Abstract
Introduction:
Clostridium difficile (C. difficile) is currently the leading cause of healthcare-associated diarrhea, but almost nothing is known about the extent of C. difficile infection (CDI) in Bosnia and Herzegovina.
Goal:
We aimed to retrospectively analyze CDI in hospitalized patients at University Clinical Center (UCC) Tuzla, Bosnia and Herzegovina from January 2009 through June 2012.
Methods:
We analyzed all patients (except children ages 0-2), diagnosed with CDI based on anamnestic and epidemiological, clinical picture and microbiological tests (proof of toxins in the stool by enzyme-linked immunosorbent assay).
Results:
From a total of 989 patients tested for C. difficile toxin (60.2 per 10,000 inpatient days) 347 (35.08%) were positives. The mean incidence rate of CDI was 2.23 per 10,000 inpatient days (range 1.32-2.87). Annual rates of hospitalization were 15.68 per 10,000 admissions (range 8.99-20.35). Most patients had a previously identified risk profile of old age, comorbidity and recent use of antibiotics. 41/276 (14.86%) patients had died, and 11/41 (26.82%) were CDI-associated deaths. Complicated CDI were registered in 53/276 (19.21%) patients, and recurrent infections in 65/276 (23.55%).
Conclusion:
Our data suggest that CDI is largely present in our setting which represents a serious problem and points to the importance of international surveillance, detection and control of CDI.
Key words: Clostridium difficile infection, healthcare-associated, incidence rate, diarrhea.
1. INTRODUCTION
Clostridium difficile (C. difficile) is currently the leading cause of healthcare-associated diarrhea with potentially fatal outcomes. The incidence and severity of C. difficile infection (CDI) throughout the world has increased in the last 20 years due to the emergence of hypervirulent strains, increased use and misuse of antibiotics, as well as increased susceptibility in a population at-risk, and other risk factors. Various studies in Canada, the United States (U.S.) and Europe recorded an increase of 2 to 4-fold in CDI incidence (1, 2). As the incidence of CDI increased, CDI mortality and colectomy rates increased as well (3). Recent data from the European studies show a mean incidence rate of healthcare-associated CDI of 4.1 per 10,000 inpatient days, but ranged from 0.0 to 36.3 (4).
There is abundance of data on CDI prevalence in Europe, however little is known about the prevalence of CDI in Eastern Europe, and almost nothing about prevalence in Bosnia and Herzegovina. Therefore we aimed to retrospectively analyze epidemiological, clinical and microbiological characteristics of patients with CDI hospitalized in clinics at University Clinical Center (UCC) Tuzla, Bosnia and Herzegovina from January 2009 through June 2012.
2. METHODS
Study design and patients
We retrospectively collected and analyzed epidemiological, clinical and microbiological data for 276 patients, who were during hospitalization in clinics at UCC Tuzla, in the period from 1.1.2009 to 30.6.2012, diagnosed and treated for CDI. The diagnosis of CDI was determined based on anamnestic data, epidemiological data, clinical picture (basic clinical symptoms and signs), microbiological tests (enzyme-linked immunosorbent assay (ELISA) for detection of C. difficile toxin A and B; Serazym Clostridium difficile Toxin A+B, Seramun Diagnostica GmBH, Heidesee, Germany), and according to the definition of guidelines for CDI treatment by the European Association of Clinical Microbiology and Infectious Diseases (ESCMID) (5). We also collected data from the Institute of Microbiology UCC Tuzla; the total number of toxin-positive cases and the number of patients tested, as well as the data from the Office of Planning and Analysis UCC Tuzla; the number of hospitalized patients and the number of inpatient days.
Statistical analysis
All statistical analyses were performed using SPSS 15.0 (SPSS, Chicago, Illinois, USA). Descriptive statistical parameters have been used for the determination of baseline characteristics. We calculated intra hospital prevalence based on 10,000 inpatient days. A statistical level of 95% (P<0.05) was considered significant for all performed tests.
3. RESULTS
From January 2009 through June 2012, 347 patients were admitted and treated for CDI at UCC Tuzla. The total number of toxin-positive cases amounted to 347 out of 989 patients tested with suspected CDI (35.08%) (Table 1). The number of patients tested per 10,000 inpatient days was 60.2 (Table 1). 276/347 (79.53%) patients were included in this study: who had toxin-positive-stool samples and available clinical and epidemiological data. Most cases were healthcare-associated infections 256/276 (92.75%), while 20/276 (7.25%) were the outpatient cases. Healthcare-associated infections were registered in 15/19 (78.94%) clinics at UCC Tuzla, while in the remaining four clinics (21.06%) there were no patients with CDI. The incidence rate of CDI varied across clinics, weighted mean 2.23 per 10,000 inpatient days (range 1.32-2.87) (Table 1).
Table 1.
Summary of Clostridium difficile infection in clinics University Clinical Center Tuzla 2009.–2012
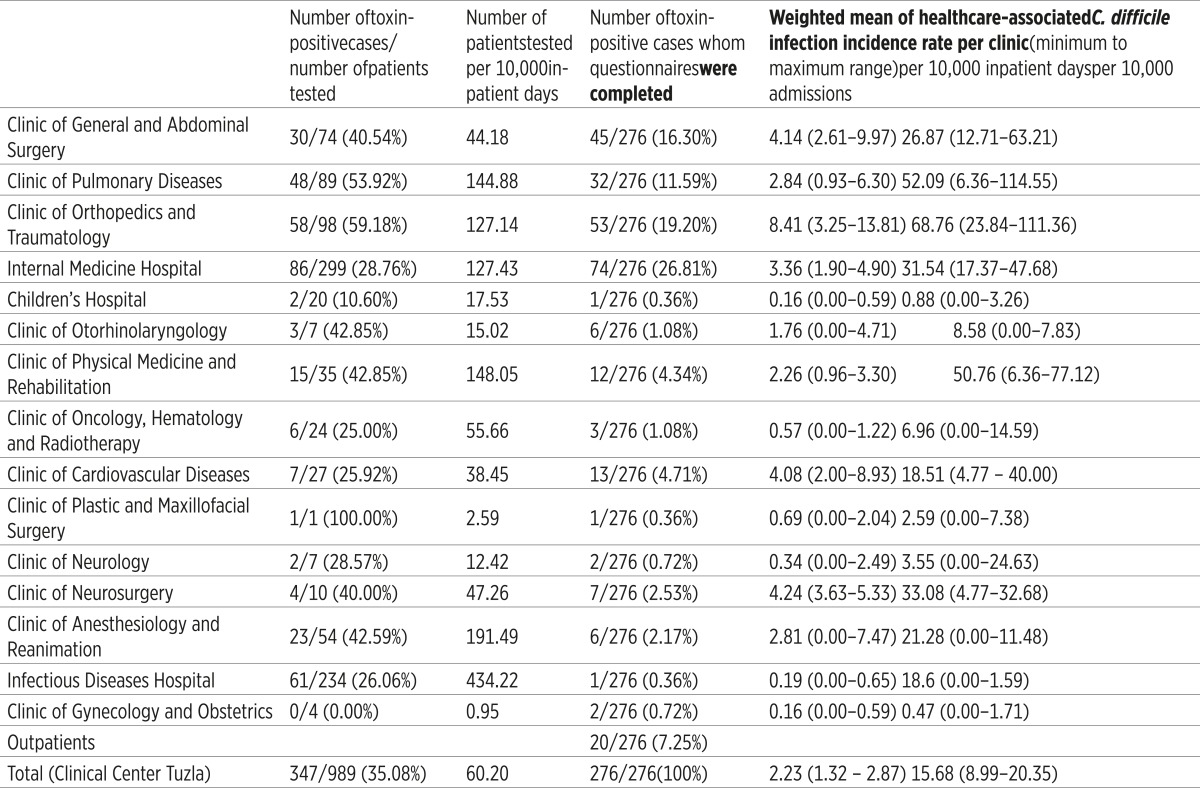 |
Three leading clinics with the highest incidence rate of CDI per 10,000 inpatient days were: the Clinic of Orthopedics and Traumatology, weighted mean 8.41 (range 3.25-13.81), the Clinic of General and Abdominal Surgery, weighted mean 4.14 (range 2.61-9.97) and the Clinic of Cardiovascular Diseases, weighted mean 4.08 (range 2.00- 8.93).
Our research has shown that the annual rates of hospitalization with CDI during were 15.68 per 10,000 admissions (range 8.99-20.35). Three leading clinics with registered and hospitalized patients with CDI were: the Clinic of Orthopedics and Traumatology, weighted mean 68.76 per 10,000 admissions (range 23.84-111.36), then the Clinic of Pulmonary Diseases and Tuberculosis, 52.09 (range 6.36- 114.55), and the Clinic of Physical Medicine and Rehabilitation, 50.76 (range 6.36-77.12). The largest number of hospitalized patients after evidence of healthcare-associated CDI infection was transferred to the Infectious Diseases Hospital 142/276 (51.44%), for further treatment.
Most patients fit the previously determined risk profile: 174 (63.04%) were older than 65 years, 148 (53.62%) had severe comorbidity, 139 (49.64%) had a surgical procedure in the previous 3 months, and most of them, 256 (92.5%), received antibiotics 3 months prior to infection; usually cephalosporines (71.02%), quinolones (32.97%) or different combinations of antibiotics (Table 2).
Table 2.
Characteristics of patients with Clostridium difficile infection
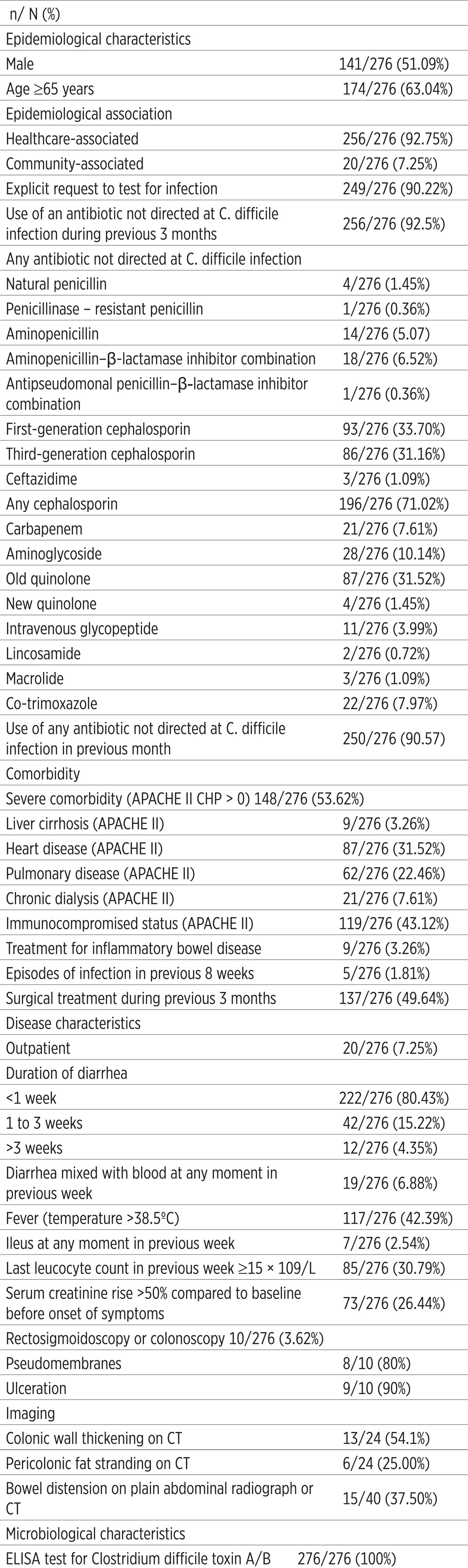 |
250/276 (90.57%) CDI patients were receiving antibiotics within 1 month before the onset of symptoms. 5/276 (1.81%) patients had recurrent CDI at inclusion. Before hospital admission, 20/276 (7.25%) patients were infected with C. difficile in the outpatient setting. In 222/276 (80.43%) patients, CDI was clinically manifested as a short diarrheal disease before taking stool samples, less than 1 week. Furthermore, 117/276 (42.93%) patients had fever, diarrhea mixed with blood 19/276 (6.88%), ileus 7/276 (2.54%) in the week before taking stool samples (Table 2).
In 73/276 (26.44%) patients, the most recent laboratory results of serum creatinine before taking stool samples were increased by more than 50% compared with the last values before the onset of CDI symptoms. Leukocyte count during the week prior to sampling was ≥ 15 × 109/L in 85/276 (30.79%) patients. In moderate and severe cases of CDI, the disease was manifested with colitis, a large number of stools (at times mucous and bloody), dehydration, sometimes severe abdominal pain and meteorism. 10/276 (3.62%) patients underwent rectosigmoidoscopy or colonoscopy. Thus, in 8/10 (80%) patients we detected pseudo membranes and ulcerations in 9/10 (90%). Also, in the first radiography or computed tomography (CT) image bowel distension was observed in 15/40 (37.50%) patients.
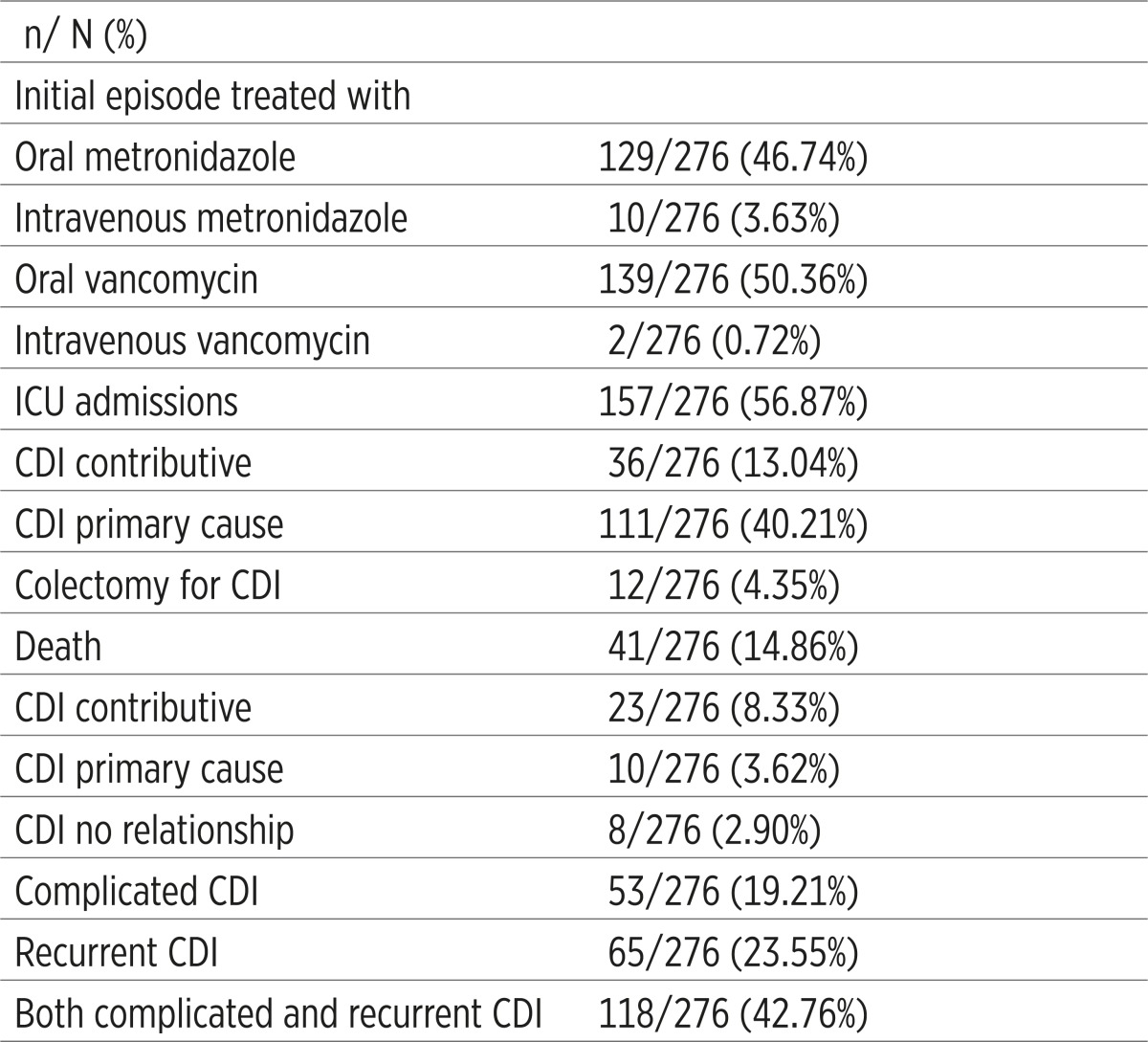
In the course of this analysis, of the total number of CDI patients, hospitalized and confirmed by ELISA for C. difficile toxins, treatment and outcome data were collected for 276/347 (79.5%). From 41/276 (14.86%) patients who died, in 11/41 (26.82%) death was CDI-associated (Table 3).
Table 3.
Treatment and outcome characteristics of patients with Clostridium difficile infection
 |
All 11 patients whose death was due primarily to C. difficile infection were between 70 to 91 years old, and their infection was healthcare-associated. They all had severe comorbidity, 1 to 3 different diseases, and 6 of them besides comorbidity had previous surgical interventions within 3 months before the onset of symptoms.
Colectomy was performed in 12/276 (4.35%) patients. Complicated CDI were registered in 53/276 (19.21%), while recurrent infections in 65/276 (23.55%) patients. Treatment of CDI patients consisted of discontinuation of antimicrobial therapy, changing antimicrobial agent, diet and rehydration, and other supportive therapies. The initial episode of CDI in our patients was treated with oral metronidazole in 129/276 (50.36%). Since CDI infections were mainly healthcare-associated, occurred in different, most clinics at UCC Tuzla and represented a serious problem in the past, a large percentage of patients with CDI were isolated and treated in the intensive care unit, a total of 157/276 (56.8%).
4. DISCUSSION
Reports from many European countries (Finland, Denmark, and Germany) (6-8), and elsewhere (2, 9, 10), record the increment in CDI incidence. Some authors report reduction in CDI incidence and CDI-associated mortality due to improved surveillance, reporting and prevention (11). According to reports from the U.S., the rate of pediatric CDI hospitalizations increased from 7.2 to 12.80 from 1997 through 2006; the lowest rate was for children under age 1 [9]. Approximately 11 – 28 % of CDI patients acquired infection in the community, which seems consistent in different countries (12, 13). It is known that the main cause of CDI is exposure to antibiotics or chemotherapeutics (antineoplastic agents), then high age, comorbidity and hospital stay. Just recently additional risk factors for acquiring CDI have been reported: inflammatory bowel disease – ulcerative colitis and Crohn’s disease, pharmacological blockade of gastric acid secretion caused primarily by proton pump inhibitors, organ transplantation, previous gastrointestinal surgery and others (1, 14, 15).
C. difficile causes pathogenic diseases of the gastrointestinal tract by secreting 2 exotoxins, enterotoxin A and cytotoxin B, which cause diarrhea and colitis. For a long time, it has been thought that C. difficile produces both toxins, but the latest research suggests the existence of strains that produce only toxin B. It is estimated that the changes in the flora of the colon and intestinal motility dysfunction represent a suitable basis for the development of infection (16, 17).
C. difficile is considered to be responsible for a spectrum of diseases ranging from asymptomatic colonization to diarrhea of varying severity to life-threatening pseudomembranous colitis. Diseases that it causes are known as C. difficile-associated diseases (CDAD). Typical manifestations of CDAD are abdominal pain and cramps, profuse diarrhea (mucous, greenish, watery, foul-smelling stool), with fever and leukocytosis (10). A case of CDI is defined by the presence of symptoms (usually diarrhea) and either a positive stool test for CDI toxins, polymerase chain reaction (PCR) or colonoscopic findings revealing the presence of pseudomembranous colitis (2, 5).
Briefly, according to the definition of guidelines for CDI treatment by ESCMID, CDI is divided into hospital cases (those that occurred in hospitals, or nursing homes 48 hours after admission, or within 4 weeks after discharge from these institutions); and outpatient cases (those that occurred outside hospital, before hospital admission, and the patient has not been hospitalized in the past 12 weeks) (5). The increase in CDI mortality rate which has been recorded since 2000 is associated with the emergence of hypervirulent strain of C. difficile ribotype 027 which is characterized by much stronger production of toxins A and B, resistance to fluoroquinolones and production of a binary toxin (18). It is also unfavorable that a large number of adults at-risk no longer present typical predisposing factors that contribute to CDI development. Additional problems are recurrent CDI infections; 20% of those who recover from the first CDI infection will suffer again (18).
We have shown that CDI is indeed present in our setting and it was the leading cause of healthcare-associated diarrhea with sometimes fatal consequences. From a total of 347 patients admitted and treated for CDI, we were able to collect data for 276/347 (79.5%) of them, but due to technical and financial constrains molecular diagnosis of CDI was not performed. However, these data are from a hospital center with limited resources and budget, and are a true representation of our condition. Thus, our research has shown that the mean incidence of CDI at UCC Tuzla was 2.23 per 10,000 inpatient days, similar to numbers reported by Barbut et al. (19). These authors reviewed the mean incidence of healthcare-associated CDI in 23 European hospitals and reported European CDI average of 2.45 per 10,000 inpatient days (range 0.1-7.1). This is slightly lower than the incidence reported by Bauer et al. (4) with a mean incidence of healthcare-associated CDI in 34 European hospitals of 4.1 per 10,000 inpatient days (range 0.0-36.3). In several departments at UCC Tuzla, the mean incidence of healthcare-associated CDI was substantially higher; e.g. the Clinic of Orthopedics and Traumatology, 8.4 per 10,000 inpatient days. The reasons are many, including a somewhat injudicious use of antibiotics and poor on-ward prophylaxis.
It was shown that the annual rates of CDI hospitalization were 15.68 per 10,000 admissions at UCC Tuzla, in conjunction with a rise in annual rates since 2009 to 2012. The increasing rate of CDI hospitalizations in hospitalized patients has been also reported by other studies (9, 20-22). Most of our patients fit the previously determined risk profile such as age over 65, presence of severe comorbidity (heart disease, lung disease, immunodeficiency, previous surgical intervention), and most of them were receiving antibiotics within 3 months, or within 1 month prior to infection; usually cephalosporines, quinolones or different combinations of antibiotics (1, 2, 4, 10). Most studies have shown that the vast majority of patients with CDI had previously taken antimicrobial drugs, which is practically a conditio sine qua non for the empirical diagnosis (2, 23). Wide use of antimicrobials and tendency to polypragmasia implies that accurate quantification of CDI risk associated with a particular antibiotic is very difficult. A number of applied antimicrobials, a larger number of doses and longer duration of administration were associated with an increased risk for CDI.
Our research has shown that almost a half of our patients with CDI had previous surgery within 3 months and not only gastrointestinal but also other types of surgeries, which is different from other reports where gastrointestinal surgery was the prominent risk factor for CDI (1). However, most of these patients also had other risk factors for CDI.
A relatively small ratio of patients (7.25%) was infected with C. difficile in the outpatient setting. This is somewhat lower than in other reports which reported 11-28% patients with community-associated CDI (12, 24, 25). Whether this is due to lower exposure to C. difficile in the community or a lower incidence of antibiotic therapy predisposing for CDI remains to be explored.
In historical terms, the mortality of CDI is low, whether it is a direct or indirect result of the infection, and its value is less than 2% (2). The increase in deaths attributed to CDI, which has been recorded since 2000, is associated with the emergence of hypervirulent strain of C. difficile ribotype 027 which is characterized by much stronger production of toxins A and B, the resistance to fluroquinolones and production of a binary toxin (26). In our study out of all patients who died, in 11/41 (26.82%) deaths were CDI-associated. These results were similar to the study by Bauer et al. (4), where the mortality rate caused by hypervirulent strain of C. difficile at three-month follow-up in 34 European hospitals was 22%, and direct mortality from CDI was 40%. Technical inability to perform molecular diagnosis of CDI hampered a detailed analysis of the cause of death of our patients. All 11 patients in our study, whose death was a direct result of CDI, had similar risk factors (over 70 years old, healthcare-associated infection, comorbidity), similar to other studies (2, 4, 5).
Complicated CDI were reported in 1/5 patients, and recurrent infections in ¼, as in other reports (2, 4, 12, 17). Recurrent CDI are a particular problem because besides additional costs to the healthcare system due to repeated hospitalizations and increasing costs of treatment, they also are ongoing frustration for patients. The treatment of our CDI patients was guided by relevant guidelines: Clinical Practice Guidelines for Clostridium difficile Infection in Adults: 2010 Update by the Society for Healthcare Epidemiology of America (SHEA) and Infectious Diseases Society of America (IDSA) and ESCMID’s algorithm for the treatment (2, 5).
5. CONCLUSION
CDI in our hospital center is a much bigger problem than it was thought, although probably not only in our hospital but rather throughout Bosnia and Herzegovina. This research has important practical significance as it will help us to be more aware that the CDI is widely present in our setting. We have to give a serious thought to this clinical problem and more efficiently carry out the diagnosis, treatment, control and prevention of this infection. Our data also point to the importance and necessity of introducing molecular diagnostics of this infection, because prevalence data on PCR ribotypes in European and hospitals worldwide indicate the need for international monitoring of detection, treatment and control of CDI.
Conflict of interest
None declared.
REFERENCES
- 1.Lo Vecchio A, Zacur GM. Clostridium difficile infection: an update on epidemiology, risk factors, and therapeutic options. Curr Opin Gastroenterol. 2012;28(1):1–9. doi: 10.1097/MOG.0b013e32834bc9a9. [DOI] [PubMed] [Google Scholar]
- 2.Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31(5):431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 3.Kelly CP, LaMont JT. Clostridium difficile-more difficult than ever. N Engl J Med. 2008;359(18):1932–1940. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 4.Bauer MP, Notermans DW, van Benthem BH, Brazier JS, Wilcox MH, Rupnik M, et al. ECDIS Study Group. Clostridium difficile infection in Europe: a hospital-based survey. Lancet. 2011;377(9759):63–73. doi: 10.1016/S0140-6736(10)61266-4. [DOI] [PubMed] [Google Scholar]
- 5.Bauer MP, Kuijper EJ, van Dissel JT European Society of Clinical Microbiology and Infectious Diseases. European Society of Clinical Microbiology and Infectious Diseases (ESCMID): treatment guidance document for Clostridium difficile infection (CDI) Clin Microbiol Infect. 2009;15(12):1067–1079. doi: 10.1111/j.1469-0691.2009.03099.x. [DOI] [PubMed] [Google Scholar]
- 6.Søes L, Mølbak K, Strøbaek S, Truberg Jensen K, Torpdahl M, Persson S, et al. The emergence of Clostridium difficile PCR ribotype 027 in Denmark-a possible link with the increased consumption of fluoroquinolones and cephalosporines? Euro Surveill. 2009;14(15):19176. [PubMed] [Google Scholar]
- 7.Lyytikäinen O, Turunen H, Sund R, Rasinperä M, Kӧnӧnen E, Ruutu P, et al. Hospitalizations and deaths associated with Clostridium difficile infection, Finland, 1996-2004. Emerg Infect Dis. 2009;15:761–765. doi: 10.3201/eid1505.081154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vonberg RP, Schwab F, Gastmejer P. Clostridium difficile in discharged inpatients, Germany. Emerg Infect Dis. 2007;13(1):179–180. doi: 10.3201/eid1301.060611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marya D, Zilberberg MD, Tillotson GS, McDonald LC. Clostridium difficile infections among hospitalized children, United States, 1997-2006. Emerg Infect Dis. 2010;16(4):604–609. doi: 10.3201/eid1604.090680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuijper EJ, Coignard B, Tüll P. Emergence of Clostridium difficileassociated disease in North America and Europe. Clin Microbiol Infect Dis. 2006;12(Suppl 6):2–18. doi: 10.1111/j.1469-0691.2006.01580.x. [DOI] [PubMed] [Google Scholar]
- 11.Freeman J, Bauer MP, Baines SD, Corver J, Fawley WN, Goorhuis B, et al. The changing epidemiology of Clostridium difficile infections. Clin Microbiol Rev. 2010;23(3):529–549. doi: 10.1128/CMR.00082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Nispen tot Pannerden CM, Verbon A, Kuipers EJ. Recurrent Clostridium difficile infection: what are the treatment options? Drugs. 2011;71(7):853–868. doi: 10.2165/11591230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Aslam S, Hamill RJ, Musher DM. Treatment of Clostridium difficile-associated disease: old therapies and new strategies. Lancet Infect Dis. 2005;5(9):549–557. doi: 10.1016/S1473-3099(05)70215-2. [DOI] [PubMed] [Google Scholar]
- 14.Barbut F, Richard A, Hamadi K, Chomette V, Burghoffer B, Petit JC. Epidemiology of recurrences or reinfections of Clostridium difficile-associated diarrhea. J Clin Microbiol. 2000;38(6):2386–2388. doi: 10.1093/gao/9781884446054.article.t031141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yearsley KA, Gilby LJ, Ramadas AV, Kubiak EM, Fone DL, Allison MC. Proton pump inhibitor therapy is a risk factor for Clostridium difficile-associated diarrhoea. Aliment Pharmacol Ther. 2006;24(4):613–619. doi: 10.1111/j.1365-2036.2006.03015.x. [DOI] [PubMed] [Google Scholar]
- 16.Borriello SP. Pathogenesis of Clostridium difficile infection. J Antimicrob Chemother. 1998;41(Suppl C):13–19. doi: 10.1093/jac/41.suppl_3.13. [DOI] [PubMed] [Google Scholar]
- 17.Antun B. [Recurrent Clostridium difficile infections: meaning and therapy] Infektološki Glasnik. 2011;31(3):155–161. [Google Scholar]
- 18.Stabler RA, Dawson LF, Phua LT, Wren BW. Comparative analysis of BI/NAP1/027 hypervirulent strains reveals novel toxin B-encoding gene (tcdB) sequences. J Med Microbiol. 2008;57(Pt 6):771–775. doi: 10.1099/jmm.0.47743-0. [DOI] [PubMed] [Google Scholar]
- 19.Barbut F, Mastrantonio P, Delmée M, Brazier J, Kuijper E, Poxton I European Study Group on Clostridium difficile (ESGCD) Prospective study of Clostridium difficile infections in Europe with phenotypic and genotypic characterisation of the isolates. Clin Microbiol Infect. 2007;13(11):1048–1057. doi: 10.1111/j.1469-0691.2007.01824.x. [DOI] [PubMed] [Google Scholar]
- 20.Hookman P, Barkin JS. Clostridium difficile associated infection, diarrhea and colitis. World J Gastroenterol. 2009;15(13):1554–1580. doi: 10.3748/wjg.15.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drudy D, Harnedy N, Fanning S, Hannan M, Kyne L. Emergence and control of fluoroquinolone-resistant, toxin A-negative, toxin Bpositive Clostridium difficile. Infect Control Hosp Epidemiol. 2007;28(8):932–940. doi: 10.1086/519181. [DOI] [PubMed] [Google Scholar]
- 22.Miller MA, Hyland M, Ofner-Agostini M, Gourdeau M, Ishak M Canadian Hospital Epidemiology Committee. Morbidity, mortality, and healthcare burden of nosocomial Clostridium difficile-associated diarrhea in Canadian hospitals. Infect Control Hosp Epidemiol. 2002;23(3):137–140. doi: 10.1086/502023. [DOI] [PubMed] [Google Scholar]
- 23.Chang HT, Krezolek D, Johnson S, Parada JP, Evans CT, Gerding DN. Onset of symptoms and time to diagnosis of Clostridium difficile-associated disease following discharge from an acute care hospital. Infect Control Hosp Epidemiol. 2007;28(8):926–931. doi: 10.1086/519178. [DOI] [PubMed] [Google Scholar]
- 24.Kutty PK, Woods CW, Sena AC, Benoit SR, Naggie S, Frederick J, et al. Risk factors for and estimated incidence of community-associated Clostridium difficile infection, North Carolina, USA. Emerg Infect Dis. 2010;16(2):197–204. doi: 10.3201/eid1602.090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC) Severe Clostridium difficile associated disease in populations previously at low risk-four states, 2005. MMWR Morb Mortal Wki Rep. 2005;54(47):1201–1205. [PubMed] [Google Scholar]
- 26.McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97(7):1769–1775. doi: 10.1111/j.1572-0241.2002.05839.x. [DOI] [PubMed] [Google Scholar]


