and the midbrain extrapyramidal area in locomotion elicited from the ventral pallidum. Behav Brain Res. 1999;104:63–71. doi: 10.1016/s0166-4328(99)00051-0. [DOI] [PubMed] [Google Scholar]
- Dallvechia-Adams S, Kuhar MJ, Smith Y. Cocaine- and amphetamine-regulated transcript peptide projections in the ventral midbrain: Colocalization with gamma-aminobutyric acid, melanin-concentrating hormone, dynorphin, and synaptic interactions with dopamine neurons. The Journal of comparative neurology. 2002;448:360–372. doi: 10.1002/cne.10268. [DOI] [PubMed] [Google Scholar]
- Dominguez G, Vicentic A, Del Giudice EM, Jaworski J, Hunter RG, Kuhar MJ. CART peptides: modulators of mesolimbic dopamine, feeding, and stress. Ann N Y Acad Sci. 2004;1025:363–369. doi: 10.1196/annals.1316.044. [DOI] [PubMed] [Google Scholar]
- Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15:2471–2481. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller TA, Russchen FT, Price JL. Sources of presumptive glutamergic/aspartergic afferents to the rat ventral striatopallidal region. J Comp Neurol. 1987;258:317–338. doi: 10.1002/cne.902580302. [DOI] [PubMed] [Google Scholar]
- Gong W, Justice JB, Jr., Neill D. Dissociation of locomotor and conditioned place preference responses following manipulation of GABA-A and AMPA receptors in ventral pallidum. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:839–852. doi: 10.1016/s0278-5846(97)00084-5. [DOI] [PubMed] [Google Scholar]
- Gong W, Neill D, Justice JB., Jr. Conditioned place preference and locomotor activation produced by injection of psychostimulants into ventral pallidum. Brain Res. 1996;707:64–74. doi: 10.1016/0006-8993(95)01222-2. [DOI] [PubMed] [Google Scholar]
- Gong W, Neill DB, Lynn M, Justice JB., Jr. Dopamine D1/D2 agonists injected into nucleus accumbens and ventral pallidum differentially affect locomotor activity depending on site. Neuroscience. 1999;93:1349–1358. doi: 10.1016/s0306-4522(99)00235-3. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Haber SN. Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience. 1993;57:113–142. doi: 10.1016/0306-4522(93)90115-v. [DOI] [PubMed] [Google Scholar]
- Heimer L. A new anatomical framework for neuropsychiatric disorders and drug abuse. Am J Psychiatry. 2003;160:1726–1739. doi: 10.1176/appi.ajp.160.10.1726. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Kalivas PW. Involvement of dopamine and excitatory amino acid transmission in novelty-induced motor activity. J Pharmacol Exp Ther. 1994;269:976–988. [PubMed] [Google Scholar]
- Hubert GW, Jones DC, Moffett MC, Rogge G, Kuhar MJ. CART peptides as modulators of dopamine and psychostimulants and interactions with the mesolimbic dopaminergic system. Biochem Pharmacol. 2008;75:57–62. doi: 10.1016/j.bcp.2007.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert GW, Kuhar MJ. Colocalization of CART with substance P but not enkephalin in the rat nucleus accumbens. Brain research. 2005;1050:8–14. doi: 10.1016/j.brainres.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Hubert GW, Kuhar MJ. Colocalization of CART peptide with prodynorphin and dopamine D1 receptors in the rat nucleus accumbens. Neuropeptides. 2006;40:409–415. doi: 10.1016/j.npep.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Hubert GW, Kuhar MJ. Cocaine administration increases the fraction of CART cells in the rat nucleus accumbens that co-immunostain for c-Fos. Neuropeptides. 2008 doi: 10.1016/j.npep.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert GW, Paquet M, Smith Y. Differential subcellular localization of mGluR1a and mGluR5 in the rat and monkey Substantia nigra. Journal of Neuroscience. 2001;21:1838–1847. doi: 10.1523/JNEUROSCI.21-06-01838.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RG, Kuhar MJ. CART peptides as targets for CNS drug development. Curr Drug Target CNS Neurol Disord. 2003;2:201–205. doi: 10.2174/1568007033482896. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Svensson P, Ponten M. The role of dopamine, dynorphin, and CART systems in the ventral striatum and amygdala in cocaine abuse. Annals of the New York Academy of Sciences. 1999;877:499–506. doi: 10.1111/j.1749-6632.1999.tb09285.x. [DOI] [PubMed] [Google Scholar]
- Jaworski JN, Hansen ST, Kuhar MJ, Mark GP. Injection of CART (cocaine- and amphetamine-regulated transcript) peptide into the nucleus accumbens reduces cocaine self-administration in rats. Behav Brain Res. 2008;191:266–271. doi: 10.1016/j.bbr.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski JN, Kimmel HL, Mitrano DA, Tallarida RJ, Kuhar MJ. Intra-VTA CART 55–102 reduces the locomotor effect of systemic cocaine in rats: an isobolographic analysis. Neuropeptides. 2007;41:65–72. doi: 10.1016/j.npep.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski JN, Kozel MA, Philpot KB, Kuhar MJ. Intra-accumbal injection of CART (cocaine-amphetamine regulated transcript) peptide reduces cocaine-induced locomotor activity. The Journal of pharmacology and experimental therapeutics. 2003a;307:1038–1044. doi: 10.1124/jpet.103.052332. [DOI] [PubMed] [Google Scholar]
- Jaworski JN, Vicentic A, Hunter RG, Kimmel HL, Kuhar MJ. CART peptides are modulators of mesolimbic dopamine and psychostimulants. Life sciences. 2003b;73:741–747. doi: 10.1016/s0024-3205(03)00394-1. [DOI] [PubMed] [Google Scholar]
- Kim JH, Creekmore E, Vezina P. Microinjection of CART peptide 55–102 into the nucleus accumbens blocks amphetamine-induced locomotion. Neuropeptides. 2003;37:369–373. doi: 10.1016/j.npep.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Klitenick MA, Deutch AY, Churchill L, Kalivas PW. Topography and functional role of dopaminergic projections from the ventral mesencephalic tegmentum to the ventral pallidum. Neuroscience. 1992;50:371–386. doi: 10.1016/0306-4522(92)90430-a. [DOI] [PubMed] [Google Scholar]
- Koylu EO, Couceyro PR, Lambert PD, Kuhar MJ. Cocaine- and amphetamine-regulated transcript peptide immunohistochemical localization in the rat brain. J Comp Neurol. 1998;391:115–132. [PubMed] [Google Scholar]
- Koylu EO, Couceyro PR, Lambert PD, Ling NC, DeSouza EB, Kuhar MJ. Immunohistochemical localization of novel CART peptides in rat hypothalamus, pituitary and adrenal gland. J Neuroendocrinol. 1997;9:823–833. doi: 10.1046/j.1365-2826.1997.00651.x. [DOI] [PubMed] [Google Scholar]
- Kretschmer BD. Functional aspects of the ventral pallidum. Amino Acids. 2000;19:201–210. doi: 10.1007/s007260070050. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Jaworski JN, Hubert GW, Philpot KB, Dominguez G. Cocaine- and amphetamine-regulated transcript peptides play a role in drug abuse and are potential therapeutic targets. Aaps J. 2005;7:E259–E265. doi: 10.1208/aapsj070125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PJ, Tang-Christensen M, Vrang N. New therapeutic developments in the regulation of food intake by neuropeptides. Ann Endocrinol (Paris) 2002;63:171–175. [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Yang CR. The contribution of basal forebrain to limbic-motor integration and the mediation of motivation to action. Adv Exp Med Biol. 1991;295:267–290. doi: 10.1007/978-1-4757-0145-6_14. [DOI] [PubMed] [Google Scholar]
- Murphy KG. Dissecting the role of cocaine- and amphetamine-regulated transcript (CART) in the control of appetite. Brief Funct Genomic Proteomic. 2005;4:95–111. doi: 10.1093/bfgp/4.2.95. [DOI] [PubMed] [Google Scholar]
- Napier TC, Chrobak JJ. Evaluations of ventral pallidal dopamine receptor activation in behaving rats. Neuroreport. 1992;3:609–611. doi: 10.1097/00001756-199207000-00016. [DOI] [PubMed] [Google Scholar]
- Nikolaus S, Huston JP, Hasenohrl RU. Reinforcing effects of neurokinin substance P in the ventral pallidum: mediation by the tachykinin NK1 receptor. Eur J Pharmacol. 1999;370:93–99. doi: 10.1016/s0014-2999(99)00105-3. [DOI] [PubMed] [Google Scholar]
- Panagis G, Miliaressis E, Anagnostakis Y, Spyraki C. Ventral pallidum self-stimulation: a moveable electrode mapping study. Behavioural brain research. 1995;68:165–172. doi: 10.1016/0166-4328(94)00169-g. [DOI] [PubMed] [Google Scholar]
- Panagis G, Spyraki C. Neuropharmacological evidence for the role of dopamine in ventral pallidum self-stimulation. Psychopharmacology. 1996;123:280–288. doi: 10.1007/BF02246582. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1986. [Google Scholar]
- Reynolds E. The use of lead citrate at high pH as an electron opaque stain in electron microscopy. Journal of Cell Biology. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogge G, Jones D, Hubert GW, Lin Y, Kuhar MJ. CART peptides: regulators of body weight, reward and other functions. Nat Rev Neurosci. 2008;9:747–758. doi: 10.1038/nrn2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreve PE, Uretsky NJ. AMPA, kainic acid, and N-methyl-D-aspartic acid stimulate locomotor activity after injection into the substantia innominata/lateral preoptic area. Pharmacol Biochem Behav. 1989;34:101–106. doi: 10.1016/0091-3057(89)90360-2. [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose "liking" and food intake. J Neurosci. 2005;25:8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci. 2007;27:1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behav Brain Res. 2009;196:155–167. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess J, Villarreal J, Vale W. Isolation and sequence analysis of a somatostatin-like polypeptide from ovine hypothalamus. Biochemistry. 1981;20:1982–1988. doi: 10.1021/bi00510a038. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Models of basal ganglia function and pathophysiology of movement disorders. Neurosurgery Clinics of North America. 1998;9:223–236. [PubMed] [Google Scholar]
- Zahm DS. The ventral striatopallidal parts of the basal ganglia in the rat--II. Compartmentation of ventral pallidal efferents. Neuroscience. 1989;30:33–50. doi: 10.1016/0306-4522(89)90351-5. [DOI] [PubMed] [Google Scholar]