Abstract
The aim of the study:
The aim of the study was clinical and cytological examination of gingival changes in smokers and non-smokers. Further, specific goals of this study were health promotion in patient, particularly in smokers.
Methods:
The anamnesis was taken and clinical examination was conducted on the patients who came on Dental Clinic. During the clinical examination, plaque index (Pl)16, gingival index Löe-Silness (Gi) and the community periodontal index of treatment needs (CPITN)17 were done. After diagnosis was established, participants divided into group I –smokers, and group II – non-smokers. The gingival smears were taken for cytological analysis, dried on air, and stained by haematoxylin-eosin method.
Results:
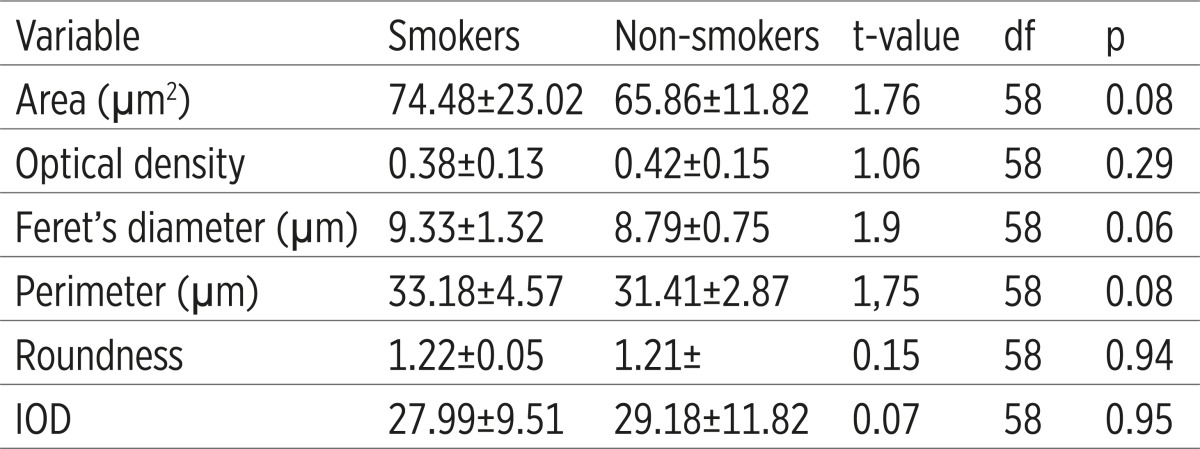
The values of gingival index (GI)- Löe-Silness and periodontal index (CPITN) were higher in the group of smokers, but plaque index was also higher with statistically significant difference of their values between examined group, with maximum level of significance (p<0,001). The size of nucleus (area, Ferret’s diameter and perimeter) was higher in the group of smokers, but differences were not statistically significant. In the group of non-smokers density of nucleus was higher than in non-smokers group, but difference was not statistically significant.
Conclusion:
The values of examined indices showed higher values in smokers group. This finding could show that the level of oral hygiene is higher in the non smokers group. The size of nucleus (area, Ferret’s diameter and perimeter) was higher in the group of smokers, but differences were not statistically significant. Teamwork of many different speciality experts is required for better periodontal health of smokers.
Key words: smoking, periodontal disease, gingival, cytological investigation.
1. INTRODUCTION
Periodontal disease is chronic, inflammatory disease followed by destruction of periodontal tissues. Oral biofilm with anaerobic microorganisms represents main etiological factor for occurrence of periodontal disease, but cigarette smoking is basic risk factor for development of chronic periodontal disease. Periodontal disease is three times more frequent in smokers than in non-smokers, regardless the level of oral hygiene (1). Disease quicker progresses in smokers than in non-smokers (2). Cigarette smoking is connected with more frequent appearance and progression of aggressive periodontal disease, with deeper periodontal pockets, alveolar bone lost and tooth lost (3). Cigarette smoking could mask an early inflammatory signs of gingivitis and periodontal disease, particularly the propensity of the gingiva to bleed on brushing, or following periodontal probing (4).
Cigarette smoking is one of the most significant risk factors for multiple diseases, including periodontal disease (5, 6). In smokers it was reported early onset of disease (7, 8) and increased rates of disease progression (9). Furthermore, clinical investigations have demonstrated that cigarette smoking may hamper the healing outcome following surgical and nonsurgical periodontal therapy (10, 11). Cigarette smoking could mask early signs of periodontal disease by suppressing of immune host response. This could cause problem in diagnosis of this disease, particularly in young people with early stage of periodontal disease. Periodontal disease is more progressive in smokers and their response on periodontal therapy is significantly weaker than in non-smokers. Group of active smokers are losing epithelium insertion, although basic treatment is correctly performed. These findings are particularly significant, because they indicate that 85-90% of patients, with periodontal disease, were group of active smokers (12).
During of inflammation of gingiva, there is deviation in size and shape of cells of stratified squamous epithelium and their nucleuses are increased independently of cell differentiation degree. Exfoliative cytology is non-invasive technique, which is very important in determination of inflammation in oral cavity, and it allows easily and painless sampling of cells, afterwards they were examined under a microscope (13, 14). Cell desquamation of stratified squamous epithelium depends on mitotic action of a basal layer, enzyme activity in cell culture and action of mechanical irritation (13). Obradović et al (15) showed that during the gingival inflammation, which is common in diabetic patients, there were deviations in size and shape of cells of stratified aqueous epithelium , and that increased their nuclei regardless of the degree of differentiation of cells.
2. AIM OF THE STUDY
The aim of the study was clinical and cytological examination of periodontal changes in smokers and non-smokers. Further, specific goals of this study were health promotion in patient, particularly in smokers.
3. PATIENTS AND METHODS
The study was conducted on Dental clinic of Medical faculty in Niš and Institute of Pathology, Medical faculty in Niš. Ethics Committee of Medical faculty Niš approved the methodology of the study. The anamnesis was taken and clinical examination was conducted on the patients who came on Dental clinic of Medical faculty in Niš. During the clinical examination indices of oral hygiene, plaque index (Pl) (16), gingival index Löe-Silness (Gi) and the community periodontal index of treatment needs (CPITN) (17) were done. After diagnosis was established, participants divided into group I –smokers, and group II – non-smokers. If participants were in group of smokers, than the smoking period and number of cigarettes per day would be registered. The study’s exclusion criteria was: patients on immunosuppressive, antibiotic or corticosteroid therapy in past six months, patients younger than 18 years, pregnant women, patients with blood disease, acute and chronic infections, autoimmune and cardiovascular disease as well as former smokers. The gingival smears were taken for cytological analysis, dried on air, and stained by haematoxylin-eosin method. Digital pictures (1280x1024x24b pixels) were taken under objective x63 at microscope NU-2 (Carl Zeiss, Jena Germany). For karyometric analysis, the ImageJ software (Wayne Rasband, National Instututes of Health, USA, http://rsb.info.nih.gov/ij) was used. Six nuclear variables were estimated: area, optical density, Ferret’s diameter, perimeter, roundness, and integrated optical density (IOD). Statistical analysis of results was performed using descriptive statistics and multivariate methods (MANOVA).
4. RESULTS
Out of ninety patients 45 were men and 45 women. The average age of the smokers was 37.72±10.99. In group of non-smokers, the average age was 42.65±13.80. That was lower than the age of group I, but the difference was not significant (Table 1).
Table 1.
Plaque index, gingival index and CPITN index of smokers and non-smokers * Higly significant difference
 |
The values of gingival index (GI)- Löe-Silness and periodontal index (CPITN) were higher in the group of smokers, but plaque index was also higher with statistically significant difference of their values between examined group, with maximum level of significance (p<0.001). The size of nucleus (area, Ferret’s diameter and perimeter) was higher in the group of smokers, but differences were not statistically significant. In the group of non-smokers density of nucleus was higher than in non-smokers group, but difference was not statistically significant (Table 2).
Table 2.
Karyometric variables on cytological material from smokers and non-smokers
 |
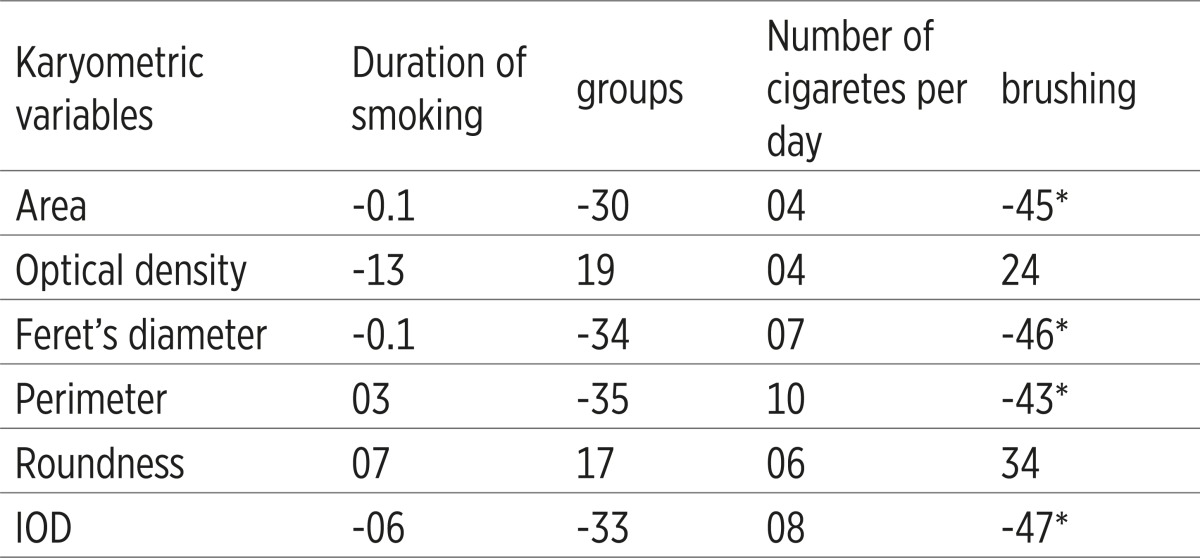
There is statistically significant negative correlation between tooth brushing and area, major axis, minor axis, middle diameter and perimeter (Table 3). The average smoking period was 15.92±9.84 years. There is negative correlation between density and period of smoking (more than 0,5). Perimeter- the size of nucleus was higher in the group of smokers. In the group of non-smokers was higher density of nucleus. There is negative correlation between tooth brushing and area, major axis, minor axis, middle diameter and perimeter.
Table 3.
Coefficients of correlation between kariometric variables and duration of smoking, number of cigarettes, and brushing. * Statistically significant corelations
 |
5. DISCUSSION
Numerous authors were established that smoking is significant risk factor for occurrence of periodontal disease (1, 2, 4, 18, 19). Comparison of clinical parameters of periodontal disease between smokers and non-smokers, could be notice that clinical symptoms were heavier and it was manifested by higher number of deep pockets, more excessive loss of epithelial insertion and presence of higher number of gingival recessions, accelerated alveolar bone loss, but gingival bleeding is reduced in these patients (1, 20, 21). Preber et al (4), were noticed that gingival bleeding, which is first sign of gingival inflammation, and periodontal disease, was lower expressed in group of smokers with regard to the longer period of smoking. Although, it was noticed that passionate smokers had significantly greater plaque index, average number of bleeding sites after periodontal probing was reduced in smokers (27%), than in non-smokers (40%). Anil (22), explained that nicotine which causes vasoconstriction of peripheral blood vessels and that reduces the objective clinical signs of gingivitis showed reduction of inflammatory clinical signs. Mavropoulos et al. (23) were consider that small recurrent vasoconstrictive attacks, during long period of smoking could lead to gingival vascular dysfunction and periodontal disease. Induced vasoconstriction could impair gingival blood vessels and could reduce amount of oxygen and blood elements that supply gingiva with nutritive elements. Nevertheless, the gingival bleeding and the amount of gingival crevicular fluid GCF was reduced in smokers compared to non-smokers (24).
Cigarette smoke contains approximately 4800 chemicals, with over 60 of them known to have adverse effects on human cells and tissues (25). Chemicals found in cigarette smoke are also highly genotoxic and lead to various forms of DNA damage (26). One of chemicals of smoke, nicotine, effects on the strength of attachment of gingival fibroblasts, and when epithelial cells were treated with nicotine than collagen and uncollagen proteins production from gingival fibroblasts was significantly disturbed (27,28). Following treatment with nicotine on human gingival fibroblasts leads to a proinflammatory cytokine production: IL-6 and IL-8 (29). According to average values of dental plaque, it could be notice that low level of oral hygiene is present in the control group-non-smokers, but it was lower in smokers where the dental plaque is covering more than one third surface of dental crown. According to results of this study it could be concluded that an increased amount of dental plaque in smokers was consequence of low level of oral hygiene. Wilson (30) was explained that an increased amount of dental plaque was consequence of smoking dental deposits, witch made dental surface uneven and because that reason dental plaque was annexed easier. According to previous findings, calculus forming was increased in smokers, due to the increased flow of saliva and concentration of calcium present in fresh saliva of smokers, immediately after smoking (31). Nicotine from cigarette smoke affects exocrine glands, primarily by increasing the secretion of salivary and bronchial glands, while it inhibits them later. According to previous clinical and epidemiological findings in different population, the authors (32,33) were suggesting that tobacco smoking was associated with increased accumulation of supra gingival and subgingival dental calculus.
The group of smokers were reacted unfavorably on periodontal therapy although in group of smokers and in group of non-smokers, the same pathogens were present (34,35). It is establish that smoking has influence on level of cytokines in host, however the specific mechanisms, which damaged periodontal tissue, stay unclear (36). Partial, the reason why smokers have more deposits than non-smokers is the maintenance of lower level of oral hygiene, or they are used to tolerate the lower level of oral hygiene.
6. CONCLUSION
The values of gingival index (GI) Löe-Silness and periodontal index (CPITN) were higher in the group of smokers, but plaque index was also higher with statistically significant difference of their values between examined group, with maximum level of significance (p<0.001). The size of nucleus (area, Ferret’s diameter and perimeter) was higher in the group of smokers, but differences were not statistically significant. In the group of non-smokers density of nucleus was higher than in non-smokers group, but difference was not statistically significant. Previous reports about influence of smoking on periodontal health, indicated that a periodontologist should educate smokers with periodontal disease, and show them the risk and consequences how this bad habit influences on periodontal tissues. Teamwork of many experts of different speciality field is required for better periodontal health of smokers.
Conflict of interest
None declared.
REFERENCES
- 1.Machuca G, Rosales I, Lacalle JR, Machuca C, Bullon P. Effects of cigarette smoking on periodontal status of healthy young adults. J Periodontol. 2000;71:73–78. doi: 10.1902/jop.2000.71.1.73. [DOI] [PubMed] [Google Scholar]
- 2.Bergstrom J, Eliasson S, Dock J. A 10-year prospective study of tobacco smoking and periodontal health. J Periodntol. 2000;71:1338–1347. doi: 10.1902/jop.2000.71.8.1338. [DOI] [PubMed] [Google Scholar]
- 3.Bergström J, Eliasson S, Dock J. Exposure to tobacco smoking and periodontal health. J Clin Periodontol. 2000;27:61–68. doi: 10.1034/j.1600-051x.2000.027001061.x. [DOI] [PubMed] [Google Scholar]
- 4.Preber H, Bergström J. Occurence of gingival bleeding in smoker and non-smoker patients. Acta Odontol Scand. 1985;43:315–320. doi: 10.3109/00016358509046515. [DOI] [PubMed] [Google Scholar]
- 5.Johnson GK, Guthmiller JM. The impact of cigarette smoking on periodontal disease and treatment. Periodontol. 2007;44:178–194. doi: 10.1111/j.1600-0757.2007.00212.x. [DOI] [PubMed] [Google Scholar]
- 6.Kibayashi M, Tanaka M, Nishida N, Kuboniwa M, Kataoka K, Nagata H, Nakayama H, Morimoto K, Shizukuishi S. Longitudinal study of the association between smoking as a periodontitis risk and salivary biomarkers related to periodontitis. J Periodontol. 2007;78:859–867. doi: 10.1902/jop.2007.060292. [DOI] [PubMed] [Google Scholar]
- 7.Mullally B. The influence of tobacco smoking on the onset of periodontitis in young persons. Tob Induc Dis. 2004;2:53–65. doi: 10.1186/1617-9625-2-2-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiau HJ, Reynolds MA. Sex differences in destructive periodontal disease: exploring the biologic basis. J Periodontol. 2010;81(11):1505–1517. doi: 10.1902/jop.2010.100045. [DOI] [PubMed] [Google Scholar]
- 9.Moimaz SA, Zina LG, Saliba O, Garbin CA. Smoking and periodontal disease: clinical evidence for an association. Oral Health Prev Dent. 2009;7(4):369–376. [PubMed] [Google Scholar]
- 10.Silva CO, Ribeiro Edel P, Sallum AW, Tatakis DN. Free gingival grafts: graft shrinkage and donor-site healing in smokers and non-smokers. J Periodontol. 2010;81(5):692–701. doi: 10.1902/jop.2010.090381. [DOI] [PubMed] [Google Scholar]
- 11.Wan CP, Leung WK, Wong MC, Wong RM, Wan P, Lo EC, Corbet EF. Effects of smoking on healing response to non surgical periodontal therapy: a multilevel modelling analysis. J Clin Periodontol. 2009;36(3):229–239. doi: 10.1111/j.1600-051X.2008.01371.x. [DOI] [PubMed] [Google Scholar]
- 12.Haber J. Cigarette smoking: a major risk factor for periodontitis. Compendium. 1994 Aug;15(8):1002. 4-8 passim; quiz 14. [PubMed] [Google Scholar]
- 13.Igić M. Evaluation of results of chronic catarrhal gingivitis treatment by hyaluronic acid, basic therapy and laser therapy in children. [dissertation] Niš: School of Medicine; 2008. (Serbian) [Google Scholar]
- 14.Anuradha A, Sivapathasundharam B. Image analysis of normal exfoliated gingival cells. Indian J Dent Res. 2007;18(2):63–66. doi: 10.4103/0970-9290.32422. [DOI] [PubMed] [Google Scholar]
- 15.Obradović R, Kesić L, Jovanović G, Petrović D, Radičević G, Mihailović D. Low power laser efficacy in the therapy of inflamed gingive in diabetics with parodontopathy. Vojnosanit pregl. 2011;68(8):684–689. doi: 10.2298/vsp1108684o. [DOI] [PubMed] [Google Scholar]
- 16.Harald Löe. The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol. 1967;38(6):610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 17.Ainamo J, Barmes D, Beagrie G, Cutress T, Martin J, Sardo-Infirri J. Development of the World Health Organization (WHO) community periodontal index of treatment needs (CPITN) Int Dent J. 1982;32(3):281–291. [PubMed] [Google Scholar]
- 18.Kraal JH, Chancellor MB, Bridges RB, Bemis KG, Hawke JE. Variations in the gingival polymorphonuclear leukocyte migration rate in dogs induced by chemotactic autologous serum and migration inhibitor from tobacco smoke. J Periodontal Res. 1977;12(4):242–249. doi: 10.1111/j.1600-0765.1977.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 19.MacFarlane GD, Herzberg MC, Wolff LF, Hardie NA. Refractory periodontitis associated with abnormal polymorphonuclear leukocyte phagocytosis and cigarette smoking. J Periodontol. 1992 Nov;63(11):908–913. doi: 10.1902/jop.1992.63.11.908. [DOI] [PubMed] [Google Scholar]
- 20.Bergstrom J, Preber H. Tobacco use as a risk factor. J Periodontol. 1994;65:545–550. doi: 10.1902/jop.1994.65.5s.545. [DOI] [PubMed] [Google Scholar]
- 21.Calsina G, Ramon J-M, Echeverria JJ. Effects of smoking on periodontal tissues. J Clin Periodontol. 2002;29:771–776. doi: 10.1034/j.1600-051x.2002.290815.x. [DOI] [PubMed] [Google Scholar]
- 22.Anil S. Study of the patterns of periodontal destruction in smokers with chronic periodontitis. Indian J Dent Res. 2008;19(2):124–128. doi: 10.4103/0970-9290.40466. [DOI] [PubMed] [Google Scholar]
- 23.Mavropoulos A, Aars H, Brodin P. Hyperaemic response to cigarette smoking in haelthy gingiva. J Clin Periodont. 2003;30:14–221. doi: 10.1034/j.1600-051x.2003.10284.x. [DOI] [PubMed] [Google Scholar]
- 24.Bergström J, Persson L, Preber H. Influence of cigatette smoking on vascular reaction during experimental gingivitis. Scan J Dent Res. 1988;96:34–39. doi: 10.1111/j.1600-0722.1988.tb01405.x. [DOI] [PubMed] [Google Scholar]
- 25.Semlali A, Chakir J, Goulet J-P, Chmielewski W, Rouabhia M. Whole cigarette smoke promotes human gingival epithelial cell apoptosis and inhibits cell repair processes. J Periodont Res. 2011;46:533–541. doi: 10.1111/j.1600-0765.2011.01370.x. [DOI] [PubMed] [Google Scholar]
- 26.Mills AL, Messer K, Gilpin EA, Pierce JP. The effect of smoke-free homes on adult smoking behavior: a review. Nicotine Tob Res. 2009;11(10):1131–1141. doi: 10.1093/ntr/ntp122. [DOI] [PubMed] [Google Scholar]
- 27.Tanur E, McQuade MJ, McPherson JC, Al-Hashimi IH, Rivera-Hidalgo F. Effects of nicotine on the strenght of attachment of gingival fibroblasts to glass and non-diseased human root surfaces. J Periodontol. 2000;71:717–722. doi: 10.1902/jop.2000.71.5.717. [DOI] [PubMed] [Google Scholar]
- 28.Giannopoulou C, Roehrich N, Mombelli A. Effect of nicotine - treated epithelial cells on the proliferation and collagen production of gingival fibroblasts. J Clin Periodontol. 2001;28:769–775. doi: 10.1034/j.1600-051x.2001.280808.x. [DOI] [PubMed] [Google Scholar]
- 29.Wndell KJ, Stein SH. Regulation of cytokine production in human gingival fibroblasts following treatment with nicotine and lipopolysaccharide. J Periodontol. 2001;72:1038–1044. doi: 10.1902/jop.2001.72.8.1038. [DOI] [PubMed] [Google Scholar]
- 30.Wilson T. Effects of smoking on periodontium. Quintessence International. 1998;29:265–266. [PubMed] [Google Scholar]
- 31.Khan GJ, Salah-ud-Din MR, Marwat FM, Haq I, Reihman J. Secretion of calcium in the saliva of long term tobacco users. J Ayub Med Col Abbott. 2005;17:453–460. [PubMed] [Google Scholar]
- 32.Anerud A, Loe H, Baysen H. Natural history and clinical course of calculus formation in man. J Clin Periodontol. 1991;18:160–170. doi: 10.1111/j.1600-051x.1991.tb01128.x. [DOI] [PubMed] [Google Scholar]
- 33.Bergström J. Tobacco smoking and supragingival dental calculus. J Clin Periodontol. 1999;26:541–547. doi: 10.1034/j.1600-051x.1999.260808.x. [DOI] [PubMed] [Google Scholar]
- 34.Boström L, Linder LE, Bergström J. Clinical expression of TNF-alpha in smoking-associated periodontal disease. J Clin Periodontol. 1998;25:767–773. doi: 10.1111/j.1600-051x.1998.tb02368.x. [DOI] [PubMed] [Google Scholar]
- 35.Buduneli N, Bazlas H, Buduneli E, Turkuglou O, Dahlen G. Evaluation of the relationship between smoking during pregnancy and subgingival microbiota. J Clin Periodontol. 2005;32:174–181. doi: 10.1111/j.1600-051X.2004.00633.x. [DOI] [PubMed] [Google Scholar]
- 36.Böstrom L, Linder LE, Bergström J. Smoking and crevicular fluid levels of IL-6 and TNF-α in periodontal disease. J Clin Periodontol. 1999;26:352–357. doi: 10.1034/j.1600-051x.1999.260604.x. [DOI] [PubMed] [Google Scholar]


