Abstract
The use of surfactant mixtures to affect both electroosmotic flow (EOF) and separation selectivity in electrophoresis with poly(dimethylsiloxane) (PDMS) substrates is reported, and capacitively coupled contactless conductivity detection (C4D) is introduced for EOF measurement on PDMS microchips. First, the EOF was measured for two nonionic surfactants (Tween 20 and Triton X-100), mixed ionic/nonionic surfactant systems (SDS/Tween 20 and SDS/Triton X-100), and finally for the first time, mixed zwitterionic/nonionic surfactant systems (TDAPS/Tween 20 and TDAPS/Triton X-100). EOF for the nonionic surfactants decreased with increasing surfactant concentration. The addition of SDS or TDAPS to a nonionic surfactant increased EOF. After establishing the EOF behavior, the separation of model catecholamines was explored to show the impact on separations. Similar analyte resolution with greater peak heights was achieved with mixed surfactant systems containing Tween 20 and TDAPS relative to the single surfactant system. Finally, the detection of catecholamine release from PC12 cells by stimulation with 80 mM K+ was performed to demonstrate the usefulness of mixed surfactant systems to provide resolution of biological compounds in complex samples.
Keywords: EOF control; Electrochemical detection; Microchip capillary electrophoresis; Mixed surfactants (ionic + nonionic, nonionic + zwitterionic); PDMS microchip; Capacitively coupled contactless conductivity detection
1 Introduction
Microchip capillary electrophoresis (MCE) has been established as an important sub-section of traditional capillary electrophoresis and has found widespread use in academic laboratories and more recently in commercial products [1–3]. While MCE provides fast separations, the short separation channels make resolving multiple compounds challenging. Our group has explored a number of methods to improve separations [4–9], including the use of mixed surfactant micelles that both expand the ability to control electroosmotic flow (EOF) and enhance resolution [10]. Here, combinations of ionic, zwitterionic, and nonionic surfactants are explored as new tools to achieve better microchip electrophoretic separations.
Because of the importance of EOF in capillary electrophoresis [11, 12], accurate and precise methods for its measurement are useful. Many EOF measurement methods have been reported for CE and MCE, including neutral marker, fluorescent marker, weight measurement, current monitoring and conductivity methods [13]. The current monitoring method is most commonly used and measures the electrophoretic current change as an electrolyte of different conductivity fills the capillary. The time required to reach a steady-state separation current is used to calculate EOF. Reported precision for EOF measured by this method range between 5% and 15% [14–17]. Based on a similar measurement principle, conductivity detection monitors the change in bulk solution conductivity between two electrodes when an analyte band passes through the electrode gap [18]. More reproducible EOF measurements (relative standard deviation (RSD) 1.9%) were reported using this method than the current monitoring method (RSD 5.9%) [19]. As an alternative to direct conductivity monitoring, capacitively coupled contactless conductivity detection (C4D) can be used [20, 21]. C4D is attractive because the detection electrodes are isolated from the electric field and can be located anywhere along the separation capillary [22]. The coupling of C4D on microfluidic systems has led to a large range of applications, including bioanalytical assays, on-chip enzymatic reactions, food analysis, and determinations of explosives, and chemical warfare agents [3, 23, 24]. In 2003, do Lago et al. demonstrated EOF measurements by coupling C4D with polyester-toner (PT) devices [25]. In this paper, simultaneous EOF measurements using both C4D and current monitoring methods were performed on poly(dimethylsiloxane) (PDMS) microchips and the comparison between these two methods discussed.
Permanent [23, 24], adsorbed/permanent [25–27], and adsorbed/dynamic coatings [28, 29] are commonly used for surface modification to control EOF in electrophoresis and have been discussed in several review papers [24, 28–30]. Adsorbed/dynamic coatings rely on the equilibrium between the solution-phase modifier and the surface, modifying the zeta potential [11, 12] and therefore the EOF. Applications of anionic [16, 31], cationic [32–34], and zwitterionic surfactants [35–37] in dynamic coatings have been published previously. Nonionic surfactants, such as polyoxyethylene ether (Brij 35), polyoxyethylene (20) sorbitan monolaurate (Tween 20), polyoxyethylene octyl phenyl ether (Triton X-100), have primarily been used for reducing analyte-wall interactions, since they create a hydrophilic, nonionic coating that is highly effective at minimizing adsorption [38]. Successful applications of these nonionic surfactants to suppress EOF and minimize surface adsorption of biomolecules in CE and microfluidic system have been reported [39–42].
Mixed surfactant systems represent an interesting alternative to single surfactant systems for both EOF control and alternative separation chemistry. For example, mixtures of zwitterionic and cationic surfactants were used to modify the EOF from nearly zero to −5×10−4 cm2 V−1 s−1 by Lucy’s group [43]. This ability to control EOF was employed to fine-tune the separation of inorganic anions [43] and to separate ammonium isotopes through EOF counterbalance [44]. In another example, mixed cationic/anionic (CTAB/SDS) surfactants demonstrated enhanced EOF stability relative to CTAB alone for separation of basic proteins [45]. The separation of proteins on PDMS-coated fused silica capillaries and glass microchips was achieved using a mixture of charged surfactants and nonionic Brij 35 to control EOF [46]. Furthermore, mixed zwitterionic (N-tetradecyl-N,N-dimethyl-3-ammonio-1-propane sulfonate (TDAPS)) and nonionic (Tween 20) surfactants were used for the direct determination of bromide and nitrate in undiluted seawater [47]. Despite these advances, the application of mixed surfactants systems for surface modification on polymeric microdevices has been limited. Guan et al. recently explored the electrophoretic separation and electrochemical detection of model catecholamines in buffer and reduced glutathione (GSH) in red blood cell lysate in PDMS microchips using a mixture of ionic/zwitterionic surfactants (SDS/TDAPS) [10]. The mixed surfactant system provided shorter analysis times and/or improved resolution when compared to the single surfactant systems. Hoeman and Culbertson used a mixture of zwitterionic stationary phases to separate fluorescently labeled amino acids as an effort to reduce the need for organic solvents for this separation [48].
Here, the use of mixed surfactant systems consisting of a non-ionic surfactant mixed with either an anionic or zwitterionic surfactant to control EOF and modify separation selectivity in PDMS microchips is reported. C4D is introduced as an alternative to the current monitoring method for EOF measurements. EOF measurements as a function of surfactant concentration were made for nonionic Tween 20 and Triton X-100 and combinations of these surfactants with anionic SDS or zwitterionic TDAPS. Next, separation and electrochemical detection of model analytes were explored using the mixed surfactant systems. The detection of catecholamine release from PC12 cells by stimulation with 80 mM K+ was used to demonstrate the utility of mixed surfactant systems to provide resolution of biological compounds in complex samples.
2 Material and methods
2.1 Reagents and solutions
Reagents used for fabrication of microchips include SU-8 2035 photoresist (Microchem, Newton, MA), Sylgard 184 elastomer and curing agent (PDMS) (Dow Corning, Midland, MI), 4-in. silicon wafers (University Wafer, South Boston, MA), and microwires made of 99.99% Pd (25 µm) and 99.99% Au (25 µm) (Goodfellow, Huntingdon, England). Aqueous solutions were prepared in 18.2 MΩ*cm water from a Millipore Milli-Q purification system (Milipore Corp., Billerica, MA). The BGEs were prepared by weighing the desired amount of N-tris[hydroxymethyl]methyl-2-aminoethanesulfonic acid (TES; Sigma-Aldrich, St. Louis, MO) or boric acid (Fisher, Pittsburgh, PA) and adjusting the pH with 2 M NaOH (Fisher). Following pH adjustment, surfactant was added to the BGE to the desired concentration. SDS (Aldrich, Milwaukee, WI, USA), TDAPS (Fluka, Buchs, Switzerland), Tween 20 (Sigma-Aldrich), and Triton X-100 (FisherBiotech, Fair Lawn, New Jersey) were selected for the present study (structures are shown in Fig. S1). 10-mM stock solutions of dopamine (DA), Norepinephrine (NE), Epinephrine (E), 3,4-dihydroxy-L-phenylalanine (L-DOPA), and catechol (CA) (Sigma-Aldrich) were prepared daily in 10 mM HCl. Samples were prepared by dilution of the stock with BGE. All chemicals were used as received without further purification. Sample preparation and analysis of Rat PC12 cells is shown in supporting information section.
2.2 Fabrication of the PDMS microchip
Depending on the experiment, two different PDMS microchips were fabricated using previously described methods [49, 50]. EOF measurements were performed in single straight channels (50 µm × 50 µm × 4.7 cm) using both current monitoring [14] and C4D methods. For experiments involving separation and electrochemical detection, a previously reported design consisting of a straight T injector and a bubble cell with its width 4× that of the separation channel width in the detection zone [51] was used and had channel width and depth of 50 µm, respectively. This design had sample and buffer channel lengths of 2.0 cm, a sample waste channel length of 4.0 cm, and a separation channel length of 10.0 cm. A Pd decoupler and Au working electrode (WE) were placed in the bubble cell using electrode alignment channels [52]. Each electrode channel was 50 µm wide and separated by 125 µm (center-to-center).
2.3 Instrumentation
A 3-channel (two positives and one negative) laboratory built high-voltage power supply was used for all the experiments involving an injection/separation step [53]. A 10-s hydrodynamic injection [54] was used for the separation of DA, NE, E, CA, and L-DOPA. The Pd decoupler and the sample waste reservoir were always held at ground to isolate the potentiostat from high voltage. During hydrodynamic injections, both sample and buffer reservoirs were grounded. Sample introduction was achieved by filling the sample reservoir with 80 µL of sample solution and the remaining reservoirs were filled with 50 µL of buffer solution. The separation was performed by applying positive potentials of 2200 V and 1850 V in the sample and buffer reservoirs, respectively, resulting in a field strength of 150 V cm−1 in the 10.0-cm long separation channel. Amperometric detection of DA, NE, E, CA, and L-DOPA was performed using a CHI 1010A Electrochemical Analyzer (CH Instruments, Austin, TX) in a two-electrode configuration. The Pd decoupler was held at ground at all times to protect detection electronics from high voltage. A gold wire (25-µm diameter) was used as the working electrode and the corresponding detection potential was optimized for each compound. A platinum wire (1-mm diameter) in the waste reservoir acted as a counter electrode.
2.4 EOF measurements
EOF measurements were performed using both current monitoring [14] and C4D methods simultaneously. The microchip with a single straight channel connected by two reservoirs (5 mm in diameter) was held in close contact with the commercial C4D microfluidic platform (eDAQ, Australia) by spring screws and the detection point was located at the center position between two detection electrodes on the platform (Fig. S2). An excitation frequency of 550 kHz and a amplitude of 60 Vpp were used. C4D detection was done in the microchip channel 2.0 cm from one BGE reservoir. For EOF measurements, the first reservoir and the channel were filled with higher concentration BGE (typically 20 mM BGE) and the second reservoir was filled with lower concentration BGE. The specific BGEs used in EOF measurements are discussed below. The separation current and conductivity signal were measured simultaneously using an analog to digital convertor controlled by PowerChrom software (eDAQ, Australia). The time required to reach a current plateau was used to calculate EOF for the current monitoring method. The time to the inflection point (mid point between the maximum and minimum of the transition region) of the conductivity trace was used to calculate EOF for the C4D method. Please see supporting information for the calculation equations for both measurement methods. All values are reported as the average from four microchips, with six replicates performed on each microchip. Reported uncertainties are the standard deviations obtained from the total of 24 measurements taken from four microchips.
3 Results and discussion
3.1 EOF measurements by C4D and current monitoring methods
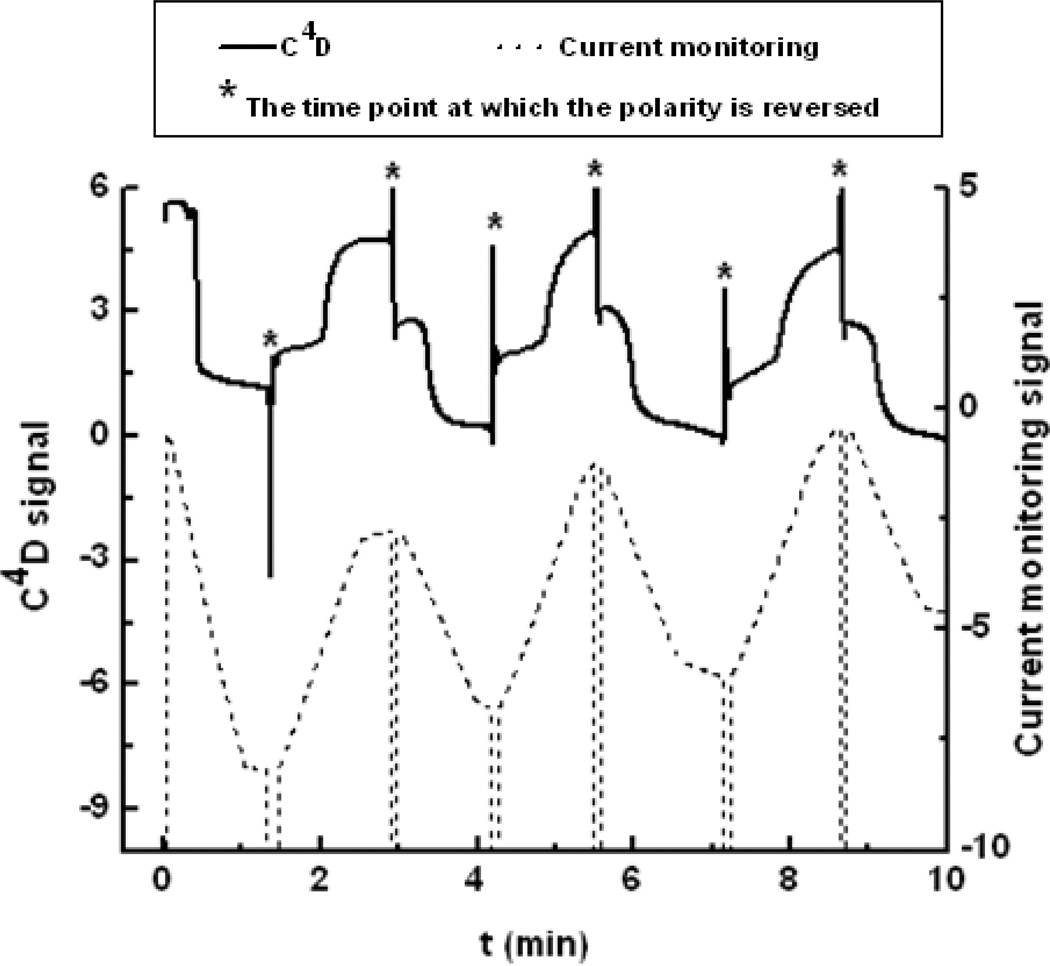
Example traces for current/conductivity signals for EOF measurements obtained simultaneously from the C4D and current monitoring methods are shown in Fig. 1. The current monitoring method measures the average conductivity along the channel and gives a gradual decrease in the current until a current plateau is reached, indicating total replacement of BGE in the channel. The C4D measures conductivity at a point along the channel, and thus there is a sudden decrease in conductivity when the lower ionic strength BGE reaches detection electrodes. To compare the two methods, the EOF reproducibility using 20 mM TES buffer (pH 7.0) combined with 5, 10, and 18 mM TES buffer (pH 7.0) as the high and low ionic strength BGEs, respectively, was established. While the two methods give statistically indistinguishable EOF values, the reproducibility of the C4D method is superior to that of the current monitoring method as evidenced by the relative standard deviations. The current monitoring method gave a relative standard deviation of 1.89%, while the C4D detector had a relative standard deviation of 1.41%, when using 20 and 18 mM TES BGEs. As the difference in ionic strength between the BGEs increased (resulting in the net ionic strength decreasing), the EOF increased slightly (from 4.52 ± 0.09 ·104 cm2 V−1s−1 when using 20 and 18 mM TES to 4.65 ± 0.31 ·104 cm2 V−1s−1 when using 20 and 5 mM TES BGEs), while the standard deviation increased significantly when using 20 and 5 mM TES BGEs. These results indicate that more precise EOF measurements can be made using BGEs with smaller differences in ionic strength in accordance with previous reports [55] and with the use of C4D. Based on these results, 20 mM and 18 mM BGEs (TES buffer at pH 7.0 or boric acid buffer at pH 9.2) were used for all remaining EOF measurements.
Figure 1.
EOF measurements using both current monitoring and C4D methods. The mark* denotes the time point at which the polarity is reversed. Field strength: 200 V/cm; BGEs: 20 mM and 18 mM TES buffer, pH 7.0.
3.2 Effect surfactant concentration on EOF
3.2.1 Single nonionic surfactant system
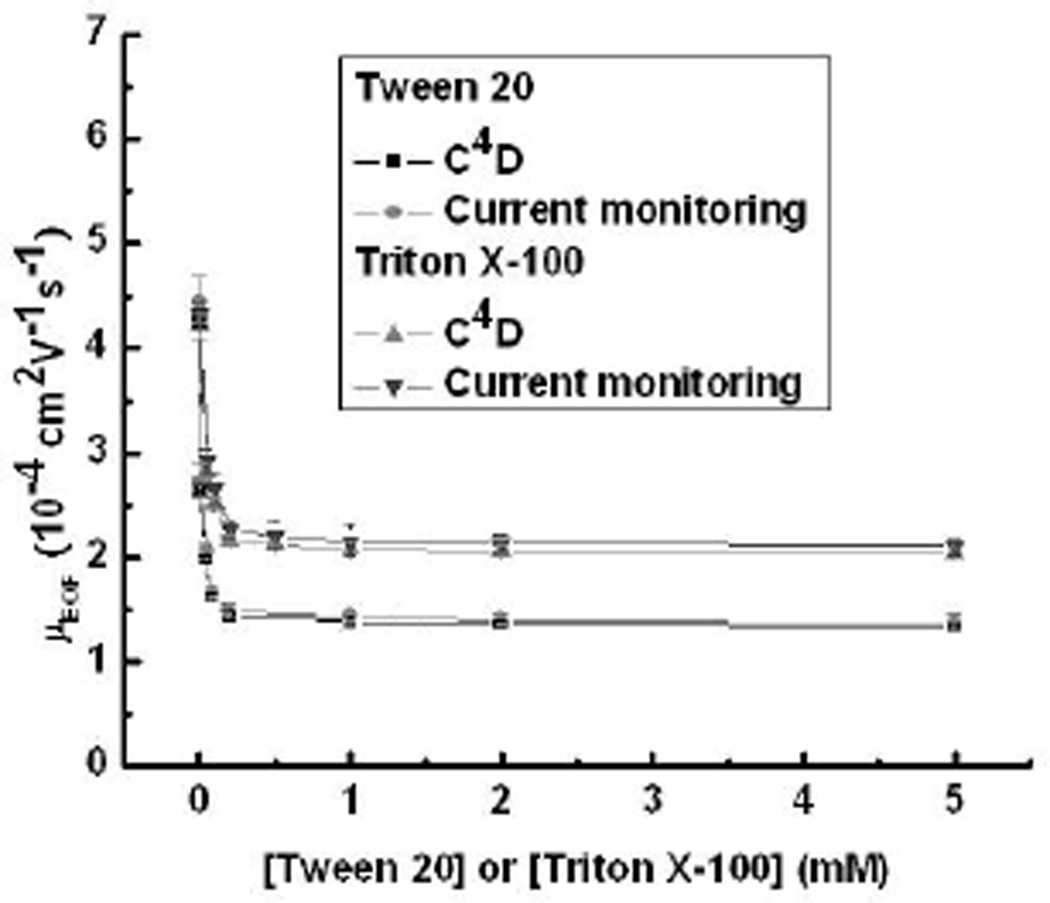
Modification of PDMS surface chemistry using nonionic surfactants has been reported by several groups who have suggested that this surfactant class interacts with the surface through their hydrophobic tails, creating an uncharged hydrophilic surface that minimizes protein adsorption and reduces EOF [39, 40]. Here, two nonionic surfactants, Tween 20 (CMC: 0.08 mM in pure water [41]) and Triton X-100 (CMC: 0.24 mM in pure water [56]), were studied. Measurement of EOF in boric acid buffer (pH 9.2) was performed using both C4D and current monitoring methods, and the results are shown in Fig. 2. The EOF decreased with increasing surfactant concentration, most likely as the result of the hydrophobic tail of the nonionic surfactant interacting with the PDMS reducing the apparent surface charge. As an example, Fig. 2 shows EOF deceasing for Tween 20, from 4.30 ± 0.08 ·10−4 cm2 V−1 s−1 at 0 mM to 1.34 ± 0.04 ·10−4 cm2 V−1 s−1 at 5 mM. The results are also in agreement with previous work presented by Chen’s group [39]. The increasing buffer viscosity in the presence of nonionic surfactants will also play a role in this behavior but is expected to be minimal relative to changes in surface charge. Similar results were found for Triton X-100, although the net change in EOF was smaller (4.22 ± 0.13 to 2.05 ± 0.16 ·10−4 cm2 V−1 s−1) than Tween 20. The reason for the difference in final EOF values is not known at this time but is most likely the result of differences in surfactant packing density on the PDMS surface.
Figure 2.
EOF as a function of concentration for Tween 20 and Triton X-100 in boric acid buffer (20 mM and 18 mM) at pH 9.2.
3.2.2 Mixed anionic/nonionic surfactant systems
Several groups have reported surface modification using mixed surfactant systems for CE. The combination of neutral and charged surfactants together provides a better means to fine tune the EOF on bare silica than individual surfactants, resulting in a larger functional mobility window [57, 58]. Additionally, by employing a mixture of charged surfactants and the nonionic surfactant, Brij 35, on PDMS-coated fused silica capillaries and glass microchips, improved control of the EOF across a larger functional mobility window was achieved for protein separations [46]. Here, mixtures of ionic (SDS) and nonionic surfactants (Tween 20 or Triton X-100) were investigated. EOF was measured using C4D at 0 to 20 mM SDS (CMC: 8.1 to 8.4 mM in pure water [62]) concentrations while the concentration of Tween 20 was fixed at 0.1 mM, 0.5 mM, 1.0 mM, or 5.0 mM in 20 mM boric acid buffer (pH 9.2) (Fig. 3A). In the absence of SDS, the EOF values measured for varying concentrations of Tween 20 matched the values shown in Fig. 2. The addition of SDS dominated the EOF behavior for all Tween 20 concentrations with only small differences in EOF obtained as a function of Tween 20 concentration [10]. For example, an EOF value of 5.79 ± 0.16 ·10−4 cm2 V−1 s−1 was measured using a mixture of 3.0 mM SDS and 1.0 mM Tween 20, which is almost three-fold higher than that using 1.0 mM Tween 20 alone (1.39 ± 0.03 ·10−4 cm2 V−1 s−1). The SDS/Tween 20 ratio was also plotted to show the relative effect (Fig. S3A). A similar EOF trend but a smaller net change in EOF was observed for mixed SDS/Triton X-100 system (2.12 ± 0.07 to 6.49 ± 0.23 ·10−4 cm2 V−1 s−1) than for mixed SDS/Tween 20 systems (1.38 ± 0.06 to 6.51 ± 0.22 ·10−4 cm2 V−1 s−1) as shown in Fig. 3B and S3B. The fact that the final EOF in both mixtures was not statistically different confirms the dominant role of SDS in this system. These results are in agreement with the EOF behaviors shown in the mixed SDS/Brij 35 surfactant system in previous work presented by Harrison’s group [46].
Figure 3.
EOF as a function of SDS concentration using (A) Tween 20 and (B) Triton X-100 in boric acid buffer (20 mM and 18 mM) at pH 9.2.
3.2.3 Mixed zwitterionic/nonionic surfactant systems
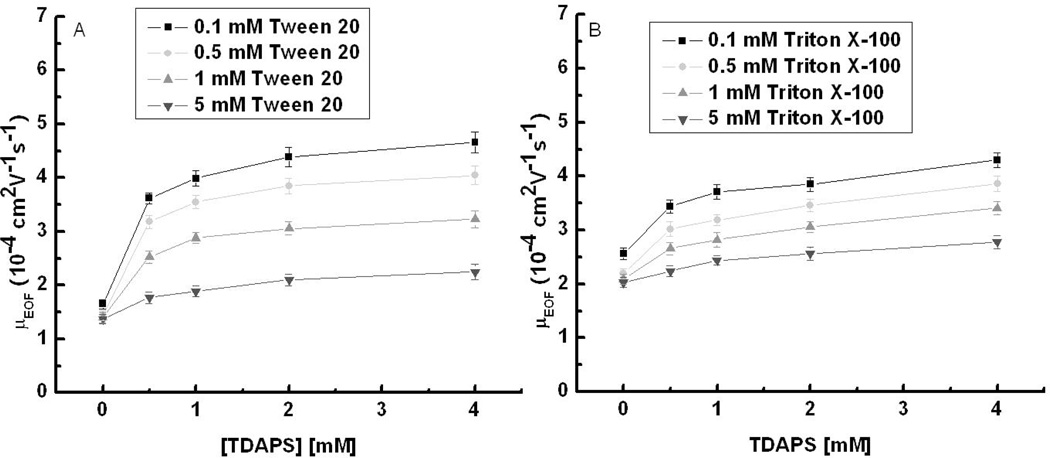
Zwitterionic surfactants represent an interesting alternative for surface modification in polymeric microchips as well as for alternative agents for micellar electrokinetic chromatography (MEKC). Our previous work explored the EOF for TDAPS (CMC: 0.1 to 0.4 mM at 20 to 25 °C in pure water [63]) and mixed SDS/TDAPS surfactant systems on PDMS and showed the mixed surfactant system yielded shorter analysis times and/or improved resolution when compared to the single surfactant [10]. Mixtures of TDAPS and Tween 20 have been employed for the direct determination of bromide and nitrate in undiluted seawater in CE [47]. Here, we measured the concentration effect of mixtures of zwitterionic surfactant TDAPS with Tween 20 and Triton X-100 on EOF. EOF measurements were performed using C4D at 0 to 4 mM TDAPS concentrations while the concentration of Tween 20 was fixed at 0.1 mM, 0.5 mM, 1 mM, or 5 mM in 20 mM boric acid buffer (pH 9.2) shown in Fig. 4A. In the absence of TDAPS, the EOF values measured for varying concentrations of Tween 20 were consistent with those obtained in pure Tween 20 system. The addition of TDAPS caused an increase in EOF for all four Tween 20 concentrations. For an example, EOF values in the mixture of 0.5 mM TDAPS/2 mM Tween 20 and 4 mM TDAPS/2 mM Tween 20 are 3.85 ± 0.12 ·10−4 and 4.05 ± 0.13 ·10−4 cm2 V−1 s−1, which are approximately two-fold higher than 1.36 ± 0.04 ·10−4 cm2 V−1 s−1 obtained using 2.0 mM Tween 20 alone. The overall EOF magnitude was dependent on the Tween 20 concentration, with 0.1 mM Tween 20 giving the highest average EOF, and 5 mM giving the lowest. One hypothesis to explain the EOF behavior of the mixed Tween 20/TDAPS system is that Tween 20 reduces the PDMS surface charge, decreasing the EOF, whereas the adsorption of TDAPS onto the surface exposes the outermost anionic sulfonate group to form a thicker cationic double layer, a larger zeta potential and thus higher EOF. A second hypothesis is that anions from the BGE (TES) adsorb to the surfactant coated surface causing a larger zeta potential and thus higher EOF. This hypothesis is supported by the work of Baryla and Lucy [59]. The same experiments were performed for the mixed Triton X-100/TDAPS system, and similar EOF behavior with a smaller EOF change (2.03 ± 0.08 to 4.30 ± 0.13 ·10−4 cm2 V−1 s−1) (Fig.4B) was observed when compared to mixed Tween 20/TDAPS system (1.37 ± 0.07 to 4.66 ± 0.15 ·10−4 cm2 V−1 s−1). These results show that mixtures of zwitterionic/nonionic surfactants give a higher EOF than nonionic surfactant alone and thus provide a larger EOF working range relative to single surfactant systems. The nonionic surfactant/TDAPS ratios were also plotted in the supporting information to show the relative effect (Fig. S4A and S4B). Finally, EOF measurements were made for the same mixtures using pH 7.0 TES (20 mM) as the BGE since these conditions are common for separation of catecholamines (Figure S5 and S6). The resulting EOF values align very closely with the values measured at pH 9.2. The combined results show that a desired EOF can be achieved in the operating range provided by the surfactants by adjusting the surfactant ratio. This should provide a better control of EOF than is presently possible with single surfactant systems.
Figure 4.
EOF as a function of TDAPS concentration using (A) Tween 20 and (B) Triton X-100 in boric acid buffer (20 mM and 18 mM) at pH 9.2.
3.3 Separation applications using mixed nonionic/zwitterionic surfactant systems
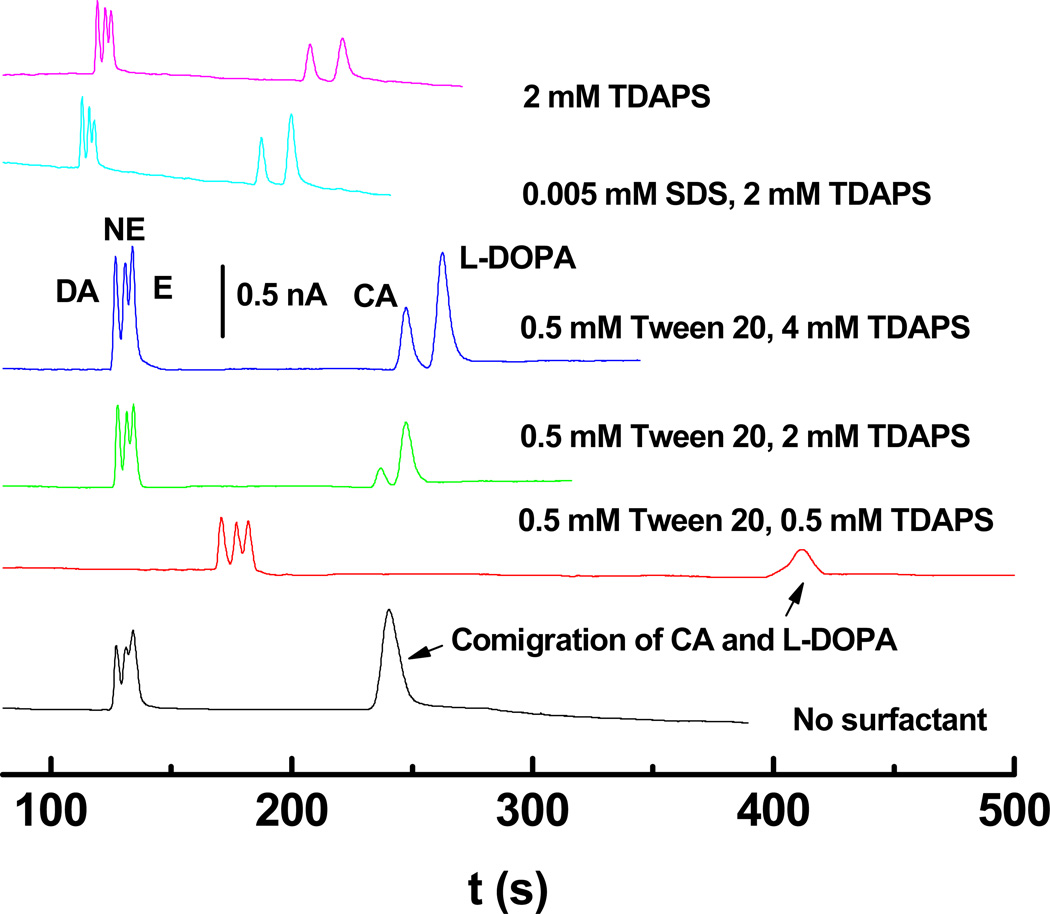
After establishing EOF behavior, the use of zwitterionic/nonionic surfactant systems was demonstrated for the separation of DA, NE, E, and their metabolic precursors L-DOPA and CA. Separations of 20 µM DA, NE, E and 40 µM CA and L-DOPA in 20 mM pH 7.0 TES buffer with various surfactant concentrations were performed (Fig. 5). The combination of 0.5 mM Tween 20 and 0.5 mM TDAPS gave the longest migration times, while the mixture of 5 µM SDS and 2 mM TDAPS gave the shortest separation times. As the TDAPS/Tween 20 ratio increases, the migration times of DA, NE and E became faster, in agreement with the EOF trend shown in Fig. 4. This result suggests that electrophoretic mobility as opposed to micelle interactions is the dominant forces dictating migration time for this set of analytes. However, CA and L-DOPA co-migrated in BGEs without surfactant or BGEs containing TDAPS/Tween 20 mixtures with low TDAPS concentration (0.5 mM). A baseline separation was obtained for CA and L-DOPA when using TDAPS alone or mixed surfactant systems containing TDAPS concentrations ≥2 mM. Here, the EOF without surfactant was very similar to that in the BGE with 2 mM TDAPS/0.5 mM Tween 20 mixture as evidenced by the similarity of migration times for DA, NE and E. As the concentration of TDAPS increased from 2 to 4 mM in the presence of 0.5 mM Tween 20, however, the migration times for CA and L-DOPA increased, as did the separation between NE and CA peaks. The slower CA and L-DOPA migration can be attributed to interactions between these two analytes and micelles formed from the surfactant mixture. Furthermore, the resolution between CA and L-DOPA also increased ((2 mM TDAPS/0.5 mM Tween 20: 1.29 ± 0.07, 4 mM TDAPS/0.5 mM Tween 20: 1.46 ± 0.08). Determining the exact nature of the interaction and the compositions of the micelles are beyond the scope of the current work, however, some insight can be gained from prior work on mixed micelles. First, the increase in migration time for both CA and L-DOPA suggest an apparent negative charge to the micelle that would result from surface exposed sulfonate groups on TDAPS. The negative charge hypothesis is supported by the fact that both CA and L-DOPA, which are neutral at pH 7.0, migrate slower than the electroosmotic flow based on their co-migration in the surfactant free electropherogram of Fig. 5. Second, prior work on the separation of cationic amines using mixtures of SDS and Tween 20 showed that increases in the Tween concentration relative to the SDS reduced the overall interaction [60]. In the results shown here, it is reasonable to conclude based on this prior work that Tween 20 moderates the interaction between the analytes and TDAPS and thus increasing the TDAPS concentrations results in greater retention by the micelles. The source of the negative charge on the micelle is most likely the result of partitioning of the BGE anion (TES in this case) into the micelle. An alternative explanation for the retention is that the Tween 20 and TDAPS form a surface coating and the resolution is based on interactions between the surface bound surfactants and the mobile phase analytes.
Figure 5.
Example electropherograms for 20 µM DA, NE, E and 40 µM CA and L-DOPA in 20 mM TES buffer at pH 7.0 as a function of surfactant composition. Field strength: 150 V/cm; 10-s hydrodynamic injection; Detection: DC Amp, Edet = 1.2 V.
The resolution between analytes for all surfactant systems was compared (Fig. S7). The mixture of 0.5 mM TDAPS and 0.5 mM Tween 20 gave the highest resolution of 1.27 ± 0.07 and 1.10 ± 0.05 (n = 3) for DA/NE and NE/E, respectively, due to the slow EOF in this surfactant system. Unfortunately, CA and L-DOPA co-migrated in this BGE. The highest resolution where all compounds were partially resolved (DA/NE: 1.18 ± 0.05, NE/E: 0.98 ± 0.05, and CA/L-DOPA: 2.34 ± 0.09, n = 3) was obtained for the BGE containing only 2 mM TDAPS. However, the resolution (DA/NE: 1.08 ± 0.06, NE/E: 0.94 ± 0.05, and CA/L-DOPA: 1.46 ± 0.08, n = 3) obtained for the BGE with 4 mM TDAPS/0.5 mM Tween 20 mixture was not statistically different from the 0.5 mM TDAPS/0.5 mM Tween BGE but provided significantly higher peaks as shown in the Fig.5.
The 4 umM TDAPS/0.5 mM Tween 20 BGE gave peak heights of 1.28 ± 0.11 nA for DA, 1.17 ± 0.12 nA for NE, 1.33 ± 0.12 nA for E, 0.66 ± 0.05 nA for CA, and 1.21± 0.13 nA for L-DOPA (n = 3), while the mixed 0.5 mM Tween 20/0.5 mM TDAPS surfactants gave the significantly lower peak heights (DA: 0.66 ± 0.06 nA, NE: 0.54 ± 0.06 nA, E: 0.57 ± 0.05 nA, CA and L-DOPA: 0.31± 0.03 nA, n = 3). Differences in peak height are unlikely to be the result of differences in injection volume because hydrodynamic injection was used and the solution viscosities are all similar. The exact mechanism is not clear at this point, however, it may be the result of enhanced solubility of the oxidized products in the 4.0 mM TDAPS/0.5 mM Tween 20 BGE. Prior work has shown similar results with pure alkyl sulfate BGEs [61]. Considering both peak height and analyte resolution the BGE composed of 4 mM TDAPS and 0.5 mM Tween 20 was chosen for analysis of catecholamines released from PC12 cells. The separation efficiencies for 20 µM DA, NE, E and 40 µM CA and L-DOPA under this separation condition were corresponding to 18200 ± 850, 13900 ± 750, 22300 ± 1200, 16000 ± 950, and 14100 ± 1100 plates. The LODs using this mixed surfactant system were 1.5 ± 0.1 µM, 1.5 ± 0.1 µM, 1.2 ± 0.1 µM, 3.5 ± 0.3 µM, and 2.5 ± 0.2 µM (n = 3) for DA, NE, E, CA and L-DOPA, respectively. Additional reductions in the concentration detection limit could be achieved by increasing the injection time, adjusting the channel dimensions, and/or application of sample stacking techniques.
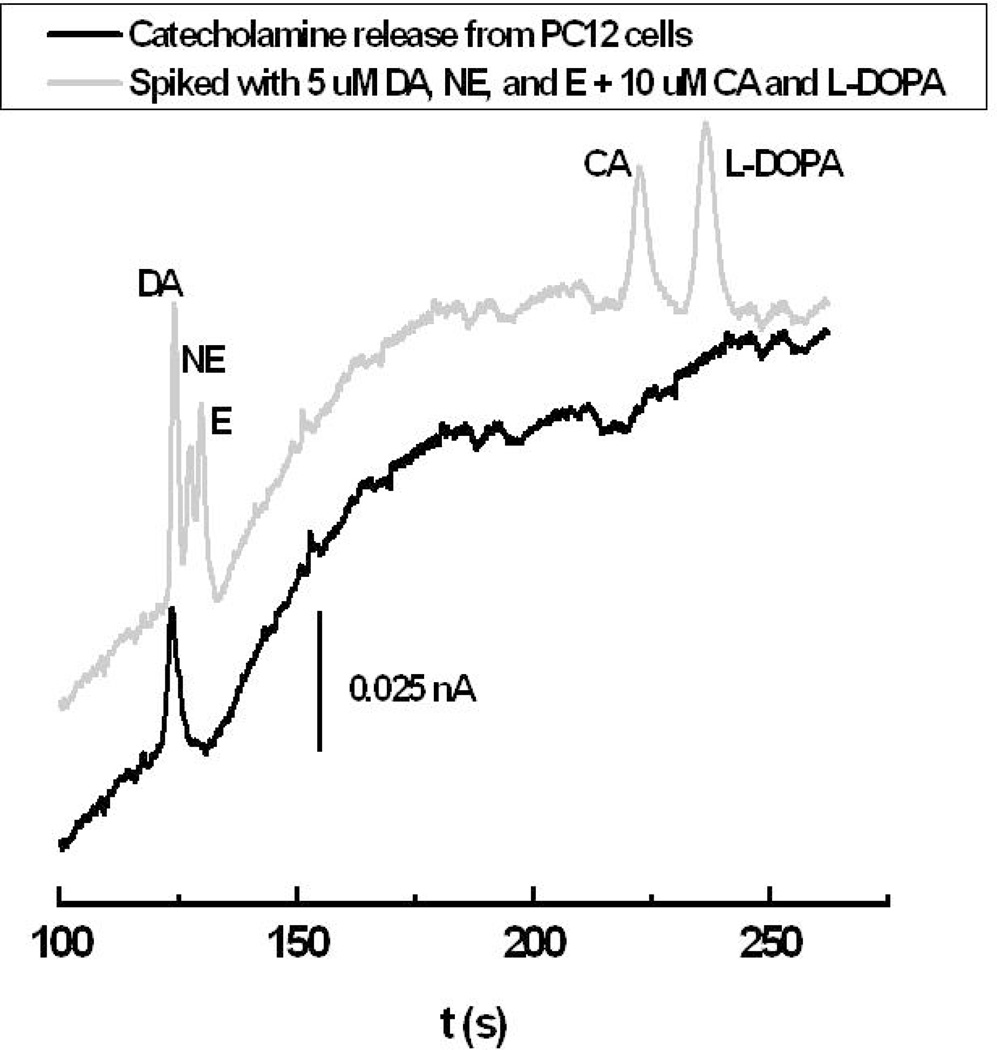
3.4. Catecholamine release from PC12 cells by stimulation with 80 mM K+
Due to their role in the brain, neurotransmitters (catecholamines: DA, NE, and E) are of considerable interest [62]. PC12 cells have been used as a model for the developing sympathetic nerve since this clone cell line exhibits many of the physiological properties of sympathetic ganglion neurons [63, 64]. The NE/DA ratio in PC12 cells varies from 0.003 to 0.53, with no detectable level of E [65, 66]. Electropherograms of catecholamine release from a PC12 cell population by stimulation with 80 mM K+ as well as the same sample spiked with standards using a BGE composed of 0.5 mM Tween 20 and 4 mM TDAPS are shown in Fig. 6. The only detectable catecholamine released from these PC12 cells is DA according to its migration time and the increased peak height observed on addition of standards. The analyte concentration was determined to be 4.96 ± 0.25 µM (n = 3) in 2×-diluted sample, corresponding to 9.92 ± 0.53 µM (64.42 ± 3.41 pM/cell) DA released in the supernatant from the PC12 cells (1.54 × 105 cells). The recovery of DA from spiking with standards is 96.3 ± 5.4%. Ewing’s group reported that PC12 cell vesicles contain an average catecholamine concentration of 110 mM and release just 0.06% of this concentration, or 67 µM (190 zmol/vesicles), during exocytotic events [67, 68]. Another publication by Martin’s group indicated that 20–160 µM DA (153–1230 pM/cell) following calcium stimulation was released from PC12 cells [69]. While the amount of catecholamine detected here is lower than previously published, it was in agreement with results (58.3 pM/cell [70]) for carbon paste electrodes modified with multi-walled carbon nanotubes.
Figure 6.
Electropherograms of catecholamine release from PC12 cells by stimulation with 80 mM K+ in 2× diluted sample and with the standard solution containing 5 µM DA, NE, E and 10 µM CA and L-DOPA. BGE: 20 mM TES, 0.5 mM Tween 20, 4 mM TDAPS, pH 7.0; Field strength: 150 V/cm; 10-s Hydrodynamic injection; Detection: DC Amp., Edet = 1.2 V.
4 Concluding remarks
The use of mixed ionic/nonionic or nonionic/zwitterionic surfactants on PDMS microchips to control EOF and alter separations was reported. C4D was introduced for EOF measurements and provided improved measurement reproducibility relative to the current monitoring method. EOF measurements as a function of the surfactant concentration were performed simultaneously using both methods for two nonionic surfactants (Tween 20 and Triton X-100), mixed ionic/nonionic surfactant systems (SDS/Tween 20 and SDS/Triton X-100), and mixed zwitterionic/nonionic surfactant systems (TDAPS/Tween 20 and TDAPS/Triton X-100). Nonionic surfactants showed a decrease in EOF as the surfactant concentration increased. Using mixed surfactants, higher EOF values and a wider tunable EOF range was obtained as compared to BGEs containing a single nonionic surfactant. Separation and electrochemical detection of model analytes was also explored using surfactant mixtures. Analyte resolution was maintained and peak height was increased in mixed surfactant BGEs containing the nonionic surfactant relative to the single surfactant system. Finally, using a BGE composed of 0.5 mM Tween 20 and 4 mM TDAPS, the catecholamine released from PC12 cells by stimulation with 80 mM K+ was determined as DA at a concentration of 64.42 ± 3.41 pM/cell, with a recovery of 96.3 ± 5.4%. This result demonstrates the usefulness of mixed surfactant systems to provide resolution of biological compounds in complex samples.
Supplementary Material
Acknowledgments
Funding for this work was provided by the National Institutes of Health (2R44HL083579-02A1) through a sub-contract from Advanced MicroLabs, LLC. SDN was partially funded by STAR Research Assistance Agreement No. F08B10308 awarded by the U.S. Environmental Protection Agency. This work has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors, and the EPA does not endorse any products or commercial services mentioned in this publication.
Abbreviations
- CA
catechol
- C4D
Capactively coupled contactless conductivity detection
- DA
dopamine
- E
epinephrine
- ECD
electrochemical detection
- L-DOPA
3, 4-dihydroxy-L-phenylalanine
- NE
norepinephrine
- PAD
pulsed amperometric detection
- PC12
rat pheochromocytoma
- RBC
red blood cell
- TES
N-tris[hydroxymethyl]methyl-2-aminoethanesulfonic acid
- TDAPS
N-tetradecyl-N,N-dimethyl-3-ammonio-1-propansulfonate
- Triton X-100
polyoxyethylene octyl phenyl ether
- Tween 20
polyoxyethylene (20) sorbitan monolaurate
Footnotes
The authors have declared no conflict of interest.
References
- 1.Reyes DR, Iossifidis D, Auroux PA, Manz A. Analytical Chemistry. 2002;74:2623–2636. doi: 10.1021/ac0202435. [DOI] [PubMed] [Google Scholar]
- 2.Auroux PA, Iossifidis D, Reyes DR, Manz A. Analytical Chemistry. 2002;74:2637–2652. doi: 10.1021/ac020239t. [DOI] [PubMed] [Google Scholar]
- 3.Vandaveer WR, Pasas SA, Martin RS, Lunte SM. Electrophoresis. 2002;23:3667–3677. doi: 10.1002/1522-2683(200211)23:21<3667::AID-ELPS3667>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 4.Vickers JA, Dressen BM, Weston MC, Boonsong K, Chailapakul O, Cropek DM, Henry CS. Electrophoresis. 2007;28:1123–1129. doi: 10.1002/elps.200600445. [DOI] [PubMed] [Google Scholar]
- 5.Vickers JA, Caulum MM, Henry CS. Analytical Chemistry. 2006;78:7446–7452. doi: 10.1021/ac0609632. [DOI] [PubMed] [Google Scholar]
- 6.Holcomb RE, Kraly JR, Henry CS. The Analyst. 2009;134:486–492. doi: 10.1039/b816289a. [DOI] [PubMed] [Google Scholar]
- 7.Garcia CD, Dressen BM, Henderson A, Henry CS. Electrophoresis. 2005;26:703–709. doi: 10.1002/elps.200410290. [DOI] [PubMed] [Google Scholar]
- 8.Gertsch JC, Noblitt SD, Cropek DM, Henry CS. Anal Chem. 2010;82:3426–3429. doi: 10.1021/ac9029086. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Fanguy JC, Bledsoe JM, Henry CS. Anal Chem. 2000;72:5939–5944. doi: 10.1021/ac000932l. [DOI] [PubMed] [Google Scholar]
- 10.Guan Q, Noblitt SD, Henry CS. Electrophoresis. 2012;33:379–387. doi: 10.1002/elps.201100259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirby BJ, Hasselbrink EF., Jr Electrophoresis. 2004;25:187–202. doi: 10.1002/elps.200305754. [DOI] [PubMed] [Google Scholar]
- 12.Kirby BJ, Hasselbrink EF. Electrophoresis. 2004;25:203–213. doi: 10.1002/elps.200305755. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Zhou F, Zhao L, Zhang JR, Zhu JJ. J Chromatogr A. 2007;1170:1–8. doi: 10.1016/j.chroma.2007.08.083. [DOI] [PubMed] [Google Scholar]
- 14.Huang XH, Gordon MJ, Zare RN. Analytical Chemistry. 1988;60:1837–1838. [Google Scholar]
- 15.Lee CS, Mcmanigill D, Wu CT, Patel B. Analytical Chemistry. 1991;63:1519–1523. [Google Scholar]
- 16.Ocvirk G, Munroe M, Tang T, Oleschuk R, Westra K, Harrison DJ. Electrophoresis. 2000;21:107–115. doi: 10.1002/(SICI)1522-2683(20000101)21:1<107::AID-ELPS107>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 17.Locascio LE, Perso CE, Lee CS. J Chromatogr A. 1999;857:275–284. doi: 10.1016/s0021-9673(99)00774-8. [DOI] [PubMed] [Google Scholar]
- 18.Wanders BJ, Vandegoor TAAM, Everaerts FM. Journal of Chromatography A. 1993;652:291–294. [Google Scholar]
- 19.Liu Y, Wipf DO, Henry CS. The Analyst. 2001;126:1248–1251. doi: 10.1039/b101479j. [DOI] [PubMed] [Google Scholar]
- 20.Zemann AJ, Schnell E, Volgger D, Bonn GK. Analytical Chemistry. 1998;70:563–567. doi: 10.1021/ac9707592. [DOI] [PubMed] [Google Scholar]
- 21.da Silva JAF, do Lago CL. Analytical Chemistry. 1998;70:4339–4343. [Google Scholar]
- 22.Zemann AJ. Electrophoresis. 2003;24:2125–2137. doi: 10.1002/elps.200305476. [DOI] [PubMed] [Google Scholar]
- 23.Schmalzing D, Piggee CA, Foret F, Carrilho E, Karger BL. J Chromatogr A. 1993;652:149–159. doi: 10.1016/0021-9673(93)80655-R. [DOI] [PubMed] [Google Scholar]
- 24.Dolnik V. Electrophoresis. 2004;25:3589–3601. doi: 10.1002/elps.200406113. [DOI] [PubMed] [Google Scholar]
- 25.Decher G. Science. 1997;277:1232–1237. [Google Scholar]
- 26.Katayama H, Ishihama Y, Asakawa N. Analytical Chemistry. 1998;70:5272–5277. doi: 10.1021/ac980522l. [DOI] [PubMed] [Google Scholar]
- 27.Katayama H, Ishihama Y, Asakawa N. Analytical Chemistry. 1998;70:2254–2260. doi: 10.1021/ac9708755. [DOI] [PubMed] [Google Scholar]
- 28.Pallandre A, de Lambert B, Attia R, Jonas AM, Viovy JL. Electrophoresis. 2006;27:584–610. doi: 10.1002/elps.200500761. [DOI] [PubMed] [Google Scholar]
- 29.Belder D, Ludwig M. Electrophoresis. 2003;24:3595–3606. doi: 10.1002/elps.200305648. [DOI] [PubMed] [Google Scholar]
- 30.Ludwig M, Belder D. Electrophoresis. 2003;24:2481–2486. doi: 10.1002/elps.200305498. [DOI] [PubMed] [Google Scholar]
- 31.Roman GT, Carroll S, McDaniel K, Culbertson CT. Electrophoresis. 2006;27:2933–2939. doi: 10.1002/elps.200500795. [DOI] [PubMed] [Google Scholar]
- 32.Vrouwe EX, Luttge R, Olthuis W, van den Berg A. J Chromatogr A. 2006;1102:287–293. doi: 10.1016/j.chroma.2005.10.064. [DOI] [PubMed] [Google Scholar]
- 33.Wang W, Zhao L, Zhang JR, Zhu JJ. J Chromatogr A. 2007;1142:209–213. doi: 10.1016/j.chroma.2006.12.092. [DOI] [PubMed] [Google Scholar]
- 34.Han B, Xu Y, Zhang L, Yang X, Wang E. Talanta. 2009;79:959–962. doi: 10.1016/j.talanta.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 35.Noblitt SD, Schwandner FM, Hering SV, Collett JL, Jr, Henry CS. J Chromatogr A. 2009;1216:1503–1510. doi: 10.1016/j.chroma.2008.12.084. [DOI] [PubMed] [Google Scholar]
- 36.Gertsch JC, Noblitt SD, Cropek DM, Henry CS. Analytical Chemistry. 2010;82:3426–3429. doi: 10.1021/ac9029086. [DOI] [PubMed] [Google Scholar]
- 37.Wei W, Ju H. Electrophoresis. 2005;26:586–592. doi: 10.1002/elps.200410273. [DOI] [PubMed] [Google Scholar]
- 38.Lucy CA, MacDonald AM, Gulcev MD. Journal of chromatography. A. 2008;1184:81–105. doi: 10.1016/j.chroma.2007.10.114. [DOI] [PubMed] [Google Scholar]
- 39.Wang AJ, Xu JJ, Chen HY. Analytica Chimica Acta. 2006;569:188–194. [Google Scholar]
- 40.Xu Y, Jiang H, Wang E. Electrophoresis. 2007;28:4597–4605. doi: 10.1002/elps.200700261. [DOI] [PubMed] [Google Scholar]
- 41.Towns JK, Regnier FE. Analytical Chemistry. 1991;63:1126–1132. doi: 10.1021/ac00011a013. [DOI] [PubMed] [Google Scholar]
- 42.Dou YH, Bao N, Xu JJ, Meng F, Chen HY. Electrophoresis. 2004;25:3024–3031. doi: 10.1002/elps.200405986. [DOI] [PubMed] [Google Scholar]
- 43.Yeung KK, Lucy CA. Analytical Chemistry. 1998;70:3286–3290. doi: 10.1021/ac9801566. [DOI] [PubMed] [Google Scholar]
- 44.Yeung KK, Lucy CA. Electrophoresis. 1999;20:2554–2559. doi: 10.1002/(SICI)1522-2683(19990801)20:12<2554::AID-ELPS2554>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 45.Wang C, Lucy CA. Electrophoresis. 2004;25:825–832. doi: 10.1002/elps.200305760. [DOI] [PubMed] [Google Scholar]
- 46.Badal MY, Wong M, Chiem N, Salimi-Moosavi H, Harrison DJ. J Chromatogr A. 2002;947:277–286. doi: 10.1016/s0021-9673(01)01601-6. [DOI] [PubMed] [Google Scholar]
- 47.Mori M, Hu WZ, Haddad PR, Fritz JS, Tanaka K, Tsue H, Tanaka S. Anal Bioanal Chem. 2002;372:181–186. doi: 10.1007/s00216-001-1199-1. [DOI] [PubMed] [Google Scholar]
- 48.Hoeman KW, Culbertson CT. Electrophoresis. 2008;29:4900–4905. doi: 10.1002/elps.200800463. [DOI] [PubMed] [Google Scholar]
- 49.Duffy DC, McDonald JC, Schueller OJA, Whitesides GM. Analytical Chemistry. 1998;70:4974–4984. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- 50.Liu Y, Vickers JA, Henry CS. Analytical Chemistry. 2004;76:1513–1517. doi: 10.1021/ac0350357. [DOI] [PubMed] [Google Scholar]
- 51.Guan Q, Henry CS. Electrophoresis. 2009;30:3339–3346. doi: 10.1002/elps.200900316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vickers JA, Henry CS. Electrophoresis. 2005;26:4641–4647. doi: 10.1002/elps.200500508. [DOI] [PubMed] [Google Scholar]
- 53.Garcia CD, Liu Y, Anderson P, Henry CS. Lab Chip. 2003;3:324–328. doi: 10.1039/b309339e. [DOI] [PubMed] [Google Scholar]
- 54.Lin CC, Chen CC, Lin CE, Chen SH. J Chromatogr A. 2004;1051:69–74. [PubMed] [Google Scholar]
- 55.Pittman JL, Henry CS, Gilman SD. Analytical Chemistry. 2003;75:361–370. doi: 10.1021/ac026132n. [DOI] [PubMed] [Google Scholar]
- 56.Morandat S, El Kirat K. Langmuir : the ACS journal of surfaces and colloids. 2006;22:5786–5791. doi: 10.1021/la0604228. [DOI] [PubMed] [Google Scholar]
- 57.Yeung KKC, Lucy CA. Journal of Chromatography A. 1998;804:319–325. [Google Scholar]
- 58.Lucy CA, Underhill RS. Analytical Chemistry. 1998;70:1045. doi: 10.1021/ac971476c. [DOI] [PubMed] [Google Scholar]
- 59.Baryla NE, Lucy CA. Anal Chem. 2000;72:2280–2284. doi: 10.1021/ac991191v. [DOI] [PubMed] [Google Scholar]
- 60.Esaka Y, Tanaka K, Uno B, Goto M, Kano K. Analytical Chemistry. 1997;69:1332–1338. doi: 10.1021/ac960731a. [DOI] [PubMed] [Google Scholar]
- 61.Ding Y, Mora MF, Merrill GN, Garcia CD. The Analyst. 2007;132:997–1004. doi: 10.1039/b704364c. [DOI] [PubMed] [Google Scholar]
- 62.Adams RN. Analytical Chemistry. 1976;48:1126A–1138A. doi: 10.1021/ac50008a001. [DOI] [PubMed] [Google Scholar]
- 63.Greene LA, Tischler AS. Proceedings of the National Academy of Sciences of the United States of America. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shafer TJ, Atchison WD. Neurotoxicology. 1991;12:473–492. [PubMed] [Google Scholar]
- 65.Takashima A, Koike T. Biochim Biophys Acta. 1985;847:101–107. doi: 10.1016/0167-4889(85)90159-4. [DOI] [PubMed] [Google Scholar]
- 66.Clift-O'Grady L, Linstedt AD, Lowe AW, Grote E, Kelly RB. J Cell Biol. 1990;110:1693–1703. doi: 10.1083/jcb.110.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen TK, Luo GO, Ewing AG. Analytical Chemistry. 1994;66:3031–3035. doi: 10.1021/ac00091a007. [DOI] [PubMed] [Google Scholar]
- 68.Kozminski KD, Gutman DA, Davila V, Sulzer D, Ewing AG. Analytical Chemistry. 1998;70:3123–3130. doi: 10.1021/ac980129f. [DOI] [PubMed] [Google Scholar]
- 69.Li MW, Spence DM, Martin RS. Electroanal. 2005;17:1171–1180. [Google Scholar]
- 70.Sameenoi Y, Mensack MM, Boonsong K, Ewing R, Dungchai W, Chailapakul O, Cropek DM, Henry CS. Analyst. 2011;136:3177–3184. doi: 10.1039/c1an15335h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.