Abstract
The story of oxytocin (OXT) began long ago in evolutionary terms with its recognition as a classical neurohypophyseal hormone important for lactation and uterine contraction. With the recent discovery of its local actions in the brain, its previously-unappreciated diverse functions in regulating social behaviors and metabolic physiology are emerging. In light of metabolic control, OXT has been shown to induce feeding restriction and body weight lowering through acting on brain regulatory regions, in particular the hypothalamus. Studies from pharmacologic interventions and genetic manipulations demonstrated that OXT can play significant roles in affecting glucose metabolism as well as insulin secretion and lipolysis, many of those functions being regulated both centrally and peripherally. Also excitingly, recent therapeutic success was obtained in clinical endeavor showing that OXT nasal spray effectively induced weight loss and metabolic improvement in human patients with obesity, thus further indicating OXT as a tangible drug target for treating obesity and metabolic complications. In addition to the native form, OXT-derived analogues have been found effective in inducing body weight control and glucose balance. Altogether, all recent advances in studying OXT and metabolic regulation has promoted a promising foundation for the therapeutic strategy of developing innovative OXT peptidyl drugs for the treatment of obesity and related metabolic diseases.
Introduction
The increasing prevalence of obesity and the associated metabolic syndrome disorders have made them challenging public health problems, and identifying effective therapeutic strategies is more imperative than ever. Although, quite a few anti-obesity drugs have been launched in the market over the past decades, they have either failed or been red flagged due to severe side effects. Alternatively, bariatric surgery has proved effective in lowering body weight and other metabolic complications under severe obesity conditions, but on the other hand, they may pose serious risks and side effects, and evaluations of long-term outcomes still remain to be followed up. Of interest, the continuing quest for newer and better therapeutic approaches have resulted in recent discovery of peptide-based dugs such as intestinal peptide glucagon-like peptide-1 and dipeptidyl peptidase-4 inhibitors in controlling obesity as well as diabetes [1,2]. The promising clinical success of peptide therapeutics is seemingly suggesting a research direction towards the therapeutic development of neuropeptides which are known to regulate body weight and glucose homeostasis. Indeed, compelling evidences have been documented to implicate that obesity and metabolic syndrome development are significantly attributed to the dysregulation of the central nervous system (CNS). In light of the hypothalamus, recent research has conclusively recognized that disruption of various hypothalamic pathways can be responsible for disordered feeding and energy balance which underlie the development of obesity and related health problems [3–7]. Taking lead from these findings, research has made efforts to target the neural components for therapeutic options, based on hypothalamic neural circuits and neuropeptides that are critical for maintaining energy and body weight balance, and OXT is one such neuropeptide. In addition to the emerging appreciated neuropsychiatric functions of OXT [8–11], OXT was recently revealed to be critical for metabolic regulation [12–17] and can work as a therapeutic approach for obesity and related metabolic diseases [15–19], and this review focuses on discussing the role of OXT in metabolic physiology and in particular its therapeutic potentials for obesity and diabetes.
Classical functions of OXT in reproduction
The hypothalamo-neurohypophyseal neuropeptide OXT is classically known for its functions in reproductive physiology of mammalian females, such as uterine contractions and delivery, milk ejection and maternal care. Such hormonal actions are mediated through OXT secretion into the circulation via stimulation of the OXT system which incorporates OXT and its receptor [20]. It has been shown in rats that suckling is one of most intense stimuli of the OXT system, resulting in burst activity of OXT neurons and pulsatile peptide secretion into the blood during the milk ejection reflex [20,21]. Also, exogenous OXT administration can improve sexual motivation and sexual performance in females, and further studies showed that OXT acts as a neurotransmitter that centrally regulates reproduction including parental and sexual behaviors [21]. The actions of OXT in uterine contraction and milk secretion are based on OXT synthesis in the magnocellular neurons of hypothalamic paraventricular nucleus (PVN) as well as the supra optic nucleus (SON) and its secretion into the circulation via projections of OXT neurons in the posterior pituitary. In addition to aiding female reproductive activities, central OXT pathways are recognized to have roles in sexual behavior and reproduction of males [10,21,22]. Through multiple signaling pathways in the CNS and the peripheral nerve system, OXT can work to control reproductive or sexual behaviors of males including penile erection, ejaculation, post-ejaculatory detumescence to post-orgasm refraction [21,22]. Indeed, administration of OXT antagonist centrally greatly reduces sexual interest and performance of males without affecting general locomotor activity [21], and similar effects of OXT antagonist were seen on female sexual behaviors [21,23], reinforcing the importance of central regulation on physiological functions of OXT.
Non-classical neuropsychiatric functions of OXT
In addition to its classical role in reproductive physiology, growing evidence indicates that OXT plays important roles for a wide spectrum of neuropsychiatric functions. Several lines of findings have suggested that these new functions of OXT include modulating neuroendocrine reflexes and establishing complex pro-social behaviors which range from the domains of maternal behavior, infant attachment, emotional control, pair bonding, reward, and social signals-cognition linkage, and the positive neuropsychiatric effects of OXT even encompass moral judgment, selfless decision-making and interpersonal relationships [8–11]. Promoted by this background, OXT has recently attained research attentions as a potential novel treatment for psychiatric disorders such as social anxiety, autism and borderline personality disorder and supportively, individuals with autism tend to have lower OXT levels [11,24]. Clinical studies albeit within small samples, have shown that OXT treatment improved social, aggressive and paranoid behavior of autistic patients [11]. Infusion of OXT has been shown to reduce repetitive behaviors while improving speech recognition and social cognition [11]. Also, compelling clinical trial results indicated that OXT can improve both positive and negative symptoms in schizophrenia [25]. Besides, OXT exhibits early-life neuroprotection and anti-stress properties, further stimulating research on therapeutic use of OXT in neuropsychiatric disorders [25]. All these evidences suggest that OXT and OXT-like drugs bear potentials of therapeutics for treating neuropsychiatric disorders.
Role of OXT in feeding and metabolic regulation
Feeding behavior, bodyweight and energy balance are critically regulated by the hypothalamus in the CNS [7,26–28]. Established understandings have depicted that the CNS controls feeding and energy balance through the hypothalamic actions of hormonal signals such as leptin and insulin, which modulate the production of orexigenic neuropeptide Y and agouti-related peptide as well as anorexigenic pro-opiomelanocortin derived α-melanocyte stimulating hormone and cocaine- and amphetamine-regulated transcript, and these neuropeptides are released to activate or inhibit melanocortin receptors (MC3/4R) in second-order neurons to reduce or increase food intake [3]. Of interest, some MC3/4R-expressing cells located in the PVN are OXT neurons [12], and many of classical and nonclassical actions of OXT are known to be associated with feeding changes [29–32]. Indeed, using pharmacological approaches via brain ventricular injections, the anorectic action of OXT was documented decades ago [32,33], and recent studies further linked OXT to the hypothalamus-brain stem circuits that work to inhibit feeding [12–14]. However, the physiological and disease relevance of OXT were unexplored until recently when researchers found that obesity is significantly attributed to OXT release defect, and OXT treatment was able to effectively correct overeating and obesity [15–19]. Further, it was discovered that OXT plays a vital role of OXT in integrating circadian control with metabolic regulation, and OXT treatment can amend the circadian dysregulation of metabolic physiology leading to reduction of obesity [15]. In terms of circadian physiology, there is a striking similitude between the circadian rhythm of food intake patterns and circulating OXT levels across a 24-hour period [15], suggesting that OXT is involved in circadian control of feeding by the hypothalamus. Agreeably, feeding has been associated with changes in OXT neuronal activity [34] and elevated plasma OXT levels [35]. Significance of OXT for feeding regulation was also appreciated in reproduction-related physiology, as lack of central OXT action in mid-pregnancy was shown to be a basis of maternal hyperphagia [29]. Also, the effects of OXT in feeding and body weight regulation were further supported by the observations that genetic deficiency of OXT [36,37] or OXT receptor [38] both led to hyperphagia and weight gain in mice. In addition to feeding regulation, evidence exists showing that OXT has an effect in promoting energy expenditure [15,16,18], thus providing a parallel mechanism for body weight regulation by OXT. In summary, as elucidated in Figure 1, hypothalamic oxytocinergic machinery regulates body weight homeostasis through the actions OXT on feeding and energy expenditure.
Figure 1. Role of OXT in metabolic physiology and disease intervention.
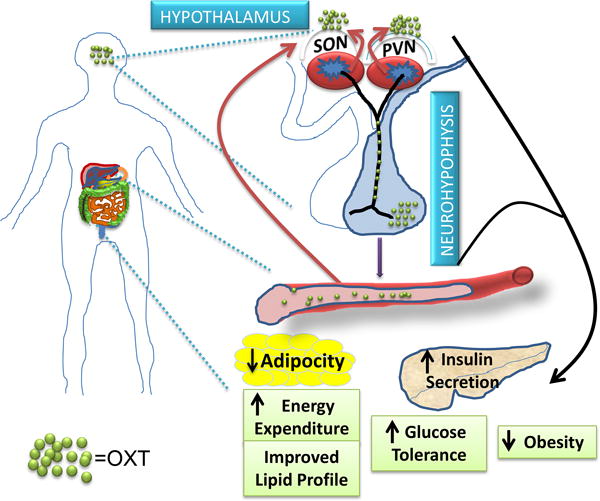
OXT is released into the brain to work on brain regulatory regions leading to control of feeding and energy balance. OXT released in the circulation and peripheral tissues, while potentially generating local effects in metabolic tissues, can employ a positive feedback loop to promote central OXT release to induce the central metabolic actions of OXT. Altogether, OXT can incorporate multiple mechanisms to regulate energy and metabolic homeostasis, and represents a new-generation peptidyl drug target for obesity and diabetes therapeutics.
Metabolic action of OXT via central vs. peripheral release
The histologically appreciated effect of OXT in appetite suppression did not immediately lead to an active query of whether it could be therapeutically useful for overnutrition-induced diseases such as obesity and diabetes. This situation was probably related to the paucity of knowledge regarding the mechanisms how OXT acts to affect metabolism, and the complex nature of OXT release represents another stumbling block which suppressed research from aiming to understand its relevance to metabolic physiology. Indeed, based on studies from basic neurobiology, OXT has dual actions mediated by its peripheral versus central release, and various stimuli can differentially determine the release of OXT from the two kinds of oxytocinergic neurons in the hypothalamus [11,31]. Depending on the quality and intensity of a given stimulus, the magnocellular neurosecretory neurons of the SON and PVN are excited to release OXT into the circulation via the neurohypophysial projections and axonal transportation, and the biological functions of this release are classically related to milk letdown and uterine contraction. OXT in the parvocellular neurons of the PVN (which have central projections) can release within the brain via direct axonal connections to the critical brain regions or by ‘volume diffusion’ effects in areas that express OXT receptor, and this intra-brain release has been related to the actions of OXT in controlling the autonomic functions which seem to also include feeding regulation [39]. In addition to food intake, activation of central or peripheral OXT receptors also regulates energy expenditure directly or indirectly [15,16,31]. Research demonstrated that OXT when delivered systemically causes reduction in fat mass and adipocyte size [19], and OXT receptor deficiency incurs an opposite effect [38]. Taken together, all these observations signify that OXT release patterns are functionally critical towards determining the bioavailability of this neuropeptide in systemic circulation or locally in the brain, while the control of OXT gene expression represents only an initial step in the whole cascade. However, despite the existing evidence about the effect of OXT in feeding, the physiological relevance and therapeutic significance of OXT in metabolic balance are still much in oblivion. Regardless, while current research has been recognizing the important role of neuropeptide release in body weight control, OXT release was identified to be relevant to obesity and metabolic syndrome both mechanically and therapeutically [15–17].
Anti-obesity effect of OXT in animal models
Few recent evidences have suggested that obesity is associated with defect of OXT signaling. Mice with haploinsuffciency of single-minded 1 (SIM1) gene have reduced OXT expression and are characterized to be hyperphagic and highly susceptible to diet-induced obesity [36,40,41]. In humans, mutations in SIM1 were associated with severe obesity; also, reduction in the number and size of OXT neurons in the PVN have been reported in patients with Prader-Willi syndrome, a genetic disorder characterized by severe hyperphagia and obesity [42]. The importance of OXT release dysregulation in feeding, body weight and obesity pathogenesis was first delineated very recently in a study using mouse model with high-fat diet (HFD)-induced obesity [16]. It was observed that HFD-induced obesity was significantly directed by downregulation of OXT release through increased vesicle binding of atypical synaptic exocytosis modulator, synaptotagmin-4 (Syt4). Syt4, which was found rich in OXT neurons of the hypothalamus [16], is a member of vesicle fusion machinery, and being Ca2+ insensitive, it is known to act as a negative regulator of neurotransmitter or neuropeptide release [43]. Zheng et al recently reported that HFD feeding led to upregulation of Syt4 in hypothalamic OXT neurons, and Syt4 knockdown can restore OXT release to protect against HFD-induced obesity [16]. While these evidences indicated that Syt4-mediated reduction of OXT release is a causal factor for the hypothalamic mechanism of obesity, the investigators further assessed if OXT could be therapeutically useful in treating obesity and related diseases. Indeed, as shown in the same study [16], data were provided demonstrating that administration of OXT provided protection against HFD-induced obesity in mouse models, and this protective effect was significantly attributed to the improved neural regulation of feeding, energy expenditure and body weight. In corroboration with these findings, Deblon et al reported that chronic central OXT infusion dose-dependently restricted weight gain and increased adipose tissue lipolysis in rats with HFD-induced obesity [18]. Of interest, studies by Zhang et al further showed that peripheral administration of OXT could also stimulate the central release of OXT to kindle the desired anti-obesity benefits in mice [15,16]. Consistently, Morton et al reported that peripheral OXT delivery induced weight loss in rats with HFD-induced obesity, and the authors mechanistically related this effect to reversal of leptin resistance through OXT's action on the satiety-related inputs in the hindbrain [44]. Taken together, all these observations clearly point to that the peripheral-central interactions mediated by OXT are involved in regulation of metabolic balance, and are opening up a new vista for drug development in obesity therapeutics (Figure 1).
Anti-diabetic effect of OXT in animal models
In addition to regulating body weight balance, OXT seems to have a role in affecting glucose and insulin homeostasis, which can be hinted by early studies reporting that OXT promotes glucose uptake and stimulate insulin secretion [45–48]. Studies using pancreatic islets or intact pancreas showed that OXT stimulated insulin secretion independently of plasma glucose changes while it also stimulated pancreatic glucagon secretion [47], which might suggest an involvement of OXT in diabetology. By focusing on diabetic pathophysiology, recent research demonstrated that OXT administration reduced obesity-related diabetic changes ranging from insulin resistance, glucose intolerance, pancreatic islet hypertrophy and hepatic steatosis in mice while obesity also reduced [15,16,19]. However, given that it was still unclear if the anti-diabetic effects were secondary to weight control, follow-up studies by Zhang et al employed acute experimental paradigms in mice with HFD-induced obesity, and found that acute treatment with OXT or OXT analogs reversed insulin resistance and glucose intolerance prior to reduction of obesity [17], thus dissecting the OXT's glucoregulatory pathways from its long-term effect on body weight. Furthermore, to directly examine the potential role of OXT in insulin secretion, Zhang et al performed treatment with OXT or OXT analogues in a streptozotocin-induced diabetic mouse model, and observed that the treatment led to improved insulin secretion so that diabetic changes were induced to a significantly less degree. Thus, while research on the therapeutic role of OXT on diabetes is still extremely limited compared to the research on obesity, the initial findings discussed above can provide a support to the conceptual model (Figure 1) that OXT may work as a therapeutic target for treating diabetes through promoting insulin sensitization and insulin secretion in addition to weight control.
Anti-obesity effect of OXT in patients
To address whether OXT and derived peptides could be the next generation anti-obesity and anti-diabetic drugs, a critical step is to examine if patients could respond to the treatment with good efficacy as did the rodents. Also, could OXT and its derivatives prove safe, unlike most weight-loss drugs marketed over the past decades which were associated with severe psychiatric and cardiovascular side effects [49]? After all, the hallmark of a successful weight-loss drug is the ability to reduce energy intake and/or increase energy expenditure without major adverse side effects. A very recent clinical trial of using OXT to treat human obesity proves to be promising and keeps the hope alive. In this clinical design, researchers considered that although OXT's effects on weight control is predicted to be largely mediated through the CNS, peripheral OXT administration can indeed stimulate the central release of OXT to induce metabolic benefits [15]. Based on this rationale, a pilot clinical study was performed to evaluate if OXT delivery through nasal spray could treat metabolic problems in human patients with obesity. In fact, OXT therapy in humans through intranasal route is already in practice for improving neuropsychiatric symptoms [9,50]. In addition to its peripheral action, intranasal delivery route may provide better feasibility for this small peptide to cross the blood brain barrier through the leaky areas and thus stimulate the central neural circuitry directly. In this study, Zhang et al found that multiple nasal sprays of OXT per day successfully lowered body weight of obese patients as opposed to placebo treatment [17]. The therapeutic benefit amplified with the increase of treatment duration from 4 weeks to 8 weeks [17]. Also as shown, the effect of weight loss by OXT treatment was reflected by decreases in waist and hip circumferences of patients thus endorsing the benefits further, as waist to hip ratio is a cogent predictor of body composition and fat distribution and is accepted as a more meaningful indicator of health outcomes. These findings are the first report of successful clinical use of OXT in lowering body weight in obese patients, and such clinical data should lead to a further expansion of research dimension towards the development of OXT-based drugs for obesity therapy in humans.
Therapeutic effects of OXT against obesity-related metabolic disorders in patients
Critical comorbidity factors associated with obesity are known to include pre-diabetic changes such as glucose intolerance, insulin resistance and hyperlipidemia. Despite that OXT is in clinical use for many decades, no thorough studies have been reported from the perspective of its possible effects on these conditions which may eventually benefit diabetic patients. In the recent clinical trial study, Zhang et al [17] demonstrated that OXT treatment in humans was found not only to reduce body weight in patients, it also improved the lipid profile of the patients by lowering serum low density lipoprotein (LDL) and cholesterol levels and a propensity for low density lipoprotein (HDL) level improvement. Also postprandial blood glucose and insulin levels in patients tended to be improved to a healthier range. Still a key outstanding question was, if these metabolic beneficial changes were just ancillary precipitates of obesity reversal? The study did not find positive correlation between these metabolic improvements and weight reduction in patients, which may indicate that OXT exerts a direct action on glucose and insulin homeostatic machinery independently of body weight control. The patients who underwent the treatment showed slight improvement in hepatic function, a possible outcome of obesity control which can reduce hepatic steatosis. Also importantly, OXT yielded all these metabolic benefits but without having the negative side effects on cardiovascular, liver or kidney functions; problems reflected by most anti-obesity drugs marketed recently. Thus, all these beneficial observations, in addition to obesity control which is discussed above, should further endorse OXT and its analogues to be significantly druggable for human obesity and diabetes.
Conclusion
Over the past years progress has been made towards understanding of the glucoregulatory action of nonapetide OXT which was classically known for its reproductive functions and its recent use for neuropsychiatric treatments. While research on animal models clearly elucidated the benefits of OXT on lowering of body weight by increasing energy expenditure, satiety signals, adiposity and decreasing food intake, recent clinical success of using OXT nasal spray for the treatment of obesity and associated metabolic symptoms in patients makes it a novel target for treating obesity and related metabolic diseases. Also, the observed metabolic benefits of OXT analogues in animal models and recent pharmaceutical focus on peptidyl innovative drug development make them promising candidates with great clinical potentials for treating obesity and related metabolic disorders. Continued research is still imperative to overcome the challenges of short half lives and limited blood brain barrier permeability of OXT or analogues, and to minimize off-target effects; nonetheless, these small peptides hold the promise of being the next generation therapeutic strategy for treatment of obesity and related metabolic diseases.
Acknowledgments
The authors sincerely thank Cai lab members for related research. D.C. is supported by NIH R01 DK078750 and R01 AG031774 and American Diabetes Association grant 1-12-BS-20. D.C. is a recipient of Irma T. Hirschl Scholarship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amori RE, et al. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298:194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- 2.Briones M, Bajaj M. Exenatide: a GLP-1 receptor agonist as novel therapy for Type 2 diabetes mellitus. Expert Opin Pharmacother. 2006;7:1055–1064. doi: 10.1517/14656566.7.8.1055. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz MW, Porte D., Jr Diabetes, obesity, and the brain. Science. 2005;307:375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- 4.Cai D, Liu T. Hypothalamic inflammation: a double-edged sword to nutritional diseases. Ann N Y Acad Sci. 2011;1243:E1–39. doi: 10.1111/j.1749-6632.2011.06388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai D, Liu T. Inflammatory cause of metabolic syndrome via brain stress and NF-kappaB. Aging (Albany NY) 2012;4:98–115. doi: 10.18632/aging.100431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elmquist JK, Flier JS. Neuroscience The fat-brain axis enters a new dimension. Science. 2004;304:63–64. doi: 10.1126/science.1096746. [DOI] [PubMed] [Google Scholar]
- 7.Flier JS, Maratos-Flier E. Obesity and the hypothalamus: novel peptides for new pathways. Cell. 1998;92:437–440. doi: 10.1016/s0092-8674(00)80937-x. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson JN, et al. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 9.Kosfeld M, et al. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 10.Argiolas A, Gessa GL. Central functions of oxytocin. Neurosci Biobehav Rev. 1991;15:217–231. doi: 10.1016/s0149-7634(05)80002-8. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald K, Macdonald TM. The peptide that binds: a systematic review of oxytocin and its prosocial effects in humans. Harv Rev Psychiatry. 2010;18:1–21. doi: 10.3109/10673220903523615. [DOI] [PubMed] [Google Scholar]
- 12.Blevins JE, et al. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol. 2004;287:R87–R96. doi: 10.1152/ajpregu.00604.2003. [DOI] [PubMed] [Google Scholar]
- 13.Blouet C, et al. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J Neurosci. 2009;29:8302–8311. doi: 10.1523/JNEUROSCI.1668-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baskin DG, et al. A new oxytocin-saporin cytotoxin for lesioning oxytocin-receptive neurons in the rat hindbrain. Endocrinology. 2010;151:4207–4213. doi: 10.1210/en.2010-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang G, Cai D. Circadian intervention of obesity development via resting-stage feeding manipulation or oxytocin treatment. Am J Physiol Endocrinol Metab. 2011;301:E1004–E1012. doi: 10.1152/ajpendo.00196.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang G, et al. Neuropeptide exocytosis involving synaptotagmin-4 and oxytocin in hypothalamic programming of body weight and energy balance. Neuron. 2011;69:523–535. doi: 10.1016/j.neuron.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, et al. Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PLoS One. 2013;8(5):e61477. doi: 10.1371/journal.pone.0061477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deblon N, et al. Mechanisms of the anti-obesity effects of oxytocin in diet-induced obese rats. PLoS One. 2011;6:e25565. doi: 10.1371/journal.pone.0025565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maejima Y, et al. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging (Albany NY) 2011;3:1169–1177. doi: 10.18632/aging.100408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 21.Insel TR, et al. Central oxytocin and reproductive behaviours. Rev Reprod. 1997;2:28–37. doi: 10.1530/ror.0.0020028. [DOI] [PubMed] [Google Scholar]
- 22.Carter CS. Oxytocin and sexual behavior. Neurosci Biobehav Rev. 1992;16:131–144. doi: 10.1016/s0149-7634(05)80176-9. [DOI] [PubMed] [Google Scholar]
- 23.Witt DM, Insel TR. A selective oxytocin antagonist attenuates progesterone facilitation of female sexual behavior. Endocrinology. 1991;128:3269–3276. doi: 10.1210/endo-128-6-3269. [DOI] [PubMed] [Google Scholar]
- 24.Meyer-Lindenberg A, et al. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 25.Feifel D. Oxytocin as a potential therapeutic target for schizophrenia and other neuropsychiatric conditions. Neuropsychopharmacology. 2012;37:304–305. doi: 10.1038/npp.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, et al. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coll AP, et al. The hormonal control of food intake. Cell. 2007;129:251–262. doi: 10.1016/j.cell.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz MW, et al. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 29.Douglas AJ, et al. Neuroendocrine mechanisms of change in food intake during pregnancy: a potential role for brain oxytocin. Physiol Behav. 2007;91:352–365. doi: 10.1016/j.physbeh.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Leng G, et al. Oxytocin and appetite. Prog Brain Res. 2008;170:137–151. doi: 10.1016/S0079-6123(08)00413-5. [DOI] [PubMed] [Google Scholar]
- 31.Ho JM, Blevins JE. Coming full circle: contributions of central and peripheral oxytocin actions to energy balance. Endocrinology. 2013;154:589–596. doi: 10.1210/en.2012-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arletti R, et al. Influence of oxytocin on feeding behavior in the rat. Peptides. 1989;10:89–93. doi: 10.1016/0196-9781(89)90082-x. [DOI] [PubMed] [Google Scholar]
- 33.Lokrantz CM, et al. Effects of central oxytocin administration on intraoral intake of glucose in deprived and nondeprived rats. Physiol Behav. 1997;62:347–352. doi: 10.1016/s0031-9384(97)00021-8. [DOI] [PubMed] [Google Scholar]
- 34.Johnstone LE, et al. Neuronal activation in the hypothalamus and brainstem during feeding in rats. Cell Metab. 2006;4:313–321. doi: 10.1016/j.cmet.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Verbalis JG, et al. Oxytocin secretion in response to cholecystokinin and food: differentiation of nausea from satiety. Science. 1986;232:1417–1419. doi: 10.1126/science.3715453. [DOI] [PubMed] [Google Scholar]
- 36.Kublaoui BM, et al. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol Endocrinol. 2008;22:1723–1734. doi: 10.1210/me.2008-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amico JA, et al. Enhanced initial and sustained intake of sucrose solution in mice with an oxytocin gene deletion. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1798–R1806. doi: 10.1152/ajpregu.00558.2005. [DOI] [PubMed] [Google Scholar]
- 38.Takayanagi Y, et al. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport. 2008;19:951–955. doi: 10.1097/WNR.0b013e3283021ca9. [DOI] [PubMed] [Google Scholar]
- 39.Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- 40.Kublaoui BM, et al. Sim1 haploinsufficiency impairs melanocortin-mediated anorexia and activation of paraventricular nucleus neurons. Mol Endocrinol. 2006;20:2483–2492. doi: 10.1210/me.2005-0483. [DOI] [PubMed] [Google Scholar]
- 41.Tolson KP, et al. Postnatal Sim1 deficiency causes hyperphagic obesity and reduced Mc4r and oxytocin expression. J Neurosci. 2010;30:3803–3812. doi: 10.1523/JNEUROSCI.5444-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swaab DF, et al. Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader-Willi syndrome: a study of five cases. J Clin Endocrinol Metab. 1995;80:573–579. doi: 10.1210/jcem.80.2.7852523. [DOI] [PubMed] [Google Scholar]
- 43.Littleton JT, et al. Synaptic function modulated by changes in the ratio of synaptotagmin I and IV. Nature. 1999;400:757–760. doi: 10.1038/23462. [DOI] [PubMed] [Google Scholar]
- 44.Morton GJ, et al. Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. Am J Physiol Endocrinol Metab. 2012;302:E134–E144. doi: 10.1152/ajpendo.00296.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knudtzon J. Acute effects of oxytocin and vasopressin on plasma levels ofglucagon, insulin and glucose in rabbits. Horm Metab Res. 1983;15:103–104. doi: 10.1055/s-2007-1018641. [DOI] [PubMed] [Google Scholar]
- 46.Stock S, Uvnas-Moberg K. l-Vasopressin inhibits oxytocin-induced increases of plasma levels of insulin in conscious dogs. Acta Physiol Scand. 1987;130:55–61. doi: 10.1111/j.1748-1716.1987.tb08111.x. [DOI] [PubMed] [Google Scholar]
- 47.Altszuler N, et al. Role of insulin and glucagon in oxytocin effects on glucose metabolism. Proc Soc Exp Biol Med. 1992;199(2):236–242. doi: 10.3181/00379727-199-43353. [DOI] [PubMed] [Google Scholar]
- 48.Paolisso G, et al. Effects of oxytocin delivery on counter-regulatory hormone response in insulin-dependent (type 1) diabetic subjects. Horm Res. 1989;31:250–255. doi: 10.1159/000181126. [DOI] [PubMed] [Google Scholar]
- 49.Adan RA. Mechanisms underlying current and future anti-obesity drugs. Trends Neurosci. 2013;36:133–140. doi: 10.1016/j.tins.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 50.Feifel D, et al. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol Psychiatry. 2010;68:678–680. doi: 10.1016/j.biopsych.2010.04.039. [DOI] [PubMed] [Google Scholar]


