Abstract
Children have lower size-normalised maximal voluntary force, speed, and power than adults. It has been hypothesised that these and other age-related performance differences are due to lesser type-II motor-unit utilisation in children. This should be manifested as slower force kinetics in explosive muscle contractions. The purpose of this study was to investigate the nature of child–adult force-kinetics differences and whether the latter could support that hypothesis. Untrained boys (n = 20) and men (n = 20) (10.1 ± 1.3 and 22.9 ± 4.4 years, respectively), performed maximal, explosive, isometric elbow flexions and knee extensions on a Biodex dynamometer. Peak torque (MVC), times to 10–100% MVC, and other kinetics parameters were determined. The boys’ body-mass-normalised knee extension MVC, peak rate of torque development, and %MVC at 100 ms were 26, 17 and 23% lower compared with the men and their times to 30% and 80% MVC were 24 and 48% longer, respectively. Elbow flexion kinetics showed similar or greater differences. The findings illuminate boys’ inherent disadvantage in tasks requiring speed or explosive force. It is demonstrated that the extent of the boys–men kinetics disparity cannot be explained by muscle-composition and/or musculo-tendinous-stiffness differences. We suggest therefore that the findings indirectly support children’s lower utilisation of type-II motor units.
Keywords: children, force, muscle, rate of force development, strength
Introduction
Compared with adults, children exhibit lower voluntary muscle strength, speed, and power, even after age- or maturity-dependent dimensional differences have been corrected for (see (Blimkie, 1989; Inbar & Bar-Or, 1986; Van Praagh & Dore, 2002) for review). One likely explanation for these differences is children’s lower level of maximal voluntary muscle activation (Grosset, Mora, Lambertz, & Perot, 2008; O’Brien, Reeves, Baltzopoulos, Jones, & Maganaris, 2009, 2010). We have further proposed that this activation deficit is more specifically due to children’s relative inability to recruit or utilise their higher-threshold, type-II motor units (Cohen et al., 2010; Dotan et al., 2012; Falk, Brunton, et al., 2009; Falk, Usselman, et al., 2009). We suggested this difference in motor-unit activation pattern between children and adults as a major underlying common factor in all the above-mentioned, as well as other child–adult, performance differences (see Dotan et al., 2012).
The existing body of evidence indirectly supporting this child–adult difference in muscle activation pattern is both diverse and extensive (Dotan et al., 2012). Nevertheless, due to technical limitations of existing tools and ethical constraints on the use of invasive procedures in children, conclusive evidence to confirm or refute the hypothesis is lacking. Electromyography (EMG) has long been a major tool in studying neuro-motor function. However, due to differences in muscle size, skin-to-muscle distance and conductivity, child–adult differences in neuro-motor function are difficult to elucidate by means of EMG.
On the other hand, due to the disparate contractile properties of type-I vs. type-II motor units, a difference in their relative involvement can markedly influence the kinetics of the muscle’s force development (Vandervoort & McComas, 1983; Viitasalo & Komi, 1978). This distinction may provide an EMG-independent insight into differential motor-unit activation patterns. For example, Gruber and Gollhofer, using sensorimotor training that presumably accentuated type-II motor-unit activation, showed a 33% increase in the rate of isometric knee-extension force development without significantly affecting maximal force (Gruber & Gollhofer, 2004). Thus, greater reliance on type-II motor units could be expected to bring about faster, more explosive force kinetics, while lesser activation of the fast-twitch motor units, as alleged in children, would result in slower kinetics.
The prevailing consensus is that child–adult strength-related differences stem from children’s smaller relative muscle size, lower level of voluntary muscle activation, or from higher levels of antagonist co-activation (Blimkie, 1989; Grosset et al., 2008; Sale & Spriet, 1996). Children’s smaller absolute muscle size clearly results in lower absolute maximal force. However, when a muscle’s rate of force development is normalised to its own peak force, smaller muscle size, in and of itself, could not explain observed differences. This is also the case for uniform, overall muscle-activation deficit or higher agonist-antagonist co-activation. In other words, these factors can be regarded as reducing the functional size of children’s musculature and they likely play a role in children’s lower size-normalised force, speed, and power generation. However, they do not affect the normalised kinetics of force development. On the other hand, lower utilisation of the type-II motor units would be expected to affect the kinetics of force development.
In view of children’s aforementioned lower physical capacities, the present study aimed to examine whether corresponding differences could be demonstrated in voluntary torque kinetics of isolated single-joint contractions. It was hypothesised that children would display significantly slower torque kinetics, thereby lending indirect support to the hypothesis of children’s lower activation of type-II motor units. Furthermore, in view of children’s greater deficit in upper- vs. lower-body strength and power (Inbar & Bar-Or, 1986; Kanehisa, Ikegawa, Tsunoda, & Fukunaga, 1995; Parker, Round, Sacco, & Jones, 1990), we chose to examine torque kinetics in frequently-used muscles of both the upper and lower extremities – elbow flexion and knee extension, respectively.
Methods and measurements
Participants
Twenty untrained boys (9–12 years) and 20 untrained men (18–25 years) participated in this study. Their mean physical characteristics are presented in Table I. No participant was involved in structured physical activity more than 2 h per week. All the boys were classified as pre- and early-pubertal, based on secondary sexual characteristics (pubic hair), as described by Tanner (1962). Those participants who had prior or present conditions that could affect muscle or neuromuscular function (e.g., muscular disease, medication use, injury to a dominant limb) were excluded from the study. All testing was reviewed and approved by the University’s Research Ethics Board. All participants, as well as the boys’ parents/guardians, were given a thorough explanation of the study’s purpose, procedures, benefits and potential risks or discomforts and signed an informed consent form prior to testing.
Table I.
Physical characteristics of the participants.
| n | Age years | Mass kg | Height cm | %Fat | |
|---|---|---|---|---|---|
| Boys | 20 | 10.1 ± 1.3 | 35.4 ± 7.7 | 141.0 ± 8.7 | 17.9 ± 5.9 |
| Men | 20 | 22.9 ± 4.4 | 80.5 ± 12.1 | 180.6 ± 7.2 | 20.4 ± 6.5 |
Procedures
The general procedures and protocols have been previously described (Cohen et al., 2010; Mitchell et al., 2011). Participants made two visits to the laboratory, 2–7 days apart. On their first visit, participants filled out medical and physical activity questionnaires and the boys self-assessed their pubertal stage (Tanner, 1962). During the first visit, anthropometric measurements were taken and participants were familiarised with the testing apparatus, instrumentation, and procedures, by performing several maximal voluntary isometric elbow flexion and knee extension contractions (MVC).
On their second visit, participants performed a contraction-specific (elbow flexion or knee extension) warm-up consisting of five isometric contractions of increasing intensity. Following 2–3 min rest, maximal repetitions were performed in two sets of five MVCs, 30 s apart, with a 2-min rest between sets. Explicit instructions were given to contract as hard and as fast as possible. The elbow flexion and knee extension tests were administered in a counterbalanced order. Participants were asked to refrain from intense physical activity for 48 hours prior to the experimental session.
Anthropometry
Height was measured using a stadiometer (Length Boards, Ellard Instrumentation, Ltd., Monroe, WA) and recorded to the nearest 0.1 cm. Body mass was measured with minimal clothing, using a digital scale (Zenith) and recorded to the nearest 0.1 kg. Triceps and subscapular skinfold thicknesses were measured in triplicate using Harpenden callipers (British Indicators, Weybridge, England) and the median value at each site was used for further calculation. Per cent body fat was estimated using age- and maturity-specific equations (Slaughter, Lohman, & Boileau, 1988).
Muscle strength
Isometric contractions were chosen primarily because they are the simplest, most fundamental form of contraction. Additionally, agonist-antagonist co-activation is minimised in isometric contractions (Calder & Gabriel, 2007), so that the measured torque can more fully be attributed to agonist action. As previously described (Cohen et al., 2010; Mitchell et al., 2011), isometric co-contraction proved both small and similar in the boys and men. Furthermore, while co-contraction may play an important role in dynamic actions, its role in force production during an isometric contraction is minimal. Also, while dynamic muscular actions are more prevalent, they introduce additional factors, such as angle, acceleration, and force-velocity interactions. These factors complicate the elucidation of possible child–adult muscle-activation differences that hitherto have not been determined in the most fundamental contraction form, i.e., isometrics.
All strength testing was performed on a Biodex System-3 dynamometer, as previously described (Cohen et al., 2010; Mitchell et al., 2011). Briefly, participants were secured onto the chair to minimise activation and movement of muscles not being tested. Two straps over the shoulders and crossing over the chest, a waist strap, and a strap crossing the tested leg at mid-thigh were used to secure participants. For knee-extension testing, the knee joint was positioned at 90° (0° = full extension) and the dynamometer’s axis of rotation was aligned with the lateral femoral epicondyle. The dynamometer’s lever arm was adjusted so that its attachment was at the participant’s ankle and was fastened to the lower leg with a padded Velcro strap. For upper-arm testing, participants sat upright in a chair with the shoulder at 90° flexion, upper arm resting on an arm rest and the elbow at 90° flexion (0° = full extension), with the hand in the neutral position. The torque axis was aligned with the lateral humeral epicondyle. Participants were instructed to contract, from a relaxed state (verified by the EMG trace), as fast and as forcefully as possible, so as to maximise torque and rate of torque development. They were verbally encouraged throughout the testing session. Participants were provided with visual feedback of their torque signal on a PC monitor. In the boys (who were less consistent than the men), intra-session, peak-torque reliability coefficient (Intra-class correlation2,1) was 0.95.
Signal recording and reduction
Torque and EMG were recorded simultaneously as previously described in detail (Cohen et al., 2010; Mitchell et al., 2011). Briefly, the torque signal was transformed with an analogue-to-digital card to a PC at 1000 Hz (Delsys EMGWorks acquisition software). The torque trace was smoothed using a 10 Hz low pass, second order Butterworth filter. The 10 trials were then scrutinised for performance irregularities and stability of both the torque and EMG baselines. The five best trials were selected, based on a composite index of peak torque and peak rate of torque development, then averaged and analysed for each participant and contraction (knee extension and elbow flexion). Torque onset was defined as the first point in time where the rate of torque development had exceeded five standard deviations of its baseline signal for > 10 ms. The average waveform was created by aligning the five chosen trials on their force onset and then averaging them point by point. Peak torque was defined as the highest torque maintained for 250 ms. The times to peak torque and peak rate of torque development were calculated as the time delays between torque onset and peak torque, or peak rate of torque development, respectively. From each waveform, the times elapsed to 10–100% peak torque (10% intervals) were calculated.
EMG was recorded for calculating the electromechanical delay (EMD), using Delsys (Boston, MA) Bagnoli EMG system and bipolar DE-2.1 differential surface electrodes. The signal was then rectified and amplified 1000-fold by a Bagnoli amplifier (20–450 Hz, CMRR 92 dB) and sampled at 1000 Hz (Delsys EMGWorks acquisition software). EMG onset was determined as the time point at which the EMG signal reached five standard deviations of the baseline signal. The EMG signal was then synchronised with that of the torque. EMD was calculated as the time difference between the two onsets.
Statistical analysis
All data are displayed as means ± 1 standard deviation. All statistical analyses were conducted using SPSS 17.0 (SPSS Inc., Chicago, IL) with α set a P ≤ 0.05. Differences in physical characteristics between group means were compared using one-sided independent t-test. Equality of variance was determined using the Levene test. In cases where the Levene test indicated that the variance differed between the two groups, the appropriate t- and P-values were utilised. In order to correct for multiple t-tests, the P value was divided by the number of comparisons (10 comparisons each for elbow flexion and knee extension). Thus, group means were considered significantly different when P < 0.005. Differences between groups in torque kinetics (i.e., time to 10, 20, 30…, 90% MVC) were analysed using analysis of variance (ANOVA) for repeated measures, with age-group as the between-participant factor and time-to-given-%MVC as the within-participant factor. Main effects (group and time) and their interaction were considered significant when P < 0.05. An LSD (least significant difference) post hoc test was used to assess pairwise differences when a main effect or an interaction between the two main effects was found.
Results
Group comparisons of peak isometric torques and the kinetic parameters characterising their attainment (EMD, torque at 100 ms, time to 30% and 80% MVC, and peak rate of torque development) for both elbow flexion and knee extension are presented in Table II. The boys’ EMD was significantly longer in elbow flexion, but the group difference in knee extension did not reach statistical significance (P = 0.064). Boys’ peak torque was significantly lower than the men’s, whether expressed in absolute values or relative to body mass. The kinetics of torque development was significantly slower in the boys (P < 0.01; Figure 1). This was true whether torque was expressed relative to peak torque (%MVC) (Figure 1, Table II), or normalised to body mass (Table II). At 100 ms, the boys could develop a much lower fraction of their peak torque compared with men, in both the elbow flexion and knee extension (Table II). The time to peak torque was 1617 ± 730 vs. 1216 ± 668 ms in elbow flexion (P = 0.04) and 1484 ± 598 vs. 1267 ± 610 ms in knee extension not significant (NS), in boys vs. men, respectively. The time to attain a given percentage of peak torque was consistently longer in boys compared with men (P < 0.05). Additionally, the group-time interaction was also significant (P < 0.01), reflecting the fact that as the contraction progressed, the boys–men difference in torque development increased. The peak rate of torque development in elbow flexion was significantly lower in boys compared with men, whether expressed in absolute values, or normalised to peak torque (Table II). For knee extension, the normalised rate of torque development was lower in the boys, although the difference did not reach statistical significance. The time to peak rate of torque development was longer in the boys compared with the men. This difference was statistically significant in elbow flexion but not in knee extension (Table II). It should be noted that the pattern of boys–men differences was similar in elbow flexion and knee extension. However, the differences were more pronounced in elbow flexion.
Table II.
Boys–men comparison of peak torque and kinetic parameters.
| EMD ms | MVC (Peak Torque)
|
Torque @ 100 ms
|
Time to %MVC ms
|
Peak RTD*
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Absolute Nm | Size-Normalised Nm · kg−1 | Relative %MVC | Size-Normalised Nm · kg−1 | 30% | 80% | Absolute Nm · s−1 | Torque-Normalised Nm · s−1 · Nm−1 | Time to peak RTD ms | ||
| Elbow Flexion | ||||||||||
| Boys (n = 20) | 73.0 ± 23.0 | 19.0 ± 4.5 | 0.54 ± 0.11 | 36.6 ± 12.3 | 0.20 ± 0.08 | 93 ± 20 | 445 ± 413 | 106 ± 51 | 5.28 ± 1.85 | 93 ± 22 |
| Men (n = 20) | 53.6 ± 10.4 | 71.4 ± 8.7 | 0.91 ± 0.17 | 52.1 ± 12.8 | 0.47 ± 0.11 | 70 ± 13 | 204 ± 55 | 567 ± 135 | 8.01 ± 2.02 | 69 ± 12 |
| Boys/Men ratio | 1.36 | 0.27 | 0.60 | 0.70 | 0.43 | 1.33 | 2.17 | 0.19 | 0.66 | 1.35 |
| Boys-Men P value | 0.002 | <0.0001 | <0.0001 | 0.0002 | <0.0001 | <0.0001 | <0.01 | <0.0001 | <0.0001 | <0.0001 |
| t value | 3.12 | 14.99 | 8.13 | 3.91 | 8.80 | 4.21 | 2.58 | 13.91 | 4.46 | 4.30 |
| Knee Extension | ||||||||||
| Boys (n = 20) | 67.1 ± 16.8 | 73.9 ± 25.4 | 2.10 ± 0.56 | 30.5 ± 9.7 | 0.65 ± 0.29 | 106 ± 25 | 364 ± 220 | 366 ± 173 | 4.96 ± 1.48 | 93 ± 22 |
| Men (n = 19) | 58.9 ± 15.1 | 226.2 ± 42.5 | 2.85 ± 0.54 | 39.6 ± 8.7 | 1.12 ± 0.33 | 85 ± 14 | 247 ± 122 | 1343 ± 355 | 5.99 ± 1.30 | 79 ± 12 |
| Boys/Men ratio | 1.14 | 0.33 | 0.74 | 0.77 | 0.58 | 1.24 | 1.48 | 0.27 | 0.83 | 1.17 |
| Boys-Men P value | 0.064 | <0.0001 | <0.0001 | 0.002 | <0.0001 | 0.002 | 0.024 | <0.0001 | 0.015 | <0.01 |
| t value | 1.56 | 13.49 | 4.25 | 3.08 | 5.11 | 3.18 | 2.07 | 10.57 | 2.25 | 2.44 |
EMD = Electro-Mechanical Delay; MVC = Maximal Voluntary Contraction (peak torque); RTD = Rate of Torque Development.
– Based on mean of best 5 contractions rather than on their average trace.
Figure 1.
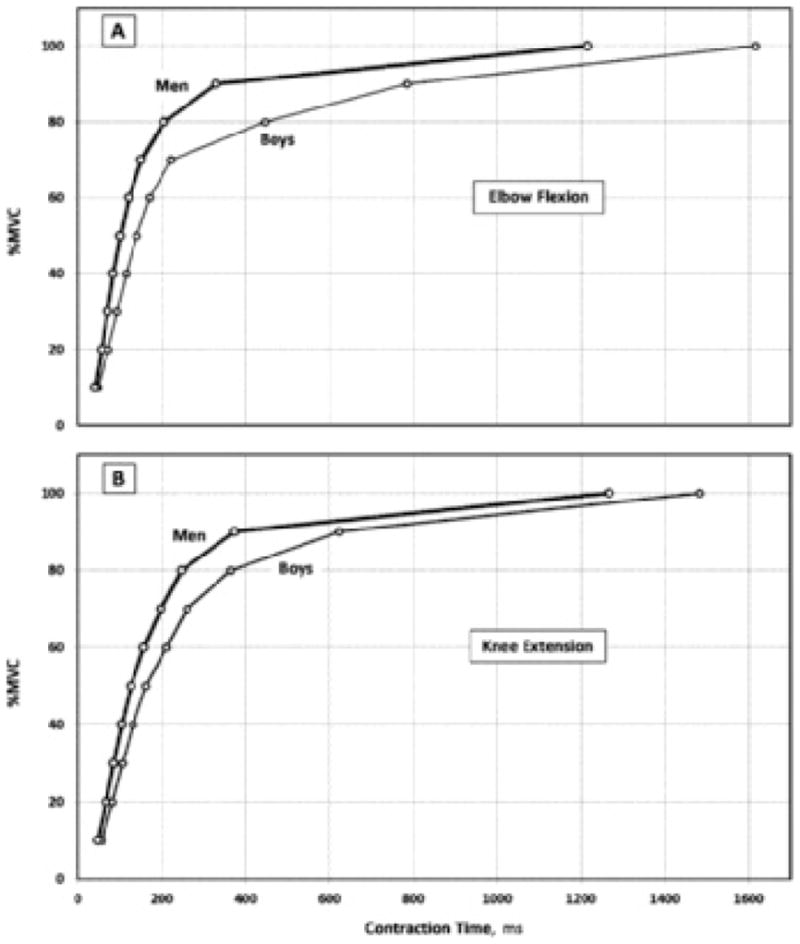
Torque kinetics during elbow flexion (A) and knee extension (B) in boys (thin line) and men (thick line). In both groups, torque is expressed as a percentage of maximal torque, thus controlling for age-related differences in maximal torque. A significant group-time interaction was observed in both contractions (P < 0.01), with pair-wise differences at all time-points up to and including 90% MVC.
Discussion
Children differ from adults in many performance and metabolic characteristics, most of which may fully or partly be explained by the differential motor-unit activation hypothesis (Dotan et al., 2012). In the present study, our pre- and early-pubertal boys demonstrated consistently slower isometric torque kinetics, compared with young men, in both the lower and upper limbs (elbow flexion, knee extension). These differences were shown not only in peak torques (absolute and normalised), but most importantly, in the fractional attainment of peak torques (%MVC; Figure 1). That is, independently of peak torques, boys demonstrated slower kinetics of torque development, consistent with the differential motor-unit activation hypothesis.
The present study is the first to directly compare normalised, voluntary torque or force kinetics of children vs. adults. Grosset, Mora, Lambertz, and Perot (2005) found involuntary (twitch) torque kinetics slower in prepubertal than in postpubertal children and adults, but did not account for differences in muscle size or maximal torque. De Ste Croix, Deighan, and Armstrong (2004) reported longer times to isometric elbow flexion (although not in knee extension) peak torque in children than in adults, but the kinetics of torque development was not examined. Therefore, the present findings extend previously reported age-related differences in time to peak torque by analysing the pattern of force development in boys and men.
To our knowledge, only Going, Massey, Hoshizaki, and Lohman (1987) investigated children’s voluntary force kinetics, albeit with no adult comparison. Their boys’ age and physical characteristics were similar to ours, as were peak rate of force development and the time to 30% MVC. However, the time to 80% MVC was 24% longer than in our study. In young men, Aagaard, Simonsen, Andersen, Magnusson, and Dyhre-Poulsen (2002) found peak isometric knee extension torque and %MVC at 100 ms that were ~30 and ~50% higher, respectively, and the time to 30% and 80% MVC that were ~40 and ~15% shorter, respectively, compared with our men. Barry, Warman, and Carson (2005) reported faster elbow flexion force kinetics than observed in our men. Thus, we are confident that our findings of slower kinetics in boys were not due to particularly slow boys, or to an exceptionally explosive men’s group.
Our findings of boys’ slower torque kinetics conform to what is expected of lower utilisation of type-II motor units. The latter is also supported by children’s lower rates of muscle activation (lower mean EMG amplitude in the first 30 ms of both elbow flexion and knee extension contractions), as previously reported (Cohen et al., 2010; Falk, Brunton, et al., 2009; Falk, Usselman, et al., 2009; Mitchell et al., 2011). It may be argued that lower type-II muscle-fibre composition and lower musculo-tendinous stiffness are two other key factors that can also negatively affect torque kinetics in children. The extent to which these factors may account for the observed differences is discussed below.
Muscle-fibre composition
Although the available data is scant and inconsistent, two studies have shown ~10% lower type-II muscle fibre composition in children (Jansson, 1996; Lexell, Sjostrom, Nordlund, & Taylor, 1992). Such compositional differences cannot be directly distinguished from lower utilisation of type-II motor units. The question then is whether a 10% type-II compositional deficit can produce the observed magnitude of torque-kinetics differences. Viitasalo and Komi correlated the time to 30% isometric knee-extension MVC with the percentage composition of the vastus-lateralis type-I fibres in adult male athletes and non-athletes (Viitasalo & Komi, 1978). Regression-line analysis showed only ~4 ms increase in the time to 30% knee-extension MVC per 10% increase in type-I composition. Interestingly, in subsequent data, based on much of the same participant cohort, the authors showed similarly ~4 ms longer EMD for the same 10% increase in type-I fibre composition (Viitasalo & Komi, 1981). Thus, muscle composition appears to similarly affect muscle contractility prior to and immediately following force onset. As our boys–men difference in the time to 30% MVC was 21 ms (Table II), it appears that possible group differences in muscle composition cannot account but for a small fraction of the observed difference in the time to 30% MVC.
Musculo-tendinous stiffness
Musculo-tendinous stiffness reflects the reciprocal of musculo-tendinous elasticity and any slack that might be present in that system at the resting state. For this reason, greater musculo-tendinous stiffness is associated with shorter EMD (Cavanagh & Komi, 1979; Grabiner, 1986; Grosset, Piscione, Lambertz, & Perot, 2009). Likewise, greater musculo-tendinous stiffness is associated with increased rate of force development at the initial stages of torque development (Wilson, Murphy, & Pryor, 1994), including peak rate of force development, which typically occurs markedly earlier than 100 ms (Cohen et al., 2010; Table II). Musculo-tendinous tension increases with the progression of contraction. Therefore, the effect of musculo-tendinous stiffness on rate of force development and force kinetics progressively diminishes. In adults, Andersen and Aagaard (2006) showed that beyond 200 ms, it was maximal torque that accounted for ~80% of rate of force development variance. That is, in the mid to late stages of contraction, muscle composition, musculo-tendinous stiffness, and muscular and neuro-motor determinants of rate of force development could collectively explain no more than ~20% of the observed rate of force development variance. Thus, while musculo-tendinous stiffness may affect the initial stages of contraction, it has little effect on contractile kinetics at the later stages.
Children have lower musculo-tendinous stiffness (Grosset, Mora, Lambertz, & Perot, 2007; Kubo, Kanehisa, Kawakami, & Fukanaga, 2001; Lambertz, Mora, Grosset, & Perot, 2003) and, correspondingly, longer EMD (Cohen et al., 2010; Falk, Brunton, et al., 2009; Falk, Usselman, et al., 2009; Grosset et al., 2005), compared with adults. The longer EMD directly contributes to children’s slower rate of force development and force kinetics (Grosset et al., 2005; Table II). The question then, is whether children’s lower musculo-tendinous stiffness (and thus, longer EMD) can explain the observed difference in time to 30% MVC (~17 ms), unaccounted for by the possibly different muscle composition, as discussed above. Although we did not measure musculo-tendinous stiffness, its effect on this time difference may be estimated.
Our boys’ mean knee extension EMD was ~8 ms longer than the men’s (Table II). Even if this difference was due only to musculo-tendinous stiffness and fully persisted to 30% MVC, it would account for < 50% of the ~17 ms unaccounted-for time-gap. However, since the musculo-tendinous stiffness effect on torque kinetics decreases as tension develops, the portion of the difference in the time to 30% MVC attributable to musculo-tendinous stiffness would also be smaller than 8 ms. Indeed, in young men Vint, McLean, and Harron (2001) demonstrated that when contractions commenced with the muscle already at 25 and 50% MVC, EMD shortened by 2.6 and 8.3 ms, respectively, compared with standard resting EMD. By interpolation, at 30% MVC the shortening would amount to ~4 ms. Similarly, in adults, Cavanagh and Komi (1979) showed that in eccentric knee extension EMD was 4.6 ms shorter than that during isometric contraction, presumably due to pre-stretching in the eccentric mode. In view of these findings, we suggest that the musculo-tendinous stiffness effect on the time to 30% MVC cannot be much greater than ~4–5 ms and as such, could explain <30% of the remaining 17-ms group difference in the time to 30% MVC. Even with EMD differences larger than the observed 8 ms (such as in elbow flexion), a possibly larger residual musculo-tendinous stiffness effect could still explain only little of the difference in the time to 30% MVC.
The above discussion refers to the early stages of contraction (time to 30% MVC), where the boys–men time-differences were relatively small. We could not find corresponding comparative data for later contraction stages. However, despite the greatly diminished musculo-tendinous stiffness effects expected at the later stages of contraction, the boys–men time difference was more than fivefold greater at 80% MVC than at 30% MVC (117 vs. 21 ms, respectively; Table II). Thus, the pattern of expanding rather than diminishing gap, even if partly due to muscle-compositional differences, suggests that an age-related difference in musculo-tendinous stiffness is insufficient to explain the observed magnitude of torque kinetics differences.
The additive effect of possible lower type-II muscle composition (~4 ms) and lower musculo-tendinous stiffness (~4–5 ms), amount to only ~8–9 ms, or ~40% of the observed 21 ms boys–men difference in the time to 30% MVC. Therefore, it is suggested that the combined difference in muscle composition and musculo-tendinous stiffness is insufficient to account for most of the observed boys–men difference in torque kinetics. We suggest then, that much of the observed boys–men torque kinetics difference may be attributed to lower utilisation of type-II motor units.
Upper- vs. lower-body kinetics
Observed boys–men differences were greater for elbow flexion than knee extension in all the measured variables (Figure 1, Table II). This is compatible with the lesser and lighter habitual use of the non-weight-bearing upper extremities and may suggest that, in children, not only maximal strength, but also explosive strength is quite trainable. Calculations for elbow flexion, comparable to those discussed above for knee extension, could not be performed for lack of suitable reference data. However, considering the consistently larger elbow-flexion boys–men differences than those in knee extension, it can reasonably be assumed that the unaccounted-for portion of the boys–men kinetics difference would be as large as shown for knee extension or larger.
Performance implications
The boys’ slower kinetics (Figure 1, Table II), offers insight into children’s characteristic inferiority in speed, force, and power activities (see Blimkie, 1989; Inbar & Bar-Or, 1986; Van Praagh & Dore, 2002 for reviews). In explosive tasks, force-application “windows” are typically only 50–150 ms wide. For example, Nummela, Rusko, and Mero (1994) found ground-contact and propulsive-phase times of 107 and 57 ms, respectively, in adult 400 m sprinters. At 100 ms, our boys had attained only ~30% of their respective knee extension MVC vs. the men’s ~40% (Δ = 23%). Corresponding elbow-flexion values were even more widely separated (Δ = 30%) (Table II). When the corresponding size-normalised torques were compared, the boys–men difference was even greater, 42% (53% in elbow flexion; Table II), due to the base differences in peak torques. Thus, at 100 ms, relative to body mass, the boys were roughly only half as strong as the men.
Body-size effect?
It should be noted that using ratio standards to partition out body-size effects has its limitations in that body mass does not fully explain the effect of body size on muscular performance. On the other hand, in weight-bearing actions, such as discussed above, the muscles need to propel the given body mass. As such, ratio standards using body mass to partial out the effect of body size have often been used in comparing muscular performance between children and adults. Importantly, in examining the kinetics of torque development, body size was partialled out by expressing the attained torque as %MVC. Thus, the age-related differences in kinetics described in this study are beyond differences in body size.
Sex differences
Previous findings in our laboratory showed girls–women differences in normalised peak torque and rate of torque development to be smaller than boys–men differences (Dotan & Falk, 2010; Falk, Brunton, et al., 2009; Falk, Usselman, et al., 2009). Thus, we opted to investigate child–adult force kinetics differences in males first. In view of the above, the generalisation of the present study’s findings to children at large needs to be supported by similar studies in females.
Conclusion
In summary, our findings demonstrate substantially slower torque kinetics in boys vs. men. The difference appears too large to be fully accounted for by known child–adult differences in musculo-tendinous stiffness, possible differences in muscle composition, or by a combination of these factors. We suggest therefore, that differential motor-unit activation pattern should be regarded an important factor in determining child–adult differences in torque kinetics and related differences in muscular performance. Future studies should attempt to better identify the mechanisms responsible for the observed differences, such as the nature of motor-unit recruitment, differential firing rates, or levels of motoneuron excitability. The findings of this study highlight important changes which occur during human growth and development. From a practical perspective, the findings can serve coaches and sports professionals in drawing attention to a fundamental underlying reason for the functional shortcomings they observe and try to affect in dealing with children. This, in turn, may impact the direction taken in training children. The characterisation of optimal training to improve force kinetics and explosive strength in children and the extent of its effectiveness should be the subject of further research.
Acknowledgments
We wish to acknowledge with gratitude the selfless contribution of all our participants and the boys’ parents, as well as the support granted by the Canadian Institute for Health Research.
References
- Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. Journal of Applied Physiology. 2002;93(4):1318–1326. doi: 10.1152/japplphysiol.00283.2002. [DOI] [PubMed] [Google Scholar]
- Andersen LL, Aagaard P. Influence of maximal muscle strength and intrinsic muscle contractile properties on contractile rate of force development. European Journal of Applied Physiology and Occupational Physiology. 2006;96(1):46–52. doi: 10.1007/s00421-005-0070-z. [DOI] [PubMed] [Google Scholar]
- Barry BK, Warman GE, Carson RG. Age-related differences in rapid muscle activation after rate of force development training of the elbow flexors. Experimental Brain Research. 2005;162(1):122–132. doi: 10.1007/s00221-004-2127-3. [DOI] [PubMed] [Google Scholar]
- Blimkie CJ. Age- and sex-associated variation in strength during childhood: Anthropometric, morphologic, neurologic, biomechanical, endocrinologic, genetic, and physical activity correlates. In: Gisolfi CV, editor. Perspectives in exercise science and sports medicine, vol. 2: Youth, exercise and sports. Indianapolis, IN: Benchmark Press; 1989. pp. 99–163. [Google Scholar]
- Calder KM, Gabriel DA. Adaptations during familiarization to resistive exercise. Journal of Electromyography and Kinesiology. 2007;17(3):328–335. doi: 10.1016/j.jelekin.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Cavanagh PR, Komi PV. Electromechanical delay in human skeletal muscle under concentric and eccentric contractions. European Journal of Applied Physiology and Occupational Physiology. 1979;42(3):159–163. doi: 10.1007/BF00431022. [DOI] [PubMed] [Google Scholar]
- Cohen R, Mitchell C, Dotan R, Gabriel D, Klentrou P, Falk B. Do neuromuscular adaptations occur in endurance-trained boys and men? Applied Physiology Nutrition and Metabolism. 2010;35(4):471–479. doi: 10.1139/H10-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ste Croix MB, Deighan MA, Armstrong N. Time to peak torque for knee and elbow extensors and flexors in children, teenagers and adults. Isokinetic Exercise Science. 2004;12:143–148. [Google Scholar]
- Dotan R, Falk B. Task-specific sex differences in muscle fatigue: Is there a common underlying cause? Exercise and Sport Sciences Reviews. 2010;38(1):36. doi: 10.1097/JES.0b013e3181c5ce0c. author reply 37. [DOI] [PubMed] [Google Scholar]
- Dotan R, Mitchell C, Cohen R, Klentrou P, Gabriel D, Falk B. Child-adult differences in muscle activation - a review. Pediatric Exercise Science. 2012;24(1):2–21. doi: 10.1123/pes.24.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk B, Brunton L, Dotan R, Usselman C, Klentrou P, Gabriel D. Muscle strength and contractile kinetics of isometric elbow flexion in girls and women. Pediatric Exercise Science. 2009;21(3):354–364. doi: 10.1123/pes.21.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk B, Usselman C, Dotan R, Brunton L, Klentrou P, Shaw J, Gabriel D. Child-adult differences in muscle strength and activation pattern during isometric elbow flexion and extension. Applied Physiology Nutrition and Metabolism. 2009;34(4):609–615. doi: 10.1139/H09-020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Going SB, Massey BH, Hoshizaki TB, Lohman TG. Maximal voluntary static force production characteristics of skeletal muscle in children 8–11 years of age. Research Quarterly for Exercise and Sport. 1987;58(2):115–123. [Google Scholar]
- Grabiner MD. Bioelectric characteristics of the electromechanical delay preceding concentric contraction. Medicine and Science in Sports and Exercise. 1986;18(1):37–43. [PubMed] [Google Scholar]
- Grosset JF, Mora I, Lambertz D, Perot C. Age-related changes in twitch properties of plantar flexor muscles in prepubertal children. Pediatric Research. 2005;58(5):966–970. doi: 10.1203/01.PDR.0000181375.61935.7D. [DOI] [PubMed] [Google Scholar]
- Grosset JF, Mora I, Lambertz D, Perot C. Changes in stretch reflexes and muscle stiffness with age in prepubescent children. Journal of Applied Physiology. 2007;102(6):2352–2360. doi: 10.1152/japplphysiol.01045.2006. [DOI] [PubMed] [Google Scholar]
- Grosset JF, Mora I, Lambertz D, Perot C. Voluntary activation of the triceps surae in prepubertal children. Journal of Electromyography and Kinesiology. 2008;18(3):455–465. doi: 10.1016/j.jelekin.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Grosset JF, Piscione J, Lambertz D, Perot C. Paired changes in electromechanical delay and musculo-tendinous stiffness after endurance or plyometric training. European Journal of Applied Physiology and Occupational Physiology. 2009;105(1):131–139. doi: 10.1007/s00421-008-0882-8. [DOI] [PubMed] [Google Scholar]
- Gruber M, Gollhofer A. Impact of sensorimotor training on the rate of force development and neural activation. European Journal of Applied Physiology and Occupational Physiology. 2004;92(1–2):98–105. doi: 10.1007/s00421-004-1080-y. [DOI] [PubMed] [Google Scholar]
- Inbar O, Bar-Or O. Anaerobic characteristics in male children and adolescents. Medicine and Science in Sports and Exercise. 1986;18(3):264–269. doi: 10.1249/00005768-198606000-00002. [DOI] [PubMed] [Google Scholar]
- Jansson E. Age-related fiber type changes in human skeletal muscle. In: Maughan RJ, Shirreffs SM, editors. Biochemistry of exercise IX. Champaign, IL: Human Kinetics; 1996. pp. 297–307. [Google Scholar]
- Kanehisa H, Ikegawa S, Tsunoda N, Fukunaga T. Strength and cross-sectional areas of reciprocal muscle groups in the upper arm and thigh during adolescence. International Journal of Sports Medicine. 1995;16(1):54–60. doi: 10.1055/s-2007-972964. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Kawakami Y, Fukanaga T. Growth changes in the elastic properties of human tendon structures. International Journal of Sports Medicine. 2001;22(2):138–143. doi: 10.1055/s-2001-11337. [DOI] [PubMed] [Google Scholar]
- Lambertz D, Mora I, Grosset JF, Perot C. Evaluation of musculotendinous stiffness in prepubertal children and adults, taking into account muscle activity. Journal of Applied Physiology. 2003;95(1):64–72. doi: 10.1152/japplphysiol.00885.2002. [DOI] [PubMed] [Google Scholar]
- Lexell J, Sjostrom M, Nordlund AS, Taylor CC. Growth and development of human muscle: A quantitative morphological study of whole vastus lateralis from childhood to adult age. Muscle and Nerve. 1992;15(3):404–409. doi: 10.1002/mus.880150323. [DOI] [PubMed] [Google Scholar]
- Mitchell C, Cohen R, Dotan R, Gabriel D, Klentrou P, Falk B. Rate of muscle activation in power- and endurance-trained boys. International Journal of Sports Physiology and Performance. 2011;6(1):94–105. doi: 10.1123/ijspp.6.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummela A, Rusko H, Mero A. EMG activities and ground reaction forces during fatigued and nonfatigued sprinting. Medicine and Science in Sports and Exercise. 1994;26(5):605–609. [PubMed] [Google Scholar]
- O’Brien TD, Reeves ND, Baltzopoulos V, Jones DA, Maganaris CN. The effects of agonist and antagonist muscle activation on the knee extension moment-angle relationship in adults and children. European Journal of Applied Physiology and Occupational Physiology. 2009;106(6):849–856. doi: 10.1007/s00421-009-1088-4. [DOI] [PubMed] [Google Scholar]
- O’Brien TD, Reeves ND, Baltzopoulos V, Jones DA, Maganaris CN. In vivo measurements of muscle specific tension in adults and children. Experimental Physiology. 2010;95(1):202–210. doi: 10.1113/expphysiol.2009.048967. [DOI] [PubMed] [Google Scholar]
- Parker DF, Round JM, Sacco P, Jones DA. A cross-sectional survey of upper and lower limb strength in boys and girls during childhood and adolescence. Annals of Human Biology. 1990;17(3):199–211. doi: 10.1080/03014469000000962. [DOI] [PubMed] [Google Scholar]
- Sale DG, Spriet LL. Skeletal muscle function and energy metabolism. In: Bar-Or DRLO, Clarkson PM, editors. Exercise and the female – A life span approach. Vol. 19. Carmel, IN: Cooper; 1996. pp. 289–359. [Google Scholar]
- Slaughter MH, Lohman TG, Boileau BA. Skinfold equations for estimation of body fatness in children and youth. Human Biology. 1988;60:709–723. [PubMed] [Google Scholar]
- Tanner JM. Growth at adolescence. 2. Oxford: Blackwell Scientific; 1962. [Google Scholar]
- Vandervoort AA, McComas AJ. A comparison of the contractile properties of the human gastrocnemius and soleus muscles. European Journal of Applied Physiology and Occupational Physiology. 1983;51(3):435–440. doi: 10.1007/BF00429079. [DOI] [PubMed] [Google Scholar]
- Van Praagh E, Dore E. Short-term muscle power during growth and maturation. Sports Medicine. 2002;32(11):701–728. doi: 10.2165/00007256-200232110-00003. [DOI] [PubMed] [Google Scholar]
- Viitasalo JT, Komi PV. Interrelationships of EMG signal characteristics at different levels of muscle tension and during fatigue. Electromyography and Clinical Neurophysiology. 1978;18(3–4):167–178. [PubMed] [Google Scholar]
- Viitasalo JT, Komi PV. Interrelationships between electromyographic, mechanical, muscle structure and reflex time measurements in man. Acta Physiologica Scandinavica. 1981;111(1):97–103. doi: 10.1111/j.1748-1716.1981.tb06710.x. [DOI] [PubMed] [Google Scholar]
- Vint PF, McLean SP, Harron GM. Electromechanical delay in isometric actions initiated from nonresting levels. Medicine and Science in Sports and Exercise. 2001;33(6):978–983. doi: 10.1097/00005768-200106000-00018. [DOI] [PubMed] [Google Scholar]
- Wilson GJ, Murphy AJ, Pryor JF. Musculo-tendinous stiffness: Its relationship to eccentric, isometric, and concentric performance. Journal of Applied Physiology. 1994;76(6):2714–2719. doi: 10.1152/jappl.1994.76.6.2714. [DOI] [PubMed] [Google Scholar]


