Abstract
Objectives
This study assessed the impact of bucindolol, a beta-blocker/sympatholytic agent, on the development of atrial fibrillation (AF) in advanced chronic heart failure with reduced left ventricular ejection fraction (HFREF) patients enrolled in the BEST (Beta-Blocker Evaluation of Survival Trial).
Background
β-Blockers have modest efficacy for AF prevention in HFREF patients. Bucindolol’s effects on HF and ventricular arrhythmic endpoints are genetically modulated by β1- and α2c-adrenergic receptor (AR) polymorphisms that can be used to subdivide HFREF populations into those with bucindolol effectiveness levels that are enhanced, unchanged, or lost.
Methods
BEST enrolled 2,708 New York Heart Association (NYHA) class III to IV HFREF patients. A substudy in which 1,040 patients’ DNA was genotyped for the β1-AR position 389 Arg/Gly and the α2c322–325 wild type (Wt)/deletion (Del) polymorphisms, and new-onset AF was assessed from adverse event case report forms or electrocardiograms at baseline and at 3 and 12 months.
Results
In the entire cohort, bucindolol reduced the rate of new-onset AF compared to placebo by 41% (hazard ratio [HR]: 0.59 [95% confidence interval (CI): 0.44 to 0.79], p = 0.0004). In the 493 β1389 arginine homozygotes (Arg/Arg) in the DNA substudy, bucindolol reduced new-onset AF by 74% (HR: 0.26 [95% CI: 0.12 to 0.57]), with no effect in β1389 Gly carriers (HR: 1.01 [95% CI: 0.56 to 1.84], interaction test = 0.008). When β1389 Gly carriers were subdivided by α2c Wt homozygotes (n = 413, HR: 0.94 [95% CI: 0.48 to 1.82], p = 0.84) or Del variant carriers (n = 134, HR: 1.33 [95% CI: 0.32 to 5.64], p = 0.70), there was a positive interaction test (p = 0.016) when analyzed with β1389 Arg homozygotes.
Conclusions
Bucindolol prevented new-onset AF; β1 and α2c polymorphisms predicted therapeutic response; and the 47% of patients who were β1389 Arg homozygotes had an enhanced effect size of 74%. (Beta-Blocker Evaluation in Survival Trial [BEST]; NCT00000560)
Keywords: arrhythmia, beta adrenergic receptors, genetics, heart failure, norepinephrine
Atrial fibrillation (AF) is the most common cardiac arrhythmia and is frequently observed in chronic heart failure/reduced ejection fraction (HFREF) populations (1), where the incidence is several-fold higher than in patients without heart failure (2). In the Framingham cohort, new-onset AF was associated with an increase in mortality in patients with heart failure (3). However, in HFREF patients, rhythm control strategies with current antiarrhythmic medications have not been associated with improved outcomes (4). This may be due to multiple adverse effects of current antiarrhythmic agents in HFREF populations (5).
A drug treatment capable of decreasing the incidence of new-onset AF with an improved safety profile would benefit HFREF patients, particularly if such therapy also favorably affected the underlying pathophysiologic mechanisms that predispose patients to AF. β-Blockers are candidates for such a therapy because they both improve heart failure outcomes (6) and have efficacy for AF prevention (7), likely due in part to reverse remodeling in both ventricular (8) and atrial (9,10) chambers. However, currently approved β-blockers exhibit only modest efficacy for reducing new-onset AF in HFREF patients (7).
In patients with HFREF, the Arg389Gly polymorphism in the β1-adrenergic receptor (−AR) (ADRB1) gene affects the therapeutic response to bucindolol, a nonselective β-AR blocker with sympatholytic properties (11). Compared to the 389 glycine (Gly) minor allele, the 389 arginine (Arg) major allele gene protein product has a 3- to 4-fold higher signal transduction capacity (11), higher affinity for agonists including norepinephrine (NE) (12), and a larger proportion of constitutively active ARs (11). In a genetic substudy of the BEST (Beta-Blocker Evaluation of Survival Trial), bucindolol exhibited β1389 Arg/Gly genotype-dependent differential effects on mortality, heart failure hospitalizations, and ventricular arrhythmias (11–13). In addition, in HFREF patients who were β1389 Gly carriers (having at least one copy of the dominant negative 389 Gly allele), an insertion/deletion polymorphism at amino acid position 322–325 of the α2c-AR, alleles commonly referred to as either wild type (Wt) or deletion (Del), affects bucindolol’s response for both heart failure (12,14) and ventricular arrhythmia (13) endpoints by regulating bucindolol’s sympatholytic effects (14–16).
We hypothesized that β1389 Arg/Gly and α2c322–325 Wt/Del AR polymorphisms may modulate bucindolol’s effects on new-onset AF in HFREF patients, as they do for heart failure (12) and serious ventricular arrhythmia endpoints (13).
Methods
Study population
The BEST was a randomized trial of bucindolol versus placebo in HFREF patients with NYHA class III to IV heart failure and left ventricular ejection fractions (LVEF) ≤0.35 (15). The current study analyzed patients who were not in AF at study entry, including 2,176 patients in sinus rhythm (SR) plus 216 patients with other rhythms to yield a study population of 2392 from the entire 2,708 patient cohort, and 925 patients from the 1,040 DNA substudy (846 SR and 79 other rhythms). In the 925 AF-free DNA bank substudy patients, the development of new-onset AF was investigated in β1389 Arg/Gly and α2c322–325 Wt/Del genetic subgroups as previously described for heart failure (12) and ventricular arrhythmic (13) endpoints. The BEST protocol, patient population, and main outcomes have been previously described (15). The DNA bank and the AR polymorphisms substudy protocols and patient populations have also been previously described (11–14). This study used the DNA substudy of BEST, a prospectively planned investigation (n = 1,040) with a separate consent form and ethical committee review designed to test the effects of AR polymorphisms on clinical outcomes. All patients signed written consent forms for both the parent BEST protocol and the DNA substudy. Although DNA analysis was performed after the trial ended, clinical data remained blinded from the investigators until the coded genetic data results were submitted to the data coordinating center and analyzed by trial statisticians.
The current substudy is a post hoc analysis investigating the incidence of new-onset AF. Cases of AF were prospectively identified from adverse event case report forms that included electrocardiograms (ECGs) and were reviewed and certified by cardiologist investigators at each site. In patients for whom no adverse event had been recorded, new-onset AF event was also obtained from study ECGs performed at baseline and at 3 and 12 months. In order to assess the total number of new-onset AF events, the separate adverse event and study ECG datasets were combined. Time of onset of the AF event was taken as the day of detection, with the duration of AF-free follow-up determined by comparison to the randomization date.
Genotyping and norepinephrine measurements
Geno-typing for β1389Arg/Gly and α2c322–325 Wt/Del polymorphisms was performed with archived DNA (11–14), and plasma norepinephrine (NE) was measured from systemic venous samples as previously described (16).
Statistical analysis
The primary analysis was the measure of time to first event of AF for patients free of AF at study entry. A log rank statistic was used to generate treatment comparison p values, and a Cox proportional hazards model was used to estimate hazard ratios (HRs) and confidence intervals (CIs) between bucindolol and placebo groups. Per the study regulatory statistical analysis plan, all analyses were adjusted for the covariates of presence/absence of coronary artery disease, LVEF ≤20% to >20%, black and non-black race, and gender, which are the four strata used in the treatment randomized assignment. Follow-up was by intention-to-treat, with censoring for cardiac transplantation, death, nonfatal lost to follow-up, or study end on July 26, 1999. For baseline characteristics, continuous variables were compared using Student t test and presented as the mean ± SD. Categorical variables were compared using the chi-square test. As previously reported (14), 66% of patients entered the DNA substudy after randomization and had DNA collection after being enrolled in the parent treatment protocol. In these “late entry” patients, postrandomization AF events that occurred prior to DNA collection were counted in the statistical analysis.
Results
Clinical characteristics of patient cohorts
Baseline characteristics for the entire 2392 BEST AF-free cohort at entry are given in Table 1, and they do not differ from previously reported characteristics of the patients in SR at study entry (17). The average follow-up of the 2392 non-AF patients was 2.0 years, with a maximum of 4.1 years. Table 1 also gives the baseline characteristics of the 925 non-AF patients in the DNA substudy (average follow-up 2.1 years) and in selected genotype groups. The 69 patient (β1389 Arg/Arg + α2c322–325 Del carrier) group contained too few events (n = 6) for analysis, and the β1389 Arg/Arg group was therefore not subdivided by α2c322–325 Wt/Del polymorphism. In the DNA substudy, there were 441 patients who were β1389 Arg homozygotes (β1389 Arg/Arg) and 484 Gly carriers (β1389 Gly/Gly or Arg/Gly). Within the β1389 Gly carrier patient group, 358 were α2c Wt homozygotes and 126 were α2c322–325 Del carriers. There were no clinically relevant differences between baseline characteristics in the DNA substudy and the entire cohort non-AF patients. As previously reported for all baseline rhythms (12,13), there were significant differences in race and hypertension history between β1389 Arg/Arg and Gly carriers groups, as well as between the two β1389 Gly carrier/α2c322–325 groups that were related to the β1389 Gly and α2c322–325, deletion alleles being more prevalent in blacks (11–14).
Table 1.
Baseline Patient Characteristics for Patients Who Were Not in AF at the Time of Study Entry, in the Entire Cohort, DNA Substudy and Within Genotype Groups
| Characteristic | Entire Cohort (n = 2,392) | DNA Substudy (n = 925) | β1389 Arg/Arg (n = 441) | β1389 Gly Carrier (n = 484) (p vs. Arg/Arg) |
β1Gly carrier + α2c Wt/Wt (n = 358) | β1 Gly carrier + α2c Del (n = 126) (p vs. Wt/Wt) |
|---|---|---|---|---|---|---|
| Age (yrs) | 59.6 ± 12.4 | 59.7 ± 12.2 | 59.7 ± 11.9 | 59.8 ± 12.4 | 60.2 ± 12.3 | 58.5 ± 12.8 |
| Male (%) | 1,829 (76%) | 718 (78%) | 345 (78%) | 373 (77%) | 281 (78%) | 92 (73%) |
| Black (%) | 586 (25%) | 198 (21%) | 63 (14%) | 135 (28%)* | 47 (13%) | 88 (70%)* |
| Resting HR (beats/min) | 82.5 ± 13.4 | 81.7 ± 13.4 | 81.5 ± 13.7 | 81.9 ± 13.2 | 81.6 ± 13.4 | 82.8 ± 12.7 |
| HTN (%) | 1,424 (60%) | 523 (57%) | 238 (54%) | 225 (59%) | 191 (53%) | 94 (75%)* |
| Diabetes (%) | 876 (37%) | 333 (36%) | 165 (38%) | 168 (35%) | 120 (34%) | 48 (38%) |
| Ischemic cause (%) | 1411 (59%) | 550 (59%) | 259 (59%) | 291 (60%) | 230 (64%) | 61 (48%)† |
| LVEF (%) | 22.9 ± 7.3 | 23.5 ± 7.1 | 23.3 ± 7.1 | 23.7 ± 7.1 | 23.9 ± 7.1 | 23.0 ± 7.1 |
| HF duration (months) | 48.2 ± 47.9 | 45.1 ± 47.4 | 47.8 ± 51.3 | 42.7 ± 43.4 | 41.5 ± 42.5 | 46.3 ± 45.7 |
| NYHA class III (%) | 2,200 (92%) | 857 (93%) | 418 (95%) | 439 (91%)‡ | 325 (91%) | 114 (90%) |
| Digoxin (%) | 2,197 (92%) | 827 (89%) | 398 (90%) | 429 (89%) | 319 (89%) | 110 (87%) |
Values are mean ± SD or n (%). p values for comparisons of genotype subgroups consist of chi-square test results for categorical variables and the Wilcoxon test results for continuous variables.
p < 0.001;
p < 0.01;
p < 0.05.
AF = atrial fibrillation; Arg = arginine; Del = deletion; Gly = glycine; HF = heart failure; HR = heart rate; HTN = history of hypertension; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; Wt = wild type.
Outcomes in the BEST cohort and DNA substudy
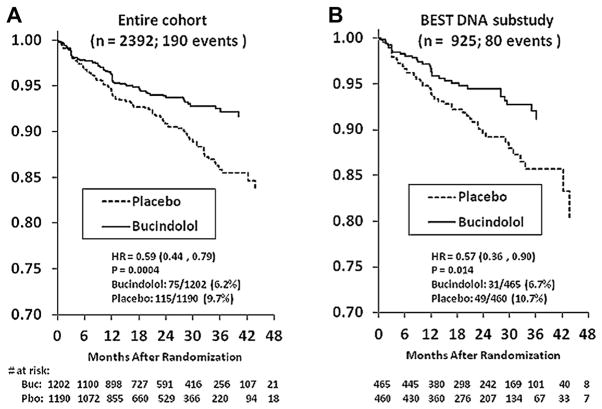
There were 190 new-onset AF events in the entire 2,392 patient cohort, for an overall event rate of 7.9%. In the 925 DNA substudy patients, there were 80 new-onset AF events (rate, 8.6%). In the entire BEST cohort, there was a lower incidence of new-onset AF in the bucindolol group than in the placebo group (n = 75 [6.2%] vs. n = 115 [9.7%] HR: 0.59 [95% CI: 0.44 to 0.79]), corresponding to a 41% risk reduction (Table 2). There was a similar decrease in the incidence of new-onset AF in the DNA substudy in the bucindolol group compared to the placebo group (n = 31 [6.7%] vs. n = 49 [10.7%]; HR: 0.57 [95% CI: 0.36 to 0.90]) (Table 2). Data presented in Table 2 indicate that 85% of events were detected from adverse event forms as opposed to routine ECGs only; thus, most of the events were symptomatic. Time to first event curves for the entire cohort and DNA substudy are given in Figure 1.
Table 2.
Prevention of New-Onset Atrial Fibrillation by Bucindolol in BEST
| Treatment Group | Patients Free of AF at Baseline Assessed by ECG | Patients With New-Onset AF Reported as AE During the Trial | Total No. of Patients With New-Onset AF During the Trial |
|---|---|---|---|
| Entire cohort
| |||
| Placebo (%) | 1,190 (88.3%) | 100 (8.4%) | 115 (9.7%) |
| Bucindolol (%) | 1,202 (89.2%) | 61 (5.1%) | 75 (6.2%) |
| Time to first event of new-onset AF | 0.55 (0.44 to 0.76), p = 0.0002 | 0.59 (0.44 to 0.79), p = 0.0004 | |
|
| |||
| DNA substudy
| |||
| Placebo (%) | 460 (88.0%) | 45 (9.8%) | 49 (10.7%) |
| Bucindolol (%) | 465 (90.6%) | 25 (5.4%) | 31 (6.7%) |
| Time to first event of new-onset AF | 0.50 (0.31 to 0.82), p = 0.005 | 0.57 (0.36 to 0.90), p = 0.014 | |
Values are n (%) or hazard ratio (95% confidence interval), p value.
AE = adverse event; AF = atrial fibrillation; BEST = Beta-Blocker Evaluation of Survival Trial.
Figure 1. Time to New-Onset AF in Bucindolol and Placebo Arms of BEST.
Time to event curves for new-onset atrial fibrillation (AF) in the BEST entire cohort (A) and the DNA substudy (B). Dashed line = placebo; solid line = bucindolol. HR = hazard ratio.
Table 3 gives the reduction in new-onset AF analyzed by event duration. AF events were classified as short duration paroxysmal (<24 h), longer duration paroxysmal (between 24 h and 7 days), or persistent (longer than 7 days). Greater than two-thirds (67.9%) of the events were persistent AF, with 23.2% of events longer paroxysmal and only 8.9% of events being short paroxysmal. By HR, bucindolol treatment effects were similar for the three AF durations, with HR of 0.51 (p = 0.183), 0.57 (p = 0.066), and 0.62 (p = 0.007) for shorter paroxysmal, longer paroxysmal, and persistent AF, respectively (Table 3). However, event rates were low in the paroxysmal groups, and the persistent AF group was the only one that attained statistical significance.
Table 3.
Duration of New-Onset Atrial Fibrillation Events in BEST
| Patient Group | Total Placebo | Total Bucindolol | Hazard Ratio (95% CI) | Logrank p Value |
|---|---|---|---|---|
| Entire cohort (n = 2392), all AF | 115/1,190 (9.7%) | 75/1,202 (6.2%) | 0.59 (0.44 to 0.79) | 0.0004 |
| <24 h (n = 17 of 190 [8.9%]) | 11/1,190 (0.9%) | 6/1,202 (0.5%) | 0.51 (0.19 to 1.39) | 0.183 |
| >24 h to ≤7 days (n = 44 of 190 [23.2%]) | 27/1,190 (2.3%) | 17/1,202 (1.4%) | 0.57 (0.31 to 1.05) | 0.066 |
| >7 days (n = 129 of 190 [67.9%]) | 77/1,190 (6.5%) | 52/1,202 (4.3%) | 0.62 (0.43 to 0.88) | 0.007 |
Values are n/N (%).
AF = atrial fibrillation; BEST = Beta-Blocker Evaluation of Survival Trial; CI = confidence interval.
Outcomes by genotype group
Table 4 gives HR data by genotype group. In the 441 β1389 Arg/Arg patients, bucindolol was associated with a marked decrease in the incidence of new-onset AF (HR: 0.26 [95% CI: 0.12 to 0.57], p = 0.0003). In contrast, bucindolol had no impact on the incidence of new-onset AF in the 484 β1389 Gly carriers (HR: 1.01 [95% CI: 0.56 to 1.84], p = 0.97). In the time to first event curves shown in Figure 2, the. 74% risk reduction by bucindolol in β1389 Arg/Arg patients was associated with an early divergence of curves. There was no reduction in new-onset AF in the β1389 Gly carriers who received bucindolol compared to placebo. These results yielded a significant statistical interaction (p = 0.008) between treatment and β1389 Arg/Gly genotypes.
Table 4.
Prevention of New-Onset AF by Bucindolol in BEST by Genotype, Total Number of Events, and Norepinephrine Change at 3 Months in Patients in Genetic Groups
| Measure | (β1389Arg/Arg + any α2C)* (P = 206, B = 235) | (β1389Gly Carrier + any α2C) (P = 254, B = 230) | (β1 Gly carrier + α2c Wt/Wt)* (P = 183, B = 175) | (β1 Gly carrier + α2c Del)* (P = 71, B = 55) |
|---|---|---|---|---|
| HR (95% CI), no. of events, p value | 0.26 (0.12 to 0.57) 36 events, p = 0.0003 | 1.01 (0.56 to 1.84) 44 events, p = 0.97 | 0.94 (0.48 to 1.82) 36 events, p = 0.84 | 1.33 (0.32 to 5.64) 8 events, p = 0.70 |
| NE change at 3 months (pg/ml) | P = 14 ± 20 | P = 31 ± 22 | P = 38 ± 25 | P = 7 ± 45 |
| B = 71 ± 22 | B = 78 ± 24 | B = 54 ± 22 | B = 164 ± 79 | |
| p = 0.0013 | p = 0.0019 | p = 0.0039 | p = 0.23 |
Data show prevention of new-onset atrial fibrillation (AF) by bucindolol in BEST by genotype (hazard ratio [R], 95% confidence intervals [CI]), total number of events, and p value. Norepinephrine (NE) change at 3 months in patients in genetic groups.
Member of three group construct tested for interaction.
B = bucindolol; Del = deletion; P = placebo; Wt = wild type. Other abbreviations as in Table 1.
Figure 2. Time to New Onset by β1389 Arg/Gly Genotype.
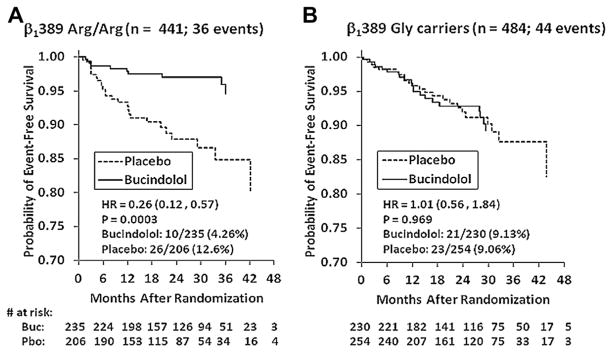
Time to event curves are shown for new-onset AF in the BEST DNA substudy by β1389 Arg/Gly genotype. There is a significant interaction between genotype and treatment. The benefit of bucindolol is seen exclusively in the β1389 Arg/Arg genotype (A), with a risk reduction of 74% compared to placebo (p = 0.008 for interaction vs. Gly carrier group). (B) There was no impact of bucindolol in the β1 Gly carriers compared to placebo. Dashed line = placebo; solid line = bucindolol. Abbreviations as in Figure 1.
For both heart failure endpoints (12) and serious ventricular arrhythmias (13), when HFREF patients are β1389 Gly carriers, the type of associated α2c322–325 Wt/Del polymorphism can alter bucindolol treatment effects. Data in Table 4 suggest this is also the case for prevention of AF, where Del carriers have a HR >1.0. Moreover, the three-genotype group construct that included β1389 Arg/Arg patients had an interaction p value of 0.016, supporting the validity of subdividing the β1389 Gly carrier group by α2c polymorphism.
Plasma norepinephrine and new-onset AF
In order to assess the relationship of adrenergic drive and outcomes, systemic venous plasma NE levels were measured at baseline and at months 3 and 12. Of the entire 2,392 patient cohort, 1,868 had baseline NE measured. Compared to patients who remained free of AF, patients who developed AF had higher baseline NE levels in the bucindolol group (581 ± 304 pg/ml vs. 514 ± 344 pg/ml, respectively, p = 0.009) and in the combined treatment groups (530 ± 231 pg/ml vs. 498 ± 326 pg/ml, respectively, p = 0.015). Bucindolol produced a significant reduction in NE levels at 3 months in patients who developed AF (by 129 ± 49 pg/ml, p = 0.0009 vs. placebo change) and in patients who remained free of AF (by 74 ± 12 pg/ml, p <0.0001 vs. placebo change), with no differences between the two groups (p = 0.23). Placebo-treated patients exhibited increases in NE in both the new-onset AF subgroup (by 88 ± 46 pg/ml) and in patients who remained free of AF (by 21 ± 11 pg/ml, p = 0.29 vs. new-onset AF). Table 4 gives NE changes at 3 months within the pharmacogenetic subgroups, where it can be observed that there are similar degrees of NE lowering in the bucindolol β1389 Arg/Arg and Gly carrier genetic groups (respectively, 71 and 78 pg/ml and both p <0.010 vs. placebo change). Within the Gly carrier group, the α2c322–325 Del carrier subgroup has a large degree of bucindolol-associated NE reduction (by 164 pg/ml) as previously reported for the full 1,040, all rhythms DNA substudy population (12), which is due to the exclusive presence of the α2c322–325 Del carrier genotype (14).
Discussion
Treatment effects of bucindolol on new-onset AF in the BEST entire cohort and the DNA substudy
The DNA substudy and the entire cohort parent populations were very similar in baseline characteristics, length of follow-up (2.0 vs. 2.1 years), overall event rates (respectively, 7.9% and 8.6%), and placebo event rates (respectively, 9.7% and 10.7%). Thus, there was no evidence that late entry of most in the DNA substudy relative to their randomization dates had any impact on the study population from the standpoint of development of new-onset AF.
For new-onset AF, bucindolol demonstrated respective risk reductions of 41% (p = 0.0004) and 43% (p = 0.014) in the entire and DNA substudy cohorts of BEST. In placebo controlled HFREF trials, the effect of β-blockade on AF episodes by event duration has not been previously reported, and we evaluated effects on both paroxysmal and persistent AF. In the entire cohort the majority (68%) of AF episodes were >7 days duration or persistent, exhibiting a 38% reduction (p = 0.007) by bucindolol. Shorter or paroxysmal episodes of AF were not significantly reduced, although they had lower HRs than in the persistent group. Thus, there was observational evidence of a bucindolol treatment effect regardless of AF duration, and statistical significance of a favorable effect in persistent AF.
Pharmacogenetic treatment effects
Reduction in new-onset AF was driven by a large bucindolol treatment effect in patients with a β1389 Arg/Arg genotype who had a 74% reduction (p = 0.0003) when treated with bucindolol compared to those treated with placebo. There was no reduction in event rate (HR: 1.01) in bucindolol patients who were β1389 Gly carriers, and the treatment × genotype group interaction p value was 0.008. Subdividing the β1389 Gly carrier genotype by α2c322–325 Wt/Del genotype appeared to further differentiate bucindolol response as it does for heart failure (12) and serious ventricular arrhythmia (13) endpoints, with a significant (p = 0.016) test for interaction when β1389 Arg/Arg patients were included in the three group analysis. Although differences in race and/or history of hypertension could have affected the analysis between genotypes, the (β1389 Gly carrier + α2c322–325 Wt/Wt) group had prevalence rates for black patients and cases of hypertension that were similar to those of the β1389 Arg/Arg group but markedly different HRs (0.94, p = 0.84 and 0.26, p = 0.0003, respectively). This indicates that the differentially enhanced treatment effect of bucindolol on AF prevention is mediated through β1389 Arg vs. Gly ARs and is not directly related to race or history of hypertension.
There appears to be a class affect of β-blockers for reduction of new-onset AF in HFREF patients. A meta-analysis by Nasr et al. (7) of new-onset AF in HFREF trials demonstrated an average 27% reduction of new-onset AF for five different β-blockers and evidence for a treatment effect for all β-blockers except nebivolol. This relatively modest reduction in new-onset AF across all β-blocker HFREF trials is in contrast to the marked 74% reduction in new-onset events in the β1389 Arg/Arg group observed in this analysis.
Role of adrenergic drive in the development of new-onset AF and the pharmacotherapeutic effects of bucindolol
Patients who developed AF had higher baseline NE levels than patients who remained free of AF, similar to data for AF development in an animal model of heart failure (18). Bucindolol’s well-known sympatholytic effects (14–16) were observed in patients who developed AF and in those who did not and to the same extent in patients with β1389 Arg/Arg and β1389 Gly carrier genotypes. Thus, NE reduction by bucindolol may play a role in its AF prevention effects, but a difference in degree of sympatholysis does not explain the highly selective therapeutic effects of bucindolol in patients with the β1389 Arg/Arg genotype. In this genotype patients express only the β1389 Arg receptor, which is the “NE receptor” in the heart (12). A reduction in NE will therefore have a selectively greater therapeutic effect in this genotype, and patients are also protected from the adverse effects of marked sympatholysis (12).
In the (β1389 Gly carrier + α2c322–325 Del carrier) group, relatively low prevalence (13.6% of the total) combination genotype that exhibited a statistically insignificant 33% numerical increase in new-onset AF, there was a large reduction in NE due to the α2c322–325 Del carrier polymorphism (12,14). The adverse affects of sympatholysis (12,14,16) may have canceled any therapeutic effect of bucindolol in β1389 Gly carriers and led to a nonsignificant increase in AF in patients with a [β1389 Gly carrier + α2c322–325 Del carrier] genotype.
Mechanisms of atrial fibrillation prevention by bucindolol as modulated by the β1389 Arg/Arg genotype
There are multiple lines of evidence linking high levels of β1-adrenergic signaling, as predicted for β1389 Arg/Arg homozygotes, to the development of AF. Higher adrenergic activity has been shown to increase the inducibility of AF in humans and dogs in a dose-dependent manner (19,20), and in a model of ischemic cardiomyopathy, dogs that developed AF had higher NE levels (18). Furthermore, in isolated human right atrial preparations, isoproterenol infusion has been shown to increase the frequency of atrial early and delayed after-depolarizations, phenomena that have been implicated in initiating AF (21). Bucindolol is especially effective in inhibiting signaling through β1389 Arg ARs, through the novel mechanisms of facilitating inactivation of constitutively active receptors (the property of inverse agonism) (11) and NE lowering (12), as well as through high-affinity competitive antagonism (6).
Study limitations
The primary limitation of the current substudy is the post hoc nature of the analysis. AF was not a prespecified efficacy endpoint, and the data were not adjudicated but rather collected from investigator-reviewed adverse event case report forms and serial ECGs, similar to the approach used by van Veldhuisen et al. (22). Thus, some AF events were likely missed, and in the case of the 15% of events that were detected by ECG, only the onset of AF could have been much earlier than the recorded date. On the other hand, using adverse event forms and ECGs to capture new-onset AF events represented a blinded, nonbiased way to assess arrhythmia occurrence with 85% of the events being symptomatic. Based on the use of adverse event case report forms and ECGs, it is likely that most AF events of more than several hours duration were detected, with the onset contemporaneous to detection in a substantial majority of cases.
Another limitation of the current analysis is the relatively small number of new-onset AF events. Although the entire cohort contained 190 events, the largest number reported in any HFREF β-blocker trial (7), the DNA substudy had only 80 events, and after pharmacogenetic subgrouping the number of events in each group was further reduced by ~50%. These limitations will be addressed in a planned study of AF prevention in β1389 Arg/Arg genotype HFREF patients who are randomized to bucindolol versus. metoprolol, a β-blocker that does not exhibit pharmacogenetic modulation of clinical therapeutic responses (23).
Conclusions
Bucindolol was associated with a significant, quantitatively large decrease in new-onset AF in the entire BEST cohort that was observed exclusively in the β1389 Arg/Arg genotype.
Acknowledgments
This study was supported by the VA Cooperative Studies Program, National Institutes of Health National Heart, Lung, and Blood Institute, and ARCA Biopharma. Dr. Bristow is CEO and a shareholder of ARCA Biopharma, Inc., which owns rights to bucindolol. Dr. Port is an employee of ARCA. Mr. Davis and Dr. Murphy are consultants to ARCA. Dr. Fiuzat is a consultant to ARCA Biopharma, Inc.
The authors thank R. Rosenberg for clerical assistance with the manuscript.
Abbreviations and Acronyms
- AF
atrial fibrillation
- AR
adrenergic receptor
- Arg/Arg
arginine homozygote
- BEST
Beta-Blocker Evaluation of Survival Trial
- Del
deletion
- HFREF
heart failure with reduced left ventricular ejection fraction
- HR
hazard ratio
- NE
plasma norepinephrine
- SR
sinus rhythm
- Wt
wild type
Footnotes
All other authors report that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Savelieva I, Camm AJ. Atrial fibrillation and heart failure: natural history and pharmacological treatment. Europace. 2004;5:S5–19. doi: 10.1016/j.eupc.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–4. [PubMed] [Google Scholar]
- 3.Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–5. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 4.Caldeira D, David C, Sampaio C. Rate versus rhythm control in atrial fibrillation and clinical outcomes: updated systematic review and meta-analysis of randomized controlled trials. Arch Cardiovasc Dis. 2012;105:226–38. doi: 10.1016/j.acvd.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Saksena S, Slee A, Waldo ALF, et al. Cardiovascular outcomes in the affirm trial (atrial fibrillation follow-up investigation of rhythm management). An assessment of individual antiarrhythmic drug therapies compared with rate control with propensity score-matched analyses. J Am Coll Cardiol. 2011;58:1975–85. doi: 10.1016/j.jacc.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bristow MR. Treatment of chronic heart failure with β-adrenergic receptor antagonists: a convergence of receptor pharmacology and clinical cardiology. Circ Res. 2011;109:1176–94. doi: 10.1161/CIRCRESAHA.111.245092. [DOI] [PubMed] [Google Scholar]
- 7.Nasr IA, Bouzamondo A, Hulot JS, Dubourg O, Le Heuzey JY, Lechat P. Prevention of atrial fibrillation onset by beta-blocker treatment in heart failure: a meta-analysis. Eur Heart J. 2007;28:457–62. doi: 10.1093/eurheartj/ehl484. [DOI] [PubMed] [Google Scholar]
- 8.Eichhorn EJ, Bristow MR. Medical therapy can improve the biologic properties of the chronically failing heart: a new era in the treatment of heart failure. Circulation. 1996;94:2285–96. doi: 10.1161/01.cir.94.9.2285. [DOI] [PubMed] [Google Scholar]
- 9.Arslan S, Erol MK, Bozkurt E, et al. Effect of beta-blocker therapy on left atrial function in patients with heart failure: comparison of meto-prolol succinate with carvedilol. Int J Cardiovasc Imaging. 2007;23:549–55. doi: 10.1007/s10554-006-9195-3. [DOI] [PubMed] [Google Scholar]
- 10.Workman AJ, Kane KA, Russell JA, Norrie J, Rankin AC. Chronic beta-adrenoceptor blockade and human atrial cell electrophysiology: evidence of pharmacological remodelling. Cardiovasc Res. 2003;58:518–25. doi: 10.1016/s0008-6363(03)00263-3. [DOI] [PubMed] [Google Scholar]
- 11.Liggett SB, Mialet-Perez J, Thaneemit-Chen S, et al. A polymorphism within a conserved beta(1)-adrenergic receptor motif alters cardiac function and beta-blocker response in human heart failure. Proc Natl Acad Sci U S A. 2006;103:11288–93. doi: 10.1073/pnas.0509937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connor CM, Fiuzat M, Carson PE, et al. Combinatorial pharmacogenetic interactions of bucindolol and β1, β2C adrenergic receptor polymorphisms. PLoS One. 2012;7:e44324. doi: 10.1371/journal.pone.0044324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aleong RG, Sauer WH, Robertson AD, Liggett SB, Bristow MR. Adrenergic receptor polymorphisms and prevention of ventricular arrhythmias with bucindolol in patients with chronic heart failure. Circ Arrhythm Electrophysiol. 2013;6:137–43. doi: 10.1161/CIRCEP.111.969618. [DOI] [PubMed] [Google Scholar]
- 14.Bristow MR, Murphy GA, Krause-Steinrauf H, et al. An alpha2C-adrenergic receptor polymorphism alters the norepinephrine lowering effects and therapeutic response of the beta blocker bucindolol in chronic heart failure. Circ Heart Fail. 2010;3:21–8. doi: 10.1161/CIRCHEARTFAILURE.109.885962. [DOI] [PubMed] [Google Scholar]
- 15.BEST Investigators. A trial of the beta-adrenergic blocker bucindolol in patients with advanced heart failure. N Engl J Med. 2001;344:1659–67. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- 16.Bristow MR, Krause-Steinrauf H, Nuzzo R, et al. Effect of baseline or changes in adrenergic activity on clinical outcomes in the beta-blocker evaluation of survival trial. Circulation. 2004;110:1437–42. doi: 10.1161/01.CIR.0000141297.50027.A4. [DOI] [PubMed] [Google Scholar]
- 17.Kao DP, Davis G, MS, Aleong R, et al. Effect of bucindolol on heart failure outcomes and heart rate response in patients with reduced ejection fraction heart failure and atrial fibrillation. Eur J Heart Fail. 2012;15:324–33. doi: 10.1093/eurjhf/hfs181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tisdale JE, Borzak S, Sabbah HN, Shimoyama H, Goldstein S. Hemodynamic and neurohormonal predictors and consequences of the development of atrial fibrillation in dogs with chronic heart failure. J Card Fail. 2006;12:747–51. doi: 10.1016/j.cardfail.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Oral H, Crawford T, Frederick M, et al. Inducibility of paroxysmal atrial fibrillation by isoproterenol and its relation to the mode of onset of atrial fibrillation. J Cardiovasc Electrophysiol. 2008;19:466–70. doi: 10.1111/j.1540-8167.2007.01089.x. [DOI] [PubMed] [Google Scholar]
- 20.Sharifov OF, Fedorov VV, Beloshapko GG, Glukhov AV, Yushmanova AV, Rosenshtraukh LV. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. J Am Coll Cardiol. 2004;43:483–90. doi: 10.1016/j.jacc.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 21.Wang C, Zhang YJ, Wang YL, et al. Effect of dipfluzine on delayed afterdepolarizations and triggered activity induced by isoprenaline in human atrial fibers. Acta Pharmaceut Sinica. 2006;41:184–7. [PubMed] [Google Scholar]
- 22.van Veldhuisen DJ, Aass H, El Allaf D, Dunselman PH, Gullestad L, Halinen M, Kjekshus J, Ohlsson L, Wedel H, Wikstrand J for the MERIT-HF Study Group. Presence and development of atrial fibrillation in chronic heart failure. Experiences from the MERIT-HF study. Eur J Heart Fail. 2006;8:539–46. doi: 10.1016/j.ejheart.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 23.White HL, de Boer RA, Maqbool A, et al. An evaluation of the beta-1 adrenergic receptor Arg389Gly polymorphism in individuals with heart failure: a MERIT-HF sub-study. Eur J Heart Fail. 2003;5:463–8. doi: 10.1016/s1388-9842(03)00044-8. [DOI] [PubMed] [Google Scholar]