Abstract
Portopulmonary hypertension (POPH), or pulmonary arterial hypertension associated with cirrhosis, carries a high mortality and often precludes liver transplantation. Many POPH patients have preserved or increased cardiac output, therefore decreasing pulmonary artery pressure rather than improving cardiac output is more important to reduce liver transplant risk, making sildenafil an attractive therapeutic option. We assessed the clinical response of patients with POPH treated with sildenafil monotherapy.
We retrospectively reviewed the charts of 10 patients with POPH and sildenafil monotherapy.
Eight of ten patients had hepatitis C virus infection. Patients took a mean (± SD) of 31 ± 14 mg thrice daily and were followed for 21 ± 16 months. WHO functional class improved from 3.0 ± 0.0 to 2.3 ± 0.5, at one year, p<0.05. Four of eight patients increased six minute walk distance at one year by 30m or more. Three patients became transplant eligible, one of whom underwent successful transplantation, and three patients have been stable without progression of liver disease or POPH. The remainder were not transplant candidates due to refractory POPH (n=2) or other comorbidities (n=2).
We conclude that sildenafil may be an effective therapy for POPH that can stabilize or improve hemodynamics in patients with POPH and thereby facilitate liver transplantation.
Keywords: liver transplant, portopulmonary hypertension, pulmonary arterial hypertension, sildenafil
INTRODUCTION
Pulmonary arterial hypertension (PAH) associated with portal hypertension, portopulmonary hypertension (POPH), is reported to occur in approximately 4–16% of patients with cirrhosis (1, 2). Patients with POPH are at increased risk for liver transplant-related mortality, and a systolic pulmonary artery pressure of ≥ 50mmHg has been used as a criterion for exclusion from this potentially life-saving treatment (3, 4). However, in those POPH patients who survive transplantation, it has been shown that the pulmonary vascular disorder may be reversible (3, 5–7). Thus there is a need to aggressively treat patients with POPH severe enough to preclude transplantation so that they can become eligible for listing. It has been shown that hemodynamic improvement in POPH prior to liver transplantation is associated with post-transplant survival similar to that observed in transplantation for other indications (8).
In the last decade important advances have been made in the treatment of PAH. Endothelin receptor antagonists and phosphodiesterase type 5 inhibitors are now widely used to treat PAH, and prostagladins continue to have an important role in the management of more severe disease. Concern about hepatotoxicity from endothelin receptor antagonists has limited their use in POPH. Generally considered the gold standard for idiopathic PAH, epoprostenol is often an unattractive treatment in POPH as a primary effect of this medication is augmentation of cardiac output, which is preserved or even elevated in mild to moderate POPH (1, 9). Indeed, epoprostenol has been shown to have direct inotropic effects on cardiac muscle contraction (10). Thus direct pulmonary vasodilation with phosphodiesterase 5 inhibitors such as sildenafil, which has an excellent safety profile, proven efficacy in PAH, and minimal effects on cardiac output are an attractive choice for treatment of POPH (11).
Sildenafil use has been reported in limited numbers of patients with POPH (12–14). However, in most of these case series or reports patients were treated with prostanoids as well and only a minority of patients with cirrhosis resulting from viral infection. Here we report the largest series of patients with POPH related primarily to hepatitis C virus treated with sildenafil monotherapy including three patients in whom treatment allowed listing for liver transplant.
METHODS
All patients referred to the Pulmonary Hypertension Clinic at Vanderbilt University Medical Center from April 2003 to March 2006 who were diagnosed with POPH were screened via retrospective chart review. POPH was defined by the known diagnosis of cirrhotic liver disease and a mean pulmonary artery pressure of ≥25 mmHg with normal left heart filling pressure on right heart catheterization. Patients with severe disease and right heart failure were treated with epoprostenol often in combination with sildenafil (n=4), while those with normal to elevated cardiac index or no evidence of right heart failure were treated with sildenafil alone. Patients, from all functional classes, treated with sildenafil monotherapy were eligible for inclusion in this analysis.
Patient charts were analyzed with respect to demographic characteristics, underlying cause of liver disease and outcome. In addition, WHO functional class (FC), 6 minute walk, and right heart catheterization results at baseline and at approximately one year following institution of sildenafil therapy were analyzed. The Vanderbilt University IRB authorized a waiver of approval for this retrospective chart review.
One-way ANOVA with Tukey's post test was performed using GraphPad Prism version 4.00 for Macintosh (GraphPad Software, San Diego California USA, www.graphpad.com). Results are presented as mean ± SD. P<0.05 was considered statistically significant.
RESULTS
Ten patients had POPH and were treated with sildenafil alone in the 48 month period of analysis. The mean age of patients in this cohort 51 ± 7 years. The patients were obese with a mean BMI of 31 ± 5. All of the patients were Caucasian and half were female. Eight of the ten patients had hepatitis C infection as their underlying cause for liver disease. One of these patients had co-existing alcohol abuse. One patient had primary biliary cirrhosis and one had alpha-1 antitrypsin deficiency without significant obstructive lung disease. No patient had severe depression in right ventricular function or severe right ventricular dilation on baseline echocardiography. Two patients (1 and 7 in Table I) refused prostaglandin therapy. MELD scores were available for 7 patients with a mean of 14.3 ± 3.3. POPH was the limiting factor in preventing listing for liver transplantation in three patients; the remainder either did not require transplantation yet (n=3) or were not considered candidates due to other co-morbidities or refractory POPH (n=4, one with POPH developing post liver transplant, one with opiate abuse, and two with persistent POPH). Baseline hemodynamic data is shown in Table I.
Table I.
Baseline Hemodynamic Data
| Patient | RAP (mmHg) |
PAs (mmHg) |
PAd (mmHg) |
mPAP (mmHg) |
PCWP (mmHg) |
CO (l/min) |
CI (l/min/m2) |
PVR (WU) |
PA sat (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 11 | 86 | 30 | 51 | 9 | 7.83 | 3.93 | 11.41 | 80 |
| 2 | 5 | 54 | 24 | 35 | 12 | 6.81 | 3.51 | 3.89 | 74 |
| 3 | 8 | 82 | 43 | 59 | 7 | 4.03 | 2.04 | 12.90 | 65 |
| 4 | 3 | 58 | 28 | 38 | 5 | 7.40 | 3.20 | 4.60 | 74 |
| 5 | 12 | 95 | 50 | 66 | 11 | 4.90 | 2.30 | 11.20 | 70 |
| 6 | 4 | 54 | 23 | 36 | 7 | 7.93 | 3.87 | 3.95 | 79 |
| 7 | 15 | 100 | 39 | 65 | 13 | 5.19 | 2.76 | 10.03 | NR |
| 8 | 2 | 65 | 17 | 38 | 8 | 9.97 | 5.57 | 3.01 | 78 |
| 9 | 13 | 58 | 26 | 40 | 13 | 7.51 | 4.58 | 4.83 | 67 |
| 10 | 2 | 83 | 28 | 50 | 3 | 5.84 | 2.69 | 16.95 | 61 |
RAP=right atrial pressure, PAs=systolic pulmonary artery pressure, PAd=diastolic pulmonary artery pressure, mPAP=mean pulmonary artery pressure, PCWP=pulmonary capillary wedge pressure, CO= cardiac output, CI=cardiac index, PVR=pulmonary vascular resistance, PA sat = oxygen saturation pulmonary artery, NR=not recorded.
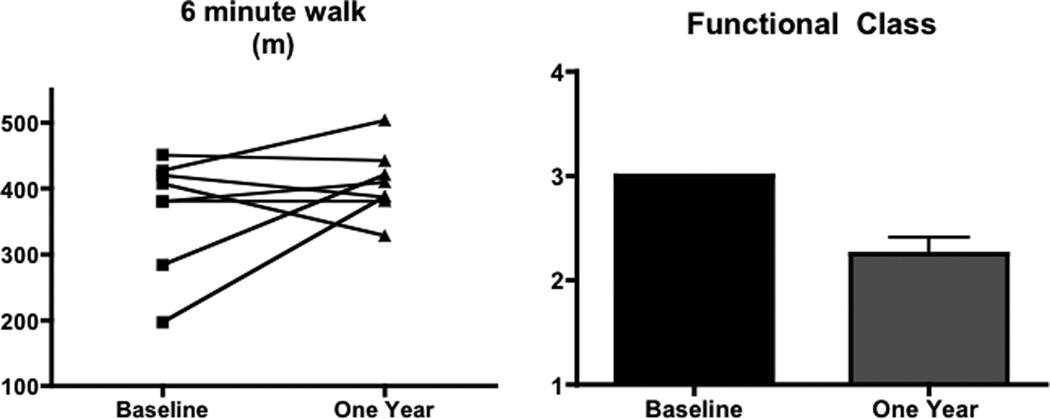
Sildenafil was well tolerated in this cohort without significant side effects including hypotension or changes in liver function or blood counts. The mean dose of sildenafil administered was 31± 14 mg thrice daily (range 20–50 mg TID), with patients 2, 6, and 7 receiving 50 mg dose and the remainder receiving 20–25 mg dependent on use prior to or after FDA approval of sildenafil for PAH. At one year, 8 patients were alive and returned to clinic (one died and one did not return to clinic). All underwent a six minute walk test (Figure 1). Six minute walk distance increased from 369 ± 86 m to 408 ± 51 m, (p=0.29). The mean increase in 6 minute walk distance was 26 ± 82 m at one year, with four of eight patients having an improvement of 30 or more meters at this time point (range −80 to 191m). There were no statistically significant differences between heart rate and oxygen saturation at one or two years after six minute walk test. There was a statistically significant improvement in FC at one year, improving from 3.0 ± 0.0 to 2.3 ± 0.5 (p<0.05, Figure 1). In the three patients seen at two years, functional class was two in two patients and three in one patient. The one patient seen at three years was stable at class two.
Figure 1.
Six minute walk distance in meters in patients with POPH treated with sildenafil alone at baseline and one year (n=8). Although there was a substantial trend towards improvement at each time point, this did not reach statistical significance. WHO functional class in the same patients at baseline and one year (*p<0.05 baseline vs. 1 year).
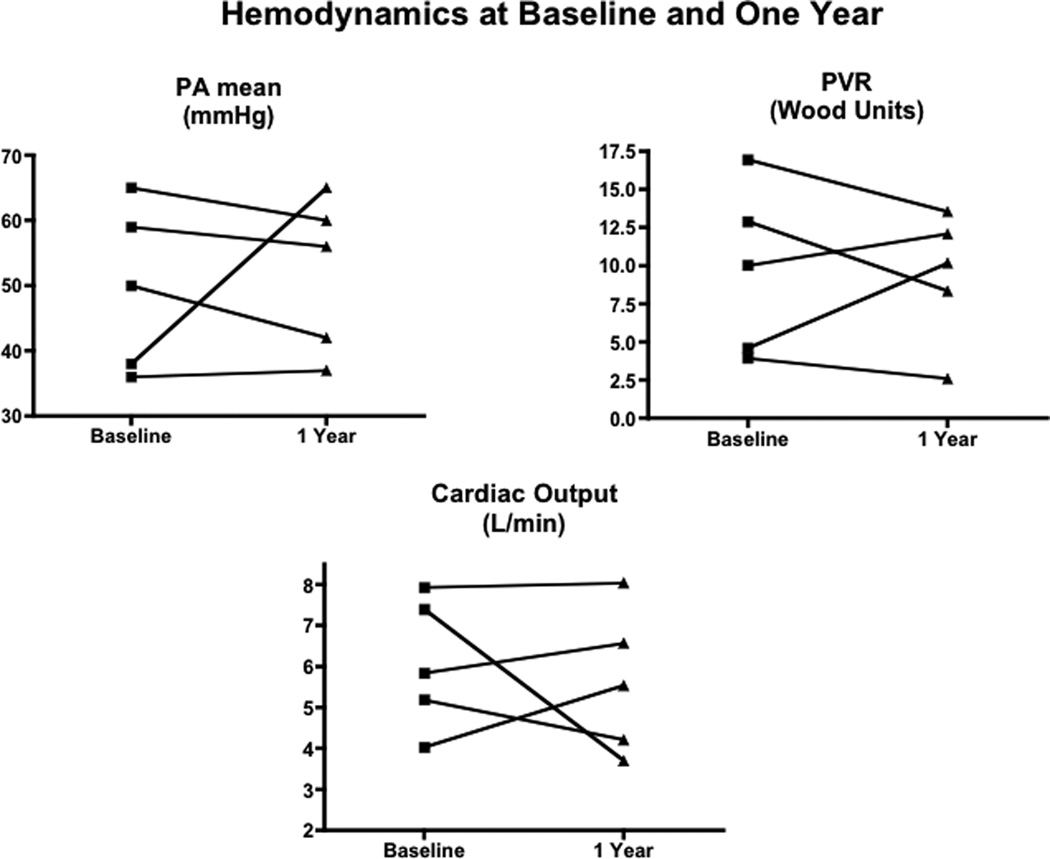
Baseline right heart catheterization was obtained in all 10 patients (Table I). No patients exhibited acute vasoreactivity to inhaled nitric oxide. Five patients underwent repeat hemodynamic measurement within one year. Two patients had right heart catheterization at two years. Results of baseline and one year follow up catheterization in those patients who underwent evaluation at both time points are shown in Figure 2 (patients 3,4,6,7,10). Although there was no statistically significant difference in hemodynamic data over the one year period, mean pulmonary artery pressure decreased or remained unchanged in four of five patients, three of five had decreased pulmonary vascular resistance decreased in three patients, and cardiac index was not significantly increased in any patients. Patient 2, had an increase in mPAP and PVR with a decline in CI at one year and was started on epoprostenol.
Figure 2.
Invasive hemodynamic data obtained at right heart catheterization at baseline and within 12 months (n=5, patients 3,4,6,7,10). There were no statistically significant differences in any of the temporal comparisons. One patient, patient 2, had an increase in mPAP and PVR with a decline in CI at one year. This patient required epoprostenol administration.
Three patients with hepatitis C associated cirrhosis have been stable on sildenafil and medical treatment for liver disease. Liver transplant has not been recommended for these patients. They have been followed for up to 3 years. One patient who did not return for follow up died 14 months after beginning sildenafil. Three other patients who were not transplant candidates due to other co-morbidities died of causes other than POPH. Mean time to death in the non-transplant candidates was 15 ± 12 months. Of the three patients who were potentially transplant eligible after sildenafil (patients 2, 4, and 6 in Table I), one was deactivated due to multifocal hepatocellular carcinoma 11 months after sildenafil initiation, another is presently awaiting transplantation, 35 months after being treated with sildenafil, and one patient successfully underwent liver transplantation 14 months after beginning sildenafil. He had a follow up echocardiogram prior to listing for transplantation showing normal right heart size and function with minimal tricuspid regurgitation. The systolic PA pressure at the time of transplant was less than 50 mmHg. This patient had worsening of pulmonary hypertension six months post transplant necessitating the addition of epoprostenol titrated to a dose of 20 ng/kg/min, which was weaned off after 10 months to sildenafil alone after 10 months. He remains alive at the time of this publication, 48 months after treatment with sildenafil monotherapy with a most recent right heart catheterization showing mPAP of 29 mmHg, right atrial pressure of 4 mmHg, and cardiac index of 3.64 l/min/m2.
DISCUSSION
We found sildenafil to be well tolerated in patients with POPH and associated with a significant improvement in functional class. Six minute walk test improved in nearly half of the patients at follow up. Furthermore, sildenafil decreased pulmonary artery pressure enough to allow listing for two patients who were initially ineligible POPH. A third patient had a good hemodynamic response to sildenafil, but died of hepatocellular carcinoma that developed after treatment of POPH; this case highlights the importance of early, aggressive treatment of POPH prior to development of catastrophic complications in order allow transplantation. Three patients have had stable liver and pulmonary vascular disease and have not required escalation of therapy. Overall, there was no significant improvement in hemodynamic parameters; however, the sample was small and individual patients had impressive hemodynamic improvements with more than half of those tested at one year with a decrease in mean pulmonary artery pressure and pulmonary vascular resistance. Interestingly, despite all patients being functional class III at initiation of sildenafil, there was significant variability in hemodynamic data, highlighting the potential limitations to the use of this classification in patients with POPH. Of note, three patients were treated with sildenafil at 50 mg TID, similar to the dose used in other reports in POPH and suggests that in some cases 20 mg TID may be insufficient and that dose escalation is indicated (13). In contrast to previously published literature on POPH, the majority of patients reported here had viral-induced cirrhosis, which may reflect an important shift in cirrhosis epidemiology (15). Regardless of the etiology, sildenafil appears to be efficacious in patients with POPH.
Significant liver disease is generally an exclusion criteria in multicenter treatment trials in PAH (11, 16). There have been case reports and case series published on vasoactive therapy for POPH, but despite the recent data that 15% of patients at a PAH referral center had POPH there is relatively limited information to guide clinicians’ treatment for patients with POPH, particularly those with preserved cardiac output (12–14, 17, 18). Bosentan, which has potential hepatotoxicity, has been used in patients with cirrhosis without worsening of liver disease; however the number of patients in these reports may be too low to detect this complication (19, 20). Others have reported using epoprostenol in POPH, with hemodynamic improvement defined by decreased pulmonary vascular resistance and mean pulmonary artery pressure with increase in cardiac output (21). In 10 year review of 66 patients with POPH, the mean CO was 6.1 L/min. Concern has been raised about increasing an already elevated CO in patients with POPH, potentially precipitating high output failure and decompensation of portal hypertension related to increased blood delivery (21).
Sildenafil is not associated with hepatotoxicity and is relatively pulmonary-selective due to the high concentration of phosphodiesterase 5 in lung tissue (22). Thus sildenafil is an attractive option for treatment of POPH with preserved cardiac function. While the hyperdynamic circulatory state of liver disease may be due to increased nitric oxide in the mesenteric circulation, this molecule has a very short half life. Therefore, mesenteric nitric oxide excess in cirrhosis is likely not affecting the pulmonary vasculature and inhibition of phosphodiesterase 5 with resultant increase in nitric oxide’s second messenger cGMP may be beneficial in POPH. However, the exclusion of POPH in the largest trial of sildenafil leaves many unanswered questions about the utility of phosphodiesase 5 inhibition in POPH. Other studies have reported on combination therapy of iloprost and sildenafil as well as sildenafil monotherapy in patients with depressed cardiac output. There are also case reports of sildenafil monotherapy, but as yet there is no report of the results of large numbers of POPH patients treated with sildenafil alone and it is unlikely there will be a study in this population (12–14). The preserved cardiac output in the patients reported here is a unique clinical challenge as the successful lowering of pulmonary artery pressure, rather than augmentation of cardiac function, can allow listing for liver transplantation. Furthermore, we have shown that sildenafil is well tolerated in POPH with no systemic hypotension or alteration in hepatic function or blood counts.
The limitations of this study are its retrospective and observational nature as well as the small sample size. There may be additional benefit to combination vasoactive therapy, but this effect is not explored here.
We conclude that sildenafil therapy for POPH may be effective in improving function class and may also allow liver transplantation in POPH patients with mild to moderate pulmonary vascular disease. This is an important therapeutic option for patients with preserved cardiac output in POPH. Further study of optimal drug therapy is needed in this sizeable subclass of PAH.
Table II.
Follow Up Data
| Follow up time (months, mean ± SD) | 21 ± 16 |
| Dead | 5/10 |
| Progressive POPH and cirrhosis | 2 |
| Hepatocellular carcinoma | 1 |
| Unknown cause | 2 |
| Transplant required | 3 |
| Transplant eligible | 2/3 |
| Successful transplant | 1 |
| Awaiting transplant | 1 |
| Alive, not requiring transplant | 3/10 |
ACKNOWLEDGEMENTS
We gratefully acknowledge the help of Cynthia A. Fink in data acquisition for this manuscript.
Grant Support for Dr. Hemnes: NIH 7 F32 HL082132-02
Abbreviations Used
- PAH
pulmonary arterial hypertension
- POPH
portopulmonary hypertension
References
- 1.Castro M, Krowka MJ, Schroeder DR, Beck KC, Plevak DJ, Rettke SR, Cortese DA, Wiesner RH. Frequency and clinical implications of increased pulmonary artery pressures in liver transplant patients. Mayo Clin Proc. 1996;71(6):543–551. doi: 10.4065/71.6.543. [DOI] [PubMed] [Google Scholar]
- 2.Benjaminov FS, Prentice M, Sniderman KW, Siu S, Liu P, Wong F. Portopulmonary hypertension in decompensated cirrhosis with refractory ascites. Gut. 2003;52(9):1355–1362. doi: 10.1136/gut.52.9.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krowka MJ, Plevak DJ, Findlay JY, Rosen CB, Wiesner RH, Krom RA. Pulmonary hemodynamics and perioperative cardiopulmonary-related mortality in patients with portopulmonary hypertension undergoing liver transplantation. Liver Transpl. 2000;6(4):443–450. doi: 10.1053/jlts.2000.6356. [DOI] [PubMed] [Google Scholar]
- 4.Starkel P, Vera A, Gunson B, Mutimer D. Outcome of liver transplantation for patients with pulmonary hypertension. Liver Transpl. 2002;8(4):382–388. doi: 10.1053/jlts.2002.31343. [DOI] [PubMed] [Google Scholar]
- 5.Schott R, Chaouat A, Launoy A, Pottecher T, Weitzenblum E. Improvement of pulmonary hypertension after liver transplantation. Chest. 1999;115(6):1748–1749. doi: 10.1378/chest.115.6.1748. [DOI] [PubMed] [Google Scholar]
- 6.Csete M. Intraoperative management of liver transplant patients with pulmonary hypertension. Liver Transpl Surg. 1997;3(4):454–455. doi: 10.1002/lt.500030422. [DOI] [PubMed] [Google Scholar]
- 7.Scott V, De Wolf A, Kang Y, Martin M, Selby R, Fung J, Doyle H, Ziady G, Paradis I, Miro A, et al. Reversibility of pulmonary hypertension after liver transplantation: a case report. Transplant Proc. 1993;25(2):1789–1790. [PubMed] [Google Scholar]
- 8.Ashfaq M, Chinnakotla S, Rogers L, Ausloos K, Saadeh S, Klintmalm GB, Ramsay M, Davis GL. The impact of treatment of portopulmonary hypertension on survival following liver transplantation. Am J Transplant. 2007;7(5):1258–1264. doi: 10.1111/j.1600-6143.2006.01701.x. [DOI] [PubMed] [Google Scholar]
- 9.Hinderliter AL, Willis PWt, Barst RJ, Rich S, Rubin LJ, Badesch DB, Groves BM, McGoon MD, Tapson VF, Bourge RC, Brundage BH, Koerner SK, Langleben D, Keller CA, Murali S, Uretsky BF, Koch G, Li S, Clayton LM, Jobsis MM, Blackburn SD, Jr, Crow JW, Long WA. Effects of long-term infusion of prostacyclin (epoprostenol) on echocardiographic measures of right ventricular structure and function in primary pulmonary hypertension. Primary Pulmonary Hypertension Study Group. Circulation. 1997;95(6):1479–1486. doi: 10.1161/01.cir.95.6.1479. [DOI] [PubMed] [Google Scholar]
- 10.Pavlovic M, Petkovic D, Cvetkovic M, Zdjelar K, Starcevic V, Bosnic O. Study of the mechanism of prostacyclin (PgI2) action on myocardial contractility. Agents Actions Suppl. 1992;37:171–175. doi: 10.1007/978-3-0348-7262-1_23. [DOI] [PubMed] [Google Scholar]
- 11.Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353(20):2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 12.Callejas Rubio JL, Salmeron Escobar J, Gonzalez-Calvin J, Ortego Centeno N. Successful treatment of severe portopulmonary hypertension in a patient with Child C cirrhosis by sildenafil. Liver Transpl. 2006;12(4):690–691. doi: 10.1002/lt.20748. [DOI] [PubMed] [Google Scholar]
- 13.Reichenberger F, Voswinckel R, Steveling E, Enke B, Kreckel A, Olschewski H, Grimminger F, Seeger W, Ghofrani HA. Sildenafil treatment for portopulmonary hypertension. Eur Respir J. 2006;28(3):563–567. doi: 10.1183/09031936.06.00030206. [DOI] [PubMed] [Google Scholar]
- 14.Makisalo H, Koivusalo A, Vakkuri A, Hockerstedt K. Sildenafil for portopulmonary hypertension in a patient undergoing liver transplantation. Liver Transpl. 2004;10(7):945–950. doi: 10.1002/lt.20153. [DOI] [PubMed] [Google Scholar]
- 15.Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13(17):2436–2441. doi: 10.3748/wjg.v13.i17.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, Landzberg M, Simonneau G. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346(12):896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 17.Deibert P, Bremer H, Roessle M, Kurz-Schmieg AK, Kreisel W. PDE-5 inhibitors lower portal and pulmonary pressure in portopulmonary hypertension. Eur Respir J. 2007;29(1):220–221. doi: 10.1183/09031936.00110006. [DOI] [PubMed] [Google Scholar]
- 18.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Simonneau G. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173(9):1023–1030. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 19.Grander W, Eller P, Fuschelberger R, Tilg H. Bosentan treatment of portopulmonary hypertension related to liver cirrhosis owing to hepatitis C. Eur J Clin Invest. 2006;36(Suppl 3):67–70. doi: 10.1111/j.1365-2362.2006.01687.x. [DOI] [PubMed] [Google Scholar]
- 20.Hoeper MM, Halank M, Marx C, Hoeffken G, Seyfarth HJ, Schauer J, Niedermeyer J, Winkler J. Bosentan therapy for portopulmonary hypertension. Eur Respir J. 2005;25(3):502–508. doi: 10.1183/09031936.05.00080804. [DOI] [PubMed] [Google Scholar]
- 21.Krowka MJ, Frantz RP, McGoon MD, Severson C, Plevak DJ, Wiesner RH. Improvement in pulmonary hemodynamics during intravenous epoprostenol (prostacyclin): A study of 15 patients with moderate to severe portopulmonary hypertension. Hepatology. 1999;30(3):641–648. doi: 10.1002/hep.510300307. [DOI] [PubMed] [Google Scholar]
- 22.Corbin JD, Beasley A, Blount MA, Francis SH. High lung PDE5: a strong basis for treating pulmonary hypertension with PDE5 inhibitors. Biochem Biophys Res Commun. 2005;334(3):930–938. doi: 10.1016/j.bbrc.2005.06.183. [DOI] [PubMed] [Google Scholar]