Abstract
CART (cocaine- and amphetamine-regulated transcript) peptides (CART 55-102 and CART 62-102) are peptidergic neurotransmitters that are widely but specifically distributed throughout the brain, gut and other parts of the body. They are found in many brain regions associated with drug addiction including the nucleus accumbens, ventral tegmental area and ventral pallidum. Injections of CART 55-102 into the nucleus accumbens have no effect on basal locomotor activity. However, an injection of CART just before an i.p. injection of cocaine reduces the locomotor activating effects of cocaine. These and other data suggest that CART in the accumbens blunts the effects of cocaine. A hypothesis is that CART is homeostatic in the accumbens and tends to oppose large increases in dopamine signaling. These actions would therefore be able to regulate the effects of some abused drugs such as the psychostimulants.
CART: Discovery of an addiction-related peptide
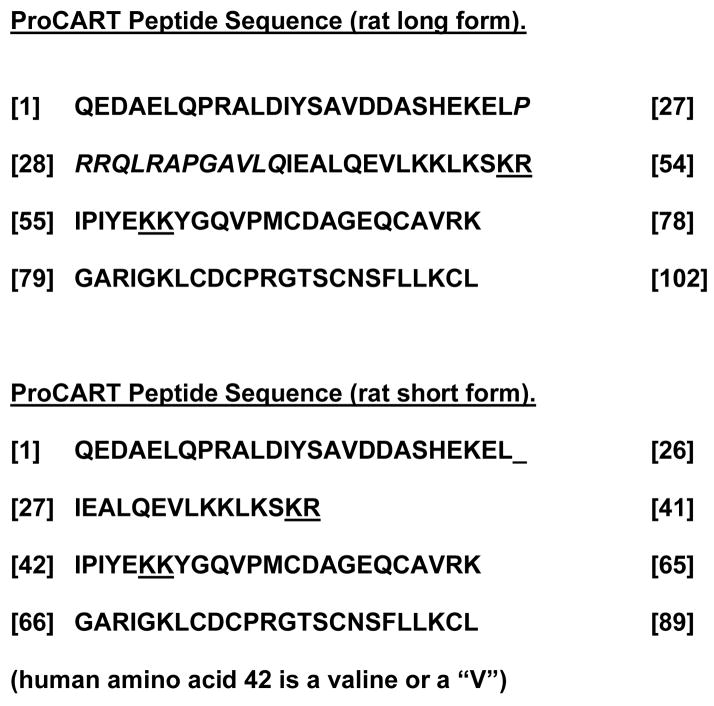
In 1980, Speiss, et al.[1], obtained evidence that a CART peptide existed. In an extract from hypothalamus, they found a peptide with a new, previously unknown sequence that later was understood to be part of one of the CART peptides. Speiss, et al. [1], did not know the function or the exact localization of the peptide and referred to it as an unknown peptide. In 1995, Douglass, et al. [2], using differential display techniques, found an mRNA was upregulated after cocaine and amphetamine, but not after morphine, in the rat striatal region. This mRNA coded for a gene product that contained the peptide previously identified by Speiss, et al [1]. This lead to the naming of the peptide with the acronym, CART, which stands for cocaine and amphetamine regulated transcript [2]. Processing of proCART into active species, rat CART 55-102 and CART 62-102, has been described [3–5]. The amino acid sequence of rat CART peptide is shown in Figure 1. The human peptide is numbered differently since it is all in the spliced form (Figure 1).
Figure 1.
Sequence of the ProCART peptide. In the rat, there are 2 splice variants; amino acids 27–39 (in italics) are spliced out from the long form of 102 amino acids to give the short form of 89 amino acids. The fragments of the long form that have been reliably shown to be active are CART 55-102 and CART 61-102 (in the short form the numbers are CART 42-89 and CART 49-89). In humans, the splicing is complete and only the short 89 amino acid form exists, with some changes in amino acids. Position 42 which is isoleucine in the rat is a valine in the human. Underlined pairs of basic amino acids indicate sites of processing by prohormone convertases.
Most of the general requirements needed to show that CART is a peptide neurotransmitter have been shown and summarized [1, 6–9]. CART peptide has been shown to be produced by neurons [1]. In addition, CART is packaged in large, dense-cored vesicles and present in nerve terminals that appose postsynaptic elements in the nucleus accumbens [7]. CART has also been shown to effect changes in postsynaptic cells, specifically in the ERK pathway, which is affected by a number of G protein-coupled receptors [9, 10]. No specific clearance mechanism has been found for CART, though it is possible that synaptic CART levels are controlled by diffusion and peptidases. One major obstacle in investigating the functionality of CART is the lack of success in identifying a receptor devoted to CART neurotransmission.
Surprisingly, the work of Douglass, et al. [2], has not always been reproduced [11]. Nevertheless, there have been confirmatory reports using binge versus acute doses of cocaine [12, 13], and it is unclear why the increase in mRNA levels after psychostimulant injection is somewhat variable and not always found, although it may involve stress and a need for corticosterone [12]. More recent studies in animals have shown that CART mRNA levels increased after injection of methamphetamine or alcohol [14, 15]. Furthermore, in the post mortem human brain, CART mRNA levels are changed in the VTA and nucleus accumbens of cocaine overdose victims [16, 17]. Also a mutation in the CART gene is associated with alcoholism and not schizophrenia [18].
There is additional evidence that CART may be involved in addiction, but it is less direct. For example, over expression of CREB in the accumbens results in a reduction in rewarding effects of cocaine [19]. If CART peptide is inhibiting the effects of cocaine, then CREB over expression should increase CART in the accumbens. In fact, it has been shown that CART is regulated by CREB and that CREB over expression increases CART levels [20–22]. Thus, there is much evidence that does indeed suggest that CART is involved in modulating the action of psychostimulants and dopamine (DA).
CART – DA interactions in the mesolimbic system
Since a commonality of many addictive drugs is activation of the mesolimbic DA system, one would expect that CART and DA would interact, especially in light of CART’s interactions with cocaine. Electron microscopy shows that tyrosine hydroxylase-containing nerve terminals synapse on CART-containing neurons in the nucleus accumbens [6, 23]. Moreover, injection of D3 dopamine receptor agonists lowers levels of CART mRNA in the accumbens [24], and DA receptors are found on CART neurons [25, 26]. Injection of CART 55-102 into the ventricles causes an increased turnover of DA [27, 28]. CART-containing neurons in the nucleus accumbens core project to the substantia nigra zona compacta, while projections from the shell seem to be located more medially in the ventral tegmental area [29]. Injection of CART into the VTA, where cell bodies of the mesolimbic system are located, produces weak psychostimulant-like effects and causes an efflux of dopamine in the nucleus accumbens [30, 31]. However, a recent study found that injections of CART 55-102 into the VTA decreased locomotor counts following systemic administration of cocaine, especially at high doses of CART [32]. In summary, there are neurochemical data showing complex CART-DA interactions as pertains to the mesolimbic dopaminergic projection, and anatomical studies provide a physical basis to support this idea as well.
Effects of CART peptide on the locomotor effects of cocaine and dopamine. in the nucleus accumbens
It is well known that i.p. injection of cocaine into a rodent results in an increase in locomotor activity (LMA). When attempting to identify an effect of CART on cocaine, we had injected CART peptide into the nucleus accumbens and measured LMA. Injection of CART 55-102 alone into the nucleus accumbens had no effect, which was puzzling [33]. However, when CART was injected into the nucleus accumbens immediately after an i.p. injection of cocaine, it was found that the LMA effects of cocaine were blunted (Table 1). This effect of CART has also been reported with respect to amphetamine-induced LMA [34]. So by itself, CART has no apparent effect when injected into the accumbens, but when combined with psychostimulants, it will blunt drug-stimulated LMA [33].
Table 1.
Effect of CART on Cocaine-induced LMA
| Cocaine Dose (mg/kg) | Cocaine LMA (cm) | Coc + CART LMA (cm) |
|---|---|---|
| 20 | 26,000 | 12,000 |
| 30 | 33,000 | 16,000 |
CART 55-102 peptide (2.5 ug each side into the nucleus accumbens) blunts the LMA induced by cocaine. CART 1-27, and inactive CART peptide, had no effect. LMA – locomotor activity. Data from Jaworski et al 2003.
To explore this phenomenon further, dopamine was injected into the nucleus accumbens, and it produced the increases in LMA that have been observed previously using psychostimulants. When CART 55-102 and dopamine were co-administered in the same injection, the effects of dopamine were reduced [33]. Because cocaine acts through dopamine in producing LMA, the data suggest that CART tends to oppose the actions of dopamine and cocaine in the accumbens. Moreover, these findings suggest that the action of CART peptide is downstream of dopamine receptor activation. This could be due to many mechanisms, including a competition for intracellular signaling pathways [33].
A major reason why we chose to study the nucleus accumbens with injections of CART 55-102 is that the nucleus accumbens has a very high concentration of CART peptide [35]. CART neurons in the accumbens have recurrent collaterals and collateral innervations of CART neurons to both CART-containing and non CART-containing neurons. Electron microscopic studies show a substantial number of CART peptide-containing nerve terminals in the nucleus accumbens [7]. In addition, several areas that have CART peptide-containing cell bodies are known to project to the nucleus accumbens [36, 37]. Accordingly, because of the clear innervation of the nucleus accumbens by CART, it is concluded that the nucleus accumbens contains CART receptors, and that these receptors mediate the effects of CART peptides in the accumbens discussed above.
CART and sensitization to psychomotor stimulants
It is a well-documented characteristic of psychomotor stimulants to produce a sensitization of locomotor activity with repeated doses. In studies in CART knockout mice, repeated doses of amphetamine produce a much smaller and delayed locomotor sensitization than in controls [38]. This is in direct contrast to a separate study, using different CART knockout mice, that found no change in the sensitization to cocaine in CART knockouts [39]. Moreover, repeated injection of CART 55-102 into the VTA of rats, an area critical to the development of sensitization to amphetamine, is not sufficient to induce sensitization to cocaine or amphetamine [30]. These data imply that CART in and of itself is not sufficient to produce psychomotor stimulant sensitization, but that increased levels of dopamine are necessary.
Studies using CART knockout mice
Currently, there are two separate strains of knockout mice available for research. One that deletes exons I and II [40] and one that deletes all three exons [41]. For review, see Moffett et al. [42]. CART knockout mice typically feed more than wild type, and increased body weight is especially evident when the mice are given a high caloric diet [40]. In addition, the knockout mice generated by Wierup et al. [41], also show increased weight as well as impaired pancreatic function, which may be the cause of altered body weight.
Various studies disagree on the effects of the absence of CART on the function of systems affected by psychomotor stimulants. Couceyro et al. [38], using the mice generated by Wierup et al. [41] reports that locomotor activity was decreased after cocaine and that vertical and stereotypic activity were decreased after amphetamine in the CART knockout group. In addition, responding for intravenous cocaine and total cocaine consumption were reduced in CART knockouts. CART knockout mice also did not show any sensitization to the effects of amphetamine. The same mice, however showed no inhibition of open field activity and sucrose preference, indicating that the elimination of the CART gene did not effect locomotor ability or taste preference [38]. In contrast, separate studies using the different strain of CART knockout mice did not detect a difference in cocaine-induced locomotor activity, cocaine self-administration or sensitization to the behavioral effects of cocaine [39, 42]. Additional studies will be needed to reliably ascertain effects of knocking out CART.
A search for the CART receptor
When CART was first identified as an important brain peptide in the late 1990s, many laboratories prepared radiolabeled CART peptide and attempted to find the receptors for CART in binding studies. Surprisingly, these studies all failed for unknown reasons. It seemed possible that the CART receptor had a low affinity for radiolabeled CART peptide, and therefore had a rapid rate of unbinding which precluded the identification of specific binding in a binding approach. Alternatively, the receptor number could be very low – below the limits of detection, or, the number of non-specific binding sites could be overwhelmingly high. In any case, until a couple of years ago, the CART receptor was largely uncharacterized.
After taking a new approach, that is exploring cell lines for CART-induced signaling events, it was found that application of CART55-102 to AtT20 cells resulted in an increase in P-ERK-1 and P-ERK-2 [9]. Shortly thereafter, Vicentic et al. [10] identified specific binding in the same cell line under the same conditions (Figure 2). Thus, CART-specific receptor binding has been identified. Moreover, the binding of the agonist CART peptide was reduced in the presence of excess GTP analogue, but not in the presence of excess ATP analogue, indicating that the CART receptor being studied in the AtT20 cells was a G protein-coupled receptor. Furthermore, pertussis toxin inhibited CART signaling indicating that the GPCR was coupled with a Gi or a Go protein [9]. More recently, another laboratory found CART receptor binding in differentiated PC12 cells [43]. These breakthroughs are likely to lead to additional studies on CART receptor binding and to the identification of the gene or genes for the CART receptor or receptors. This will not only lead to a new understanding of CART systems in brain and other tissues, but will also facilitate drug screening for potential CART agonists and antagonists.
Figure 2.
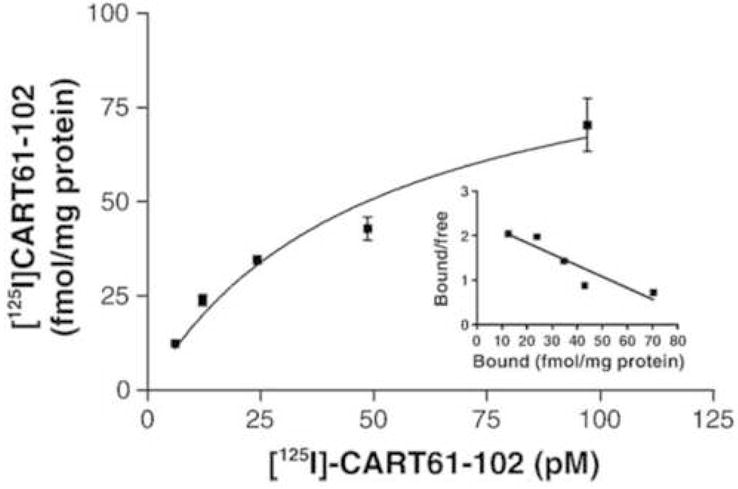
Identification of specific saturable binding of radiolabeled CARTpeptide to AtT20 cells. The Kd is subnanomolar, and the binding is specific for CART 55-12 and CART 62-102; no other tested peptide or substance inhibited the binding. Reproduced from Vicentic et al., 2005.
CART in other processes
One of the most interesting things about CART peptides is that they appear to be involved in a number of physiological functions including feeding and body weight [36, 44]. One of the first experiments carried out after the discovery of CART were injections of CART peptide into the ventricles of rodents, which resulted in an inhibition of feeding, while an injection of antibodies resulted in an increase in feeding ([45–47]. Human genetic studies also support this hypothesis. A missense mutation has been associated with early onset obesity [48] and another mutation associates with increased weight [49] The issue of CART and body weight has been critically reviewed [44].
Changes in CART levels have been associated with stress [50, 51]), and CART 55-102 has antinociceptive effects [52–54]. Evidence is increasing for a role for CART in endocrine regulation [27, 55–57]. Also CART has neurotrophic [58, 59] as well as vasoconstrictor properties [60–62].
Hypothesis: CART is a homeostatic regulator in the nucleus accumbens
A consideration of available data suggests that CART 55-102 (or CART 62-102) is a peptide neurotransmitter that functions as a homeostatic regulator in the nucleus accumbens. In other words, as dopamine signaling in the nucleus accumbens becomes great, CART peptide is released and tends to oppose these actions of dopamine (Figure 3). It is not surprising that such a regulatory system(s) could exist in the nucleus accumbens. The major evidence for this are the LMA studies noted above (Table 1) and some recent studies appearing in an abstract [63] which suggest that accumbal CART peptide reduces the rewarding effects of cocaine by inhibiting cocaine self-administration patterns.
Figure 3.
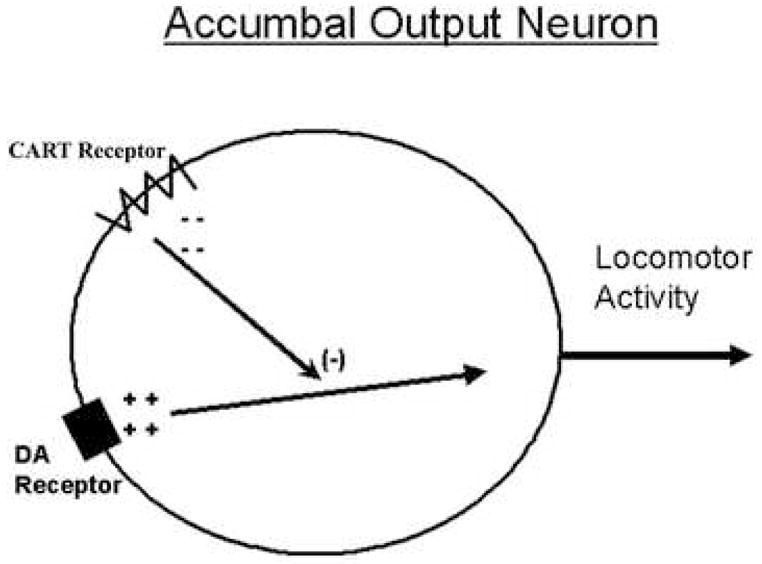
A hypothesis in schematic form about a function of CART peptide in the Nucleus accumbens (Acc). Its action is to oppose the changes induced by cocaine. When CART is given alone into the Acc, it has no effect, but when coadministered with DA or cocaine, it tends to block the locomotor effects of those compounds. While the cellular mechanisms of these CART-DA receptor interactions have not yet been clarified, perhaps candidates would be heterologous desensitization and/or interfering downstream signaling pathways from CART and DA receptors.
There are, however, some difficulties with this theory. It is difficult to fully understand the action of a substance when the only approach available is to increase levels of the transmitter in question. Studies of CART knockout mice in the context of drug abuse have been conducted, but these studies have produced conflicting results [42]. Other ways of manipulating CART systems will have to be developed to fully explore the functions of CART peptides.
Acknowledgments
The authors acknowledge the support if grants RR00165, DA10732, DA015040, DA00418, and individual NRSAs: DA021056, 5F31DA0219 and DA20312.
Abbreviations
- CART
cocaine- and amphetamine-regulated transcript
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spiess J, Villarreal J, Vale W. Isolation and sequence analysis of a somatostatin-like polypeptide from ovine hypothalamus. Biochemistry. 1981;20:1982–8. doi: 10.1021/bi00510a038. [DOI] [PubMed] [Google Scholar]
- 2.Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15:2471–81. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thim L, Kristensen P, Larsen PJ, Wulff BS. CART, a new anorectic peptide. Int J Biochem Cell Biol. 1998;30:1281–4. doi: 10.1016/s1357-2725(98)00110-1. [DOI] [PubMed] [Google Scholar]
- 4.Kuhar MJ, Yoho LL. CART peptide analysis by Western blotting. Synapse. 1999;33:163–71. doi: 10.1002/(SICI)1098-2396(19990901)33:3<163::AID-SYN1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 5.Dey A, Xhu X, Carroll R, Turck CW, Stein J, Steiner DF. Biological processing of the cocaine and amphetamine-regulated transcript precursors by prohormone convertases, PC2 and PC1/3. Journal of Biological Chemistry. 2003;278:15007–14. doi: 10.1074/jbc.M212128200. [DOI] [PubMed] [Google Scholar]
- 6.Smith Y, Kieval J, Couceyro PR, Kuhar MJ. CART peptide-immunoreactive neurones in the nucleus accumbens in monkeys: ultrastructural analysis, colocalization studies, and synaptic interactions with dopaminergic afferents. J Comp Neurol. 1999;407:491–511. doi: 10.1002/(sici)1096-9861(19990517)407:4<491::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 7.Smith Y, Koylu EO, Couceyro P, Kuhar MJ. Ultrastructural localization of CART (cocaine- and amphetamine-regulated transcript) peptides in the nucleus accumbens of monkeys. Synapse. 1997;27:90–4. doi: 10.1002/(SICI)1098-2396(199709)27:1<90::AID-SYN10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 8.Vicentic A, Lakatos A, Jones D. The CART receptors: background and recent advances. Peptides. 2006;27:1934–7. doi: 10.1016/j.peptides.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 9.Lakatos A, Prinster S, Vicentic A, Hall RA, Kuhar MJ. Cocaine- and amphetamine-regulated transcript (CART) peptide activates the extracellular signal-regulated kinase (ERK) pathway in AtT20 cells via putative G-protein coupled receptors. Neurosci Lett. 2005;384:198–202. doi: 10.1016/j.neulet.2005.04.072. [DOI] [PubMed] [Google Scholar]
- 10.Vicentic A, Lakatos A, Kuhar MJ. CART (cocaine- and amphetamine-regulated transcript) peptide receptors: specific binding in AtT20 cells. Eur J Pharmacol. 2005;528:188–9. doi: 10.1016/j.ejphar.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 11.Vrang N, Larsen PJ, Kristensen P. Cocaine-amphetamine regulated transcript (CART) expression is not regulated by amphetamine. Neuroreport. 2002;13:1215–8. doi: 10.1097/00001756-200207020-00029. [DOI] [PubMed] [Google Scholar]
- 12.Hunter RG, Vicentic A, Rogge G, Kuhar MJ. The effects of cocaine on CART expression in the rat nucleus accumbens: a possible role for corticosterone. Eur J Pharmacol. 2005;517:45–50. doi: 10.1016/j.ejphar.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 13.Fagergren P, Hurd YL. Mesolimbic gender differences in peptide CART mRNA expression: effects of cocaine. Neuroreport. 1999;10:3449–52. doi: 10.1097/00001756-199911080-00034. [DOI] [PubMed] [Google Scholar]
- 14.Ogden CA, Rich ME, Schork NJ, Paulus MP, Geyer MA, Lohr JB, et al. Candidate genes, pathways and mechanisms for bipolar (manic-depressive) and related disorders: an expanded convergent functional genomics approach. Mol Psychiatry. 2004;9:1007–29. doi: 10.1038/sj.mp.4001547. [DOI] [PubMed] [Google Scholar]
- 15.Salinas A, Wilde JD, Maldve RE. Ethanol enhancement of cocaine- and amphetamine-regulated transcript mRNA and peptide expression in the nucleus accumbens. J Neurochem. 2006;97:408–15. doi: 10.1111/j.1471-4159.2006.03745.x. [DOI] [PubMed] [Google Scholar]
- 16.Albertson DN, Pruetz B, Schmidt CJ, Kuhn DM, Kapatos G, Bannon MJ. Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J Neurochem. 2004;88:1211–9. doi: 10.1046/j.1471-4159.2003.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang WX, Fasulo WH, Mash DC, Hemby SE. Molecular profiling of midbrain dopamine regions in cocaine overdose victims. J Neurochem. 2003;85:911–24. doi: 10.1046/j.1471-4159.2003.01740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung SK, Hong MS, Suh GJ, Jin SY, Lee HJ, Kim BS, et al. Association between polymorphism in intron 1 of cocaine- and amphetamine-regulated transcript gene with alcoholism, but not with bipolar disorder and schizophrenia in Korean population. Neurosci Lett. 2004;365:54–7. doi: 10.1016/j.neulet.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 19.Carlezon WA, Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, et al. Regulation of cocaine reward by CREB. Science. 1998;282:2272–5. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- 20.Dominguez G, Kuhar MJ. Transcriptional regulation of the CART promoter in CATH. a cells Brain Res Mol Brain Res. 2004;126:22–9. doi: 10.1016/j.molbrainres.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 21.Dominguez G, Lakatos A, Kuhar MJ. Characterization of the cocaine- and amphetamine-regulated transcript (CART) peptide gene promoter and its activation by a cyclic AMP-dependent signaling pathway in GH3 cells. J Neurochem. 2002;80:885–93. doi: 10.1046/j.0022-3042.2002.00775.x. [DOI] [PubMed] [Google Scholar]
- 22.Lakatos A, Dominguez G, Kuhar MJ. CART promoter CRE site binds phosphorylated CREB. Brain Res Mol Brain Res. 2002;104:81–5. doi: 10.1016/s0169-328x(02)00321-2. [DOI] [PubMed] [Google Scholar]
- 23.Smith Y, Pare JF, Koylu E, Couceyro P, Ince E, Levey AI, et al. CART peptide immunoreactivity in the nucleus accumbens of monkeys: Electron microscopic analysis and co-localization studies. Society for Neuroscience Abstracts. 1997;23:384.6. [Google Scholar]
- 24.Hunter RG, Jones D, Vicentic A, Hue G, Rye D, Kuhar MJ. Regulation of CART mRNA in the rat nucleus accumbens via D3 dopamine receptors. Neuropharmacology. 2006;50:858–64. doi: 10.1016/j.neuropharm.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Beaudry G, Zekki H, Rouillard C, Levesque D. Clozapine and dopamine D3 receptor antisense reduce cocaine- and amphetamine-regulated transcript expression in the rat nucleus accumbens shell. Synapse. 2004;51:233–40. doi: 10.1002/syn.10302. [DOI] [PubMed] [Google Scholar]
- 26.Hubert GW, Kuhar MJ. Colocalization of CART peptide with prodynorphin and dopamine D1 receptors in the rat nucleus accumbens. Neuropeptides. 2006;40:409–15. doi: 10.1016/j.npep.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Shieh KR. Effects of the cocaine- and amphetamine-regulated transcript peptide on the turnover of central dopaminergic neurons. Neuropharmacology. 2003;44:940–8. doi: 10.1016/s0028-3908(03)00095-9. [DOI] [PubMed] [Google Scholar]
- 28.Yang G, Chun J, Arakawa-Uramoto H, Gawinowicz MA, Zhao K, Landry DW. Anti-cocaine catalytic antibodies: A synthetic solution to improved diversity. J Am Chem Soc. 1996;118:5881–90. [Google Scholar]
- 29.Dallvechia-Adams S, Smith Y, Kuhar MJ. CART peptide-immunoreactive projection from the nucleus accumbens targets substantia nigra pars reticulata neurons in the rat. J Comp Neurol. 2001;434:29–39. doi: 10.1002/cne.1162. [DOI] [PubMed] [Google Scholar]
- 30.Kimmel HL, Gong W, Vechia SD, Hunter RG, Kuhar MJ. Intra-ventral tegmental area injection of rat cocaine and amphetamine-regulated transcript peptide 55-102 induces locomotor activity and promotes conditioned place preference. J Pharmacol Exp Ther. 2000;294:784–92. [PubMed] [Google Scholar]
- 31.Kuhar MJ, Jaworski JN, Hubert GW, Philpot KB, Dominguez G. Cocaine- and amphetamine-regulated transcript peptides play a role in drug abuse and are potential therapeutic targets. Aaps J. 2005;7:E259–65. doi: 10.1208/aapsj070125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaworski JN, Kimmel HL, Mitrano DA, Tallarida RJ, Kuhar MJ. Intra-VTA CART 55-102 reduces the locomotor effect of systemic cocaine in rats: an isobolographic analysis. Neuropeptides. 2007;41:65–72. doi: 10.1016/j.npep.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaworski JN, Kozel MA, Philpot KB, Kuhar MJ. Intra-accumbal injection of CART (cocaine-amphetamine regulated transcript) peptide reduces cocaine-induced locomotor activity. J Pharmacol Exp Ther. 2003;307:1038–44. doi: 10.1124/jpet.103.052332. [DOI] [PubMed] [Google Scholar]
- 34.Kim JH, Creekmore E, Vezina P. Microinjection of CART peptide 55-102 into the nucleus accumbens blocks amphetamine-induced locomotion. Neuropeptides. 2003;37:369–73. doi: 10.1016/j.npep.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Koylu EO, Couceyro PR, Lambert PD, Kuhar MJ. Cocaine- and amphetamine-regulated transcript peptide immunohistochemical localization in the rat brain. J Comp Neurol. 1998;391:115–32. [PubMed] [Google Scholar]
- 36.Yang SC, Shieh KR, Li HY. Cocaine- and amphetamine-regulated transcript in the nucleus accumbens participates in the regulation of feeding behavior in rats. Neuroscience. 2005;133:841–51. doi: 10.1016/j.neuroscience.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 37.Philpot K, Smith Y. CART peptide and the mesolimbic dopamine system. Peptides. 2006;27:1987–92. doi: 10.1016/j.peptides.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 38.Couceyro PR, Evans C, McKinzie A, Mitchell D, Dube M, Hagshenas L, et al. Cocaine- and amphetamine-regulated transcript (CART) peptides modulate the locomotor and motivational properties of psychostimulants. J Pharmacol Exp Ther. 2005;315:1091–100. doi: 10.1124/jpet.105.091678. [DOI] [PubMed] [Google Scholar]
- 39.Steiner RC, Hsiung HM, Picciotto MR. Cocaine self-administration and locomotor sensitization are not altered in CART knockout mice. Behav Brain Res. 2006;171:56–62. doi: 10.1016/j.bbr.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 40.Asnicar MA, Smith DP, Yang DD, Heiman ML, Fox N, Chen YF, et al. Absence of cocaine- and amphetamine-regulated transcript results in obesity in mice fed a high caloric diet. Endocrinology. 2001;142:4394–400. doi: 10.1210/endo.142.10.8416. [DOI] [PubMed] [Google Scholar]
- 41.Wierup N, Richards WG, Bannon AW, Kuhar MJ, Ahren B, Sundler F. CART knock out mice have impaired insulin secretion and glucose intolerance, altered beta cell morphology and increased body weight. Regul Pept. 2005;129:203–11. doi: 10.1016/j.regpep.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 42.Moffett M, Stanek L, Harley J, Rogge G, Asnicar M, Hsiung H, et al. Studies of cocaine- and amphetamine-regulated transcript (CART) knockout mice. Peptides. 2006;27:2037–45. doi: 10.1016/j.peptides.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 43.Maletinska L, Maixnerova J, Matyskova R, Haugvicova R, Sloncova E, Elbert T, et al. Cocaine- and amphetamine-regulated transcript (CART) peptide specific binding in pheochromocytoma cells PC12. Eur J Pharmacol. 2007;559:109–14. doi: 10.1016/j.ejphar.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 44.Hunter RG, Philpot K, Vicentic A, Dominguez G, Hubert GW, Kuhar MJ. CART in feeding and obesity. Trends Endocrinol Metab. 2004;15:454–9. doi: 10.1016/j.tem.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 45.Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393:72–6. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- 46.Lambert P, Couceyro P, Koylu E, Ling N, DeSouza E, Kuhar M. A role for novel CART peptide fragments in the central control of food intake. Neuropeptides. 1997;31:620–1. [Google Scholar]
- 47.Lambert PD, Couceyro PR, McGirr KM, Dall Vechia SE, Smith Y, Kuhar MJ. CART peptides in the central control of feeding and interactions with neuropeptide Y. Synapse. 1998;29:293–8. doi: 10.1002/(SICI)1098-2396(199808)29:4<293::AID-SYN1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 48.del Giudice EM, Santoro N, Cirillo G, D’Urso L, Di Toro R, Perrone L. Mutational screening of the CART gene in obese children: identifying a mutation (Leu34Phe) associated with reduced resting energy expenditure and cosegregating with obesity phenotype in a large family. Diabetes. 2001;50:2157–60. doi: 10.2337/diabetes.50.9.2157. [DOI] [PubMed] [Google Scholar]
- 49.Yamada K, Yuan X, Otabe S, Koyanagi A, Koyama W, Makita Z. Sequencing of the putative promoter region of the cocaine- and amphetamine-regulated-transcript gene and identification of polymorphic sites associated with obesity. Int J Obes Relat Metab Disord. 2002;26:132–6. doi: 10.1038/sj.ijo.0801848. [DOI] [PubMed] [Google Scholar]
- 50.Balkan B, Koylu E, Pogun S, Kuhar MJ. Effects of adrenalectomy on CART expression in the rat arcuate nucleus. Synapse. 2003;50:14–9. doi: 10.1002/syn.10213. [DOI] [PubMed] [Google Scholar]
- 51.Balkan B, Koylu EO, Kuhar MJ, Pogun S. The effect of adrenalectomy on cocaine and amphetamine-regulated transcript (CART) expression in the hypothalamic nuclei of the rat. Brain Res. 2001;917:15–20. doi: 10.1016/s0006-8993(01)02899-2. [DOI] [PubMed] [Google Scholar]
- 52.Damaj MI, Hunter RG, Martin BR, Kuhar MJ. Intrathecal CART (55-102) enhances the spinal analgesic actions of morphine in mice. Brain Res. 2004;1024:146–9. doi: 10.1016/j.brainres.2004.07.058. [DOI] [PubMed] [Google Scholar]
- 53.Damaj MI, Martin BR, Kuhar MJ. Antinociceptive effects of supraspinal rat cart (55-102) peptide in mice. Brain Res. 2003;983:233–6. doi: 10.1016/s0006-8993(03)03094-4. [DOI] [PubMed] [Google Scholar]
- 54.Ohsawa M, Dun SL, Tseng LF, Chang J, Dun NJ. Decrease of hindpaw withdrawal latency by cocaine- and amphetamine-regulated transcript peptide to the mouse spinal cord. Eur J Pharmacol. 2000;399:165–9. doi: 10.1016/s0014-2999(00)00374-5. [DOI] [PubMed] [Google Scholar]
- 55.Baranowska B, Wolinska-Witort E, Chmielowska M, Martynska L, Baranowska-Bik A. Direct effects of cocaine-amphetamine-regulated transcript (CART) on pituitary hormone release in pituitary cell culture. Neuroendocrinol Lett. 2003;24:224–6. [PubMed] [Google Scholar]
- 56.Larsen PJ, Seier V, Fink-Jensen A, Holst JJ, Warberg J, Vrang N. Cocaine- and amphetamine-regulated transcript is present in hypothalamic neuroendocrine neurones and is released to the hypothalamic-pituitary portal circuit. Journal of Neuroendocrinology. 2003;15:219–26. doi: 10.1046/j.1365-2826.2003.00960.x. [DOI] [PubMed] [Google Scholar]
- 57.Kuriyama G, Takekoshi S, Tojo K, Nakai Y, Kuhar MJ, Osamura RY. Cocaine-and Amphetamine-Regulated Transcript Peptide in the Rat Anterior Pituitary Gland Is Localized in Gonadotrophs and Suppresses Prolactin Secretion. Endocrinology. 2004;145:2542–50. doi: 10.1210/en.2003-0845. [DOI] [PubMed] [Google Scholar]
- 58.Louis JCM. Methods of preventing neuron degeneration and promoting neuron regeneration. Amgen, International Patent Application. 1996 Publication #WO96/34619. [Google Scholar]
- 59.Xu Y, Zhang W, Klaus J, Young J, Koerner I, Sheldahl LC, et al. Role of cocaine-and amphetamine-regulated transcript in estradiol-mediated neuroprotection. Proc Natl Acad Sci U S A. 2006;103:14489–94. doi: 10.1073/pnas.0602932103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iliff JJ, Alkayed NJ, Gloshani KJ, Traystman RJ, West GA. Cocaine- and amphetamine-regulated transcript (CART) peptide: a vasoactive role in the cerebral circulation. J Cereb Blood Flow Metab. 2005;25:1376–85. doi: 10.1038/sj.jcbfm.9600136. [DOI] [PubMed] [Google Scholar]
- 61.Scruggs P, Dun SL, Dun NJ. Cocaine- and amphetamine-regulated transcript peptide attenuates phenylephrine-induced bradycardia in anesthetized rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1496–503. doi: 10.1152/ajpregu.00183.2003. [DOI] [PubMed] [Google Scholar]
- 62.Matsumura K, Tsuchihashi T, Abe I. Central human cocaine- and amphetamine-regulated transcript peptide 55-102 increases arterial pressure in conscious rabbits. Hypertension. 2001;38:1096–100. doi: 10.1161/hy1101.092968. [DOI] [PubMed] [Google Scholar]
- 63.Jaworski J, Hansen ST, Kuhar MJ, Mark GP. Intra-accumbal Injection of Cocaine- and Amphetamine-Regulated Transcript Peptide Alters Cocaine Self-Administration. Abstracts of the Society for Neuroscience. 2006:195.9. [Google Scholar]