Summary
Drug delivery systems (DDSs) face several challenges including site-specific delivery, stability, and the programmed release of drugs. Engineered nanoparticle (NP) surfaces with responsive moieties can enhance the efficacy of DDSs for in vitro and in vivo systems. This triggering process can be achieved through both endogenous (biologically controlled release) and exogenous (external stimuli controlled release) activation. In this review, we will highlight recent examples of the use of triggered release strategies of engineered nanomaterials for in vitro and in vivo applications.
Keywords: Engineered nanoparticles, drug delivery system, in vitro, in vivo, triggered release
Introduction
Engineered nanomaterials provide important tools for improving the effectiveness of chemotherapeutics [1]. An ideal drug delivery system (DDS) would limit therapeutic activity to diseased tissues and organs, maximizing efficacy while minimizing collateral damage [2]. While DDSs provide a means of overcoming the drawbacks of free drugs, tissue penetration and homogeneous distribution within the target tissues and organs still remain formidable challenges [3,4]. Nanomaterials provide a promising platform for DDSs, possessing biologically relevant size, molecular tunability, and high surface to volume ratios [5,6,7,8]. Modification of the surfaces of nanoparticles (NPs) can be tuned to influence the pharmacological behavior of DDSs [9,10] and the ligand density on drug delivery vehicle surfaces can be controlled to attain desired tissue penetration and cellular uptake [11,12]. Ligand design can also enhance DDS efficacy by selectively binding to targeted tissues and causing a conformational change of the vehicle, releasing its payload [13,14]. The engineering of nanoparticle (NP) surfaces can endow DDSs with enhanced targeting, improved solubility, extended half-life, reduced immunogenicity, and improved biodistribution [15,16,17].
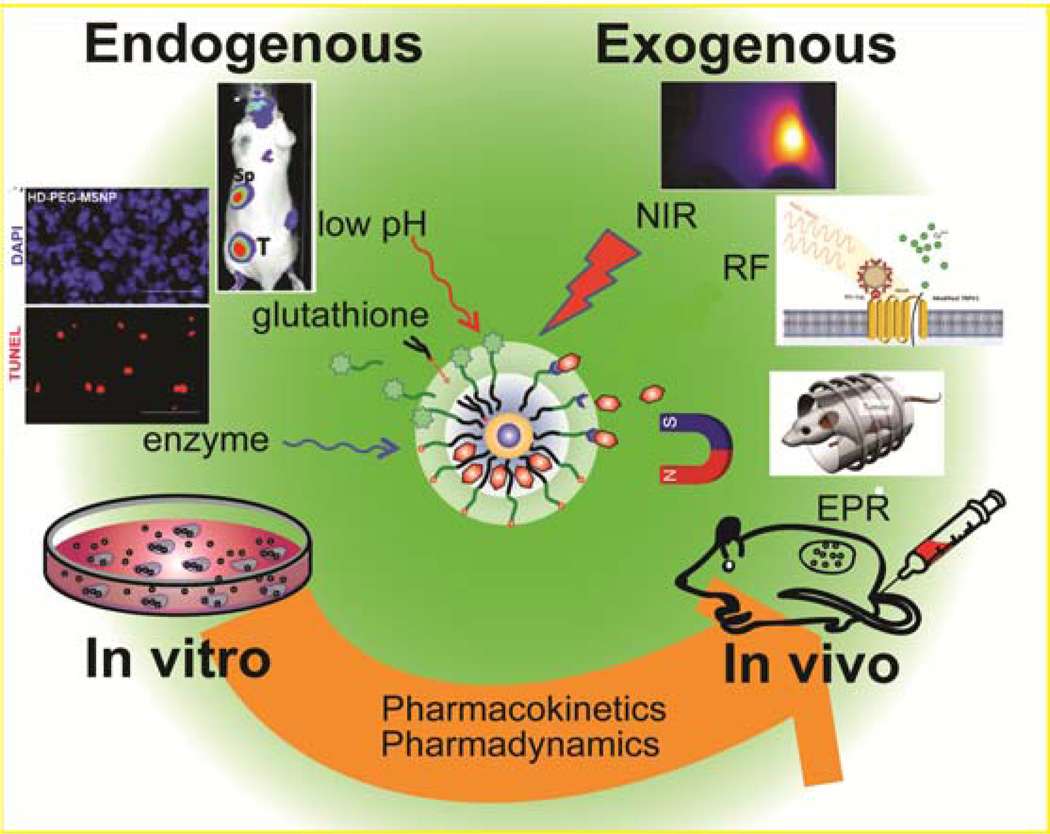
A further strategy for creating enhanced DDSs is the activation of therapeutics at the desired site to provide spatiotemporal localization of treatment. NPs that can be activated either exogenously (i.e., remotely) or endogenously (i.e., naturally) at the targeted site provide platforms for enhanced therapeutics (Figure 1) [18,19]. These attributes can be further coupled with imaging and therapeutic moieties to generate theragnostic systems [20]. This review will highlight selected strategies for triggered activation, providing examples of studies in both in vitro and in vivo systems.
Figure 1.
Triggered nanomaterials for endogenous and exogenous drug release in vitro and in vivo.
Exogenous activation
Exogenous activation is the triggered onset of therapy through the use of an external stimuli such as light or an alternating magnetic field. This external input provides the researcher or clinician with non-invasive spatial and temporal modulation of therapeutics at the molecular level, enhancing therapeutic efficacy.
Radio Frequency (RF) activation
The ability of RF radiation to penetrate deep into tissues with minimal energy loss has made RF activation a promising therapeutic strategy [21]. NPs can be designed to absorb and convert this energy into heat for therapeutic effects [22,23]. Mukherjee et al. observed gold NPs (AuNPs) conjugated with anti-EGFR (epidermal growth factor receptor) moieties induced cytotoxicity in Panc-1 cells when exposed to a RF field [24]. These fluorescently tagged theragnostic particles featured targeting, therapeutic properties, and imaging capabilities. More recently, a modified temperature-sensitive channel (TRPV1)-iron oxide NP complex was developed to control insulin production as an initial step toward non-invasive regulation of protein production [25]. The iron oxide NPs generated heat when exposed to a low frequency RF field, opening the TRPV1 channel and causing an influx of Ca2+ ions that triggered downstream pathways. Their system provided on-demand insulin production under RF stimulation, reducing the glucose level in mouse models.
Magnetic field induction
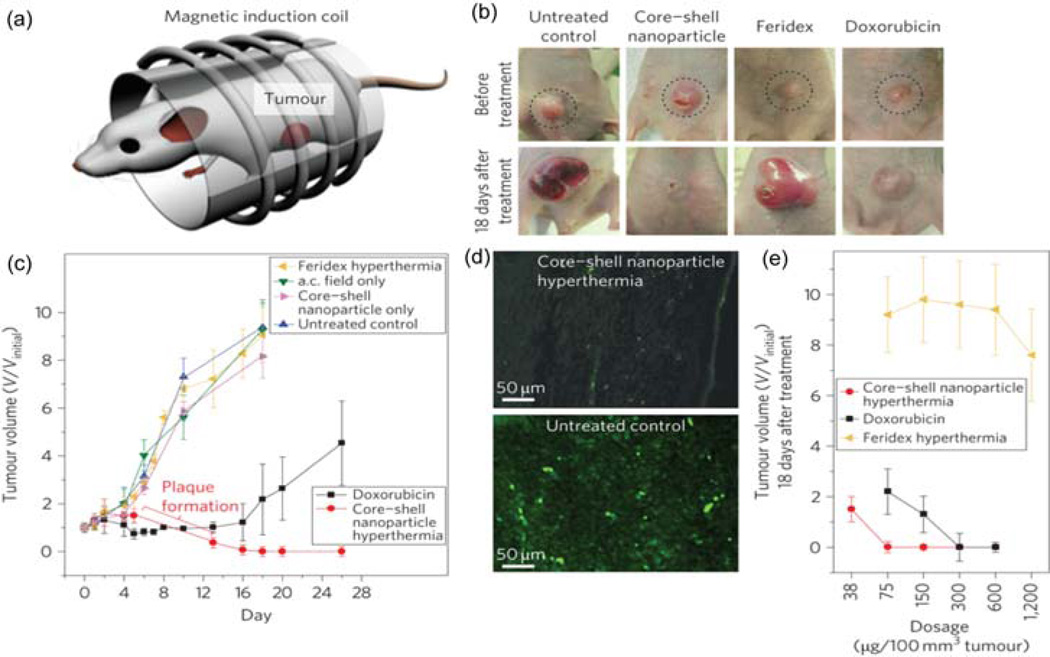
Analogous to RF fields, alternating magnetic fields (AMF) can be used to generate heat from NPs featuring appropriate magnetically-active core materials [26]. AMFs readily penetrate tissues and organs, making them excellent exogenous inducers. Recently, NPs with a core-shell structure were synthesized to optimize heating properties, with efficacy demonstrated by in vivo hyperthermia treatment of nude mice xenografted with human brain cancer cells (U87MG) (Figure 2(a),(b)) [27]. Over one month, the hyperthermia treated group of mice showed the elimination of the tumor tissue, while rapid tumor growth was observed using either control conditions or by using doxorubicin (Dox) (Figure 2(c)). A magnetic field gradient has also been used to selectively enhance the accumulation of anticancer drug-coated iron oxide NPs in cancer tissues [28]. Parak and co-workers reported that strong magnetic field gradients zhelp accumulate iron oxide NPs bound with mitoxantrone (an antineoplastic drug) at the tumor location using only 20% of the systemic dose required for complete remission of the tumors [29].
Figure 2.
(a) Magnetic hyperthermia treatment apparatus. (b) Mice xenografted with U87MG cancer cells before treatment (upper row, dotted circle) and 18 days after treatment (lower row). (c) Treatment with core–shell NP hyperthermia, Dox, Feridex hyperthermia, alternating current (a.c.) field only, core–shell NPs only or untreated control versus tumor volume (V/Vinitial). (d) Immunofluorescence images of the tumor region after nanoparticle mediated hyperthermia treatment (upper image) and the control tumor region (lower image). (e) Dose versus tumor volume measured 18 days after treatment. Reprinted with permission from [27].
Photothermal therapy
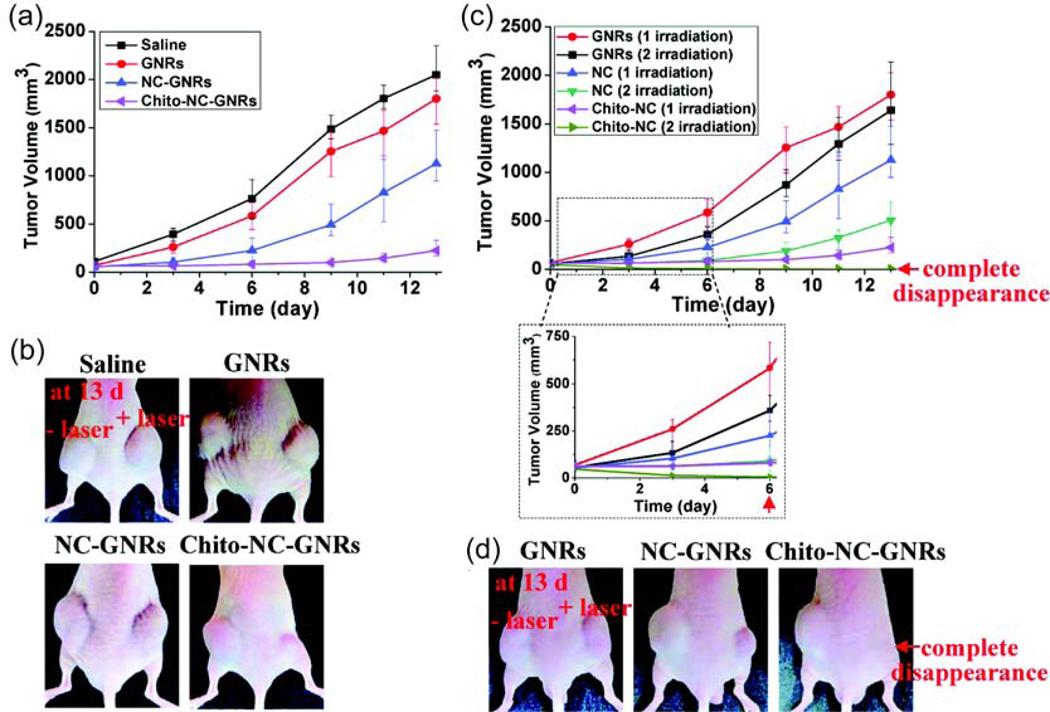
As shown above, hyperthermic strategies provide a promising tool for exogenous particle activation. However, most current hyperthermia methods require high-energy outputs and can cause significant collateral damage. Light activation by engineered NPs provides very rapid and hence localized heating. [30]. In one example, Tae and coworkers created a gold nanorod based photothermal therapy using tumor bearing mice (Figure 3) [31]. Gold nanorod loaded nanocarrier injections showed significant suppression of tumor growth when paired with NIR laser irradiation. Complete tumor disappearance was possible after two NIR laser irradiation treatments done at 24 hrs and 48 hrs after intravenous injection of the nanorods.
Figure 3.
Change in tumor size (a) and corresponding image (b) after one NIR laser irradiation (808 nm, 4W/cm2) at 24 hrs after injection with gold nanorods (GNR), polymer conjugated nanocarrier GNRs (NC-GNRs), and chitosan conjugated NC-GNRs (Chito-NC-GNRs). Change in tumor size (c) and corresponding image (d) after irradiation at both 24 hrs and 48 hrs after nanorod injection. Reprinted with permission from [31].
Photodynamic therapy
Photodynamic therapy (PDT) is a cancer therapy that uses the light activation of a photosensitizer to convert endogenous oxygen to reactive oxygen species (ROS) capable of killing cells [32,33]. Most of the efficient photosensitizers, however, are hydrophobic, resulting in solubility and biodistribution issues that hinder therapeutic efficiency [34]. In a recent report, a non-covalent hydrophobic silicon phthalocyanine photosensitizer was encapsulated into engineered amphiphilic poly(ethylene glycol) (PEG) coated AuNPs. Through passive targeting via the enhanced permeation and retention effect, the system showed rapid drug release and deep penetration of the drug into tumors within hours in mouse models [35].
Light activation
Radiation with light can also be used to break photo-cleavable bonds and produce chemical responses from DDS materials [36,37]. Horiike et al. developed a photo-responsive DDS for amine-containing biomolecules [38]. Irradiating these modified AuNPs with near-UV irradiation released conjugated histamine molecules, eliciting a biological response. Rotello et al. furthered this concept by using an engineered AuNP platform with a mixed monolayer of zwitterionic moieties and photocaged chemotherapeutics [39]. The zwitterionic ligands promoted solubility and limited cellular uptake while the photocaged ligands released the anti-cancer drug (5-fluorouracil) upon irradiation with near-UV (365 nm), with no significant cytotoxicity observed in the cells treated with only light or only AuNPs.
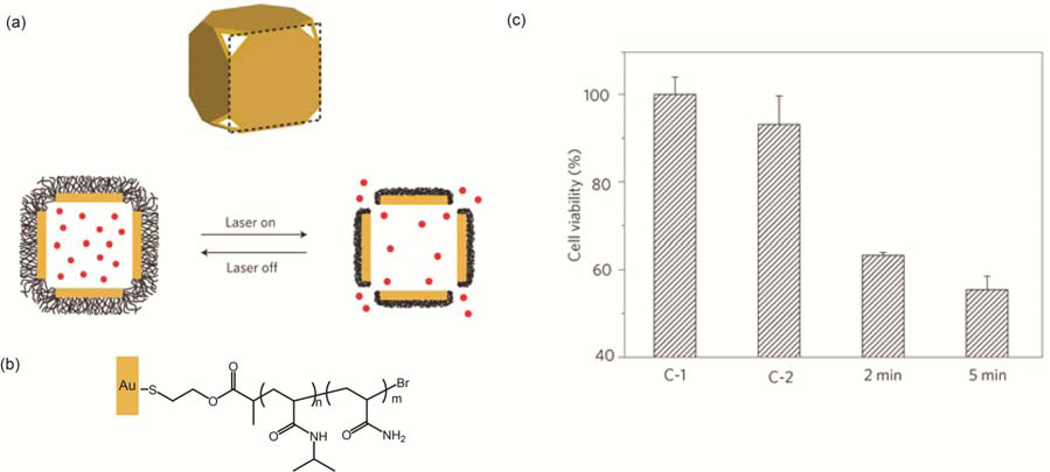
NPs with absorbance in the near-infrared (NIR) are particularly promising photoactivated DDSs due to the deep tissue penetration and photothermal conversion of NIR light [40,41]. Hamad-Schifferli el al. demonstrated the controlled release of DNA oligonucleotides from surface engineered gold nanorods using ultrafast laser irradiation at the longitudinal surface plasmon resonance peaks of the nanorods [42]. Xia and coworkers developed an Au nanocage/polymer DDS that was responsive to NIR irradiation (Figure 4(a)) [43]. The Au nanocages were functionalized with thiol terminated polymers (Figure 4(b)). Nisopropylacrylamide (NIPAAm) and acrylamide (AAm) were copolymerized controlling the ratio of NIPAAm to AAm to tune the release profile of the polymer shell. Upon NIR irradiation, the nanocage heated the local environment causing the polymer to collapse, releasing the encapsulated Dox through the pores of the nanocages (Figure 4(c)).
Figure 4.
Polymer coated Au nanocages for the controlled release of encapsulated Dox. (a) Structure of Au nanocage with thermally responsive polymer upon laser irradiation. (b) Molecular structure of p(NIPAAm-co-AAm) co-polymer on Au nanocage surface. (c) Cell viability of SK-BR-3 breast cancer cells exposed to: (C-1) 2 min of NIR laser pulse in the absence of nanocages, (C-2) 2 min of NIR laser pulse in the presence of Dox-free nanocages, and 2/5 min of NIR irradiation in presence of Dox-loaded nanocages. Reprinted with permission from [43].
Supramolecular release
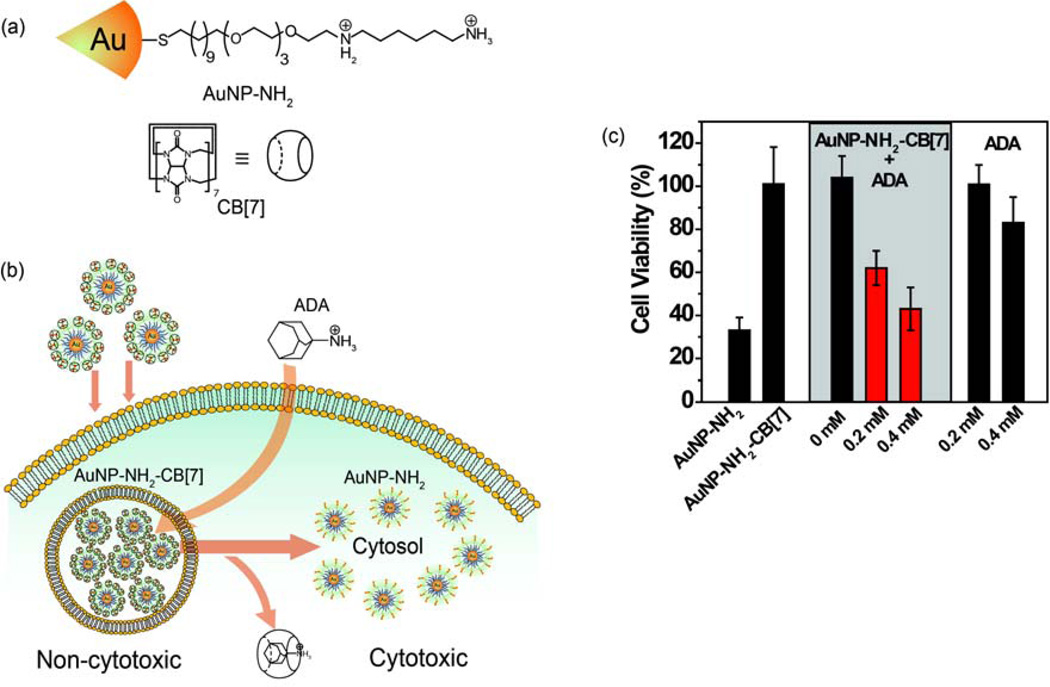
Functionalized nanoparticles can be engineered to assemble and disassemble spontaneously in response to a range of supramolecular triggers. Rotello et al. used a supramolecular host-guest system and an exogenous activator to provide triggered NP toxicity [44]. Toxic diaminohexane-terminated AuNPs (Figure 5(a)) were incubated with cucurbit[7]uril (CB[7]) forming a host-guest complex that significantly reduced the toxicity of the NPs. These NPs were rapidly taken up into MCF-7 cells, where they were trapped in endosomes and remained harmless. Treatment with 1-adamantylamine (ADA) displaced the CB[7], resulting in the NPs regaining their previous cytotoxicity (Figure 5(b), (c)).
Figure 5.
An exogenously controlled supramolecular nanotherapeutic. (a) Structure of AuNP-NH2 and CB[7]. (b) Triggered cytotoxicity by the dethreading of CB[7] from the AuNP-NH2 by ADA. (c) Exogenous triggering of cytotoxicity using ADA. To MCF-7 cells incubated with AuNP– NH2–CB[7] complexes, different concentrations (0, 0.2 and 0.4 mM) of ADA in medium were added generating cytotoxic AuNP-NH2. Reprinted with permission from [44].
Endogenous activation
Endogenous activation harnesses chemical differences between the heterogeneous environments within living systems to trigger therapeutic activity. These differences occur in healthy tissues; however tumors present multiple environments (e.g. pH, enzyme expression, and hypoxia) distinct from normal tissues. Endogenous activation can provide direct tumor targeting to precisely deliver drugs at a molecular level to the tumor site [45].
pH-based release mechanisms
Nanomaterials are generally taken up through endocytic pathways and as a result are exposed to the acidic environment of early and late endosomes and ultimately lysosomes [46]. This increase in acidity has been used therapeutically to provide efficient release of nitric oxide (NO) using AuNPs as carriers for the NO precursors [47,48]. NO was released through the decomposition of N-diazeniumdiolate functionalities present on the surface of the NPs. Further studies by Schoenfisch et al. went on to demonstrate the effective suppression of tumor growth in ovarian cancer cells [49].
In addition to the low pH values of the endocytic pathway, the limited delivery of oxygen and nutrients to solid tumors located away from blood vessels leads to a lower extracellular pH through the excess production of lactic and carbonic acid [50, 11]. Amiji et al. used paclitaxel encapsulated in pH-sensitive poly(ethylene oxide) modified poly(beta-amino ester) NPs to treat human ovarian adenocarcinoma bearing mice [51]. The NPs showed significant tumor growth inhibition when compared to free paclitaxel. In another study, Hammond and coworkers designed a pH sensitive layer-by-layer (LbL) NP based DDS [52]. Quantum dots (QD) were encompassed in trilayer architecture where a pH sensitive neutravidin layer is sandwiched between poly-l-lysine modified with iminobiotin and PEG modified with biotin. The iminobiotin-neutravidin interaction is highly pH dependent and the outer stealth PEG layer was removed in acidic conditions revealing a positively charged NP. This LbL thin film technique was used to control the retention time of NPs for in vitro and in vivo applications (Figure 6). The NPs readily accumulated in tumor tissue and demonstrated the ability to target hypoxic tumor environment through pH responsiveness.
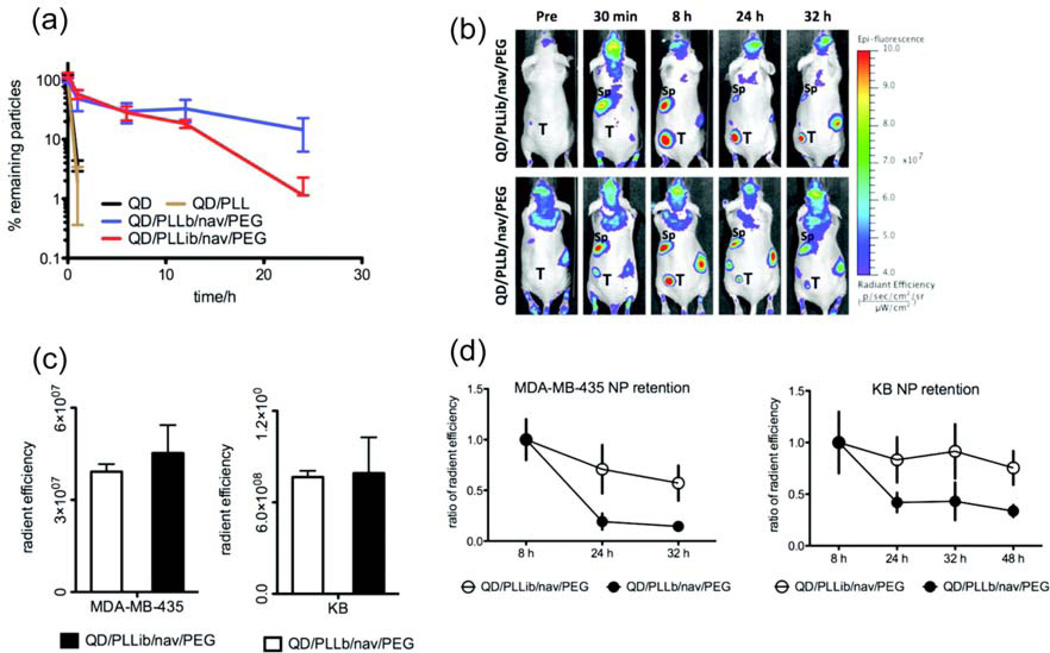
Figure 6.
Circulation profiles of pH sensitive NPs. (a) Amount of remaining NPs in circulation over time. Quantum dot (QD), poly-l-lysine (PLL), PPL modified with iminobiotin (PPLib), PLL functionalized with biotin (PLLb), neutravidin (nav), biotin end-functionalized PEG (PEG). (b) Accumulation and clearance of LbL NPs in mice with induced MDA-MB-435 tumors (left hind flank). Tumor (T), spleen (Sp). (c) LbL NP accumulation levels in tumors at 8 h. (d) Rate of clearance of NPs from tumors. Reprinted with permission from [52].
Enzymatic release
Tumor environments also have markedly different levels of certain enzymes than those found in healthy tissues [53]. For example, a matrix metalloproteinase (MMP) can be used not only to overcome elevated interstitial fluid pressure (IFP) but also to enhance NP uptake in solid tumors. The lack of functional lymphatic vessels and the vascular hyperpermeability inside solid tumors, increases IFP levels, interfering with the uptake of cancer therapeutics [54]. Fukumura et al. proposed a MMP-2 (high level of MMP-2 in tumor) responsive system where 100 nm NPs aggregates are broken down to 10 nm particles, overcoming the elevated IFP in solid tumors and allowing penetration deep into the tumor parenchyma [55]. MMPs can also be used to enhance the uptake of NPs coated with neutralized cell-penetrating peptides (CPPs) by activating the CPPs in the tumor parenchyma [56]. In an alternate strategy using phosphodiesterases, Shieh et al. conjugated the anticancer drug paclitaxel to Fe3O4 NPs through phosphate linkers as an enzymatically responsive prodrug [57]. Phosphodiesterases present in human cancer cells (OECM1) cleaved the linker releasing free paclitaxel. The use of these linkers was also expanded in the report to include other nanomaterials including AuNPs. In another report, Xing et al. presented a DDS using a protease-responsive, Dox–peptide coated, magnetic silica NP conjugate for intracellular drug delivery in vitro [58]. Using cancer cell line HT-29 that highly expresses protease cathepsin B, results showed that cells incubated with these particles had a significant decrease in cell viability as compared to the control NIH/3T3.
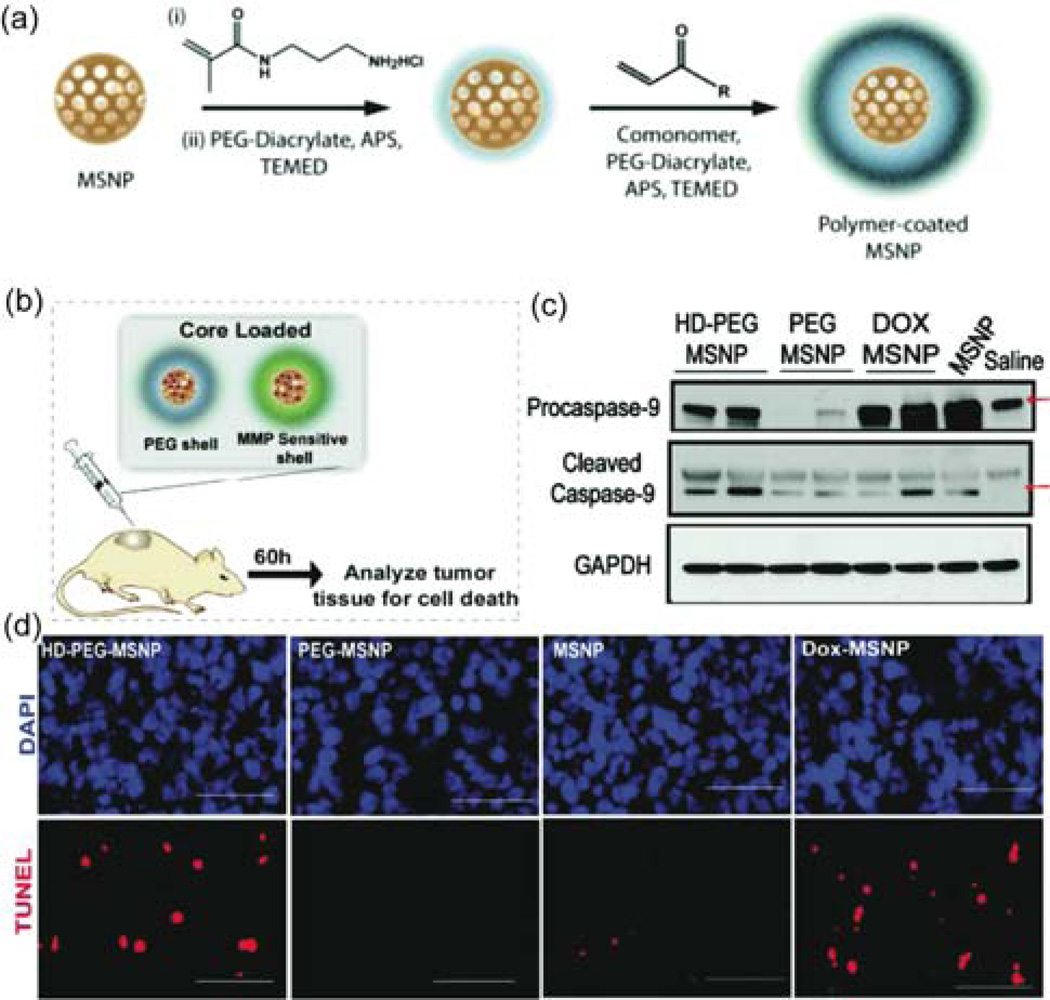
Bhatia et al. engineered biocompatible polymer-coated mesoporous silica NPs to release Dox in response to proteases present at tumor sites in an in vivo model (Figure 7(a),(b)) [59]. To determine efficacy of treatment the tumors were harvested and the tumor cell lysates were analyzed for caspase expression and cells were stained with terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and 4',6-diamidino-2-phenylindole (DAPI) (Figure 7 (c),(d)). The caspase levels and TUNEL staining of the tumor section showed the protease-sensitive polymer shell particle (HD-MMP) generated higher Dox related cell death in vivo in comparison to the controls.
Figure 7.
(a) Synthetic scheme for the NPs. (b) Protease triggered release from the loaded mesoporous silica NPs. (c) Immunoblots for caspase protein levels as well as the GAPDH from tumor lysates of animals 60 h after injection. (d) Staining for apoptotic cells in the collected tumor sections. Scale bars are 50 µm. Reprinted with permission from [59].
Glutathione-mediated release
Free thiol concentration inside the cell (1–10 mM of predominately glutathione (GSH)) is 100–1000 fold higher than that found in the extracellular environment (cysteine 8 µM, GSH 2 µM) [60]. DDSs based on GSH mediated release mechanisms are promising vectors for selective intracellular release [61,62,63]. Rotello and coworkers explored GSH mediated monolayer release from the surface of AuNPs [64]. The AuNPs with mixed monolayers of cationic ligands to improve cellular uptake and fluorescent BODIPY conjugated ligands were engineered to monitor thiol exchange in vitro. The fluorescence of BODIPY moieties is quenched when in close proximity to the core of the AuNP [65]. Upon incubation with human liver cells (Hep G2), BODIPY fluorescence was observed in a dose dependent manner to free GSH. Further studies probed the role of surface charge on GSH exchange showing that NPs with cationic mixed monolayers had greater exchange levels relatively to their anionic analogues [66].
In a later study, Rotello, Vachet, and colleagues measured the intracellular stability of thiols bound to QDs using a combination of mass spectrometric techniques (Figure 8(a)) [67]. QDs with different core sizes were synthesized and then functionalized with the same cationic ligand via a place exchange reaction [68]. These QDs were incubated with HeLa cells and the stability of their monolayers was determined by comparing the intracellular concentration of cadmium (determined by inductively coupled plasma mass spectroscopy) versus the amount of ionized ligands (determined by laser desorption ionization mass spectrometry). The results showed the larger the surface area of the QD, the less stable the thiol ligand was to intracellular exchange (Figure 8(b)). Furthermore, bidentate ligands showed enhanced stability over the monodentate analogue.
Figure 8.
Intracellular stability of QD monolayers determined by mass spectrometry. (a) Structure of QDs studied. (b) Percentage of monolayers retained within HeLa cells at 3, 6, 9, and 24 hrs. Reprinted with permission from [67].
In research that integrates covalent and supramolecular approaches, Kim et al. engineered a non-covalently loaded DDS through the use of cyclodextrin functionalized AuNPs [69]. The surface of the AuNPs was further functionalized with anti-EGFR antibodies and PEG ligands to target the DDS and prevent premature degradation of the DDS, respectively. The anti-cancer drug β-lapachone, was loaded into the cyclodextrin moieties that decorated the surface of the AuNPs and were released from the surface upon exposure to GSH. In another study, Kotov et al. designed a 6-mercaptopurine-9-β-D-ribofuranoside conjugated AuNP based prodrug DDS [70]. The antiproliferative effects of the drug were observed in K-562 leukemia cells after GSH-mediated release.
There is a large gap between the behavior of cells in the 2D environment found in cell culture techniques and the complex heterogeneous environment found in tissues and tumors [71]. Three-dimensional, multicellular tumor cylindroids that mimic the poor vascularization of tumors in vivo provide a useful in vitro testbed to predict DDS properties in vivo [72]. Real time observation of glutathione mediated release within these tumor cylindroids was observed with Dox and fluorescein functionalized AuNPs [73]. These studies showed that cationic NPs were taken into cells preferentially while anionic particles showed deeper cylindroid penetration. This result provides an indication of how influential the role surface engineering of NPs can be in determining tissue penetration of NPs. Pharmacokinetic (PK) and pharmacodynamic (PD) factors also need to be considered when using tumor cylindroids. Harashima and coworkers investigated the discrepancies between in vitro and in vivo systems in terms of different PKs and PDs using octaarginine (R8) modified engineered lipid NPs in mice for siRNA delivery [74]. These results showed a remarkable difference in the PKs between the two types of systems. This contrast indicates that while in vitro approaches containing in vivo parameters may be helpful during the early stages of preclinical development to eliminate potentially dangerous engineered NPs, there is no simple test to predict the toxicity of an engineered NP in clinical applications [75,76]. Therefore, advances in cell culture systems reflecting in vivo parameters (e.g., microfluidic technologies that mimic the in vivo conditions) are required [77].
Conclusions
Designing nanomaterials for triggered release through exogenous and/or endogenous activation is a powerful strategy for the development of highly effective DDSs. The spatial and temporal control yielded from triggered DDSs provides a potent platform for personalized medicine. While the studies shown above are promising, many challenges remain, including exogenous activation strategies with deep tissue penetration. Furthermore, the highly heterogeneous environments found in tumors present a formidable obstacle for controlled drug distribution and targeting efficiency. Nanomaterials are uniquely poised to address these issues. Through appropriate engineering, NPs can control interactions with biological systems and influence the pharmacodynamics and pharmacokinetics of therapeutics. Further research into the structure of tumor environments, homogeneous tissue penetration, and cellular uptake will expand the possible strategies to improve delivery and overall patient health. Coupling of this enhanced understanding of cancer biology and physiology with our increasing ability to engineer nanomaterials will be paramount in the development of personalized chemotherapies for cancer and other diseases.
Highlights.
Triggered nanoparticles provide therapeutic benefits
Exogenously mediated release of therapeutics from nanoparticle surfaces using external stimuli is discussed
Endogenously mediated release using environmental differences in living systems is overviewed.
Developing multifunctional nanoparticles allows for theragnostic applications
Acknowledgements
This work was supported by grants from the NIH (GM077173 and EB014277).
Biographies

Chang Soo Kim received his B.S. (2001) from Sung Kyun Kwan University, South Korea, M.S.(2004) from California State University, Long Beach and Ph.D (2010) from University of California, Irvine under the supervision of Prof. Young Jik Kwon and Zhongping Chen in Chemical Engineering. Currently, he is working as a postdoctoral researcher in the Department of Chemistry at the University of Massachusetts-Amherst under the guidance of Professor Vincent M. Rotello. His current research is focused on therapeutic nanocapsule synthesis and imaging application with in vivo system.

Bradley Duncan received his H.A.B. degree in Chemistry from Saint Anselm College in 2009. Currently, he is a Ph.D. student in the Department of Chemistry at the University of Massachusetts-Amherst under the guidance of Professor Vincent M. Rotello. His research focuses on the self-assembly of nanoparticles at the liquid–liquid interface and the fabrication of colloidal micro/nanocapsules for delivery applications.

Brian Creran received his A.B in Chemistry from the College of the Holy Cross in Worcester, MA in 2004. Presently, he is a graduate student in the Department of Chemistry at the University of Massachusetts at Amherst, under the guidance of Professor Vincent M. Rotello. His current research uses nanoparticle inkjet printing to create substrates for biological and materials applications.

Vincent M. Rotello is the Goessmann Professor of Chemistry at the University of Massachusetts. He has received the NSF CAREER, Cottrell Scholar, Dreyfus Teacher-Scholar, Sloan Fellowship, and the Langmuir Lectureship, and is a Fellow of the American Association for the Advancement of Science and the Royal Society of Chemistry (U.K.). He is an Executive Editor for Advanced Drug Delivery Reviews. His research focuses on using chemistry to engineer the interface between hard and soft materials.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yu X, Zhang Y, Chen C, Yao Q, Li M. Biochim. Biophys. Acta. 2010;1805:97–104. doi: 10.1016/j.bbcan.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schroeder A, Heller DA, Winslow MM, Dahlman JE, Pratt GW, Langer R, Jacks T, Anderson DG. Nat. Rev. Cancer. 2012;12:39–50. doi: 10.1038/nrc3180. [DOI] [PubMed] [Google Scholar]
- 3.Allen TM, Cullis PR. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 4.Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A, Natl J. Cancer Inst. 2006;98:335–344. doi: 10.1093/jnci/djj070. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh P, Han G, De M, Kim CK, Rotello VM. Adv. Drug Deliv. Rev. 2008;60:1307–1315. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 7.Sawant RM, Hurley JP, Salmaso S, Kale A, Tolcheva E, Levchenko TS, Torchilin VP. Bioconjug. Chem. 2006;17:943–949. doi: 10.1021/bc060080h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho EC, Zhang Q, Xia YN. Nat. Nanotechnol. 2011;6:385–391. doi: 10.1038/nnano.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lees P, Cunningham FM, Elliott J, Vet J. Pharmacol. Ther. 2004;27:397–414. doi: 10.1111/j.1365-2885.2004.00620.x. [DOI] [PubMed] [Google Scholar]
- 10.Bryant B, Knights K, Salerno E. Pharmacology for health professionals. 1st ed. Mosby, Sydney, Australia: 2003. [Google Scholar]
- 11.Farokhzad OC, Langer R. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 12.Albanese A, Tang PS, Chan WC. Annu. Rev. Biomed. Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 13.Kang S, Park T, Chen X, Dickens G, Lee B, Lu K, Rakhilin N, Daniel S, Jin MM. Biomaterials. 2011;32:3487–3498. doi: 10.1016/j.biomaterials.2011.01.046. [DOI] [PubMed] [Google Scholar]
- 14.Peer D, Zhu P, Carman CV, Lieberman J, Shimaoka M. Proc. Natl. Acad. Sci. U. S. A. 2007;104:4095–4100. doi: 10.1073/pnas.0608491104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petros RA, DeSimone JM. Nat. Rev. Drug. Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 16.Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, Tamanoi F, Zink JI. ACS Nano. 2008;2:889–896. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu ZJ, Carboni R, Quercio MJ, Jr, Yan B, Miranda OR, Anderton DL, Arcaro KF, Rotello VM, Vachet RW. Small. 6:2261–2265. doi: 10.1002/smll.201000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ge J, Neofytou E, Cahill TJ, 3rd, Beygui RE, Zare RN. ACS Nano. 2012;6:227–233. doi: 10.1021/nn203430m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nam J, Won N, Jin H, Chung H, Kim S. J. Am. Chem. Soc. 2009;131:13639–13645. doi: 10.1021/ja902062j. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy JR, Weissleder R. Adv. Drug. Deliv. Rev. 2008;60:1241–1251. doi: 10.1016/j.addr.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cherukuri P, Glazer ES, Curley SA. Adv. Drug Delivery Rev. 2010;62:339–345. doi: 10.1016/j.addr.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young JH, Wang MT, Brezovich IA. Electron. Lett. 1980;16:358–359. [Google Scholar]
- 23.Richardson HH, Hickman ZN, Govorov AO, Thomas AC, Zhang W, Kordesch ME. Nano Lett. 2006;6:783–788. doi: 10.1021/nl060105l. [DOI] [PubMed] [Google Scholar]
- 24.Curley SA, Cherukuri P, Briggs K, Patra CR, Upton M, Dolson E, Mukherjee P. J. Exp. Ther. Oncol. 2008;7:313–326. [PubMed] [Google Scholar]
- 25.Stanley SA, Gagner JE, Damanpour S, Yoshida M, Dordick JS, Friedman JM. Science. 2012;336:604–608. doi: 10.1126/science.1216753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu TY, Hu SH, Liu DM, Chen SY, Chen IW. Nano Today. 2009;4:52–65. [Google Scholar]
- 27.Lee JH, Jang JT, Choi JS, Moon SH, Noh SH, Kim JW, Kim JG, Kim IS, Park KI, Cheon J. Nat. Nanotechnol. 2011;6:418–422. doi: 10.1038/nnano.2011.95. [DOI] [PubMed] [Google Scholar]
- 28.Cheng Z, Al Zaki A, Hui JZ, Muzykantov VR, Tsourkas A. Science. 2012;338:903–910. doi: 10.1126/science.1226338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexiou C, Schmid RJ, Jurgons R, Kremer M, Wanner G, Bergemann C, Huenges E, Nawroth T, Arnold W, Parak FG. Eur Biophys J. 2006;35:446–450. doi: 10.1007/s00249-006-0042-1. [DOI] [PubMed] [Google Scholar]
- 30.Hauck TS, Jennings TL, Yatsenko T, Kumaradas JC, Chan WCW. Adv. Mater. 2008;20:3832–3838. [Google Scholar]
- 31.Choi WI, Kim JY, Kang C, Byeon CC, Kim YH, Tae G. ACS Nano. 2011;5:1995–2003. doi: 10.1021/nn103047r. [DOI] [PubMed] [Google Scholar]
- 32.Castano AP, Mroz P, Hamblin MR. Nat. Rev. Cancer. 2006;6:535–545. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dolmans DE, Fukumura D, Jain RK. Nat. Rev. Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 34.Konan YN, Gurny R, Allemann E, Photochem J. Photobiol. B. 2002;66:89–106. doi: 10.1016/s1011-1344(01)00267-6. [DOI] [PubMed] [Google Scholar]
- 35.Cheng Y, Meyers JD, Broome AM, Kenney ME, Basilion JP, Burda C. J. Am. Chem. Soc. 2011;133:2583–2591. doi: 10.1021/ja108846h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCoy CP, Rooney C, Edwards CR, Jones DS, Gorman SP. J. Am. Chem. Soc. 2007;129:9572–9573. doi: 10.1021/ja073053q. [DOI] [PubMed] [Google Scholar]
- 37.Mayer G, Heckel A. Angew. Chem. Int. Ed. Engl. 2006;45:4900–4921. doi: 10.1002/anie.200600387. [DOI] [PubMed] [Google Scholar]
- 38.Nakanishi J, Nakayama H, Shimizu T, Ishida H, Kikuchi Y, Yamaguchi K, Horiike Y. J. Am. Chem. Soc. 2009;131:3822–3823. doi: 10.1021/ja809236a. [DOI] [PubMed] [Google Scholar]
- 39.Agasti SS, Chompoosor A, You CC, Ghosh P, Kim CK, Rotello VM. J. Am. Chem. Soc. 2009;131:5728–5729. doi: 10.1021/ja900591t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weissleder R. Nat. Biotechnol. 2001;19:316–317. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- 41.von Maltzahn G, Park JH, Lin KY, Singh N, Schwöppe C, Mesters R, Berdel WE, Ruoslahti E, Sailor MJ, Bhatia SN. Nat. Mater. 2011;10:545–552. doi: 10.1038/nmat3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wijaya A, Schaffer SB, Pallares IG, Hamad-Schifferli K. ACS Nano. 2009;3:80–86. doi: 10.1021/nn800702n. [DOI] [PubMed] [Google Scholar]
- 43.Yavuz MS, Cheng Y, Chen J, Cobley CM, Zhang Q, Rycenga M, Xie J, Kim C, Song KH, Schwartz AG, Wang LV, Xia Y. Nat. Mater. 2009;8:935–939. doi: 10.1038/nmat2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim C, Agasti SS, Zhu Z, Isaacs L, Rotello VM. Nat. Chem. 2010;2:962–966. doi: 10.1038/nchem.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu C, Chen C, Lai J, Chen J, Mu X, Zheng J, Zhao Y. Chem Commun (Camb) 2008:2662–2664. doi: 10.1039/b804886j. [DOI] [PubMed] [Google Scholar]
- 46.Schwagler M, Nowak C, Hoffmann J, Schartl W. J. Phys. Chem. C. 2009;113:15124–15132. [Google Scholar]
- 47.Polizzi MA, Stasko NA, Schoenfisch MH. Langmuir. 2007;23:4938–4943. doi: 10.1021/la0633841. [DOI] [PubMed] [Google Scholar]
- 48.Rothrock AR, Donkers RL, Schoenfisch MH. J. Am. Chem. Soc. 2005;127:9362–9363. doi: 10.1021/ja052027u. [DOI] [PubMed] [Google Scholar]
- 49.Stevens EV, Carpenter AW, Shin JH, Liu JS, Der CJ, Schoenfisch MH. Molecular Pharmaceutics. 2010;7:775–785. doi: 10.1021/mp9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Minchinton AI, Tannock IF. Nat. Rev. Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 51.Devalapally H, Shenoy D, Little S, Langer R, Amiji M. Cancer Chemother. Pharmacol. 2007;59:477–484. doi: 10.1007/s00280-006-0287-5. [DOI] [PubMed] [Google Scholar]
- 52.Poon Z, Chang D, Zhao XY, Hammond PT. ACS Nano. 2011;5:4284–4292. doi: 10.1021/nn200876f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koblinski JE, Ahram M, Sloane BF. Clin. Chim. Acta. 2000;291:113–135. doi: 10.1016/s0009-8981(99)00224-7. [DOI] [PubMed] [Google Scholar]
- 54.Jain RK, Stylianopoulos T. Nat. Rev. Clin. Oncol. 2010;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wong C, Stylianopoulos T, Cui JA, Martin J, Chauhan VP, Jiang W, Popovic Z, Jain RK, Bawendi MG, Fukumura D. Proc. Natl. Acad. Sci. U. S. A. 2011;108:2426–2431. doi: 10.1073/pnas.1018382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olson ES, Jiang T, Aguilera TA, Nguyen QT, Ellies LG, Scadeng M, Tsien RY. Proc. Natl. Acad. Sci. U. S. A. 2010;107:4311–4316. doi: 10.1073/pnas.0910283107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hwu JR, Lin YS, Josephrajan T, Hsu MH, Cheng FY, Yeh CS, Su WC, Shieh DB. J. Am. Chem. Soc. 2009;131:66–68. doi: 10.1021/ja804947u. [DOI] [PubMed] [Google Scholar]
- 58.Yang Y, Aw J, Chen K, Liu F, Padmanabhan P, Hou Y, Cheng Z, Ying B. Chem.--Asian J. 2011;6:1381–1389. doi: 10.1002/asia.201000905. [DOI] [PubMed] [Google Scholar]
- 59.Singh N, Karambelkar A, Gu L, Lin K, Miller JS, Chen CS, Sailor MJ, Bhatia SN. J. Am. Chem. Soc. 2011;133:19582–19585. doi: 10.1021/ja206998x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jones DP, Carlson JL, Samiec PS, Sternberg P, Jr, Mody VC, Jr, Reed RL, Brown LA. Clin. Chim. Acta. 1998;275:175–184. doi: 10.1016/s0009-8981(98)00089-8. [DOI] [PubMed] [Google Scholar]
- 61.Anderson ME. Chem. Biol. Interact. 1998;111–112:1–14. doi: 10.1016/s0009-2797(97)00146-4. [DOI] [PubMed] [Google Scholar]
- 62.Sies H. Free Radic. Biol. Med. 1999;27:916–921. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 63.Jones DP, Carlson JL, Mody VC, Cai J, Lynn MJ, Sternberg P. Free Radic. Bio.l Med. 2000;28:625–635. doi: 10.1016/s0891-5849(99)00275-0. [DOI] [PubMed] [Google Scholar]
- 64.Hong R, Han G, Fernandez JM, Kim BJ, Forbes NS, Rotello VM. J. Am. Chem. Soc. 2006;128:1078–1079. doi: 10.1021/ja056726i. [DOI] [PubMed] [Google Scholar]
- 65.Sapsford KE, Berti L, Medintz IL. Angew. Chem. Int. Ed. Engl. 2006;45:4562–4589. doi: 10.1002/anie.200503873. [DOI] [PubMed] [Google Scholar]
- 66.Chompoosor A, Han G, Rotello VM. Bioconjug. Chem. 2008;19:1342–1345. doi: 10.1021/bc8000694. [DOI] [PubMed] [Google Scholar]
- 67.Zhu Z-J, Yeh Y-C, Tang R, Yan B, Tamayo J, Vachet RW, Rotello VM. Nat. Chem. 2011;3:963–968. doi: 10.1038/nchem.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yeh Y-C, Patra D, Yan B, Saha K, Miranda OR, Kim CK, Rotello VM. Chem. Commun. 2011;47:3069–3071. doi: 10.1039/c0cc04975a. [DOI] [PubMed] [Google Scholar]
- 69.Park C, Youn H, Kim H, Noh T, Kook YH, Oh ET, Park HJ, Kim C, Mater J. Chem. 2009;19:2310–2315. [Google Scholar]
- 70.Podsiadlo P, Sinani VA, Bahng JH, Kam NWS, Lee J, Kotov NA. Langmuir. 2007;24(2):568–574. doi: 10.1021/la702782k. [DOI] [PubMed] [Google Scholar]
- 71.Yamada KM, Cukierman E. Cell. 2007;130:601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 72.Kim BJ, Forbes NS. Biotechnol. Bioeng. 2008;101:797–810. doi: 10.1002/bit.21985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim B, Han G, Toley BJ, Kim CK, Rotello VM, Forbes NS. Nat. Nanotechnol. 2010;5:465–472. doi: 10.1038/nnano.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hayashi Y, Noguchi Y, Harashima H. Control Release. 2012;161:757–762. doi: 10.1016/j.jconrel.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 75.Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE. Mol. Pharm. 2008;5:487–495. doi: 10.1021/mp800032f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lilienblum W, Dekant W, Foth H, Gebel T, Hengstler JG, Kahl R, Kramer PJ, Schweinfurth H, Wollin KM. Arch. Toxicol. 2008;82:211–236. doi: 10.1007/s00204-008-0279-9. [DOI] [PubMed] [Google Scholar]
- 77.Valencia PM, Farokhzad OC, Karnik R, Langer R. Nat. Nanotechnol. 2012;7:623–629. doi: 10.1038/nnano.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]