Abstract
A new sesquiterpene lactone, rufescenolide C (1), the first furanoheliangolide dimer, was isolated from the leaves of Piptocoma rufescens, collected in the Dominican Republic. Its structure was determined by analysis of its spectroscopic data, with the absolute configuration being established by analysis of the CD spectrum. A plausible biogenesis of this dimer is proposed. This compound showed potent cytotoxicity with an IC50 value of 150 nM, when tested against HT-29 human colon cancer cells.
Keywords: Piptocoma rufescens, Furanoheliangolide dimer, Cytotoxicity
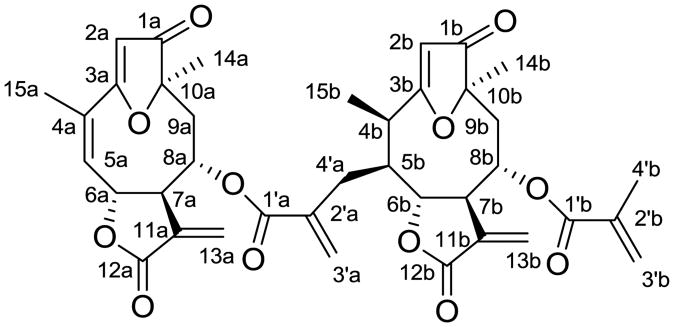
Piptocoma is a small genus of the plant family Asteraceae, occurring in the Western Hemisphere. A previous investigation of the plant Piptocoma rufescens Cass., collected in the Dominican Republic, resulted in the isolation and characterization of several sesquiterpene lactones.1 Further isolation work (Supplementary data) on this species has led to purification and structure elucidation of a new dimeric goyazensolide-type sesquiterpene lactone, rufescenolide C (1, Figure 1). The structure of this new compound was determined by analysis of its spectroscopic data, and the absolute configuration was established using its CD spectrum. The cytotoxicity toward the HT-29 human colon cancer cell line was determined.
Figure 1.
Structure of rufescenolide C (1).
The ground leaves of Piptocoma rufescens were extracted with MeOH. The MeOH extract was partitioned with n-hexane and then CHCl3. The chloroform-soluble extract was found to be active when evaluated by a cytotoxicity assay.1 Repeated chromatography of the active chloroform-soluble extract over silica gel monitored by cytotoxicity toward HT-29 cells afforded rufescenolide C (1).2
Compound 1 was isolated as an amorphous white powder. It showed UV (λmax 214 and 263 nm) and IR [νmax 1770 and 1654 (α,β-unsaturated γ-lactone), 1712 and 1629 (α,β-unsaturated ester), 1712 and 1587 (dihydrofuran-3-one ring) cm−1] absorptions typical for a furan ring-containing germacranolide.1 This compound could be proposed as being a dimeric furanoheliangolide, as indicated by its similar UV and IR spectra to those of 15-deoxygoyazensolide1 and its molecular formula of C38H40O12 (positive HRESIMS m/z 711.2407, calcd for C38H40O12Na, 711.2417), as supported by the 13C NMR spectroscopic data (Table 1), which were comparable to those of 15-deoxygoyazensolide (subunit a),1 and 4,5-dihydro-15-deoxygoyazensolide (subunit b).3
Table 1. NMR spectroscopic data for rufescenolide C (1) in CDCl3.
| Positiona | δC(mult.)b | δH(mult., J, Hz)c | COSY (H→H)d | HMBC (H→C)e | NOESY (H→H)f |
|---|---|---|---|---|---|
| 1a | 205.0 C | ||||
| 2a | 104.9 CH | 5.70 s | 1a, 3a, 10a | 8a | |
| 3a | 187.2 C | ||||
| 4a | 130.6 C | ||||
| 5a | 135.0 CH | 5.98 br s | 6a | 15a | 15a |
| 6a | 81.9 CH | 5.25 m | 7a | 8a | 8a |
| 7a | 51.3 CH | 3.70 m | 6a, 8a | 6a, 9a-b, 13a-a | |
| 8a | 74.2 CH | 4.47 dt (2.0, 12.0) | 7a, 9a | 6a, 10a, 1′a | 6a, 9a-a, 9a-b |
| 9a-a | 44.2 CH2 | 2.26 m | 8a, 9a-b | 1a, 8a, 10a, 14a | 8a |
| 9a-b | 2.50 t (12.0) | 8a, 9a-a | 1a, 8a, 10a, 14a | 7a, 8a, 14a | |
| 10a | 89.8 C | ||||
| 11a | 133.8 C | ||||
| 12a | 168.8 C | ||||
| 13a-a | 124.5 CH2 | 5.42 d (2.4) | 7a, 12a | 7a | |
| 13a-b | 6.23 d (3.2) | 7a, 12a | |||
| 14a | 20.9 CH3 | 1.52 s | 1a, 9a, 10a | ||
| 15a | 20.5 CH3 | 2.07 s | 3a, 4a, 5a | 5a | |
| 1′a | 166.3 C | ||||
| 2′a | 137.9 C | ||||
| 3′a-a | 128.5 CH2 | 5.76 br s | 1′a, 2′a, 4′a | 4′a-a, 4′a-b | |
| 3′a-b | 6.12 br s | 1′a, 2′a, 4′a | |||
| 4′a-a | 30.8 CH2 | 2.22 m | 5b | 1′a, 2′a, 3′a, 4b, 5b, 6b | 3′a-a, 15b |
| 4′a-b | 2.73 m | 5b | 1′a, 2′a, 3a′, 4b, 5b, 6b | 3′a-a | |
| 1b | 205.7 C | ||||
| 2b | 105.9 CH | 5.70 s | 1b, 3b, 10b | 6b, 8b, 15b | |
| 3b | 193.8 C | ||||
| 4b | 36.3 CH | 2.85 m | 5b, 15b | 3b, 5b, 6b, 15b | |
| 5b | 46.3 CH | 2.63 m | 4′a, 6b | 6b, 7b, 15b, 2′a | 7b, 14b |
| 6b | 81.0 CH | 4.28 dd (3.6, 7.2) | 5b, 7b | 8b, 11b, 12b, 13b | 2b, 7b, 15b |
| 7b | 55.9 CH | 3.40 m | 6b, 8b | 5b, 6b, 8b, 13b-a | |
| 8b | 72.4 CH | 4.36 dt (2.0, 11.2) | 7b, 9b | 6b, 10b, 1′b | 7b, 9b-b |
| 9b-a | 45.8 CH2 | 2.35 m | 8b, 9b-b | 1b, 8b, 10b, 14b | |
| 9b-b | 2.63 m | 8b, 9b-a | 1b, 8b, 10b, 14b | 8b | |
| 10b | 90.1 C | ||||
| 11b | 133.3 C | ||||
| 12b | 169.2 C | ||||
| 13b-a | 125.2 CH2 | 5.47 d (2.4) | 7b, 12b | 7b | |
| 13b-b | 6.17 d (2.8) | 7b, 12b | |||
| 14b | 21.2 CH3 | 1.51 s | 1b, 9b | 5b | |
| 15b | 9.9 CH | 1.25 (overlap) | 4b | 3b, 4b, 5b | 2b, 6b, 4′a-a |
| 1′b | 166.9 C | ||||
| 2′b | 135.7 C | ||||
| 3′b-a | 126.4 CH2 | 5.51 br s | 1′b, 2′b, 4′b | 4′b | |
| 3′b-b | 5.98 brs | 1′b, 2′b, 4′b | |||
| 4′b | 18.1 CH3 | 1.81 s | 1′b, 2′b, 3′b | 3′b-a |
Assigned by analysis of 1H, 13C, DEPT 90, DEPT 135, COSY, HSQC, and HMBC NMR spectra.
Recorded at 100.6 MHz and referenced to residual CDCl3 at δ 77.16.4 CH3, CH2, CH, and C determined by DEPT 90 and DEPT 135 and HSQC experiments.
Recorded at 400.1 MHz and referenced to residual CDCl3 at δ 7.26.4 The overlapped signals were assigned by 1H–1H COSY, HSQC, and HMBC spectra are presented without designating multiplicity.
Recorded at 400.1 MHz and referenced to residual CDCl3 at δ 7.26 with proton showing COSY correlation to indicated proton.
Recorded at 800.1 MHz with proton showing HMBC correlation to indicated carbon.
Recorded at 800.1 MHz and referenced to residual CDCl3 at δ 7.26 with proton showing NOESY correlation to indicated proton.
A ten-membered ring fused with a furan ring at the C-3 and C-10 positions was suggested for subunits a and b of 1, respectively, from the 1H–1H COSY sequences of H-5a/H-6a (δH 5.24 m)/H-7a/H-8a/H2-9a and H-15b/H-4b/H-5b/H-6b [δH 4.28 dd (3.6, 7.2)]/H-7b/H-8b/H2-9b (Table 1).4 A cyclic lactone ring containing an exomethylene group was proposed at the C-6 and C-7 positions for both subunits a and b, as supported by HMBC correlations between H-13a/C-7a and C-12a and H-13b/C-7b and C-12b (Table 1). In addition, the 1H and 13C NMR spectra of 1 revealed the presence of a methacrylate group for both subunits a and b, as characterized by two methylene multiplets at δH 2.22 (H-4′a-a) and δH 2.73 (H-4′a-b) and two broad singlets at δH 5.76 (H-3′a-a) and 6.12 (H-3′a-b) for subunit a, and a methyl singlet at δH 1.81 (H-4′b) and broad singlets at δH 5.51 (H-3′b-a) and 5.98 (H-3′b-b) for subunit b in the 1H NMR spectrum. In addition, eight signals appeared at δC 166.3 (C-1′a), 137.9 (C-2′a), 128.5 (C-3′a), and 30.8 (C-4′a) for subunit a and at δC 166.9 (C-1′b), 135.7 (C-2′b), 126.4 (C-3′b), and 18.1 (C-4′b) for subunit b in the 13C NMR spectrum of 1.1 These methacrylate groups were assigned to the C-8a and C-8b positions, respectively, as supported by HMBC correlations between H-8a/C-1′a and H-8b/C-1′b (Table 1 and Figure S8, Supplementary data).
Subunit a of 1 contained a structural unit of 15-deoxygoyazensolide, as indicated by comparison of the 1H and 13C NMR data of this part of the molecule (Table 1) with those of 15-deoxygoyazensolide.1 This subunit showed closely comparable signals for the sesquiterpene lactone core to those of 15-deoxygoyazensolide but different signals for the ester residue at the C-8 position, which appeared at δ 166.7 (C-1′), 135.5 (C-2′), 126.4 (C-3′), and 18.0 (C-4′) for 15-deoxygoyazensolide.1 Also, a signal at δ 18.0 for a methyl group of C-4′ of 15-deoxygoyazensolide appeared as a signal at δ 30.7 for the C-4′a methylene group of subunit a of 1, indicating this subunit to be linked to subunit b at its C-4′a position. This elucidation was confirmed by HMBC correlations between H-2a/C-1a, -3a, and -10a, H-8a/C-6a, -10a, and 1′a, H-9a/C-1a, -8a, -10a and -14a, H-14a/C-1a, -9a, and -10a, and H-4′a/C-1′a, 2′a, 3′a, -4b, -5b, and 6b, respectively (Table 1).
Subunit b of 1 was proposed as being based on 4,5-dihydro-15-deoxygoyazensolide.3 Comparison of the NMR data of this subunit with literature data indicated that it exhibited identical signals for the ester residue at the C-8 position to those of 4,5-dihydro-15-deoxygoyazensolide but different signals for the sesquiterpene lactone core, especially at the C-3, -4, -5, -6, -7, -8, and -15 positions, which appeared at δ 192.6 (C-3), 33.6 (C-4), 42.4 (C-5), 82.1 (C-6), 54.4 (C-7), 71.9 (C-8), and 18.5 (C-15) for 4,5-dihydro-15-deoxygoyazensolide,3 and at δ 193.8 (C-3b), 36.3 (C-4b), 46.3 (C-5b), 81.0 (C-6b), 55.9 (C-7b), 72.4 (C-8b), and 9.9 (C-15b) for subunit b of 1. A signal at δ 9.9 was assigned to a C-15 methyl group connected to the C-4 position of a goyazensolide core containing a substituent at the C-5 position.1 The signal at δ 42.4 for the C-5 methylene group of 4,5-dihydro-15-deoxygoyazensolide3 was observed at δ 46.3 for a methine group at C-5b of subunit b of 1, indicating that this subunit is linked to subunit a at the C-5 position. This was confirmed by HMBC correlations, in turn, between H-2b/C-1b, -3b, and -10b, H-5b/C-6b, -7b, -15b, and -2′a, H-8b/C-6b, -10b, and 1′b, H-9b/C-1b, -8b, -10b and -14b (Table 1). Based on this spectroscopic evidence, compound 1 was determined as 15-deoxygoyazensolide-(4′→5)-4,5-dihydro-15-deoxygoyazensolide.
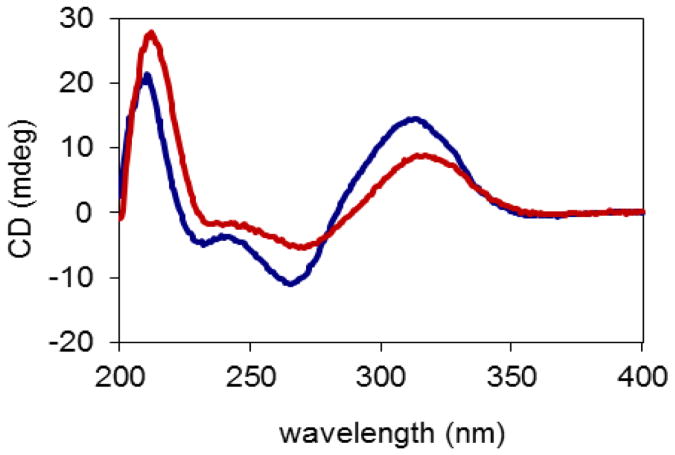
The relative configuration of 1 was established by NOESY correlations in combination with comparison of its NMR data with those of both 15-deoxygoyazensolide and 4,5-dihydro-15-deoxygoyazensolide.1,3 In turn, the absolute configuration of 1 was determined by analysis of the CD spectrum. According to the determination of absolute configuration of goyazensolide-type sesquiterpene lactones,1 the negative Cotton effects at 235 and 270 nm exhibited in the CD spectrum (Figure 2) of 1 indicated 7aR and 7bR configurations, and the positive Cotton effects at 212 and 317.5 nm supported 10aR and 10bR configurations. The NOESY correlations between H-2a/H-8a and H-6a/H-8a indicated 6aR and 8aS configurations, as supported by the similar NMR data of this part with those of 15-deoxygoyazensolide.1 The NOESY correlations between H-2b/H-6b, H-8b, and H-15b indicated 6bR and 8bS configurations, as supported by the similar coupling constants for H-6b to those of H-6 of rufescenolides A and B1 and the similar coupling constant for H-8b to that of H-8a, together with the consistent CD spectra with those of rufescenolide A (Figure 2). The NOESY correlations between H-5b/H-7b and H-14b suggested a 5bS configuration, and the NOESY correlation between H-15b/H-6b suggested 6bR and 4bR configurations in 1. Determination of the absolute configurations at C-4b, -5b, -6b, -7b, -8b, and -10b were supported by the consistent NMR data of 1 with those of 4,5-dihydro-15-deoxygoyazensolide,3 but not with those of zexbrevin.5 Therefore, the structure and absolute configuration of compound 1 was proposed as (6aR,7aR,8aS,10aR)-1-oxo-3,10-epoxy-8-methacryloyloxygermacra-2,4,11(13)-trien-6,12-olide-(4′→5)-(4bR,5bS,6bR,7bR,8bS,10bR)-1-oxo-3,10-epoxy-8-methacryloyloxygermacra-2,11(13)-dien-6,12-olide,1 which has been accorded the trivial name, rufescenolide C.
Figure 2.
CD spectra of rufescenolide C (1, red) and rufescenolide A (blue). The CD data were obtained in MeOH corrected by subtracting a spectrum of the appropriate solution in the absence of the samples recorded under identical conditions.
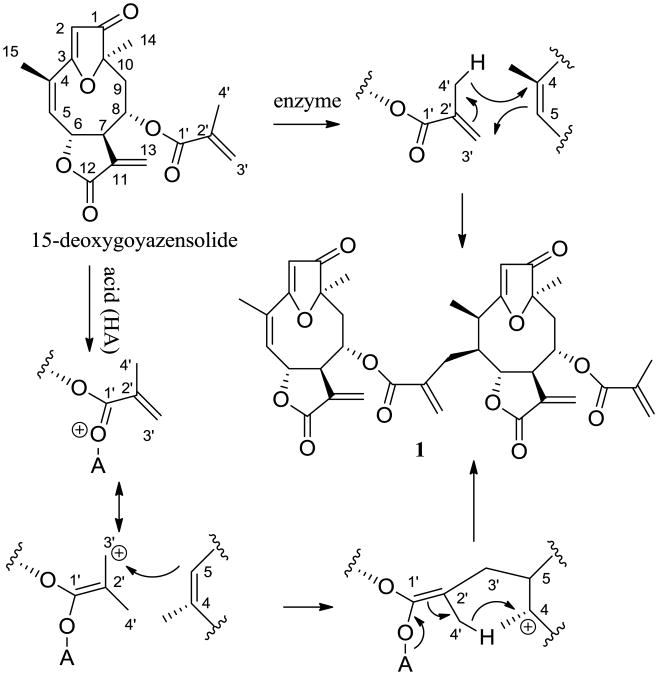
As shown in Scheme 1, it is proposed that rufescenolide C (1) may be formed from either an enzyme- or an acid-catalyzed ene-type reaction of 15-deoxygoyazensolide, which was isolated previously from Piptocoma rufescens in a high yield,1 with the C-2′, 3′ and 4′ positions of this molecule as an ene and the C-4 and C-5 positions of the same molecule as an enophile.6
Scheme 1.
Proposed biogenesis of rufescenolide C (1) from an ene-type reaction of 15-deoxygoyazensolide.
Rufescenolide C (1) was tested in terms of its cytotoxicity against the HT-29 human colon cancer cell line by a previous procedure,1 using paclitaxel as positive control (IC50, 0.10 nM). It showed high cytotoxicity toward the HT-29 cell line, with an IC50 value of 150 nM.
Dimeric sesquiterpene lactones are rare natural products discovered mainly from the family Asteraceae and exhibit a number of structural types. The most common members of this compound family are symmetrical dimers. Double-linked guaianolide dimers containing either a non-spiro or a spiro linkage are more prevalent than their single-linked variants,7–10 with dimers having a single ether oxygen bridge being unusual.11 The connectivity of the monomers of dimeric eudesmanolides may occur either as a C-11-spiro-double linkage or as a single linkage,12,13 while dimeric eremophilanolides tend to occur in non-spiro-double-linked or single-linked forms.14,15 These compounds, together with the small member of known germacranolide dimers,16,17 as well dimeric xanthanolides and elemanolides,18,19 and the several unsymmetrical sesquiterpene lactone dimers,20–23 exhibit considerable chemical diversity. It is proposed that doubly-linked dimeric sesquiterpene lactones are formed by Diels-Alder additions,9,20,22 which has been supported by the subsequent synthesis of several representatives of this compound type using such a synthetic strategy.24 Also, this same hypothesis was proposed for the biosynthesis of singly-linked sesquiterpene dimers,10 but supportive evidence for such a proposal is limited.
Dimeric sesquiterpene lactones are known to exhibit many types of biological activities, including cytotoxicity,9,21,25 anti-HIV potency,7 antidiabetic activity,11 antiprotozoal effects,10,18 anti-inflammatory efficacy,26 and inhibition of LPS-induced NO production.8,23 In addition, several guaianolide dimers showed more potent cytotoxicity toward a panel of human cancer cell lines than their monomer.25 The dimeric guaianolide, microlenin, was found to suppress Walker 256 carcinosarcoma growth in vivo,9 and the antitumor potency of artemisinin, a sesquiterpene lactone endoperoxide, has been improved considerably by dimerization of this molecule.27,28 Consistent with these previous studies, the present study showed that rufescenolide C (1) exhibits more potent cytotoxicity against HT-29 human colon cancer cells than its monomeric analogues,1 indicating that this compound might be an enhanced antitumor lead for further investigation.
Supplementary Material
Acknowledgments
This investigation was supported by grants U01 CA52956 and P01 CA125066, funded by the National Cancer Institute, NIH, Bethesda, MD. The leaf sample of Piptocoma rufescens was collected under a collaborative agreement between the University of Illinois at Chicago and the Jardín Botánico Nacional “Dr. Rafael Ma. Moscoso”, Santo Domingo, Dominican Republic. We thank Dr. David Hart, Department of Chemistry and Biochemistry, The Ohio State University, for his kind suggestions regarding the biogenesis of rufescenolide C. We thank Dr. Kari Green-Church, of the Mass Spectrometry and Proteomics of the Campus Chemical Instrument Center (CCIC), The Ohio State University (OSU), for assistance with the MS measurements, and Dr. Chunhua Yuan (CCIC, OSU) and Jack Fowble (College of Pharmacy, OSU) for access to the NMR instrumentation used in this investigation.
Footnotes
Supplementary data. Supplementary data about experimental procedures, MS and NMR spectra of rufescenolide C (1) associated with this article can be found in the online version at http://dx.doi.org/tetlet.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ren Y, Acuña U, Jiménez F, García R, Mejía M, Chai H, Gallucci JC, Farnsworth NR, Soejarto DD, Carcache de Blanco EJ, Kinghorn AD. Tetrahedron. 2012;68:2671–2678. doi: 10.1016/j.tet.2012.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selected data for 1: Amorphous colorless powder (n-hexane) showing a dark color under UV light at 254 nm; [α]20D +58.9 (c 0.09, MeOH); [α]20D +44.0 (c 0.1, CH2Cl2); UV (MeOH) λmax (log ε) 214 (4.71), 263 (4.66) nm; CD (MeOH, nm) λmax (Δε) 212 (+25.97), 235 (-1.74), 270 (-5.08), 317.5 (+8.24); IR (dried film) νmax 1770, 1712, 1654, 1629, 1587, 814 cm−1; 1H and 13C NMR data, see Table 1; positive HRESIMS m/z 711.2407, calcd for C38H40O12Na, 711.2417.
- 3.Vichnewski W, Takahashi AM, Nasi AMT, Goncalves DCRG, Dias DA, Lopes JNC, Goedken VL, Gutierrez AB, Herz W. Phytochemistry. 1989;28:1441–1451. [Google Scholar]
- 4.Gottlieb HE, Kotlyar V, Nudelman A. J Org Chem. 1997;62:7512–7515. doi: 10.1021/jo971176v. [DOI] [PubMed] [Google Scholar]
- 5.Herz W, Kumar N. Phytochemistry. 1980;19:593–597. [Google Scholar]
- 6.(a) Snider BB. Acc Chem Res. 1980;13:426–432. [Google Scholar]; (b) Paderes GD, Jorgensen WL. J Org Chem. 1992;57:1904–1916. [Google Scholar]
- 7.(a) Beauhaire J, Chiaroni A, Fourrey JL, Riche C. Tetrahedron Lett. 1983;24:4417–4418. [Google Scholar]; (b) Beauhaire J, Fourrey JL, Guittet E. Tetrahedron Lett. 1984;25:2751–2754. [Google Scholar]; (c) Ma CM, Nakamura N, Hattori M, Zhu S, Komatsu K. J Nat Prod. 2000;63:1626–1629. doi: 10.1021/np000005+. [DOI] [PubMed] [Google Scholar]
- 8.(a) Bohlmann F, Ahmed M, Jakupovic J, King RM, Robinson H. Phytochemistry. 1983;22:191–195. [Google Scholar]; (b) Wu ZJ, Xu XK, Shen YH, Su J, Tian JM, Liang S, Li HL, Liu RH, Zhang WD. Org Lett. 2008;10:2397–2400. doi: 10.1021/ol800656q. [DOI] [PubMed] [Google Scholar]
- 9.(a) Lee KH, Imakura Y, Sims D, McPhail AT, Onan KD. J Chem Soc Chem Commun. 1976:341–342. [Google Scholar]; (b) Romo de Vivar A, Delgado G. Tetrahedron Lett. 1985;26:579–582. [Google Scholar]; (c) Nagashima F, Murakami M, Takaoka S, Asakawa Y. Chem Pharm Bull. 2004;52:949–952. doi: 10.1248/cpb.52.949. [DOI] [PubMed] [Google Scholar]; (d) Trendafilova A, Todorova M, Mikhova B, Vitkova A, Duddeck H. Phytochemistry. 2006;67:764–770. doi: 10.1016/j.phytochem.2006.01.033. [DOI] [PubMed] [Google Scholar]; (e) Wen J, Shi H, Xu Z, Chang H, Jia C, Zan K, Jiang Y, Tu P. J Nat Prod. 2010;73:67–70. doi: 10.1021/np900462u. [DOI] [PubMed] [Google Scholar]
- 10.(a) Ali MS, Ahmed W, Armstrong AF, Ibrahim SA, Ahmed S, Parvez M. Chem Pharm Bull. 2006;54:1235–1238. doi: 10.1248/cpb.54.1235. [DOI] [PubMed] [Google Scholar]; (b) Ali MS, Ibrahim SA, Ahmed S, Lobkovsky E. Chem Biodivers. 2007;4:98–104. doi: 10.1002/cbdv.200790011. [DOI] [PubMed] [Google Scholar]; (c) Maas M, Hensel A, Batista da Costa F, Brun R, Kaiser M, Schmidt TJ. Phytochemistry. 2011;72:635–644. doi: 10.1016/j.phytochem.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 11.Hou CC, Lin SJ, Cheng JT, Hsu FL. J Nat Prod. 2003;66:625–629. doi: 10.1021/np0205349. [DOI] [PubMed] [Google Scholar]
- 12.(a) Jakupovic J, Schuster A, Bohlmann F, Dillon MO. Phytochemistry. 1988;27:1113–1120. [Google Scholar]; (b) Jiang HL, Chen J, Jin XJ, Yang JL, Li Y, Yao XJ, Wu QX. Tetrahedron. 2011;67:9193–9198. [Google Scholar]
- 13.(a) Kraut L, Mues R, Sim-Sim M. Phytochemistry. 1994;37:1337–1346. [Google Scholar]; (b) Lin Y, Jin T, Wu X, Huang Z, Fan J, Chan WL. J Nat Prod. 1997;60:27–28. [Google Scholar]; (c) Rosquete C, Del Olmo E, Sanz F, San Feliciano A. Chem Pharm Bull. 2002;50:964–965. doi: 10.1248/cpb.50.964. [DOI] [PubMed] [Google Scholar]
- 14.(a) Liu X, Wu QX, Wei XN, Shi YP. Helv Chim Acta. 2007;90:1802–1810. [Google Scholar]; (b) Huang HL, Xu YJ, Liu HL, Liu XQ, Shang JN, Han GT, Yao MJ, Yuan CS. Phytochemistry. 2011;72:514–517. doi: 10.1016/j.phytochem.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Liu JQ, Zhang M, Zhang CF, Qi HY, Bashall A, Bligh SWA, Wang ZT. Phytochemistry. 2008;69:2231–2236. doi: 10.1016/j.phytochem.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 16.Macías FA, López A, Varela RM, Molinillo JMG, Alves PLCA, Torres A. Tetrahedron Lett. 2004;45:6567–6570. [Google Scholar]
- 17.(a) Bohlmann F, Adler A, Jakupovic J, King RM, Robinson H. Phytochemistry. 1982;21:1349–1355. [Google Scholar]; (b) Song Q, Gomez-Barrios ML, Fronczek FR, Vargas D, Thien LB, Fischer NH. Phytochemistry. 1998;47:221–226. [Google Scholar]
- 18.Nour AMM, Khalid SA, Kaiser M, Brun R, Abdallah WE, Schmidt TJ. Planta Med. 2009;75:1363–1368. doi: 10.1055/s-0029-1185676. [DOI] [PubMed] [Google Scholar]
- 19.(a) Fu B, Su BN, Takaishi Y, Honda G, Ito M, Takeda Y, Kodzhimatov OK, Ashurmetov O. Phytochemistry. 2001;58:1121–1128. doi: 10.1016/s0031-9422(01)00334-x. [DOI] [PubMed] [Google Scholar]; (b) Liu Y, Nugroho AE, Hirasawa Y, Nakata A, Kaneda T, Uchiyama N, Goda Y, Shirota O, Morita H, Aisa HA. Tetrahedron Lett. 2010;51:6584–6587. [Google Scholar]
- 20.(a) Jakupovic J, Zdero C, Grenz M, Tsichritzis F, Lehmann L, Hashemi-Nejad SM, Bohlmann F. Phytochemistry. 1989;28:1119–1131. [Google Scholar]; (b) Zdero C, Bohlmann F. Phytochemistry. 1989;28:3105–3120. [Google Scholar]; (c) Qin JJ, Huang Y, Wang D, Cheng XR, Zeng Q, Zhang SD, Hu ZL, Jin HZ, Zhang WD. RSC Adv. 2012;2:1307–1309. [Google Scholar]
- 21.(a) Qin JJ, Jin HZ, Fu JJ, Hu XJ, Wang Y, Yan SK, Zhang WD. Bioorg Med Chem Lett. 2009;19:710–713. doi: 10.1016/j.bmcl.2008.12.043. [DOI] [PubMed] [Google Scholar]; (b) Qin JJ, Jin HZ, Zhu JX, Fu JJ, Hu XJ, Liu XH, Zhu Y, Yan SK, Zhang WD. Planta Med. 2010;76:278–283. doi: 10.1055/s-0029-1186065. [DOI] [PubMed] [Google Scholar]
- 22.Su BN, Takaishi Y, Tori M, Takaoka S, Honda G, Itoh M, Takeda Y, Kodzhimatov OK, Ashurmetov O. Tetrahedron Lett. 2000;41:1475–1479. doi: 10.1021/ol990401n. [DOI] [PubMed] [Google Scholar]
- 23.Qin JJ, Wang LY, Zhu JX, Jin HZ, Fu JJ, Liu XF, Li HL, Zhang WD. Chem Commun. 2011;47:1222–1224. doi: 10.1039/c0cc03572f. [DOI] [PubMed] [Google Scholar]
- 24.(a) Zhang W, Luo S, Fang F, Chen Q, Hu H, Jia X, Zhai H. J Am Chem Soc. 2005;127:18–19. doi: 10.1021/ja0439219. [DOI] [PubMed] [Google Scholar]; (b) Li C, Dian L, Zhang W, Lei X. J Am Chem Soc. 2012;134:12414–12417. doi: 10.1021/ja305464s. [DOI] [PubMed] [Google Scholar]
- 25.Strapasson RLB, Cervi AC, Carvalho JE, Ruiz ALTG, Salvador MJ, Stefanello MEA. Phytother Res. 2012;26:1053–1056. doi: 10.1002/ptr.3693. [DOI] [PubMed] [Google Scholar]
- 26.Hu Z, Qin J, Zhang H, Wang D, Hua Y, Ding J, Shan L, Jin H, Zhang J, Zhang W. Biochem Pharmacol. 2012;84:1482–1491. doi: 10.1016/j.bcp.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 27.Rosenthal AS, Chen X, Liu JO, West DC, Hergenrother PJ, Shapiro TA, Posner GH. J Med Chem. 2009;52:1198–1203. doi: 10.1021/jm801484v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He R, Mott BT, Rosenthal AS, Genna DT, Posner GH, Arav-Boger R. PLoS ONE. 2011;6:e24334. doi: 10.1371/journal.pone.0024334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.