Abstract
In recent years, the roles of chronic stress and depression as an independent risk factor for decreased insulin sensitivity and the development of diabetes have been increasingly recognized. However, an understanding and the mechanisms linking insulin resistance and acute psychological stress are very limited. We hypothesized that acute psychological stress may cause the development of insulin resistance, which may be a risk factor in developing type 2 diabetes. We tested the hypothesis in a well-established mouse model using 180 episodes of inescapable foot shock (IES), followed by a behavioral escape test. In this study, mice that received IES treatment were tested for acute insulin resistance by measuring glucose metabolism and insulin signaling. When compared to normal and sham mice, mice that were exposed to IES resulting in escape failure (defined as IES with behavioral escape failure) displayed elevated blood glucose levels in both glucose tolerance and insulin tolerance tests. Furthermore, mice with IES exposure and behavioral escape failure exhibited impaired hepatic insulin signaling via the insulin-induced insulin receptor/insulin receptor substrate 1/Akt pathway, without affecting similar pathways in skeletal muscle, adipose tissue and brain. Additionally, a rise in murine growth-related oncogene KC/GRO was associated with impaired glucose metabolism in IES mice, suggesting a mechanism by which psychological stress by IES may influence glucose metabolism. The present results indicate that psychological stress induced by IES can acutely alter hepatic responsiveness to insulin and affect whole-body glucose metabolism.
Keywords: Psychological stress, Inescapable foot shocks, Insulin signaling, Glucose metabolism, Acute insulin resistance
Introduction
Population-based surveys have found the prevalence of depression is 8%-25% in diabetic patients and up to 40%–80% in diabetic patients with complications (Peyrot & Rubin, 1997, Katon et al. 2004, Sullivan et al. 2012). Symptomatic depressed patients have a higher incidence of hyperinsulinemia and hyperglycemia (Lewis et al. 1983, Winokur et al. 1988, Chen et al. 2007, Hung et al. 2007), which are characteristic of an insulin resistant state, defined as a decreased response to insulin (Lillioja et al. 1988, Shulman 2000, Pessin & Saltiel 2000, Saltiel & Kahn 2001). Insulin resistance can occur through multiple mechanisms, including a change of insulin receptor (IR) number or function or defects in insulin signaling pathways (Lillioja et al. 1988, Shulman 2000, Pessin & Saltiel 2000, Saltiel & Kahn 2001). Insulin resistance is present not only in peripheral tissues such as the liver, muscle and adipose but also in brain where central actions of insulin include regulation of food intake, body weight, learning and memory (Zhao et al. 2001, Zhao et al. 2004). Following insulin binding to its receptor, endogenous tyrosine kinase is activated, resulting in the phosphorylation of insulin receptor substrates (IRS) and the activation of cytosolic proteins, including phosphatidylinositol 3-kinase (PI3K). Activated PI3K phosphorylates/activates its substrates involved in glucose metabolism, such as glycogen synthase kinase-3 (GSK-3) and p70 S6 kinase (Pessin & Saltiel 2000, Saltiel & Kahn 2001). There are multiple proposed pathophysiological mechanisms for the association between chronic psychological stress or depression and insulin resistance or diabetes (Lewis et al. 1983, Winokur et al. 1988, Peyrot & Rubin, 1997, Katon et al. 2004, Chen et al. 2007, Hung et al. 2007, Sullivan et al. 2012). One mechanism involves hyperstimulation of the hypothalamic-pituitary-adrenal (HPA) axis due to stress or depression (Heim et al. 2008, Guerry & Hastings 2011). Another includes increased proinflammatory cytokines, including interleukin-1β (IL-1β), IL-6 and tumor necrosis factor α (TNFα), which can be elevated in patients with depression in the absence of obvious inflammatory diseases (Miller et al. 2009, Shelton et al. 2011, Soczynska et al. 2011). Chronically increased glucocorticoid levels or a systemic inflammatory state can result in insulin resistance (Goldberg 2009, Bardini et al. 2010, Solomon et al. 2010). Several antidepressants have anti-inflammatory effects, which may augment their clinical efficacy in patients with major depressive disorder (Song et al. 2009, De et al. 2010, Warner-Schmidt et al. 2011), while others can cause or exacerbate insulin resistance and diabetes (Andersohn et al. 2009, Kivimaki et al. 2004). Thus, in patients with depression it can be difficult to discern whether the depression caused the inflammatory state or whether the heightened inflammatory state contributed to the depression. Since insulin is anti-inflammatory (Jeschke et al. 2004, Bortoff et al. 2010) it can be difficult to determine whether the insulin resistance was caused by inflammation or the insulin resistant state contributed to the heightened inflammatory state.
Psychological stress can interfere with carbohydrate metabolism, especially in the liver and skeletal muscle, and can lead to insulin resistance, but the mechanisms remain unclear (Depke et al. 2008). Most studies have included models of chronic psychological stress, with few studies on the effects of acute psychological stress on metabolic dysfunction and insulin resistance (Depke et al. 2008, Tamashiro et al. 2011, Finger et al. 2012).
We hypothesized that acute psychological stress could induce the acute development of insulin resistance which was tested in an established animal model of psychological stress consisting of 180 inescapable foot shocks (IES) followed by a behavioral escape test. Our results indicate that acute psychological stress induces impaired glucose metabolism and acute hepatic insulin resistance, concomitant with increased murine growth-related oncogene (KC/GRO), a human IL-8 homolog.
Methods
Animals and acute psychological stress induced by inescapable shock (IES)
Experiments were performed in accordance with the guidelines of the Care and Use of Laboratory Animals and the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. Adult male C57BL/6 mice (10–12 weeks old, 23.3 ± 2.8 g, Fredrick Cancer Research, Fredrick, MD) were housed 2 per cage with free access to chow and water in a 12-hour light/dark cycle animal facility for at least one week before the experiments. Mice were not fasted unless indicated.
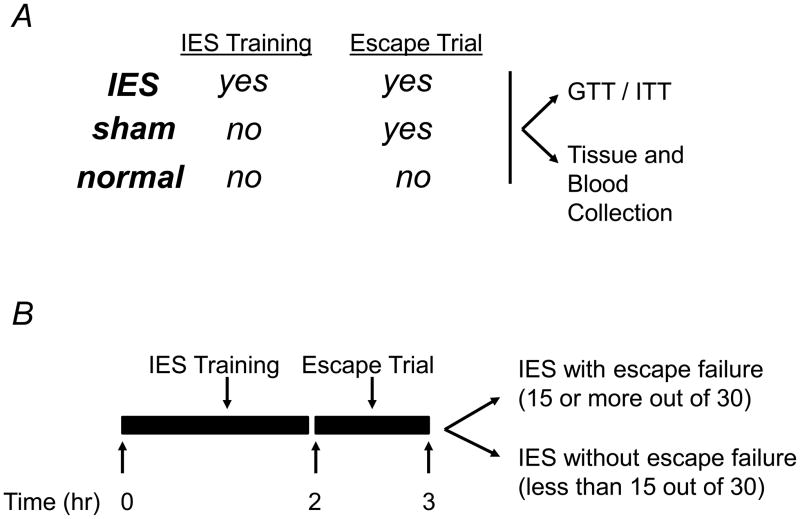
A murine model of inescapable foot shock (IES), similar to that previously described (Anisman & Merali 2001, Caldarone et al. 2003), was used in the present study to induce acute psychological stress. The procedure consisted of 180 IES followed by a behavioral escape test. On the experimental day, mice were placed in one side of a Gemini avoidance system shuttle box (San Diego Instruments, San Diego, CA) with the gate between the chambers closed. Electrical foot shocks were delivered 180 times at an amplitude of 0.3 mA, a duration of 3–5 sec per shock, and a randomized inter-shock interval of 5–45 sec. Sham mice were exposed to the same chamber for the same time, but without foot shocks (Fig. 1A). The IES or sham training was immediately followed by the behavioral escape test (Fig. 1B), in which the IES-treated and sham mice were returned to the shuttle box and given 30 escape trials. Each escape trial consisted of an electrical foot shock (0.3 mA) for 24 sec, with a fixed inter-shock interval of 30 sec. At the time of each foot shock, the chamber door opened to allow mice to escape. Latency to escape from the shock was recorded using Gemini software, and trials in which a mouse did not escape within the 24 sec time limit were counted as escape failures. Most IES-treated mice had 15 or more failures out of the 30 escape trials and were assigned to IES with escape failure group (Fig. 1B). A small fraction of IES-treated mice successfully escaped in more than 15 of the 30 escape trials, and were assigned to IES-treated but without escape failure group (Fig. 1B). In addition to IES-treated and sham mice, normal mice which were not placed in the foot shock apparatus, were also studied to control for any potential effect of the foot shock apparatus or behavioral testing (Fig. 1A).
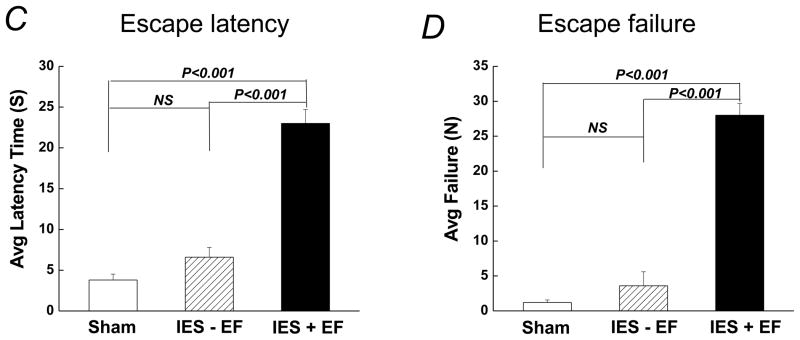
Figure 1. Inescapable foot shocks (IES)-induced behavioral changes in mice.
(A) Animal groups included in the study and different treatments. (B) Schedule for the IES training, escape test, blood and tissue collection, and insulin/glucose tolerance tests. (C and D) Mice were tested for behavioral changes, which was assessed by calculating escape latency and escape failure. Mice that were exposed to IES and had 15 or more than 15 out of the 30 escape trials were IES with escape failure group (IES+EF), had less than 15 out of the 30 escape trials were IES without escape failure group (IES−EF). There are 16 mice in sham group, 4 mice in IES without escape failure group (IES−EF group) and 20 mice in IES with escape failure group (IES+EF group).
Tissue and blood harvesting procedures
Immediately following the behavioral escape test, insulin (5 U/kg in 100 μl saline, Sigma-Aldrich, St. Louis, MO) or saline was delivered via intraperitoneal injection. Twenty minutes after the injection, mice were sacrificed by decapitation. Just before tissue collection, blood was collected after decapitation from the trunk into dry tubes, and centrifuged at 5,000 × g for 15 min. The plasma was stored at −80°C for later analysis. The peripheral tissues including the liver, epididymal adipose tissue and triceps were removed and quickly frozen in liquid nitrogen for future analysis. The brain tissues including hypothalamus, amygdala and hippocampus were removed, which was followed by immediate protein preparation. The hippocampus and amygdala are known to be important in emotional regulation and hypothalamus is critical for the hypothalamic-pituitary-adrenal (HPA) axis. For consistency, mice were sacrificed in the morning at approximately 11:30 am.
Tissue extraction and immunoblotting analysis
Liver, adipose tissue and triceps from each animal were homogenized in extraction buffer, as described previously, and stored at−80°C until use (Ma et al, 2003, Li et al, 2009, Williams et al. 2012). The brain tissues were homogenized in ice-cold lysis buffer containing 10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 0.5% NP-40, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 5 μg/ml pepstatin A, 0.1 mM β-glycerophosphate, 1 mM phenylmethanesulfonyl fluoride, 1 mM sodium vanadate, and 100 nM okadaic acid. The lysate was collected after the homogenate was centrifuged at 20,000 × g for 10 min to remove insoluble debris (Zhou et al. 2011). The protein concentrations of tissue lysates were determined by the BCA method (Pierce, Rockford, IL).
For immunoblotting, protein was separated by SDS-PAGE and analyzed by specific antibodies including anti-phospho threonine (PT) 308-AKT, anti-phospho serine (PS) 473-AKT, anti-phospho tyrosine (PY) 972-IR, anti-phospho tyrosine (PY) 612- IRS1, and either total-AKT, total-IR, total-IRS1 or total-extracellular signal-regulated kinases (ERK, Cell Signaling, Danvers, MA) as loading controls. Western blot results are representative of at least three independent experiments with similar results. The Precision Plus protein kaleidoscope standards were used to indicate the molecular weight of the loaded proteins (Bio-Rad Laboratories. Inc, Hercules, CA)
Glucose tolerance and insulin tolerance tests
A separate group of mice were used to perform glucose tolerance and insulin tolerance tests. Mice that were used for insulin tolerance test (ITT) were fasted for 4 h. Mice that were treated with the IES and exhibited 15 or more failures out of the 30 escape trials were assigned to IES with escape failure group (Fig. 1A and B). Basal blood glucose levels (0 min) were measured followed by injection of insulin (2 U/kg) into the peritoneum, and blood glucose levels were measured at 15, 30, 60, 90 and 120 min by portable glucose meter (Abbott Diabetes Care Inc., Alameda, CA) using tail vein blood.
Mice that were subjected to glucose tolerance test (GTT) were fasted overnight (16 h). Mice that were treated with the IES and had 15 or more failures out of the 30 escape trials were assigned to IES with escape failure group (Fig. 1A and B). Basal levels of glucose (0 min) were measured from the tail vein, followed by injection of glucose (1 g/kg) into the peritoneum. Blood glucose levels were measured at the same time points as in the ITT above. Total area under the curves (AUC) in response to glucose or insulin administration was calculated using Sigmaplot software (Creation Engine Inc., Mountain View, CA).
Measurement of cytokines levels and corticosterone levels
The plasma levels of IL-1β, IL-6, IL-10, IL-12, TNFα, interferon gamma (IFNγ), and KC/GRO were measured using a Meso Scale Discovery multiplex spot assay and analyzed with MSD Discovery Workbench software (Meso Scale Discovery, Gaithersburg, MD). The levels of corticosterone were determined via a RIA kit (MP Biomedicals, Orangeburg, NY).
Densitometric and statistical analysis
Enhanced chemiluminescent images of immunoblots were scanned and quantified using Zero D-Scan (Scanalytics Corp., Fairfax, VA). All data are presented as means ± standard error (S.E). The data were analyzed by t-test or one-way ANOVA (a normality test was performed before one-way ANOVA) using the InStat statistical program by GraphPad Software, Inc (San Diego, CA). Values of P<0.05 were considered significant.
Results
Effects of acute psychological stress on mouse behaviors
The IES-treated mice were divided into two groups based on the number of escape failures out of the 30 escape trials, i.e. IES with escape failure (failed more than 15 out of the 30 escape trials) and IES without escape failure (failed less than 15 out of 30 escape trials; Fig. 1B). Compared to the sham group, the IES with escape failure group showed a longer escape latency (22.9 ± 1.0 sec vs. 3.7 ± 0.7 sec, Fig. 1C) and a much higher number of escape failures (27.8 ± 2.2 in IES with escape failure mice vs. 1.2 ± 0.4 in sham mice, Fig. 1D). In contrast, escape latency and escape failures in mice exposed to the IES but learned to escape (i.e., IES without escape failure group) were not statistically different from the sham group in escape latency and statistically shorter than the IES with escape failure group (Fig. 1 C and D).
Decreased insulin signaling in the liver following acute psychological stress
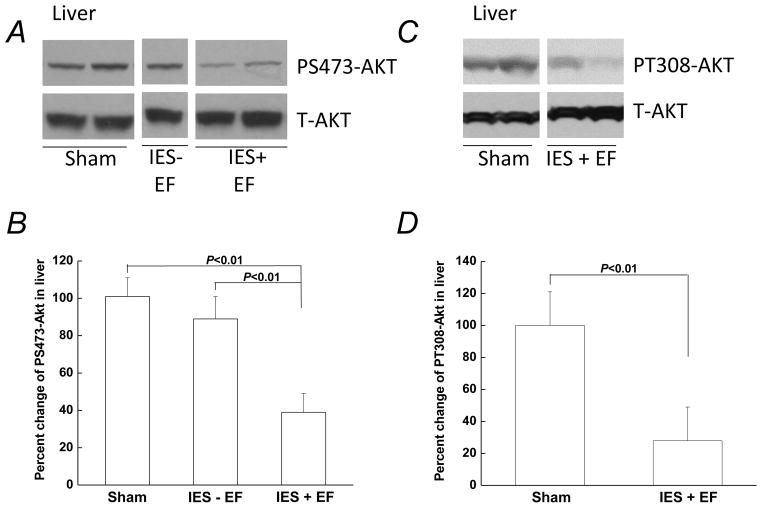
Experiments were then performed to measure any changes in insulin signaling in mice following acute psychological stress induced by IES. Basal AKT phosphorylation, without exogenous insulin injection, was too low to be detected (data not shown). Twenty minutes after insulin injection (5 U/kg), compared with basal AKT phosphorylation, there was an increase in the phosphorylation of serine 473 of AKT (PS473-AKT; Fig. 2A) in all three groups including sham, IES without escape failure, and IES with escape failure. However, when the induction of PS473-AKT by exogenous insulin was measured, the response in mice exposed to IES with escape failure was significantly less compared to sham mice (Fig. 2B). When mice exposed to IES with escape failure were compared with IES mice without escape failure, the insulin-induced PS473-AKT was also significantly lower (Fig. 2B). The IES without escape failure group was smaller in number since this was a relatively rare occurrence (4 out of 24 mice exposed to IES resulted in no escape failure). Although efforts were made attempting to increase the size of this particular group, it was unsuccessful. Considering the relatively small number of mice in the IES without escape failure group, this group was not systematically studied. The phosphorylation of threonine 308 in AKT (PT308-AKT), another insulin-responsive AKT site of phosphorylation, was found to be similarly decreased in IES with escape failure mice (Fig. 2C and D). The reduced responses of PS473-AKT and PT308-AKT to exogenous insulin indicate an impairment of hepatic insulin signaling in the IES with escape failure group.
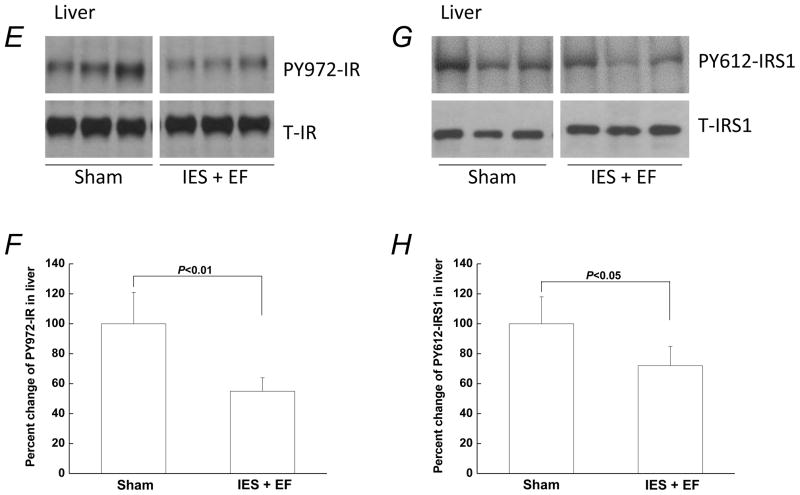
Figure 2. Decreased insulin signaling following acute psychological stress in the liver.
Mice that were not exposed to IES but had escape test are sham group (sham). At the end of the behavioral test, 5U/kg insulin was administered via intraperitoneal injection and the liver was removed 20 min after the insulin injection. Tissue lysates, 30 μg per lane, were subjected to Western blot analysis with antibodies specific for phospho-serine (PS) 473-AKT, phospho-threonine (PT) 308-AKT, total AKT, phospho-tyrosine (PY)-IRS1, total IRS1, PY-IR, or total IR. Representative Western blots are presented in A, C, E and G. (B, D, F and H) Autoradiographs from different groups (corresponding to A, C, E and G, respectively) were quantified by scanning densitometry. The data are presented as the mean ± S.E of 4 mice in IES without escape failure group (IES-EF group), 10 mice in IES with escape failure group (IES+EF group) and 10 mice in sham group. The phosphorylated protein levels in the sham group were arbitrarily set to 100%. White gap in A, C, E and G indicates grouping of lanes from different parts of the same gel.
Alterations of AKT phosphorylation may be due to changes in the activation of upstream components of the insulin signaling pathway. To test this possibility, experiments were performed to determine any changes of activating tyrosine phosphorylation of the IR and IRS1 in liver. Phosphorylation of IR tyrosine 972 (PY972-IR) and phosphorylation of IRS1 tyrosine 612 (PY612-IRS1) are critical for the activation of insulin-induced IR/IRS1/PI3K/AKT pathway (Pessin & Saltiel 2000, Shulman 2000, Saltiel & Kahn 2001). When examined in the liver, insulin-induced PY972-IR and PY612-IRS1 were decreased following acute psychological stress in IES mice with escape failure (Fig. 2E–H).
Insulin-induced P-AKT in skeletal muscle and adipose tissue was unchanged following acute psychological stress
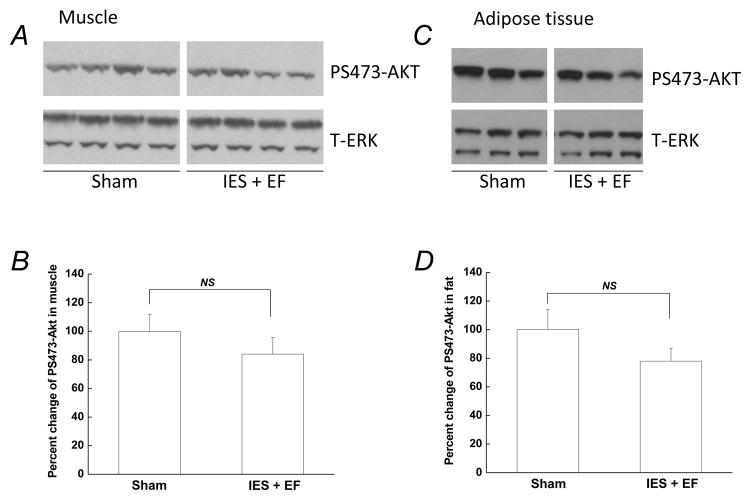
Since skeletal muscle and adipose tissue are two other important insulin target tissues, and account for over 80% of insulin-induced glucose disposal (DeFronzo et al. 1981), insulin signaling was also examined in skeletal muscle and adipose tissue to determine if they also become insulin resistant following acute psychological stress. Although there were decreased levels of insulin-induced PS473-AKT in the IES with escape failure mice in skeletal muscle and adipose tissue, the changes were not significant compared with that in sham mice (Fig. 3 A–D). This indicates that an acute insulin resistant state was not present in skeletal muscle and adipose tissue following acute psychological stress.
Figure 3. Insulin-induced phosphorylation of AKT was not affected by acute psychological stress in skeletal muscle and adipose tissue.
At the end of the behavioral test, 5U/kg insulin was injected and skeletal muscle and adipose tissue were removed 20 min after injection. Tissue lysates, 30 μg per lane, were subjected to Western blotting with antibodies specific for PS473-AKT or total ERK. Representative Western blots are presented in A and C. (B and D) Autoradiographs were quantified by scanning densitometry and the data are presented as the mean ± S.E of 10 mice in IES with escape failure group (IES+EF group) and 10 mice in sham group. The phosphorylated protein level in the sham group was arbitrarily set to 100%. White gap in A and C indicates grouping of lanes from different parts of the same gel.
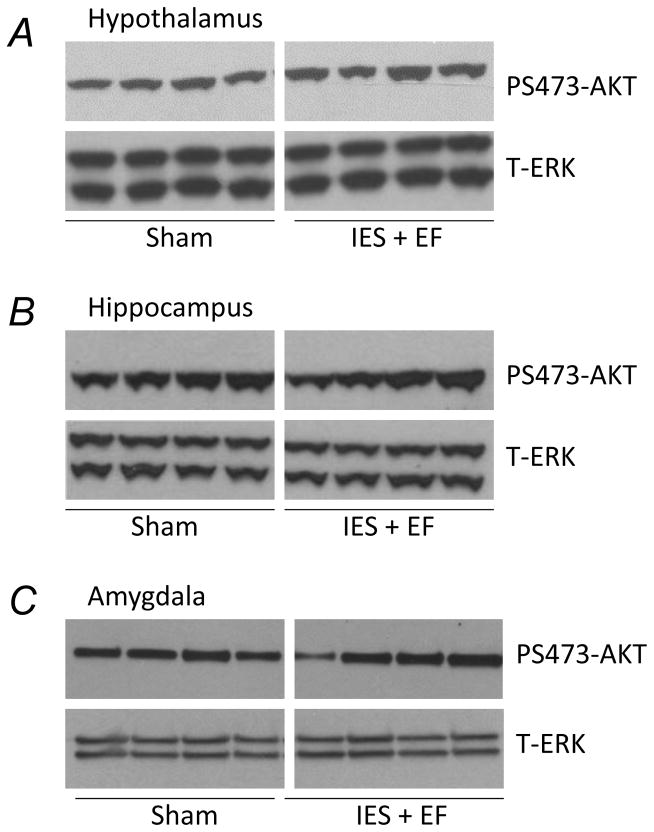
Insulin-induced P-AKT in the hippocampus, hypothalamus and amygdala was unchanged following acute psychological stress
The insulin receptor is widely expressed in the central nervous system. The effects of IES on insulin signaling were therefore measured in several insulin receptor containing areas of the mouse brain, including the hypothalamus, hippocampus and amygdala. There were no obvious changes of insulin-induced PS473-AKT in these areas after IES exposure (Fig. 4A–C). Total ERK (T-ERK) was used as a loading control.
Figure 4. Effects of acute psychological stress on the insulin signaling in central nervous system.
At the end of the behavioral test, 5U/kg insulin was injected and 20 min later the hypothalamus, hippocampus and amygdala were removed. Tissue lysates, 20 μg per lane, were subjected to Western blot analysis with antibodies specific for PS473-AKT or total ERK. There were 10 mice in IES with escape failure group (IES+EF group) and 10 mice in sham group. Representative Western blots are presented in A, B and C. White gap in A, B and C indicates grouping of lanes from different parts of the same gel.
Acute psychological stress impairs glucose tolerance and insulin sensitivity
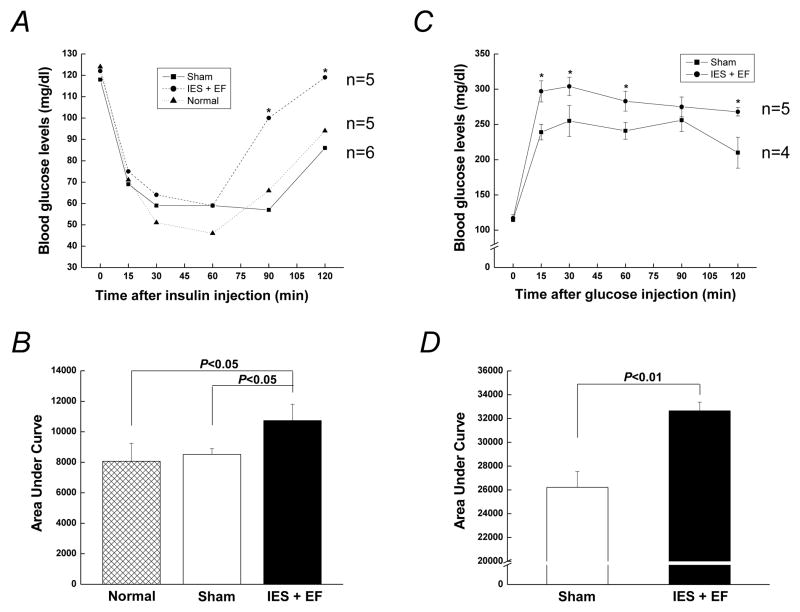
We next tested if acute psychological stress induced by IES could affect whole body glucose homeostasis. For insulin tolerance tests, exogenous insulin was injected immediately following the behavioral tests (Fig. 1A). Blood glucose levels dropped similarly upon insulin injection in the three groups including normal, sham and IES with escape failure mice (Fig. 5A and B). However, blood glucose levels in the IES with escape failure mice rose significantly after 60 min following the insulin challenge, while the normal and sham mice increased more slowly. Incremental area under the curve (iAUC, concentration × time) for glucose throughout the insulin tolerance test was calculated (Fig. 5B), and the iAUC in IES with escape failure mice was greater than that in either normal or sham mice, which were indistinguishable from each other. This suggests the sham protocol was not sufficiently stressful to alter whole body insulin sensitivity, compared to normal mice. Thus, normal animals were not further pursued for the other analysis. More importantly, insulin sensitivity in the IES mice with escape failure was reduced compared with normal and sham animals, indicating the presence of impaired insulin action in this group.
Figure 5. Effects of acute psychological stress on insulin sensitivity and glucose disposal.
(A) Mice were fasted 4 h for insulin sensitivity tests and 2U/kg insulin was administered through intraperitoneal injection. (C) For glucose tolerance tests, mice were fasted 16 h and 1g/kg glucose was administered through intraperitoneal injection. Data are presented as the mean ± S.E in each group. (B and D) Bar graphs of the area under the curve of blood glucose levels in insulin tolerance tests and glucose tolerance tests, respectively. Animal numbers in each group were indicated in the figures. IES+EF, IES with escape failure group.
Whole body glucose tolerance in response to acute psychological stress was performed next. Immediately after glucose injection, there was a greater increase of glucose levels in the IES with escape failure mice compared to the sham mice (Fig. 5C). Glucose levels in both IES with escape failure and sham groups reached the peak by 30 min and glucose levels in the IES with escape failure mice were higher than those in the sham animals at almost every time point. The iAUC curve in the IES with escape failure mice was significantly greater than sham group (Fig. 5D). Thus, compared with sham mice, mice exposed to IES with escape failure had impaired glucose tolerance.
Analysis of serum inflammatory markers and corticosterone
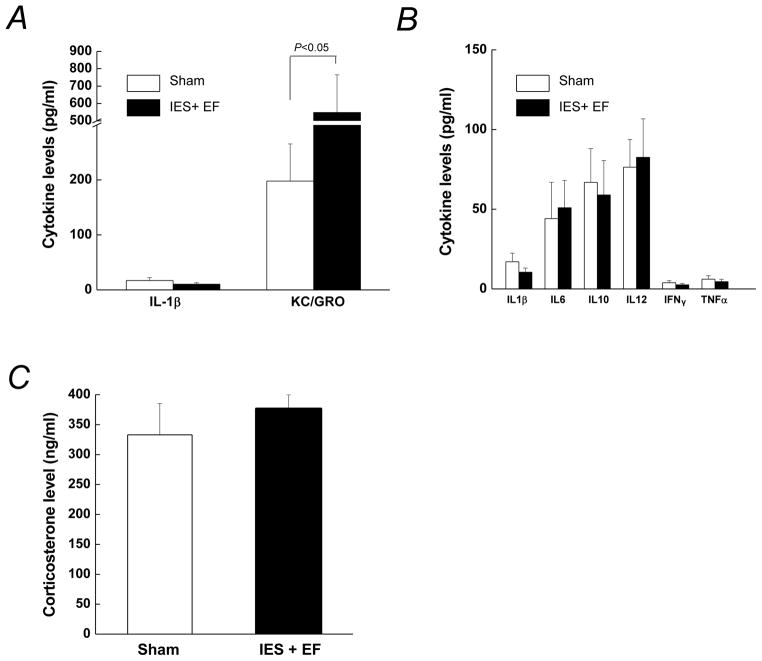
Inflammation and increased glucocorticoids (corticosterone is the main corticosteroid in rodents) are thought to contribute to the development of insulin resistance, and inflammatory markers are induced after various stresses (Pessin & Saltiel 2000, Saltiel & Kahn 2001, Heim et al. 2008, Miller et al. 2009, Guerry & Hastings 2011, Shelton et al. 2011, Soczynska et al. 2011). However, it is not known whether glucocorticoids and inflammatory markers would be increased rapidly enough to contribute to the acute insulin resistance that occurs shortly after the IES. Thus, we measured several cytokines that increase in inflammatory states. In IES with escape failure mice, serum levels of KC/GRO were increased compared to that in sham mice (Fig. 6A). The levels of other inflammatory markers including TNFα, IFNγ, IL-1β, IL-6, IL-10 and IL-12 were not affected by acute psychological stress in mice (Fig. 6B). The levels of corticosterone were also measured, and their levels were not significantly induced in the IES with escape failure mice compared to sham mice (Fig. 6C).
Figure 6. Plasma levels of inflammatory cytokines and corticosterone.
The mice were sacrificed at the end of the escape test and blood was collected for the determination of plasma inflammatory markers levels and corticosterone levels. Each value represents the mean ± S.E of 10 mice in IES with escape failure group (IES+EF group) and 10 mice in sham group.
Discussion
Recent findings indicate that psychological stress and depression are independent risk factors for new-onset type 2 diabetes (Eaton et al. 1996, Peyrot & Rubin 1997, Katon et al. 2004, Campayo et al. 2011, Sullivan et al. 2012). Depression is associated with significantly decreased insulin sensitivity, which predisposes the depressed individuals to diabetes (Peyrot & Rubin 1997, Katon et al. 2004, Sullivan et al. 2012). Neuroimaging studies in depressed patients using positron emission tomography found regional alterations in brain metabolic activity at rest and following emotional stress (Germain et al. 2007). However, the cellular mechanisms explaining the vulnerability of patients with depression to glucose dysregulation or the increased development of type 2 diabetes have been inadequately explored. In addition, almost nothing is known about the development of insulin resistance that occurs following acute psychological stress. The present study demonstrated the development of acute insulin and glucose intolerance with impairment of hepatic insulin signaling in a widely used animal model of acute psychological stress.
Acute insulin resistance occurred in the liver, but not in adipose tissue and skeletal muscle, following acute psychological stress. There are a few possibilities for this differential development of acute insulin resistance in insulin target tissues. First, the present studies may indicate that the effects of acute psychological stress may only affect the liver. There is a well-known vagal control of liver metabolism (Li et al. 2009, Li et al. 2011) which may be activated by the stress of IES and it is possible that the liver is the only tissue affected. However, the present study only examined a single time point, in which mice were sacrificed immediately after the IES exposure and behavioral tests. Acute insulin resistance in adipose tissue and skeletal muscle may take more time to develop. Similar to adipose tissue and skeletal muscle, acute insulin resistance did not develop following acute psychological stress in the hippocampus, amygdala and hypothalamus at the time point studied. It is not known whether insulin resistance will develop in these brain regions, but if it does it may take longer to develop or require more chronic psychological stress. Thus, further research may be needed to determine if insulin resistance in adipose tissue, skeletal muscle and specific areas of the brain develops at other time points following acute psychological stress.
The HPA axis is highly responsive to physical and psychological stress (Heim et al. 2008, Guerry & Hastings 2011) and is well known to play a role in the development of insulin resistance (Li et al. 2009, Ursache et al. 2012). However, the data is contradictory concerning the role of the HPA activity in psychological stress, with some studies reporting increased activation upon psychological stress, and others reporting the opposite (Li et al. 2009, Licht et al. 2010, Ursache et al. 2012). One meta-analysis showed that much of the variability is attributable to experimental conditions and especially the timing, which is a critical element since HPA activity can increase rapidly following stress onset, but can also decrease over time (Miller et al. 2007). In the present work, corticosterone levels were not significantly increased at the time point measured following the IES treatment. Again, only one time point was measured so the HPA may be activated at other time points following psychological stress, but from our current data we think it is unlikely that the HPA plays a significant role in the acute development of hepatic insulin resistance.
The association between inflammation and insulin resistance has been extensively documented, but the exact role that inflammation plays in the development of insulin resistance is highly variable, and the underlying mechanisms are not fully understood (Hung et al. 2007, Xu et al. 2008, Goldberg 2009, Bardini et al. 2010, Solomon et al. 2010). In this study, murine KC/GRO, a member of the chemoattractant cytokine family that includes human IL-8, was elevated in IES mice with escape failure. In humans plasma IL-8 or IL-8 gene expression is elevated in patients with mood disorders compared to healthy controls (Marland et al. 2007, Shelton et al. 2011). Elevated KC/GRO or IL-8 levels are also associated with insulin resistance, particularly in obese individuals (Bremer et al. 2011, Jiala et al. 2012). Our data indicate that KC/GRO or IL-8, and possibly other inflammatory markers not yet studied, may be markers of acute psychological stress, with the possibility that KC/GRO or IL-8 could be a link associating acute psychological stress with impaired insulin sensitivity. Conversely, our study did not find an increase in TNFα and IL-6, suggesting that they may not contribute to the development of acute insulin resistance following acute psychological stress (at least at the time point tested) as has been indicated in patients with depression.
Little is known about the acute behavioral and metabolic effects soon after IES, and our study is the first to indicate an association between acute psychological stress and acute impairment of insulin sensitivity. However, future experiments will need to be performed to determine how long the acute hepatic insulin resistance lasts following IES exposure as well as to examine a dynamic change of corticosterone levels and cytokine levels. In our study, the overwhelming majority of IES treated mice presented with increased escape latency and escape failure. These IES with escape failure mice also developed impaired insulin and glucose tolerance as well as decreased hepatic insulin signaling. A minority of the mice did not develop escape deficits following IES and did not develop insulin resistance. Thus, a minority of mice seem to be more resilient and may be similar to those humans who maintain relatively normal glucose metabolism in stressful situations. However, these results should be interpreted with caution because of the small sample size. A much larger beginning sample size will need to be obtained, to allow for collection of a significant number of mice following IES who do not develop escape failure so they can be properly studied.
In conclusion, we have characterized the effects of the acute psychological stress, using an established animal model of the IES, and found an acute development of insulin and glucose intolerance and hepatic insulin resistance. The information gained from these initial studies suggest that a better understanding of the initial steps in the association between acute psychological stress and glucose metabolism need to be pursued.
Acknowledgments
This research is supported by intramural grant from the department of Psychiatry and Behavioral Neurobiology to L. Li, and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-62071 and Veterans Administration Merit Review to J. L. Messina. We thank Drs. Richard Shelton, Rita Cowell, James Meador-Woodruff and Robert McCullumsmith for scientific advice, and the laboratory members in Drs. Xiaohua Li’s and Joseph Messina’s labs at the University of Alabama at Birmingham for technical support.
Footnotes
Financial Disclosures:
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- 1.Andersohn F, Schade R, Suissa S, Garbe E. Long-term use of anti-depressants for depression disorders and the risk of diabetes. American Journal of Psychiatry. 2009;166:591–598. doi: 10.1176/appi.ajp.2008.08071065. [DOI] [PubMed] [Google Scholar]
- 2.Anisman H, Merali Z. Rodent model of depression: Learned helplessness induced in mice. Current Protocols in Neuroscience. 2001;Chapter 8(Unit 8):10C. doi: 10.1002/0471142301.ns0810cs14. [DOI] [PubMed] [Google Scholar]
- 3.Bardini G, Dicembrini I, Cresci B, Rotella CM. Inflammation markers and metabolic characteristics of subjects with 1-h plasma glucose levels. Diabetes Care. 2010;33:411–413. doi: 10.2337/dc09-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bortoff KD, Keeton AB, Franklin JL, Messina JL. Anti-inflammatory action of insulin via induction of Gadd45-beta transcription by the mTOR signaling pathway. Hepatic Medicine. 2010;2:79–85. doi: 10.2147/HMER.S7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bremer AA, Devaraj S, Afify A, Jiala I. Adipose tissue dysregulation in patients with metabolic syndrome. Journal of Clinical Endocrinology & Metabolism. 2011;96:E1782–1788. doi: 10.1210/jc.2011-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campayo A, Gomez-Biel CH, Lobo A. Diabetes and depression. Current Psychiatry Reports. 2011;13:26–30. doi: 10.1007/s11920-010-0165-z. [DOI] [PubMed] [Google Scholar]
- 7.Caldarone BJ, Karthigeyan K, Harrist A, Hunsberger JG, Wittmack E. Sex differences in response to oral amitriptyline in three animal models of depression in C57BL/6J mice. Psychopharmacology (Berl) 2003;170:94–101. doi: 10.1007/s00213-003-1518-7. [DOI] [PubMed] [Google Scholar]
- 8.Chen YC, Shen YC, Hung YJ, Chou CH, Yeh CB. Comparisons of glucose-insulin homeostasis following maprotiline and fluoxetine treatment in depressed males. Journal of Affective Disorders. 2007;10:257–261. doi: 10.1016/j.jad.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 9.De BD, Conti CM, Serroni N, Moschetta FS, Olivieri L. The effect of newer serotonin-noradrenalin antidepressants on cytokine production: a review of the current literature. International Journal of Immunopathology and Pharmacology. 2010;23:417–422. doi: 10.1177/039463201002300204. [DOI] [PubMed] [Google Scholar]
- 10.DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 11.Depke M, Fusch G, Domanska G, Geffers R, Volker H. Hypermetabolic syndrome as a consequence of repeated psychological stress in mice. Endocrinology. 2008;149:2714–2723. doi: 10.1210/en.2008-0038. [DOI] [PubMed] [Google Scholar]
- 12.Eaton WW, Armenian H, Gallo J, Pratt L, Ford DE. Depression and risk for onset of type II diabetes. A prospective population-based study. Diabetes Care. 1996;19:1097–1102. doi: 10.2337/diacare.19.10.1097. [DOI] [PubMed] [Google Scholar]
- 13.Finger BC, Dinan TG, Cryan JF. The temporal impact of chronic intermittent psychosocial stress on high-fat diet-induced alterations in body weight. Psychoneuroendocrinology. 2012;37:729–741. doi: 10.1016/j.psyneuen.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Germain A, Nofzinger EA, Meltzer CC, Wood A, Kupfer DJ. Diurnal variation in regional brain glucose metabolism in depression. Biological Psychiatry. 2007;62:438–445. doi: 10.1016/j.biopsych.2006.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg RB. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. Journal of Clinical Endocrinology & Metabolism. 2009;94:3171–3182. doi: 10.1210/jc.2008-2534. [DOI] [PubMed] [Google Scholar]
- 16.Guerry JD, Hastings PD. In search of HPA axis dysregulation in child and adolescent depression. Clinical Child and Fam Psychology Review. 2011;14:135–160. doi: 10.1007/s10567-011-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heim C, Newport DJ, Meltzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies inn humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Hung YJ, Hsieh CH, Chen YJ, Pei D, Kuo SW. Insulin sensitivity, proinflammatory markers and adiponectin in young males with different subtypes of depressive disorder. Clinical Endocrinology (Oxf) 2007;67:784–789. doi: 10.1111/j.1365-2265.2007.02963.x. [DOI] [PubMed] [Google Scholar]
- 19.Jeschke MG, Klein D, Herndon DN. Insulin treatment improves the systemic inflammatory reaction to severe trauma. Annals of Surgery. 2004;239:553–560. doi: 10.1097/01.sla.0000118569.10289.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiala I, Huet BA, Kaur H, Chien A, Devaraj S. Increased toll-like receptor activity in patients with metabolic syndrome. Diabetes Care. 2012;35:900–904. doi: 10.2337/dc11-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katon W, von KM, Ciechanowski P, Russo J, Lin E. Behavioral and clinical factors associated with depression among individuals with diabetes. Diabetes Care. 2004;27:914–920. doi: 10.2337/diacare.27.4.914. [DOI] [PubMed] [Google Scholar]
- 22.Kivimaki M, Hamer M, Batty GD. Antidepressant medication use, weight gain and risk of type 2 diabetes: a population-based study. Diabetes care. 2010;33:2611–2616. doi: 10.2337/dc10-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis DA, Kathol RG, Sherman BM, Winokur G, Schlesser MA. Differentiation of depressive subtypes by insulin insensitivity in the recovered phase. Archives of General Psychiatry. 1983;40:167–170. doi: 10.1001/archpsyc.1983.01790020061005. [DOI] [PubMed] [Google Scholar]
- 24.Li JH, Gautam D, Han Sj, Guettier JM, Cui Y. Hepatic muscarinic acetylcholine receptors are not critically involved in maintaining glucose homeostasis in mice. Diabetes. 2009;58:2776–2787. doi: 10.2337/db09-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L, Thompson LH, Zhao L, Messina JL. Tissue-specific difference in the molecular mechanisms for the development of acute insulin resistance after injury. Endocrinology. 2009;150:24–32. doi: 10.1210/en.2008-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Wu X, Camacho R, Schwartz GJ, LeRoith D. Intracerebroventricular leptin infusion improves glucose homeostasis in lean type 2 diabetic MKR mice via hepatic vagal and non-vagal mechanisms. PLoS One. 2011;17:e17058. doi: 10.1371/journal.pone.0017058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Licht CM, Vreeburg SA, van Reedt Dortland AK, Giltay EJ, Hoogendijk WJ. Increased sympathetic and decreased parasympathetic activity rather than changes in hypothalamic-pituitary-adrenal axis activity is associated with metabolic abnormalities. Journal of Clinical Endocrinology & Metabolism. 2010;95:2458–2466. doi: 10.1210/jc.2009-2801. [DOI] [PubMed] [Google Scholar]
- 28.Lillioja S, Mott DM, Howard BV, Bennett PH, Yki-Jarvinen H. Impaired glucose tolerance as a disorder of insulin action. Longitudinal and cross-sectional studies in Pima Indians. New England Journal of Medicine. 1988;318:1217–1225. doi: 10.1056/NEJM198805123181901. [DOI] [PubMed] [Google Scholar]
- 29.Ma Y, Wang P, Kuebler JF, Chaudry IH, Messina JL. Hemorrhage induces the rapid development of hepatic insulin resistance. American Journal of Physiology: Gastrointestinal and Liver Physiology. 2003;284:G107–G115. doi: 10.1152/ajpgi.00217.2002. [DOI] [PubMed] [Google Scholar]
- 30.Marland AL, Sathanoori R, Muldoon MF, Manuck SB. Stimulated production of interleukin-8 with psychosocial risk factors for inflammatory disease among middle-aged community volunteers. Brain, Behavior and Immunity. 2007;21:218–228. doi: 10.1016/j.bbi.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biological Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 33.Pessin JE, Saltiel AR. Signaling pathways in insulin action: molecular targets of insulin resistance. Journal of Clinical Investigation. 2000;106:165–169. doi: 10.1172/JCI10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peyrot M, Rubin RR. Levels and risks of depression and anxiety symptomatology among diabetic adults. Diabetes Care. 1997;20:585–590. doi: 10.2337/diacare.20.4.585. [DOI] [PubMed] [Google Scholar]
- 35.Saltiel AR, Kahn CR. Insulin signaling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 36.Shelton RC, Clairborne J, Sidoryk-Negrzynowice M, Reddy R, Aschner M. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Molecular Psychiatry. 2011;16:751–762. doi: 10.1038/mp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shulman GI. Cellular mechanisms of insulin resistance. Journal of Clinical Investigation. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soczynska JK, Kennedy SH, Woldeyohannes HO, Liauw SS, Alsuwaidan M. Mood disorders and obesity: understanding inflammation as a pathophysiological nexus. Neuromolecular Medicine. 2011;13:93–116. doi: 10.1007/s12017-010-8140-8. [DOI] [PubMed] [Google Scholar]
- 39.Solomon SS, Odunusi O, Carrigan D, Majumdar G, Kakoola D. TNF-alpha inhibits insulin action in liver and adipose tissue: A model of metabolic syndrome. Hormone and Metabolic Research. 2010;42:115–121. doi: 10.1055/s-0029-1241834. [DOI] [PubMed] [Google Scholar]
- 40.Song C, Halbreich U, Han C, Leonard BE, Luo H. Imbalance between pro- and anti-inflammatory cytokines, and between Th1 and Th2 cytokines in depressed patients: the effect of electroacupuncture or fluoxetine treatment. Pharmacopsychiatry. 2009;42:182–188. doi: 10.1055/s-0029-1202263. [DOI] [PubMed] [Google Scholar]
- 41.Sullivan MD, O’Connor P, Feeney P, Hire D, Simmons DL. Depression predicts all-cause mortality: epidemiological evaluation from the ACCORD HRQL substudy. Diabetes Care. 2012;35:1708–1715. doi: 10.2337/dc11-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamashiro KL, Sakai RR, Shively CA, Karatsoreos IN, Rengan LP. Chronic stress, metabolism and metabolic syndrome. Stress. 2011;14:468–474. doi: 10.3109/10253890.2011.606341. [DOI] [PubMed] [Google Scholar]
- 43.Ursache A, Wedin W, Tirsi A, Convit A. Preliminary evidence for obesity and elevations in fasting insulin mediating associations between cortisol awakening response and hippocampal volumes and frontal atrophy. Psychoneuroendocrinology. 2012;37:1270–1276. doi: 10.1016/j.psyneuen.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P. Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proc Natl Acad Sci U S A. 2011;108:9262–9267. doi: 10.1073/pnas.1104836108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams VL, Martin RE, Franklin JL, Hardy RW, Messina JL. Injury-induced insulin resistance in adipose tissue. Biochemical and Biophysical Research Communications. 2012;421:442–448. doi: 10.1016/j.bbrc.2012.03.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winokur A, Maislin G, Phillips JL, Amsterdam Insulin resistance after oral glucose tolerance testing in patients with major depression. American Journal of Psychiatry. 1988;145:325–330. doi: 10.1176/ajp.145.3.325. [DOI] [PubMed] [Google Scholar]
- 47.Xu J, Kim HT, Ma Y, Zhao L, Zhai L. Trauma and hemorrhage-induced acute hepatic insulin resistance: dominant role of tumor necrosis factor-alpha. Endocrinology. 2008;149:2369–2382. doi: 10.1210/en.2007-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao WQ, Alkon DL. Role of insulin and insulin receptor in learning and memory. Molecular and Cellular Endocrinology. 2001;177:125–134. doi: 10.1016/s0303-7207(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 49.Zhao WQ, Chen H, Quon MJ, Alkon DL. Insulin and the insulin receptor in experimental models of learning and memory. European Journal of Pharmacology. 2004;490:71–81. doi: 10.1016/j.ejphar.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 50.Zhou W, Chen L, Yang S, Li F, Li X. Behavioral Stress-induced Activation of FoxO3a in the Cerebral Cortex of Mice. Biological Psychiatry. 2011;71:583–592. doi: 10.1016/j.biopsych.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]