SUMMARY
Malignant gliomas are one of the most treatment-refractory cancers. Development of resistance to chemo- and radio-therapies contributes to these tumors’ aggressive phenotypes. Elevated lipid levels in gliomas have been reported for the last 50 years. However, the molecular mechanisms of how tumor tissues obtain lipids and utilize them are not well understood. Recently, the oncogenic signaling EGFR/PI3K/Akt pathway has been shown to enhance lipid synthesis and uptake by upregulating SREBP-1, a master transcriptional factor, to control lipid metabolism. This article discusses the analytical chemistry results of lipid components in glioma tissues from different research groups. The molecular mechanisms that link oncogenes with lipid programming, and identification of the key molecular targets and development of effective drugs to inhibit lipid metabolism in malignant gliomas will be discussed.
Practice Points.
Unsaturated fatty acid levels are rich in human intracranial tumors.
Cholesterol esters are present in glioma tissues.
Phosphatidylcholine levels are high in gliomas.
In vivo magnetic resonance spectroscopy is capable of detecting lipid metabolism changes in gliomas.
Receptor tyrosine kinase/PI3K/Akt signaling upregulates SREBP-1-mediated fatty acid synthesis and cholesterol uptake.
Malignant gliomas are among the most lethal of human malignancies, and are highly resistant to chemo- and radio-therapies, contributing to the frequent recurrences 6–7 months after surgery [1,2]. In the past decade, significant progress has been made in treating gliomas. Patient overall survival was reported to closely correlate with the methylation status of O-6-methylguanine-DNA methyltransferase when patients were treated with radiation plus temozolomide [3,4]. With regard to tumor biology, oncogenic signaling pathways, such as receptor tyrosine kinase (RTK)/PI3K/Akt, and angiogenesis are hyperactivated in glioma patients [5]. However, targeting the RTK/PI3K/Akt/mTOR signaling pathway and blocking VEGF signaling by using its antibody bevacizumab have been shown to produce modest responses in this patient population [6–11]. Resistance to these targeted therapies is possibly derived from tumor heterogeneity or a redundant oncogenic signaling pathway in tumor cells [12]. To significantly enhance overall survival in glioma patients, it is essential to comprehensively understand the biology of malignant gliomas and identify the critical molecules involved in regulating tumor growth in order to develop more effective drugs to treat patients.
Recently, an emerging renaissance within the field of cancer metabolism has opened new windows for cancer treatment. Tumor cells have been revealed to reprogram their metabolic networks in order to satisfy their demand for rapid growth and division [13–15]. Enhanced glycolysis under normoxic conditions (the Warburg effect) and increased glutamine metabolism are the main characteristics of malignant tumors [16–19]. In addition, exacerbated lipogenesis has been demonstrated to be one of the main features of cancer [20], which has been shown to directly correlate with enhanced glucose and glutamine metabolism [21–23]. Taken together, growing evidence shows that metabolism reprogramming may play an important role in malignant tumor growth. Therefore, targeting altered cellular metabolism has become an intriguing strategy to treat cancer.
In malignant gliomas, tumor tissues’ lipid levels have been reported to be higher compared with normal tissues [24,25]. Recently, a series of investigations have revealed the underlying molecular mechanisms of how tumor cells upregulate their lipid metabolism. This article will discuss recent advances in understanding the underlying molecular mechanisms of lipid metabolism reprogramming and also introduce how oncogenic signaling pathways contribute to this metabolic alteration. Finally, the authors will discuss the potential of targeting lipid metabolism as a novel therapeutic strategy for malignant gliomas.
Lipids act as signaling molecules
Lipids, such as phospholipids, fatty acids, cholesterol, triglycerides and cholesterol esters, and sphingolipids, are important components of cells. The main biological functions of lipids are as structural components of cell membrances and energy storage [26]. However, lipids also play important roles in mediating signaling transduction to regulate cell response to a various stimuli. For example, diacylglycerol and the phosphatidylinositol phosphates are involved in PKC activation [27,28]; sphingosine-1-phosphate, a sphingolipid derived from ceramide, is involved in cell growth and apoptosis [29]; prostaglandins, which are fatty acid-derived eicosanoids, are involved in immunity and inflammation [30]; and steroid hormones, estrogen, cortisol and testosterone, which are derivatives of cholesterol, regulate metabolism and reproduction [31].
Lipid levels in glioma.
Rapid growth and division are among the major characteristics of malignant tumors. Given the important role of lipids in cell membrane formation and signaling transduction, identification of the differences in lipid composition between tumor and normal tissues, in order to find possible diagnostic and prognostic biomarkers for cancer patients, has been a long-term endeavor for biochemists.
▪ Unsaturated fatty acid levels are high in human intracranial tumors
In 1949, Brante analyzed the general lipid composition of human intracranial tumors in 11 cases of gliomas. He found that total lipids accounted for 15–35% of the dry weight of tissues, and a quarter of cholesterol was not found in its free form. By contrast, Brante showed that normal adult brain tissue contained 35% total lipids of the dry weight of the tissue. Interestingly, he found that in normal brain tissue all of the cholesterol was in its free form [32]. In 1963, Gopal et al. reported a comprehensive analysis of fatty acid distribution in several intracranial tumors in comparison with normal adult brain specimens using gas–liquid chromatography. They found that free fatty acid levels were higher in glioma, meningioma, neurinoma and carcinoma tissue than normal brain tissue. Furthermore, they showed that the levels of polyunsaturated fatty acids, particularly linoleic acids, were much higher in gliomas, meningiomas and neurinomas than normal brain tissue [24]. In addition, this group analyzed the sterol–ester fatty acid composition. They reported that more than 68% of fatty acids in sterol esters were unsaturated acids in gliomas, 46% in neurinomas and 61% in carcinomas compared with 35% in normal brain tissue. There were no striking fatty acid differences in glycerol esters between tumors and normal brain tissue [24].
By using 1H- and 13C-nuclear magnetic resonance spectroscopy (NMR), Tugnoli and colleagues showed that triglycerides were present in two biopsies from glioblastomas (GBM) that had no treatment, but absent in healthy adult brain tissues [33]. Given that triglycerides contain three fatty acids and act as energy storage in addition to unsaturated fatty acids prominent in high-grade intracranial tumors, it appears that gliomas have developed an altered metabolism for fatty acids. Since high levels of polyunsaturated fatty acids are present in gliomas, interruption of the conversion of saturated fatty acids to unsaturated acids may significantly inhibit tumor growth.
▪ Cholesterol esters are present in glioma tissues
Since NMR gives specific information on the neurochemistry of human tissues, characterization of the total lipid fraction of healthy and neoplastic human brain tissues using NMR has, therefore, been conducted by many research groups. By using 1H- and 13C-NMR, cholesterol esters, formed by the esterification of cholesterol with long-chain fatty acids, have been shown to only be present in high-grade gliomas [25,33]. In addition to gliomas, cholesterol esters have been shown to be present in malignant renal cell carcinomas [34,35] and human urothelial carcinoma [36], but absent in the corresponding healthy tissues. Taken together, the presence of cholesterol esters appears to be a promising biomarker for diagnosis of malignancy [37]. However, why neoplastic tissues form and accumulate cholesterol esters, and how tumor cells utilize this portion of lipids is unknown. Given that the levels of free cholesterol are strictly regulated by negative feedback mechanisms, formation of cholesterol esters could be the strategy glioma cells use to store cholesterol. When cells require cholesterol, cholesterol esters could quickly release cholesterol for cell growth or survival. Since cholesterol esters are absent in healthy brain tissues, preventing cholesterol ester utilization may be a possible therapeutic strategy to inhibit malignant glioma growth.
▪ Phosphatidylcholine levels are high in gliomas
By using 31P-NMR-based qualitative and quantitative analyses of the lipid components from the tissues of primary brain tumors, several groups have shown that phosphatidylcholine is significantly higher in medulloblastomas and GBMs compared with normal brain tissues. Grade II/III gliomas patients also presented higher phosphatidylcholine levels than normal subjects [25,33]. Increased levels of choline-containing phospholipids may reflect the changes of cellular membrane structure and cell turnover rates. This change may be in response to growth stimuli or correspond to malignant cell transformation [38]. Further investigation of phospholipid metabolism, and its implications on therapeutics and prognostics in gliomas is needed.
Interestingly, using single-pulse 1H- and 31P-NMR spectroscopy, Srivastava et al. showed higher amounts of phospholipids and total cholesterol in the serum of patients with various primary brain tumors compared with normal individuals, and the concentration of these lipids increased with tumor grade [25]. Furthermore, they determined lipid components in cerebrospinal fluid in patients with different tumor types and showed that cholesterol, cholesterol esters and phospholipids were present in high concentrations in brain tumor patients, but absent in normal individuals as well as in patients with other neurological disorders (e.g., meningitis, a motor neuron disease) [25]. Thus, the presence of cholesterol esters and phospholipids in cerebrospinal fluid is potentially caused by necrosis of tumor tissues and leakage of blood vessels inside tumors. Therefore, cholesterol esters and phospholipids could be potential biomarkers for diagnosis of malignant tumors in the brain, as well as prognostic markers for therapeutic treatment.
Analysis of lipid alteration in gliomas using in vivo magnetic resonance spectroscopy
Magnetic resonance spectroscopy (MRS), also known as NMR spectroscopy, is an analytical technique that has been used to study molecular changes and provide biochemical information about tissues in a noninvasive manner [39]. In vivo MRS is capable of noninvasively detecting metabolic changes in patients with cancer, strokes or Alzheimer's disease. It has been applied to analyze the alteration of metabolites, such as detection of lactate, choline and lipids, in tumor tissues using 1H-MRS. In gliomas, lipids have been clearly detected in patient tissues using in vivo 1H-MRS [40]. 1H-MRS-visible lipids have been characterized by mobile fatty acids and cholesterol esters, which are stored in lipid droplets within the cytoplasm [40]. Interestingly, signals from 1H-MRS-visible lipids have been reported to increase in the area of tumor tissue with apoptosis or necrosis [41,42]. These reports suggests that unsaturated fatty acids are released from membrane phospholipids when cells are carrying out apoptosis and then form lipid droplets that can be detected by in vivo 1H-MRS [40,43]. In summary, in vivo 1H-MRS can provide information regarding lipid metabolism within glioma tissue, and may be able to provide information about lipid homeostasis in tumor tissues in response to drug treatment. Therefore, in vivo MRS could be of assistance for glioma diagnosis and prognosis.
Molecular mechanisms of lipid metabolism in gliomas
Neurobiochemistry analysis has clearly shown that the lipid compositions of tissues are altered in malignant gliomas compared with normal brain tissues, particularly with the exclusive presence of cholesterol esters, and significant elevation of unsaturated fatty acids and phosphatidylcholine in tumor tissues [25,37]. However, the molecular mechanisms that lead to altered lipid composition in glioma tissues, and whether lipid metabolism reprogramming facilitates tumor growth remain unclear. Recently, the key genes in the regulation of lipid metabolism have been demonstrated to be overexpressed in malignant gliomas [44,45]. Oncogenic signaling pathways have been revealed to be involved in lipid metabolism reprogramming in gliomas and other cancers [2,44].
▪ SREBP-1
SREBP-1 is the main transcription factor that regulates de novo fatty acid synthesis [46]. SREBP-1 has three isoforms, SREBP-1a, -1c and -2 that have distinguished roles in regulating lipid metabolism [46]. SREBP-1 is involved in energy metabolism, including fatty acid and glucose metabolism [47], whereas SREBP-2 preferentially activates cholesterol synthesis [48]. SREBPs are synthesized as inactive precursors bound to the endoplasmic reticulum that require cleavage by a two-step proteolytic process in order to become mature transcriptional factors and regulate lipid metabolism [49]. Upon sterol deprivation, SREBP precursors translocate to the Golgi, escorted by SCAP, where SREBPs are cleaved sequentially by S1P and S2P, membrane-bound serine proteases, to release their N-terminal domain into the nucleus where it activates their target genes [46].
Recently, the current authors’ group was the first to show that SREBP-1 is highly upregulated in GBM cell lines and its N-terminal domain is strikingly present in the nucleus of tumor cells in patients’ tissues [44]. In addition, the authors found that free fatty acid levels are high in GBM cell lines [44,50]. Pharmacologic and genetic evidence showed that inhibition or reduction of SREBP-1 significantly induced GBM cell death [44]. These results demonstrate that SREBP-1 is a very promising molecular target in GBM, and suggest that further development of its specific inhibitor for treating malignant gliomas is needed.
In addition, one of the downstream genes of SREBP-1, SCD-1, converts newly synthesized saturated fatty acids to unsaturated fatty acids [46,51,52]. Upregulation of SREBP-1 may be one of the reasons for the increased accumulation of polyunsaturated fatty acids in malignant glioma tissues [24,53]. Why tumor tissues contain high levels of unsaturated fatty acids and whether these alterations contribute to tumor progression has yet to be determined and many interesting questions arise from this cancer-specific phenotype. It would be interesting to investigate whether SCD-1 levels are elevated in GBMs.
▪ ACC & FAS
Elevated de novo fatty acid synthesis is one of the main characteristics of a variety of cancers [20]. The molecular mechanisms of how cancer cells promote fatty acid synthesis have recently been revealed. ACC, the enzyme that regulates the first step of de novo fatty acid synthesis, catalyzes the irreversible carboxylation of acetyl-CoA to malonyl-CoA [54]. FAS then integrates acetyl-CoA and malonyl-CoA to form 16C fatty acid palmitic acid, a saturated fatty acid [20,55]. Cells can further convert saturated fatty acids to mono- or poly-unsaturated fatty acids [52]. The levels of unsaturated fatty acids in phospholipids could affect cell membrane fluidity [56]. Higher levels of unsaturated fatty acids in malignant gliomas could significantly change the components of lipids in the tumor cell membrane [24], which may enhance tumor cell motility and invasion.
ACC and FAS have both been demonstrated to be highly expressed in malignant cancers, as well as being indicators of poor prognosis [20,57]. Pharmacologic and genetic inhibition of both genes has been demonstrated to significantly suppress tumor growth in vitro and in a xenograft mouse model [58–62]. In GBMs, ACC and FAS were both shown to be highly expressed in patient tissues and closely correlated with the expression levels of their transcription factor, SREBP-1 [44]. These data demonstrated that the SREBP-1/ACC/FAS-regulated de novo fatty acid synthesis pathway is highly activated in GBMs. The key roles of these molecules in the fatty acid synthesis pathway have made them attractive targets for developing drugs against GBM. Several inhibitors, such as fatostatin, TOFA and C75, of SREBP-1, ACC and FAS have shown significant inhibitory effects on tumor cell growth in vitro and in vivo [53,63–65]. Table 1 shows the drugs that have been used in vivo. Further testing of these drugs’ toxicity and side effects in mice are needed to determine whether they could be used in clinical trials.
Table 1. . Molecular targets of lipid metabolism and application of their drugs in vivo.
▪ Low-density lipoprotein receptor
Cholesterol is a major component in cell membranes. Its derivatives form steroid hormones that are involved in signal transduction [31]. Cholesterol is found at high volumes in the lipid raft, where it associates with sphingolipids to form a special structure on cellular membranes [66]. Many kinase receptors and signaling molecules, such as EGFR and Akt, are located in lipid rafts [67,68]. Thus, maintenance of cellular cholesterol levels is important for maintaining cell morphology and function.
In the human bloodstream, cholesterol is transported by lipoproteins, such as low-density lipoprotein (LDL), to the rest of the body from the liver. The LDL receptor (LDLR) is inserted in the cell surface that binds LDL and transports it into the cell via endocytosis. After LDL enters into cells, it is transported into lysosomes, where it is dissociated, and releases free cholesterol and fatty acids for the cell to use [69]. LDLR has been shown to be highly expressed in GBM and other types of cancers [45,70,71]. Its high expression ensures tumor cells obtain sufficient cholesterol for their rapid growth and division [70]. At high volumes, cholesterol is converted to cholesterol esters through esterification with fatty acids [72]. This process helps tumor cells store cholesterol and fatty acids in order to make new membranes for daughter cells during cell division. Cholesterol esters have been shown to be uniquely present in a wide range of cancers, including gliomas [37]. Then presence and enrichment may correlate with LDLR expression levels. Therefore, blocking LDLR function is a promising strategy to reduce cellular cholesterol levels, to further slow down cell proliferation and inhibit tumor growth. Cholesterol de novo synthesis regulated by SREBP-2 is another pathway in which a cell can obtain cholesterol [46]. In some cancers, inhibiting cholesterol synthesis by its inhibitor statin has been shown to suppress tumor cell growth in vitro and in vivo [73,74]. However, data from clinical studies using statin in large populations are controversial, and show that there are no significant benefits in patients with statin treatment [75]. In GBM, growth inhibition by statin was only seen in cells treated in cholesterol-free media. The data showed that uptake of cholesterol from the extracellular environment significantly alleviated the effect of inhibition of de novo synthesis [45]. Therefore, blocking both cholesterol uptake and de novo synthesis could be an attractive strategy in treating malignant gliomas.
▪ LXR
Lipid homeostasis is important for cells to maintain their structure and normal function. The nuclear receptor, LXR, plays a key role in regulating cellular cholesterol levels [76]. When cholesterol levels increase, cholesterol will be oxygenized to form oxysterols, which then bind to LXR and promote the expression of its downstream target genes ABCA1 and ABCG1 to reverse-transport cholesterol outside of the cell [77]. Thus, constitutive activation of LXR could result in the significant reduction of cellular cholesterol levels. LXR's synthetic ligands GW3965 and T0901317 have been shown to significantly reduce atherosclerotic lesions in mouse models through reducing macrophage cholesterol levels [78,79]. Activation of LXR was shown to have significant effects on Alzheimer's disease in mouse models by affecting cholesterol metabolism [80]. Activating LXR has been shown to significantly inhibit tumor cell growth in prostate and breast cancer mouse models [81–83]. Recently, GW3965 was shown to markedly kill GBM cells in GBM cell lines and slow tumor growth in a GBM xenograft model; however, the addition of cholesterol significantly rescued GBM cell growth [45]. In addition, LXR activation was shown to reduce LDLR levels by upregulation of the ubiquitin ligase E3 enzyme Idol, which promotes the degradation of LDLR and reduces cholesterol uptake [84]. Taken together, reducing cellular cholesterol levels by activation of LXR could be a promising strategy to treat GBM.
However, the side effect of LXR activation is also obvious. LXR can bind to LXR's response element on the promoter of SREBP-1c and stimulate its expression [85]. In mice, pharmacological activation of LXR has been shown to increase plasma triglyceride levels through LXR-induced upregulation of SREBP-1c gene expression [86], which indicates that these two LXR agonists may not represent effective therapeutic reagents for clinical testing. Hence, the development of novel agonists to strongly activate LXR and not stimulate SREPB-1c expression may represent a promising strategy to treat GBM. On the other hand, combining LXR agonists with inhibitors targeting the SREBP-1/ACC/FAS fatty acid synthesis pathway may overcome the side effects of LXR activation and demonstrated a synergistic effect on GBM tumor growth.
▪ RTK/PI3K/Akt signaling & lipid metabolism
Molecular mechanisms for activation of SREBPs in sterol regulation have been elegantly analyzed and described by Brown, Goldstein and colleagues [48,87,88]. However, whether other molecules or signaling pathways beyond sterol regulation are involved in SREBPs’ transcriptional activation are not yet understood. Recently, in cancer cells, PI3K/Akt/mTOR signaling has been revealed to regulate SREBP-1 expression and activation [89–92], but the detailed molecular mechanism still needs to be further investigated. In GBM, EGFR, PDGFR and Met have been shown to promote SREBP-1 activity, and be further involved in SREBP-1-regulated fatty acid synthesis and the cholesterol uptake pathway [44]. The data further showed that RTK activation upregulates SREBP-1-regulated lipid metabolism mediated by PI3K/Akt signaling. Pharmacological and genetic inhibition of SREBP-1 has been shown to preferentially kill GBM tumors expressing EGFRvIII, a constitutive EGFR mutant [44]. Taken together, these data reveal that oncogenic growth signaling hijacks SREBP-1 to reprogram lipid metabolism in order to facilitate rapid tumor growth. Therefore, disrupting the link between the oncogenic signaling pathway and lipid metabolism is a promising therapeutic approach in targeting GBM and other cancers. SREBP-1 has emerged as the best candidate to target. Further investigation of the molecular mechanism of how oncogenes regulate SREBP-1 activation will certainly promote the development of drugs against SREBP. Given that PI3K/Akt signaling has been shown to promote glucose uptake and enhance glycolysis [15,93], upregulation of SREBP-1 by the RTK-activated PI3K/Akt signaling pathway demonstrates the molecular circuit from glycolysis to lipogenesis (Figure 1). Therefore, targeting SREBP-1 will most likely also reduce glycolysis in addition to inhibition of fatty acid synthesis, thereby leading to significant suppression of tumor growth.
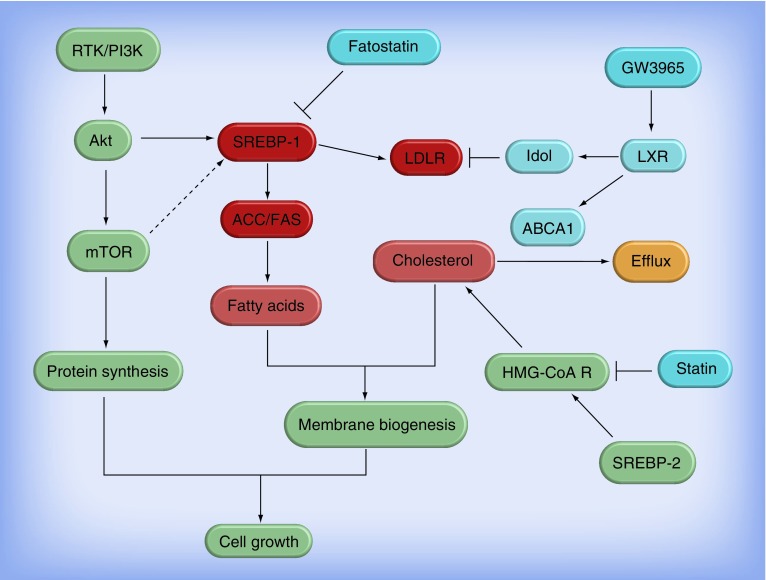
Figure 1. Regulation of lipid metabolism in glioblastoma and the therapeutic drug targets.
Receptor tyrosine kinase/PI3K/Akt signaling via upregulation of SREBP-1 promotes fatty acid synthesis and cholesterol uptake. ACC and FAS are direct downstream genes of SREBP-1 and key molecules in the regulation of de novo fatty acid synthesis. LDLR is upregulated by SREBP-1 to promote cholesterol uptake. Activation of LXR stimulates ABCA1 expression and promotes cholesterol efflux; it also reduces LDLR levels via upregulating Idol, a ubiquitin ligase E3. The light red boxes designate the molecules upregulated in glioblastoma (GBM) and could be potential therapeutic targets in GBM. The light blue boxes signify the molecules that negatively regulate GBM growth. Fatostatin is a SREBP-1 inhibitor; GW3965 is a LXR synthetic agonist; HMG-CoA R is a key enzyme in the pathway of de novo cholesterol synthesis; and statin is a HMG-CoA R inhibitor.
HMG-CoA R: HMG-CoA reductase; LDLR: Low-density lipoprotein receptor; RTK: Receptor tyrosine kinase.
▪ Hedgehog pathway & fatty acid synthesis
In medulloblastomas, elevated lipid levels in tumor tissues have been shown to be prominent. Bhatia et al. have revealed a potential molecular link between oncogenic signaling and enhanced lipid metabolism. They showed that activation of sonic hedgehog signaling promoted de novo fatty acid synthesis through upregulation of FAS expression. They further revealed that increasing FAS expression by sonic hedgehog is mediated by the Rb/E2F pathway [94]. It is interesting that activation of E2F by sonic hedgehog promotes lipogenesis in correlation with cell cycle progression initiation. The data suggest that cell proliferation requires preparation of enough lipids to support the transition of the cell cycle. Therefore, not surprisingly, inhibition of FAS dramatically reduced tumor growth [94]. Taken together, these data suggested that targeting fatty acid synthesis is also a promising therapeutic strategy to treat medulloblastoma.
Conclusion
Metabolic reprogramming has been recognized as a new hallmark of cancer [95]. In the last decade, identification of the key molecules within the metabolic network and further development of new drugs have become rapidly growing research areas. Exaggerated lipogenesis has been found in a variety of cancers [20]. Neurochemistry analyses of lipid components in tumors versus normal tissues has demonstrated that the levels of unsaturated fatty acids are enhanced in malignant gliomas [24]. Surprisingly, cholesterol esters are only present in tumor tissues and absent in normal individuals [37]. Furthermore, increased fatty acid synthesis and enhanced cholesterol uptake were shown to be characteristic of malignant gliomas [44,45,50,96]. Recently, the molecular mechanisms of regulation of lipid reprogramming in cancers have been investigated. RTK/PI3K/Akt signaling has been shown to regulate lipid metabolism through upregulation of SREBP-1 transcriptional activity [44,45]. Targeting fatty acid synthesis and reducing cellular cholesterol levels were shown to significantly inhibit GBM growth, particularly in EGFRvIII-expressing tumors [44,45]. Therefore, altered lipid metabolism is emerging as a potential therapeutic target in malignant gliomas.
Future perspective
There is growing evidence demonstrating that altered lipid metabolism plays a key role in malignant glioma growth. Directly targeting lipid metabolism was shown to be a promising approach in treating malignant gliomas [44,45]. In order to further translate lipid metabolism targeting into clinics, it is necessary to understand the underlying molecular mechanisms of how tumor cells reprogram lipid metabolism in accordance with oncogenic changes, and further identify the key molecules that link oncogenes and lipid metabolism. There is also a need to develop more effective drugs to target lipid metabolism in mice models with reduced side effects to help further translate them into clinical trials. In addition, it is possible that altered lipid metabolism may mediate the resistance of GBM to chemo- and radio-therapies. Therefore, it is worth investigating the therapeutic effects of targeting lipid metabolism in combination with chemo- or radio-therapy. Furthermore, development of targeted therapies has become a promising direction in cancer treatment. However, inhibitors that directly target EGFR, PDGFR, PI3K/mTOR and VEGF/VEGFR have shown only transient or no effects in glioma patients. Combining these targeted inhibitors with inhibition of lipid metabolism may display synergistic therapeutic effects in the clinic.
Footnotes
Financial & competing interests disclosure
D Guo has received funding from Rose DiGangi American Brain Tumor Association Translational Grant, and NIH/NINDS NS072838 and NS079701. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: ▪ of interest ▪▪ of considerable interest
- 1.Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21(21):2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]; ▪ Summarizes the genetics and biology of gliomas, and also discusses the clinical therapy options for glioblastoma (GBM).
- 2.Bell EH, Guo D. Malignant Gliomas, Radiation Medicine Rounds (Volume 3, Issue 2) Demos Medical Publishing; NY, USA: 2012. Biomarkers for malignant gliomas; pp. 389–357. [Google Scholar]; ▪▪ First publication to summarize metabolic reprogramming, including alterations of glucose, glutamine and lipid metabolism, in GBMs.
- 3.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised Phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]; ▪ Summarizes the effects of temozolomide plus radiotherapy in a Phase III trial in GBM, which is the current standard therapy.
- 4.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]; ▪▪ First publication to demonstrate that radiotherapy plus adjuvant temozolomide significantly enhances overall survial in GBM patients.
- 5.Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cloughesy TF, Yoshimoto K, Nghiemphu P, et al. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5(1):e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellingson BM, Cloughesy TF, Lai A, et al. Graded functional diffusion map-defined characteristics of apparent diffusion coefficients predict overall survival in recurrent glioblastoma treated with bevacizumab. Neuro. Oncol. 2011;13(10):1151–1161. doi: 10.1093/neuonc/nor079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang TT, Sarkaria SM, Cloughesy TF, Mischel PS. Targeted therapy for malignant glioma patients: lessons learned and the road ahead. Neurotherapeutics. 2009;6(3):500–512. doi: 10.1016/j.nurt.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai A, Tran A, Nghiemphu PL, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J. Clin. Oncol. 2011;29(2):142–148. doi: 10.1200/JCO.2010.30.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mellinghoff IK, Schultz N, Mischel PS, Cloughesy TF. Will kinase inhibitors make it as glioblastoma drugs? Curr. Top. Microbiol. Immunol. 2011;355:135–169. doi: 10.1007/82_2011_178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N. Engl. J. Med. 2005;353(19):2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 12.Glickman MS, Sawyers CL. Converting cancer therapies into cures: lessons from infectious diseases. Cell. 2012;148(6):1089–1098. doi: 10.1016/j.cell.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell. 2012;21(3):297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deberardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7(1):11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Deberardinis RJ, Thompson CB. Cellular metabolism and disease: what do metabolic outliers teach us? Cell. 2012;148(6):1132–1144. doi: 10.1016/j.cell.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qu W, Oya S, Lieberman BP, et al. Preparation and characterization of L-[5–11C]-glutamine for metabolic imaging of tumors. J. Nucl. Med. 2012;53(1):98–105. doi: 10.2967/jnumed.111.093831. [DOI] [PubMed] [Google Scholar]
- 18.Deberardinis RJ, Mancuso A, Daikhin E, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl Acad. Sci. USA. 2007;104(49):19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shanware NP, Mullen AR, Deberardinis RJ, Abraham RT. Glutamine: pleiotropic roles in tumor growth and stress resistance. J. Mol. Med. (Berl.) 2011;89(3):229–236. doi: 10.1007/s00109-011-0731-9. [DOI] [PubMed] [Google Scholar]
- 20.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Net. Rev. Cancer. 2007;7(10):763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 21.Mullen AR, Wheaton WW, Jin ES, et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2012;481(7381):385–388. doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metallo CM, Gameiro PA, Bell EL, et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2012;481(7381):380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wise DR, Ward PS, Shay JE, et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc. Natl Acad. Sci. USA. 2011;108(49):19611–19616. doi: 10.1073/pnas.1117773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gopal K, Grossi E, Paoletti P, Usardi M. Lipid composition of human intracranial tumors: a biochemical study. Acta Neurochir. (Wien) 1963;11:333–347. doi: 10.1007/BF01402012. [DOI] [PubMed] [Google Scholar]; ▪▪ Provides a comprehensive analysis of fatty acid distribution in several intracranial tumors in comparison with normal adult brain specimens by using gas–liquid chromatography, and shows that the levels of polyunsaturated fatty acids, particularly linoleic acids, are much higher in gliomas, meningiomas and neurinomas than normal brain.
- 25.Srivastava NK, Pradhan S, Gowda GA, Kumar R. In vitro, high-resolution 1H and 31P NMR based analysis of the lipid components in the tissue, serum, and CSF of the patients with primary brain tumors: one possible diagnostic view. NMR Biomed. 2010;23(2):113–122. doi: 10.1002/nbm.1427. [DOI] [PubMed] [Google Scholar]
- 26.Fahy E, Subramaniam S, Murphy RC, et al. Update of the LIPID MAPS comprehensive classification system for lipids. J. Lipid Res. 2009;50(Suppl.):S9–S14. doi: 10.1194/jlr.R800095-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein C, Malviya AN. Mechanism of nuclear calcium signaling by inositol 1,4,5-trisphosphate produced in the nucleus, nuclear located protein kinase C and cyclic AMP-dependent protein kinase. Front. Biosci. 2008;13:1206–1226. doi: 10.2741/2756. [DOI] [PubMed] [Google Scholar]
- 28.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008;9(2):112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saddoughi SA, Song P, Ogretmen B. Roles of bioactive sphingolipids in cancer biology and therapeutics. Subcell. Biochem. 2008;49:413–440. doi: 10.1007/978-1-4020-8831-5_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyce JA. Eicosanoids in asthma, allergic inflammation, and host defense. Curr. Mol. Med. 2008;8(5):335–349. doi: 10.2174/156652408785160989. [DOI] [PubMed] [Google Scholar]
- 31.Kumar R, McEwan IJ. Allosteric modulators of steroid hormone receptors: structural dynamics and gene regulation. Endocr. Rev. 2012;33(2):271–299. doi: 10.1210/er.2011-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brante G. Studies on lipids in the nervous system; with special reference to quantitative chemical determination and topical distribution. Acta Physiol. Scand. 1949;18(Suppl.):63. [Google Scholar]
- 33.Tugnoli V, Tosi MR, Tinti A, Trinchero A, Bottura G, Fini G. Characterization of lipids from human brain tissues by multinuclear magnetic resonance spectroscopy. Biopolymers. 2001;62(6):297–306. doi: 10.1002/bip.10005. [DOI] [PubMed] [Google Scholar]
- 34.Tugnoli V, Bottura G, Fini G, et al. 1H-NMR and 13C-NMR lipid profiles of human renal tissues. Biopolymers. 2003;72(2):86–95. doi: 10.1002/bip.10299. [DOI] [PubMed] [Google Scholar]
- 35.Righi V, Mucci A, Schenetti L, et al. Ex vivo HR-MAS magnetic resonance spectroscopy of normal and malignant human renal tissues. Anticancer Res. 2007;27(5A):3195–3204. [PubMed] [Google Scholar]
- 36.Tugnoli V, Tosi MR. Cholesteryl ester detection in a human urothelial carcinoma. Clin. Chim. Acta. 2005;360(1–2):208–210. doi: 10.1016/j.cccn.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Tosi MR, Tugnoli V. Cholesteryl esters in malignancy. Clin. Chim. Acta. 2005;359(1–2):27–45. doi: 10.1016/j.cccn.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Podo F. Tumour phospholipid metabolism. NMR Biomed. 1999;12(7):413–439. doi: 10.1002/(sici)1099-1492(199911)12:7<413::aid-nbm587>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 39.Bothwell JH, Griffin JL. An introduction to biological nuclear magnetic resonance spectroscopy. Biol. Rev. Camb. Philos. Soc. 2011;86(2):493–510. doi: 10.1111/j.1469-185X.2010.00157.x. [DOI] [PubMed] [Google Scholar]
- 40.Hakumaki JM, Kauppinen RA. 1H NMR visible lipids in the life and death of cells. Trends Biochem. Sci. 2000;25(8):357–362. doi: 10.1016/s0968-0004(00)01614-5. [DOI] [PubMed] [Google Scholar]
- 41.Hakumaki JM, Poptani H, Sandmair AM, Yla-Herttuala S, Kauppinen RA. 1H MRS detects polyunsaturated fatty acid accumulation during gene therapy of glioma: implications for the in vivo detection of apoptosis. Nat. Med. 1999;5(11):1323–1327. doi: 10.1038/15279. [DOI] [PubMed] [Google Scholar]
- 42.Liimatainen T, Lehtimaki K, Ala-Korpela M, Hakumaki J. Identification of mobile cholesterol compounds in experimental gliomas by (1)H MRS in vivo: effects of ganciclovir-induced apoptosis on lipids. FEBS Lett. 2006;580(19):4746–4750. doi: 10.1016/j.febslet.2006.07.062. [DOI] [PubMed] [Google Scholar]
- 43.Liimatainen T, Hakumaki JM, Kauppinen RA, Ala-Korpela M. Monitoring of gliomas in vivo by diffusion MRI and 1H MRS during gene therapy-induced apoptosis: interrelationships between water diffusion and mobile lipids. NMR Biomed. 2009;22(3):272–279. doi: 10.1002/nbm.1320. [DOI] [PubMed] [Google Scholar]
- 44.Guo D, Prins RM, Dang J, et al. EGFR signaling through an Akt-SREBP-1-dependent, rapamycin-resistant pathway sensitizes glioblastomas to antilipogenic therapy. Sci. Signal. 2009;2(101):ra82. doi: 10.1126/scisignal.2000446. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪▪ First publication to identify that tyrosine kinase receptors (receptor tyrosine kinase/EGFR, PDGFR and c-Met) upregulate SREBP-1 and its regulated fatty acid synthesis pathway through PI3K/Akt signaling, and to show that SREBP-1 is highly activated in GBM patient tissues.
- 45.Guo D, Reinitz F, Youssef M, et al. An LXR agonist promotes GBM cell death through inhibition of an EGFR/AKT/SREBP-1/LDLR-dependent pathway. Cancer Discov. 2011;1(5):442–456. doi: 10.1158/2159-8290.CD-11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪▪ First to demonstrate that EGFRvIII promotes tumor survival through PI3K/Akt/SREBP-1-dependent upregulation of low-density lipoprotein receptor, and that disturbance of cholesterol homeostasis through activating the nuclear receptor LXR is a novel therapeutic strategy to treat GBM.
- 46.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 2002;109(9):1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimano H. Sterol regulatory element-binding proteins (SREBPs): transcriptional regulators of lipid synthetic genes. Prog. Lipid Res. 2001;40(6):439–452. doi: 10.1016/s0163-7827(01)00010-8. [DOI] [PubMed] [Google Scholar]
- 48.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89(3):331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 49.Sakai J, Duncan EA, Rawson RB, Hua X, Brown MS, Goldstein JL. Sterol-regulated release of SREBP-2 from cell membranes requires two sequential cleavages, one within a transmembrane segment. Cell. 1996;85(7):1037–1046. doi: 10.1016/s0092-8674(00)81304-5. [DOI] [PubMed] [Google Scholar]
- 50.Guo D, Hildebrandt IJ, Prins RM, et al. The AMPK agonist AICAR inhibits the growth of EGFRvIII-expressing glioblastomas by inhibiting lipogenesis. Proc. Natl Acad. Sci. USA. 2009;106(31):12932–12937. doi: 10.1073/pnas.0906606106. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪▪ First publication to show that the fatty acid synthesis pathway is activated in GBMs, and to demonstrate that activation of AMPK preferentially inhibited the growth of EGFRvIII-expressing GBMs by suppressing lipogenesis.
- 51.Shimomura I, Shimano H, Horton JD, Goldstein JL, Brown MS. Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J. Clin. Invest. 1997;99(5):838–845. doi: 10.1172/JCI119247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Enoch HG, Catala A, Strittmatter P. Mechanism of rat liver microsomal stearyl-CoA desaturase. Studies of the substrate specificity, enzyme-substrate interactions, and the function of lipid. J. Biol. Chem. 1976;251(16):5095–5103. [PubMed] [Google Scholar]
- 53.Williams KJ, Argus JP, Zhu Y, et al. An essential requirement for the SCAP/SREBP signaling axis to protect cancer cells from lipotoxicity. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-13-0382-T. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gregolin C, Ryder E, Kleinschmidt AK, Warner RC, Lane MD. Molecular characteristics of liver acetyl CoA carboxylase. Proc. Natl Acad. Sci. USA. 1966;56(1):148–155. doi: 10.1073/pnas.56.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abramson HN. The lipogenesis pathway as a cancer target. J. Med. Chem. 2011;54(16):5615–5638. doi: 10.1021/jm2005805. [DOI] [PubMed] [Google Scholar]
- 56.Kim HJ, Miyazaki M, Ntambi JM. Dietary cholesterol opposes PUFA-mediated repression of the stearoyl-CoA desaturase-1 gene by SREBP-1 independent mechanism. J. Lipid Res. 2002;43(10):1750–1757. doi: 10.1194/jlr.m100433-jlr200. [DOI] [PubMed] [Google Scholar]
- 57.Menendez JA, Lupu R. Oncogenic properties of the endogenous fatty acid metabolism: molecular pathology of fatty acid synthase in cancer cells. Curr. Opin. Clin. Nutr. Metab. Care. 2006;9(4):346–357. doi: 10.1097/01.mco.0000232893.21050.15. [DOI] [PubMed] [Google Scholar]
- 58.Lupu R, Menendez JA. Pharmacological inhibitors of fatty acid synthase (FASN) – catalyzed endogenous fatty acid biogenesis: a new family of anti-cancer agents? Curr. Pharm. Biotechnol. 2006;7(6):483–493. doi: 10.2174/138920106779116928. [DOI] [PubMed] [Google Scholar]
- 59.Lupu R, Menendez JA. Targeting fatty acid synthase in breast and endometrial cancer: an alternative to selective estrogen receptor modulators? Endocrinology. 2006;147(9):4056–4066. doi: 10.1210/en.2006-0486. [DOI] [PubMed] [Google Scholar]
- 60.Menendez JA, Colomer R, Lupu R. Inhibition of fatty acid synthase-dependent neoplastic lipogenesis as the mechanism of gamma-linolenic acid-induced toxicity to tumor cells: an extension to Nwankwo's hypothesis. Med. Hypotheses. 2005;64(2):337–341. doi: 10.1016/j.mehy.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 61.Menendez JA, Vellon L, Mehmi I, et al. Inhibition of fatty acid synthase (FAS) suppresses HER2/neu (erbB-2) oncogene overexpression in cancer cells. Proc. Natl Acad. Sci. USA. 2004;101(29):10715–10720. doi: 10.1073/pnas.0403390101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beckers A, Organe S, Timmermans L, et al. Chemical inhibition of acetyl-CoA carboxylase induces growth arrest and cytotoxicity selectively in cancer cells. Cancer Res. 2007;67(17):8180–8187. doi: 10.1158/0008-5472.CAN-07-0389. [DOI] [PubMed] [Google Scholar]
- 63.Kamisuki S, Mao Q, Abu-Elheiga L, et al. A small molecule that blocks fat synthesis by inhibiting the activation of SREBP. Chem. Biol. 2009;16(8):882–892. doi: 10.1016/j.chembiol.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 64.Pizer ES, Thupari J, Han WF, et al. Malonyl-coenzyme-A is a potential mediator of cytotoxicity induced by fatty acid synthase inhibition in human breast cancer cells and xenografts. Cancer Res. 2000;60(2):213–218. [PubMed] [Google Scholar]
- 65.Thupari JN, Pinn ML, Kuhajda FP. Fatty acid synthase inhibition in human breast cancer cells leads to malonyl-CoA-induced inhibition of fatty acid oxidation and cytotoxicity. Biochem. Biophys. Res. Comm. 2001;285(2):217–223. doi: 10.1006/bbrc.2001.5146. [DOI] [PubMed] [Google Scholar]
- 66.Lingwood D, Kaiser HJ, Levental I, Simons K. Lipid rafts as functional heterogeneity in cell membranes. Biochem. Soc. Trans. 2009;37(5):955–960. doi: 10.1042/BST0370955. [DOI] [PubMed] [Google Scholar]
- 67.Freeman MR, Cinar B, Kim J, et al. Transit of hormonal and EGF receptor-dependent signals through cholesterol-rich membranes. Steroids. 2007;72(2):210–217. doi: 10.1016/j.steroids.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327(5961):46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 69.Goldstein JL, Brown MS. The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 2009;29(4):431–438. doi: 10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rudling MJ, Angelin B, Peterson CO, Collins VP. Low density lipoprotein receptor activity in human intracranial tumors and its relation to the cholesterol requirement. Cancer Res. 1990;50(3):483–487. [PubMed] [Google Scholar]
- 71.Vitols S, Angelin B, Ericsson S, et al. Uptake of low density lipoproteins by human leukemic cells in vivo: relation to plasma lipoprotein levels and possible relevance for selective chemotherapy. Proc. Natl Acad. Sci. USA. 1990;87(7):2598–2602. doi: 10.1073/pnas.87.7.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khelef N, Soe TT, Quehenberger O, Beatini N, Tabas I, Maxfield FR. Enrichment of acyl coenzyme A:cholesterol O-acyltransferase near trans-golgi network and endocytic recycling compartment. Arterioscler. Thromb. Vasc. Biol. 2000;20(7):1769–1776. doi: 10.1161/01.atv.20.7.1769. [DOI] [PubMed] [Google Scholar]
- 73.Katz MS. Therapy insight: potential of statins for cancer chemoprevention and therapy. Nat. Clin. Pract. Oncol. 2005;2(2):82–89. doi: 10.1038/ncponc0097. [DOI] [PubMed] [Google Scholar]
- 74.Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J. Clin. Invest. 2005;115(4):959–968. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bonovas S, Filioussi K, Sitaras NM. Statins are not associated with a reduced risk of pancreatic cancer at the population level, when taken at low doses for managing hypercholesterolemia: evidence from a meta-analysis of 12 studies. Am. J. Gastroenterol. 2008;103(10):2646–2651. doi: 10.1111/j.1572-0241.2008.02051.x. [DOI] [PubMed] [Google Scholar]
- 76.Calkin AC, Tontonoz P. Liver X receptor signaling pathways and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010;30(8):1513–1518. doi: 10.1161/ATVBAHA.109.191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Invest. 2006;116(3):607–614. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Joseph SB, McKilligin E, Pei L, et al. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc. Natl Acad. Sci. USA. 2002;99(11):7604–7609. doi: 10.1073/pnas.112059299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bradley MN, Hong C, Chen M, et al. Ligand activation of LXR beta reverses atherosclerosis and cellular cholesterol overload in mice lacking LXR alpha and apoE. J. Clin. Invest. 2007;117(8):2337–2346. doi: 10.1172/JCI31909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koldamova R, Lefterov I. Role of LXR and ABCA1 in the pathogenesis of Alzheimer's disease – implications for a new therapeutic approach. Curr. Alzheimer Res. 2007;4(2):171–178. doi: 10.2174/156720507780362227. [DOI] [PubMed] [Google Scholar]
- 81.Chuu CP, Hiipakka RA, Kokontis JM, Fukuchi J, Chen RY, Liao S. Inhibition of tumor growth and progression of LNCaP prostate cancer cells in athymic mice by androgen and liver X receptor agonist. Cancer Res. 2006;66(13):6482–6486. doi: 10.1158/0008-5472.CAN-06-0632. [DOI] [PubMed] [Google Scholar]
- 82.Chuu CP, Lin HP. Antiproliferative effect of LXR agonists T0901317 and 22(R)-hydroxycholesterol on multiple human cancer cell lines. Anticancer Res. 2010;30(9):3643–3648. [PubMed] [Google Scholar]
- 83.Vedin LL, Lewandowski SA, Parini P, Gustafsson JA, Steffensen KR. The oxysterol receptor LXR inhibits proliferation of human breast cancer cells. Carcinogenesis. 2009;30(4):575–579. doi: 10.1093/carcin/bgp029. [DOI] [PubMed] [Google Scholar]
- 84.Zelcer N, Hong C, Boyadjian R, Tontonoz P. LXR regulates cholesterol uptake through idol-dependent ubiquitination of the LDL receptor. Science. 2009;325(5936):100–104. doi: 10.1126/science.1168974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoshikawa T, Shimano H, Amemiya-Kudo M, et al. Identification of liver X receptor-retinoid X receptor as an activator of the sterol regulatory element-binding protein 1c gene promoter. Mol. Cell. Biol. 2001;21(9):2991–3000. doi: 10.1128/MCB.21.9.2991-3000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grefhorst A, Elzinga BM, Voshol PJ, et al. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J. Biol. Chem. 2002;277(37):34182–34190. doi: 10.1074/jbc.M204887200. [DOI] [PubMed] [Google Scholar]
- 87.Brown MS, Goldstein JL. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc. Natl Acad. Sci. USA. 1999;96(20):11041–11048. doi: 10.1073/pnas.96.20.11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nohturfft A, Debose-Boyd RA, Scheek S, Goldstein JL, Brown MS. Sterols regulate cycling of SREBP cleavage-activating protein (SCAP) between endoplasmic reticulum and golgi. Proc. Natl Acad. Sci. USA. 1999;96(20):11235–11240. doi: 10.1073/pnas.96.20.11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Porstmann T, Griffiths B, Chung YL, et al. PKB/Akt induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene. 2005;24(43):6465–6481. doi: 10.1038/sj.onc.1208802. [DOI] [PubMed] [Google Scholar]
- 90.Porstmann T, Santos CR, Griffiths B, et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8(3):224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yecies JL, Zhang HH, Menon S, et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 2011;14(1):21–32. doi: 10.1016/j.cmet.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peterson TR, Sengupta SS, Harris TE, et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146(3):408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rathmell JC, Fox CJ, Plas DR, Hammerman PS, Cinalli RM, Thompson CB. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol. Cell. Biol. 2003;23(20):7315–7328. doi: 10.1128/MCB.23.20.7315-7328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bhatia B, Hsieh M, Kenney AM, Nahle Z. Mitogenic Sonic hedgehog signaling drives E2F1-dependent lipogenesis in progenitor cells and medulloblastoma. Oncogene. 2011;30(4):410–422. doi: 10.1038/onc.2010.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 96.Guo D, Cloughesy TF, Radu CG, Mischel PS. AMPK: a metabolic checkpoint that regulates the growth of EGFR activated glioblastomas. Cell Cycle. 2010;9(2):211–212. doi: 10.4161/cc.9.2.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]