Abstract
Children differ from adults in many muscular performance attributes such as size-normalized strength and power, endurance, fatigability and the recovery from exhaustive exercise, to name just a few. Metabolic attributes, such as glycolytic capacity, substrate utilization, and VO2 kinetics also differ markedly between children and adults. Various factors, such as dimensionality, intramuscular synchronization, agonist-antagonist coactivation, level of volitional activation, or muscle composition, can explain some, but not all of the observed differences. It is hypothesized that, compared with adults, children are substantially less capable of recruiting or fully employing their higher-threshold, type-II motor units. The review presents and evaluates the wealth of information and possible alternative factors in explaining the observations. Although conclusive evidence is still lacking, only this hypothesis of differential motor-unit activation in children and adults, appears capable of accounting for all observed child—adult differences, whether on its own or in conjunction with other factors.
This review aims to substantiate the notion that child–adult differences in muscle activation constitute an underlying factor that best accounts for a comprehensive array of observed performance and metabolic child–adult differences. While other factors may partially explain some of the observed differences, the review will attempt to establish that differential motor-unit activation can solely accounted for these child-adult differences.
Muscle activation has mostly been examined in relation to maximal isometric strength (25,34,37,47–49,67). Children’s maximal volitional muscular force, contractile velocity and muscular power are notoriously lower than adults’, especially in males (see (18) for review). Although absolute differences are largely attributable to body- and muscle-size differences, they clearly persist after body dimensionality has been taken into account (see 18,53,91 for review). Thus, size differences do not suffice to fully account for the observed strength differences.
Agonist-antagonist muscle cocontraction can potentially explain some strength and power differences between children and adults. Simultaneous activation of antagonist muscles detracts from the externally measured force and power output, attributed to the examined agonist muscles. Thus, higher agonist-antagonist cocontraction could partly explain children’s lower strength and power. Some studies have indeed reported greater coactivation in children (40,47), but others have not (10,57). Notably, age-related cocontraction differences have been mostly observed in submaximal, multijoint, or dynamic contractions. However, in maximal isometric contractions, most studies show minimal or no age-related cocontraction differences (34,37,69). Thus, while cocontraction differences may account for some strength and power differences in dynamic contractions, they cannot explain differences in maximal isometric strength.
Substantially lower type-II fiber composition of children’s muscles could explain many of the observed child—adult functional, metabolic, and other differences. For primarily ethical reasons, muscle-composition data of healthy children are scant. The available data are largely derived from clinical biopsies of children with various diseases, or from cadavers. Several studies suggest similar muscle-fiber composition in children and adults (14,21,92), but others support as much as 10% or higher type-I muscle-fiber composition in prepubertal children (54,63). On the other hand, contractile characteristics such as contraction time and half-relaxation time, generally regarded as reflecting muscle composition, have been shown to be similar across age groups in numerous studies (13,26,27,65,72).
Despite their methodologically-limited and conflicting nature, the available data do not allow dismissal of possible, functionally-significant child—adult differences in muscle composition. These compositional differences could fully or partly account for most of the observed functional differences, as outlined in Table 1, but (as will be shown below) cannot explain all observed child—adult differences. Although numerous differences can be explained by multiple factors, it is the purpose of this review to present the body of evidence that supports a single underlying factor which singly, or in conjunction with other factors, explains all observed differences.
Table 1.
Evaluation of Possible Factors in Explaining the Observed Child—Adult Differences in Muscular Performance and Metabolic Response to Exercise
| Observed Differences (Dimensionally normalized; relative to adults) | Factors
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Muscle Anatomy | Muscle Metabolism | Muscle Function | ||||||
|
| ||||||||
| ↓ Relative Muscle Size | ↓ Type-II Muscle-Fiber Composition (*) | ↓ Anaerobic, ↑ Oxidative Profile | ↑ Agonist- Antagonist Co-Activation (**) | ↓ Intra-Muscular Synchronization | ↓ Volitional Muscle Activation | ↓ Type-II Motor-Unit Activation | ||
| Acute Exercise | ↓ Maximal Isometric Strength | √ | √√ | — | -- | √ | √√ | √√ |
| ↓ Short-Term Power | √ | √√ | √√ | √√ | √√ | √√ | √√ | |
| ↓ Force Kinetics | — | √√ | — | √ | √√ | — | √√ | |
| ↓ Force-Velocity Relationships | — | √√ | — | √ | √√ | — | √√ | |
| ↓ Q30 & Mean Power Frequency | — | √√ | — | — | √√ | — | √√ | |
| ↑ Muscle Endurance | — | √√ | √√ | — | √ | √ | √√ | |
| ↑ Recovery | — | √√ | √√ | — | — | √ | √√ | |
| Response to Resistance Training (non-hypertrophic) | — | — | — | √ | √ | √√ | √√ | |
| Metabolic Responses | ↓ Peak [La] | √ | √√ | √√ | — | — | √ | √√ |
| ↑ Lactate Threshold in Blood & Muscle | — | √√ | √√ | — | — | √ | √√ | |
| ↑ Intra-Cellular Pi/PCr Threshold | — | √√ | √√ | — | — | √ | √√ | |
| ↑ Intra-Cellular PCr-Recovery Kinetics | — | √√ | √√ | — | — | √ | √√ | |
| ↑ Fat, ↓ CHO Utilization | — | √√ | √√ | — | — | — | √√ | |
| ↑ VO2 Kinetics | — | √√ | √√ | — | — | — | √√ | |
‘*’ - Assuming the possibility that the type-I ber composition of prepubertal children is higher than that of adults by as much as 10–15%.
‘**’ - Differences in coactivation may account for part of the observed strength child-adult difference in multijoint and dynamic movements, but apparently, not in single-joint, isometric contractions.
‘↓’ - Lower in children
‘↑’ - Higher in children
‘—‘ - Marginal or no effect
‘√’ - Can provide partial explanation
‘√√’ - Can provide full explanation
Hypothesis
Differences in muscle function can be explained by different levels of motor-unit activation. The first to propose such a qualitative difference between children and adults was the late Erling Asmussen, in 1955 (6). Having observed that the increase in strength during childhood and adolescence is more than can be expected from the increase in body size, Asmussen proposed that children do not activate, or use their muscles to the extent typical of adults (5,6). Subsequently, in view of more supporting evidence, this view has been fostered by others (4,13,18,34,35,37,48,72).
The proposal, by Asmussen and subsequent researchers, implies that children’s maximal neuromuscular activation is generally lower. That is, children recruit a smaller percentage of their total motor-unit pool. While we endorse Asmussen’s idea, we propose to modify it with a more specific hypothesis. Namely, that the child—adult muscle functional gap is due to children’s inability to recruit, or fully use, higher-threshold (type II) motor units to the extent typical of adults. Thus, we specifically point to type-II motor-unit utilization as being the compromised portion of children’s muscle function.
Note: “Recruitment” is a dichotomic term, denoting whether motor units are neuromotorically accessed. “Utilization”, on the other hand, denotes the extent or intensity of use of recruited motor-unit, which largely depends on their firing rates.
The Evidence
Unequivocal neuromotor evidence would be needed to conclusively validate the differential motor-unit activation hypothesis. Unequivocal, direct validation would require sufficiently large samples of individual motor-units to be qualitatively monitored for activation, as well as for relative torque and timing patterns. No such evidence, or appropriate technique, currently exists. Presently, therefore, it is necessary to rely on indirect evidence to support the differential muscle activation hypothesis. Table 1 provides an overview of the evidence discussed below.
Volitional vs. Nonvolitional Force Production
The interpolated-twitch technique is frequently used to estimate the degree of motor-unit activation during volitional effort, in relation to total potential activation. During maximal contraction, an electrical stimulus is superimposed onto a muscle or its motor nerve, and the evoked interpolated twitch torque is measured. The difference (or ratio) between volitional and evoked nonvolitional maximal force is an index of the degree of muscle activation (or percentage of the motor-unit pool recruited) during a given volitional contraction. Although the technique is not free of controversy (28,84,90) and its accuracy might be limited, it has produced seemingly valid findings.
In comparative studies, the interpolated-twitch technique has generally shown lower muscle activation in children (13,18,47,72), although the difference was not always statistically significant (50,87). Belanger & McComas (13) were first to apply the technique to children and suggested that children’s motor-unit activation was lower (~94%) than adults’ (~99%). Close scrutiny of this frequently cited study reveals that boys defined as prepubertal were 6–13 yrs old (thus, not necessarily all prepubertal), and that the percentage child—adult difference in motor-unit activation was not statistically significant (p = .20). Examining just the 6–10-yr-old subgroup revealed volitional muscle activation that was much lower than the adults’ (p = .06). Indeed, the authors concluded that “younger children were less able to activate plantar flexor motoneurons than older ones and adolescents” ((13) p.566). Subsequent studies, using different indices of volitional vs. nonvolitional force production, have also demonstrated reduced motor-unit activation in children (18,47,72). Grosset et al. (47), using plantar flexion, demonstrated motor-unit activation deficits progressively decreasing with age in 7–11-yr-old children and in adults. Others demonstrated a similar trend, although the differences did not always reach statistical significance (50,87). A partial explanation for the inconsistent findings may be that the level of muscle activation is muscle-group dependent. For example, Blimkie (18) showed lower motor-unit activation in boys (78%) than in men (95%) in the knee extensors, but not in the elbow flexors.
In two recent studies, O’Brien et al. (69,70) used magnetic (rather than electrical) knee-extensor stimulation to produce an interpolated twitch. While they too found children to activate significantly fewer motor units than adults (~68–75% vs. ~85–87%, respectively), their values were considerably lower than those reported earlier by Belanger and McComas (13; 94% vs. 99%, respectively), who used electrical stimulation of the same muscles. Thus, while the true percentage of volitional motor-unit activation in both children and adults remains unclear, the interpolated twitch technique nevertheless depicts a clearly lower overall activation level in children.
These findings do not address the question of which motor-units are less activated in children. However, according to the size principle (51), it is the slower, low-threshold (type I) motor units which are recruited first. To increase force output, faster, higher-threshold (type II) motor units are recruited by increasing motoneuron firing frequency. It might be argued, therefore, that lower overall motor-unit activation, as described above, reflects lesser activation of high-threshold motor-units, since those are the ones typically activated last.
It may also be argued that children’s lower volitional/nonvolitional force ratio is a result of lesser motor-unit synchronization during volitional contraction. While this possibility cannot be dismissed, it has never been examined and is only speculative at present. Moreover, substantial synchronization differences are incongruent with other observed child—adult differences (see “Lactate response to maximal short-term exercise”, below).
Finally, it is important to underscore the fact that regardless of the exact reason, children’s lower volitional muscle activation cannot be attributed to differential muscle-fiber composition.
EMG-Derived Evidence
Much of the evidence supporting child—adult differences in muscle activation has been derived using surface EMG. Surface EMG can readily detect electrical activity within the muscle. However, the nature and amplitude of the detected signal are highly affected by muscle size and the filtering effect of the intervening skin and subcutaneous tissues (68). These factors differ between individuals, and particularly between children and adults, thus precluding direct quantitative comparisons. Some of these issues can be skirted by the use of rate- or timing-related parameters, as described below. However, while all of this EMG-related evidence is congruent with the differential motor-unit activation hypothesis, it cannot distinguish it from differences in muscle composition.
Rate of EMG Rise
The initial slope of the rectified surface-EMG trace has been thought of as reflecting the initial rate of muscle activation (25,34,37,42,44). Greater involvement of type-II motor units is expected to manifest itself by greater EMG activity immediately following neural stimulation. An index describing this is the Q30, defined as the integral (area under the curve) of the rectified EMG activity during the first 30ms. In adults, performing 400 explosive elbow-flexion repetitions over four training sessions (i.e., time period sufficient for learning, but not substantial structural or metabolic adaptations), Gabriel & Boucher (42) showed Q30 to increase with training (learning) and to be related to the speed of movement. Presumably, faster movements were attained as the subjects learned to activate their type-II motor units faster, or in a more synchronous manner. Although there are some unanswered reservations as to the validity of the Q30 index (25), its theoretical and commonly observed relationship with velocity supports its use as an indicator of the activation level of faster, higher-threshold motor-units.
Similarly, we have shown both Q30 and the rate of force development (RFD) to be significantly higher in young male gymnasts (typically trained for explosive muscular performance), than in young swimmers (mostly endurance-trained), or nonathletes (67). It may be argued that such differences reflect gymnasts’ preselection rather than true training effect. However, the observed differences were limited to the highly trained knee extensor muscles, but could not be shown in the marginally-trained knee flexors.
Along these lines, we have recently demonstrated consistently lower Q30 and RFD values, in boys compared with men (25,34). Collectively, these results suggest that children have lower initial rate of muscle activation, as reflected by the lower Q30 values. This could be due to differential motor-unit recruitment, but also to lesser motor-unit synchronization, or differential rate-coding of the higher-threshold (type II) motor units (41). Whatever the reason, children’s lower Q30 appears strongly related to their lower RFD, which in turn explains their compromised explosive power compared with adults (see below).
Electromechanical Delay (EMD)
The detection of force onset in fast, maximal muscle contractions would be expected to be slightly delayed by lesser activation of the muscle in general, and of fast-twitch motor units, in particular. Indeed, longer EMD (force-onset latency relative to EMG onset) has been consistently shown in children compared with adults (4,25,34,37), seemingly supporting the notion of children’s lower muscle activation. However, EMD has been shown to be largely and inversely related to musculo-tendinous stiffness (23) which is considerably lower in children (46,60,62). Thus, the typically reported 10–20-ms EMD differences far exceed those expected from differential motor-unit activation. Consequently, while the direction of the available data conforms to the hypothesis, it is impossible to tease out and argue the purported effect of differential motor-unit involvement.
Mean Power Frequency (MPF)
The EMG signal is characterized by different signal magnitudes and frequencies. The power spectrum density describes the relative distribution of EMG frequencies and resembles a Gaussian curve, skewed toward the lower frequencies. The MPF is the weighted mean of that distribution. It may be affected by motoneuron firing rates as well as by the nature and frequency of the resulting motor-unit action potentials (56).
In adults, MPF has been shown to be affected by fiber-type distribution (58). Similarly, it has been argued that the MPF is related to the relative utilization of type-II motor units, with higher utilization rate resulting in a right shift (to higher frequencies). As type-II motor units fatigue faster than type-I units, a decrease (left shift) in MPF is typically observed in fatiguing exercise (56). In adults, this left-shift has been shown to be greater in persons with higher type-II fiber composition (61). Interestingly, an increase in MPF was demonstrated in adults following 3 explosive-training sessions (41), suggesting that subjects were able to recruit more higher-threshold motor units after such training. Halin et al. (49) showed a greater MPF-decrease during a 25-s elbow-flexion fatigue test, in 10-yr-old power-trained male gymnasts (~21%), compared with age-matched controls (~12%). It has been suggested that this shift toward the lower-frequencies, is due to selective accumulation of metabolites in the more fatigable type-II motor units (2,41,48).
Halin et al. (48) found higher MPF during maximal contraction in men compared with boys. The authors suggested that the difference was due to a higher composition or greater utilization of type-II motor units. Moreover, during a 28-s fatigue test, the men demonstrated a much greater MPF decrease (~50%) than the boys (~16%), suggesting a greater drop-off of type-II motor units. More recently, Armatas et al. (2) examined MPF during maximal knee extension in boys and men. Although no differences could be shown during maximal contraction, MPF decreased substantially more in the men than in the boys during an isometric fatiguing task. The authors attributed the difference to a possibly greater lactic-acid accumulation in the men (not measured), a phenomenon expected more of type-II than of type-I motor units (see below).
Contractile and Power-Related Differences
Differential motor-unit activation ought to have implications that extend beyond maximal force. Because type-II motor units are faster contracting than their type-I counterparts, these differences should be observable in various parameters of whole-muscle performance, as described below. While conforming to the differential motor-unit activation hypothesis, this class of differences does not exclude differential muscle composition as an alternative or additional explanation.
Rate of Force Development (RFD)
Lower RFD should be expected if children use their fast-twitch motor units to a lesser extent than adults. Indeed, lower RFD during maximal isometric contractions has been repeatedly shown in children (4,25,34,37,45). A similar outcome could be expected if motor-unit contractility was inherently deficient in children. However, based on observations of similar twitch contractile characteristics (contraction time, half-relaxation time) in children and adults (45,72), the latter possibility seems unlikely.
Maximal force is heavily dependent on muscle mass. Fully correcting for child—adult muscle-mass difference is not trivial. Muscle mass is difficult to quantify and children’s muscle- to body-mass ratio, is variably lower than in adults. Nevertheless, when RFD is normalized to the muscle’s maximal force, it is found independent of both absolute and relative muscle mass. Thus corrected, children’s maximal RFD is still lower than that of adults (4,25,34,37).
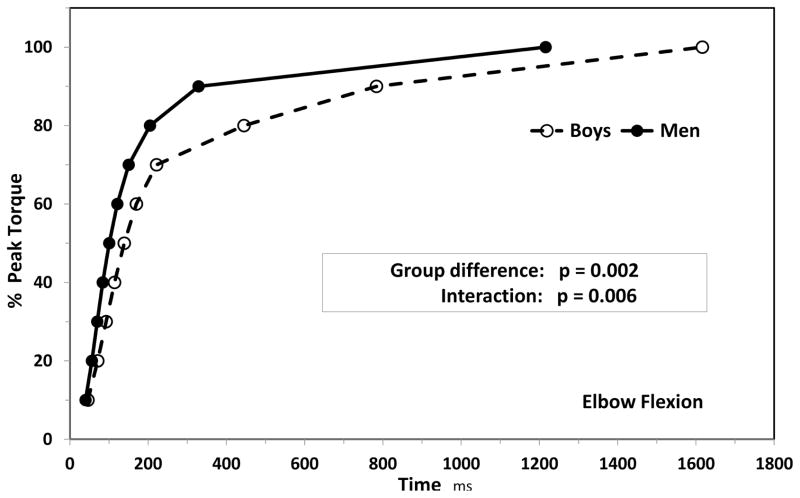
Lower RFD values may be a reflection of shorter muscles, or fewer sarcomeres in series (smaller potential shortening). At the same time, lower RFD can also be caused by lower musculo-tendinous stiffness (more elastic muscle-tendon complex). Shorter muscles are more limited in their potential elongation under load, effectively meaning lower elasticity and increased stiffness. As with electromechanical delay (see above), children’s lower musculo-tendinous stiffness (46,60,62) likely has a greater negative effect on RFD than their shorter muscles. Musculo-tendinous stiffness, in turn, has been shown to affect only the early phases of force development (<~50ms) and not the later phases (~200ms; 1, 17). We have recently demonstrated that after correcting for maximal torque, boys’ torque kinetics were still considerably slower than the men’s at both the early and late contraction phases (29; Figure 1). After ~100ms of isometric knee extension, boys attained only ~40% of their maximal torque, while men have already reached ~60%. Viewed differently, boys required >400ms to reach 80% of their maximal torque, whereas men needed <200ms.
Figure 1.
Child—adult differences in torque kinetics. Data from Dotan et al. (29).
A less synchronous motor-unit activation might also lower children’s peak RFD. Motor-unit synchronization has been shown to be higher in power-trained than in untrained adults (39), but has not been examined in children. A lower degree of synchronization could result in a lower RFD during the early contraction phase. However, as mentioned earlier, significant child—adult synchronization differences are not likely because children’s torque kinetics were shown compromised at both the early and late phases of torque development (29), and because it is incongruent with the observed lactate response to maximal short-term exercise (see below).
Muscular Endurance or Fatigability
Employing less type-II motor units (i.e., more type-I), would mean greater muscular endurance, or lower muscle fatigability in repeated contractions. Indeed, lower muscle fatigability has been shown in children during dynamic (2,59,73,96) as well as sustained (isometric) contractions (48). Armatas et al. (2) recently demonstrated that during repeated maximal knee extensions, men’s performance significantly deteriorated after completing ~20% of total repetitions, while boys comparably fatigued only after completing ~70%. This difference cannot be explained by differences in muscle size or maximal strength, since both task and fatigue were defined relative to maximal strength. Aside from muscle composition, these findings could also be explained by a differential metabolic profile (77; see Summary and Table 1). However, at the same time they fully conform to expectations from a smaller proportion of activated type-II motor units in children’s muscles.
Force-Velocity Curve
Hill’s classic force-velocity curve (52), demonstrates the inverse, curvilinear relationships between contractile force and velocity. This relationship has been repeatedly demonstrated in adults, but only to a very limited extent in children. Asai & Aoki (4), had young boys and adults perform horizontal elbow flexions, as rapidly as possible. Forearm velocity was measured at 90° elbow angle. Repeated testing under various resistance loads produced a series of force-dependent contraction velocities used to create child- and adult-specific force-velocity curves. To facilitate group comparisons, force was normalized to maximal strength (100%) and velocity to forearm length. Both men and boys exhibited Hill’s characteristic curvilinear inverse force-velocity relationship. However, at any given percentage of normalized force level, the boys’ contraction velocity was ~35% lower than the men’s.
Barrett & Harrison (7) also depict lower velocity at any force (torque) level in boys vs. men. However, for reasons that have not been elucidated, their force-velocity relationship, whether absolute or normalized, is highly incongruent with Hill’s original (52) and others’ subsequent findings (e.g., 4). That is, in spite of a wide velocity range (30–300°·s−1), children’s torque does not appreciably fall off with increasing velocity, while in adults, it drops off only slightly. Consequently, and contrary to its well-established behavior, power does not peak within this velocity range. Therefore, the apparent child—adult normalized-power identity is difficult to interpret and the conclusions hard to accept.
High-Velocity Isokinetic Torque
Maximal isometric or low-velocity force production depends on both, slow- and fast-twitch motor-unit recruitment. High-velocity force production or high power production, on the other hand, must heavily depend on the extent of fast-twitch motor-unit utilization (24). Presumably, this may be due to a significant portion of the type-I motor-unit pool either not being recruited in the first place, or more likely, being unable to effectively contribute to the fast-contracting muscle.
Seger & Thorstensson (83) longitudinally examined low- to high-velocity torque production in ~11-yr-old boys and girls until they reached ~16 years of age. Concentric and eccentric quadriceps torque was tested on an isokinetic dynamometer at 45, 90, and 180°·s−1. Among the boys, aside from the concentric, low-velocity (45°·s−1) values, all normalized torque values were significantly higher at age 16 compared with 11. More importantly, the increase in torque was larger with increasing contractile velocity. Girls exhibited similar trends, although differences did not reach statistical significance. The latter could be due to the smaller sample size. However, we too found girl—woman differences (34) to be much smaller than the corresponding ones for males (37), suggesting sex differences in muscle functional development. These findings support the idea that fast-twitch motor units are being increasingly employed as children, boys in particular, go from prepubescence to adolescence.
Maximal, Short-Term Power Output
Children’s size-normalized power output in short-term, supra-maximal exercise (e.g., Wingate Anaerobic Test), is significantly lower than that of adults (91). These differences persist even after child—adult differences in relative muscle-mass proportions are corrected for. A seeming exception is a study by Beneke et al. (15) who showed Wingate-Test size-normalized power output of 16.3-yr-old adolescents to be <13% higher than that of 10.8-yr-old children. The difference, smaller than any previously reported, was claimed to be fully accounted for by corresponding differences in relative muscle mass. However, the test’s protocol and the assumptions used for muscle-mass estimation, suggest significant underestimation of the adolescents’ power output as well as the children’s active muscle-mass.
Bedu et al. (11) also studied Wingate-Anaerobic-Test power output in children and adolescents (7 & 15-yr-old). However, body-mass-normalized power output of the adolescents was found to be roughly twice that of the children. While other studies reported smaller differences, they nevertheless suggest that neither relative muscle-mass differences, nor children’s possibly greater agonist-antagonist coactivation in cyclical, multijoint contractions (40), are sufficiently large to account for the observed differences in external power output. It appears therefore justified to suggest that children’s lower motor-unit activation must partly account for the observed child—adult differences in short-term power output.
Recovery From High-Intensity, Short-Term Exercise
Children have been repeatedly shown to recover considerably faster than adults from high-intensity, short-term exercise (35). In conjunction with children’s much lower lactate response to such exercise (see below), their relative inability to recruit or use their type-II, glycolytic motor units appears as the most likely reason for their faster recovery. Namely, children’s lower utilization of type-II motor units results in lower power and lactate production and thus, less to recover from (35).
Metabolic Evidence
Different muscle substrate and enzyme activity levels have been shown in children and adults (e.g., lower glycogen and creatine phosphokinase; higher isocitrate dehydrogenase and malate dehydrogenase, in children; 16, 31, 55). The findings depict higher oxidative and lower anaerobic/glycolytic capacities in children. Such differences have typically been attributed to lagging maturation of children’s glycolytic metabolism (20,76,77). Alternatively, these may partly or fully reflect muscle-compositional differences. However, while neither of these possibilities can be dismissed, we suggest that children’s different metabolic profile may not be the underlying cause of their lower contractile and anaerobic capacity but rather the result of their under-utilization of type-II, glycolytic motor units. Under-utilization of type-II muscle fibers would under-develop these fibers’ glycolytic capacity and relative size (cross-sectional area). At the same time, children’s type-I fibers would be relatively over-used and could be expected to manifest enhanced oxidative capacity and possibly relative hypertrophy, as well. Indeed, contrary to adults, children’s type-I fibers have been shown to be similar or even larger in diameter than their type-II counterparts (21,92).
Below are several examples of apparent child—adult metabolic differences previously attributed to children’s “metabolic immaturity”, particularly of the anaerobic pathways. We suggest these differences to be largely due to differential motor-unit activation pattern.
Lactate Response to Maximal Short-Term Exercise
While child—adult differences in short-term power output support the idea of children’s lesser overall muscle activation, differences in blood lactate responses to such exertions shed light on the issue of differential motor-unit activation. We compared 30-s Wingate- Anaerobic-Test performance and blood lactate responses of prepubertal boys and young men (30). Whereas men were 24% more powerful per unit body mass, their lactate response was 52% greater. Similar findings were reported by others (76). If the boys’ lower power output was only due to generally lower motor-unit recruitment or utilization, then the corresponding percentage difference in the lactate response would be expected to be similar in magnitude to the difference in power output. The fact that the men—boys lactate difference was more than twice as large as the corresponding power output difference, strongly suggests that men relied more heavily on glycolytic, type-II motor units.
This example serves well to put the issues of agonist-antagonist coactivation and intramuscular (motor-unit) synchronization into proper perspective. If children had substantially higher coactivation or lower intramuscular synchronization, their external power output would be compromised by the internal inefficiency. This would imply that for a given (external) power output, children would need to produce higher internal power and should, therefore, show at least similar and likely higher lactate responses compared with adults. This, however, diametrically contradicts the above (30) and other findings (78,96). Thus, children’s possibly higher coactivation and lower intramuscular synchronization are incongruent with their markedly lower lactate response. Taken together then, this strongly supports the differential motor-unit activation hypothesis.
Blood Lactate and Intracellular Thresholds
Several studies have shown that, in adolescents and adults, blood lactate threshold, or related criteria, occurs at a lower percentage of maximal or peak VO2 than in children (85,88). These observations are completely in line with children’s smaller lactate response to exercise (see above) and are likely determined by the same factors. Namely, lower glycolytic capacity, whether due to immaturity or lower utilization of type-II motor units, would result in delayed onset of blood lactate rise.
Blood lactate responses are metabolic whole-body summations of numerous lactate-producing and lactate-consuming muscles and organs and may thus obscure muscle-level physiology. Using muscle biopsies, Eriksson et al. (32) found child—adult lactate-response differences in the muscle, similar to those observed in the blood. Due to ethical limitations, these findings have been difficult to replicate. However, more recently, the advent of nuclear magnetic resonance spectroscopy (MRS) has provided researchers with a noninvasive intramuscular peek. Willcocks et al. (94) employed 31P MRS during progressive exercise of the quadriceps muscle. They found the intracellular threshold of the Pi/PCr (inorganic phosphate to phosphocreatine) ratio to occur at ~20% higher body-mass-normalized power output in boys than in men. Although, by themselves, these findings cannot distinguish differential motor-unit activation from differences in the muscle’s metabolic profile, they provide tissue-level corroboration of children’s lower lactate response.
Phosphocreatine (PCr) Recovery
Faster PCr recovery following intense muscular exercise is regarded as an indication of more oxidative / less glycolytic metabolic profile, or a greater reliance on oxidative motor units (3). Following intense exercise, PCr was shown to recover considerably faster in children (particularly boys) than in adults (particularly men; 89, 94).
Fat vs. Carbohydrate Metabolism
If children indeed employ a lower proportion of type-II motor units during exercise, they should be expected to demonstrate lower carbohydrate and higher fat metabolism. Riddell et al. (79) found that during graded exercise, fat oxidation rates peaked at ~30% VO2peak in young men, but only at 55% in 11–12-yr-old prepubertal boys. Retested yearly for 3 years, the boys’ fat oxidation rates occurred at progressively lower percentage of VO2peak, approaching the men’s values.
VO2 Kinetics
Phase II of the pulmonary VO2 kinetics, following exercise onset, is thought of as closely reflecting the oxygen uptake kinetics of the working muscles (9). At any relative exercise intensity, faster phase-II kinetics would be expected in individuals with higher relative aerobic power (maxVO2) (74), muscle oxidative capacity, or type-I muscle-fiber composition (8). If children do not use type-II motor units to the same extent as adults, their muscles would be characterized by higher functional composition of type-I muscle fibers and would be expected to have faster muscle VO2 kinetics and consequently demonstrate faster phase-II pulmonary VO2 kinetics. Indeed, in comparison with adults or adolescents of comparable or even somewhat superior aerobic power, children have been repeatedly shown to attain a given percentage of the ultimate VO2 response faster than adults (38,86,95).
Thus, children’s lower lactate response, faster PCr and VO2 kinetics and greater reliance on fat oxidation provide equivocal support to the differential metabolic profile and differential motor-unit activation hypotheses, as well as to differential muscle composition (Table 1).
Training Response
Following resistance training, children have been shown to proportionately improve their strength to an extent similar to that observed in adults (80,81). However, while strength gains in adults are typically closely tied to muscular hypertrophy, only a limited hypertrophic response has been found in adolescents (64), and none could generally be shown in prepubertal children (see (12,81) for review).
While training-induced hypertrophy in prepubertal children cannot be dismissed (66,93), it is generally agreed that, when present, it is exceedingly smaller than that observed in adults and far too small to account for the observed strength gains (12). Thus, training-induced strength gains in prepubertal children must be due to increased muscle activation (12,19,36,82). Indeed, Ramsay et al. (75) found a 13–17% trend toward increased motor-unit activation in prepubertal boys, following 20 weeks of resistance training, while Ozmun et al. (71) found a 16.8% increase in integrated EMG activity following 8 weeks of training.
This difference in the mechanism of training-induced strength-gain between children and adults is nicely supported by the findings of Faigenbaum et al. (33). The authors examined strength gains following 8 weeks of low-repetition, heavy-resistance vs. high-repetition, moderate-resistance training protocols in prepubertal children. Both training protocols resulted in increased strength. However, while in adults, greater strength gains would be expected in the low-repetition, heavy-resistance protocol, the children’s strength gains were similar or greater in the high-repetition, moderate-resistance protocol. This appears to suggest that, in children, the higher resistance could not significantly access the higher-threshold motor units, rendering that form of training less efficient than the lower-resistance, higher-repetition protocol that simply provided a more extended training stimulus.
Adults also exhibit neuro-motor adaptations to resistance training, but these are largely confined to the first few weeks of training (43). Muscle hypertrophy, likely dependent on androgen levels, typically accounts for most of the strength gains beyond the initial weeks (43). It could be expected that due to their low androgen levels, children would be limited in their training-induced gains. However, the fact that children exhibit proportionately similar or even greater (81) strength gains than adults, strongly suggests that they have considerably larger untapped motor-unit recruitment and utilization capacity. Thus, children’s nonhypertrophic but adult-comparable strength gains constitute very strong evidence in support of the differential motor-unit activation hypothesis, or a general activation deficit, and cannot be explained by differential muscle composition.
Summary and Conclusions
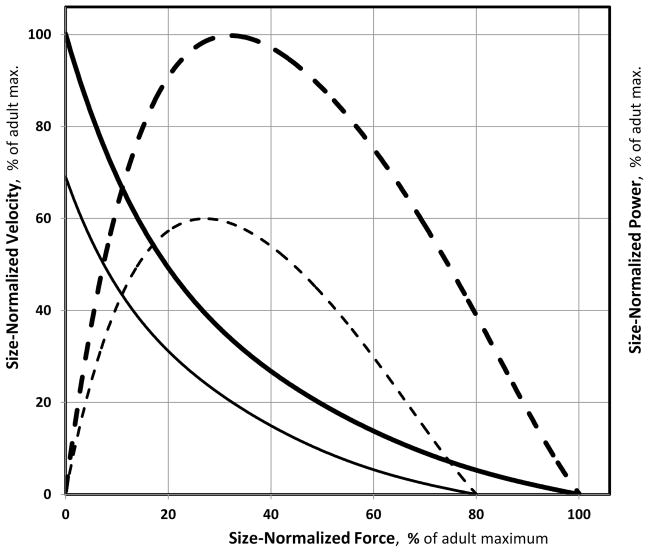
Table 1 summarizes the evidence presented in this review, indicating the likelihood of acknowledged muscle functional factors of accounting for the various known child—adult functional and metabolic differences. While some factors, notably muscle composition, can account for a considerable portion of the observed differences, only the differential motor-unit activation hypothesis can account for all of them. Figure 2 provides a schematic graphic illustration of the proposed child—adult muscle-contractile differences, as would be manifested in force-velocity and force- power relationships. This depiction is based on the evidence presented in this review, and notably that of Asai & Aoki (4). As illustrated in Figure 2, children’s size-normalized maximal force, velocity, and power are all lower than the respective adult values. Moreover, the age-related difference in maximal velocity is greater than the respective difference in maximal force. As power is a product of force and velocity, children’s maximal power is further compromised, compared with adults, than either force or velocity.
Figure 2.
Schematic representation of suggested child—adult differences in muscle contractility stemming from differential motor-unit activation. Comparisons of size-normalized muscular force, velocity (solid lines), and power (dashed lines) relationships are shown. Children’s values are presented as percentages of adults’ maximal values (100%).
Numerous factors may be involved in many of the reviewed child—adult differences. As shown in Table 1, these differences can largely or fully be explained by three main factors: muscle fiber composition, metabolic profile, and motor-unit activation. In some cases (e.g., children’s lower muscular power or greater endurance), the relative contribution of these factors cannot be untangled and the observed differences can be explained by any one, two, or all three factors. In other cases, it may be possible to dismiss metabolic profile differences, but neither of the other two factors (e.g., children’s lower instantaneous force or RFD). Differential muscle composition is impossible to dismiss in explaining all but one observation, namely, the differential response to resistance training. The nature of this response can only be explained by children’s lower level of volitional muscle activation.
The lower overall muscle activation, as suggested by volitional vs. nonvolitional force production, cannot be directly distinguished from specific lower activation of type-II motor units. This is mainly where direct, conclusive evidence is still lacking. However, the size principle of motor-unit recruitment suggests that overall lower activation is more reflective of lower type-II motor unit activation. The disproportionately-low lactate response to maximal short-term exercise is another strong if indirect evidence that higher-threshold, type-II motor units are less activated in children.
It is likely premature to speculate on the exact mechanism responsible for children’s postulated lower capacity to employ type-II motor units. A conceptual approach might involve the neuromotor impulse generation in the motor cortex. Possibly, there is a low ceiling of motoneuron impulse frequency during early development, which gradually rises with maturation. A low impulse frequency would preselect type-I motor-unit activation to the exclusion or curtailment of type-II motor-units. Contrary to what is known in adults, children’s type-II muscle-fibers were shown to be similar or even smaller in diameter / cross-sectional area than type-I fibers (21,92). This strongly suggests extensive under-use of type-II motor-units during prepubescence. Moreover, these findings may be related to those of lower type-II muscle-fiber composition during early childhood (54,63). That is, a low ceiling of neuromotor impulse frequency may in effect produce type-I phenotypes of fibers that are destined to become type-II. Indeed, early studies involving modification of neural activation in young animals indicated that it is the nature of neural activation which determines the phenotypic and contractile properties of the motor unit (22). A gradual increase in motoneuron impulse frequency during maturation could thus be the factor responsible not only for increasing utilization of type-II motor units, but for the transformation of type-I to type-II fibers during growth (54,63) as well. This then would explain findings of higher type-I muscle-fiber composition in children (54,63), and their associated effects on the muscle’s metabolic profile.
It must be reemphasized that although extensive, the presented body of evidence is inconclusive. In the future, innovative electro-myographical techniques and technology may provide novel and more refined evidence. However, the inherent technical limitations and the ethical constraints associated with pediatric testing appear to preclude the attainment of conclusive evidence from this venue. Breakthrough evidence could very well come from the fast-developing area of imaging, in general, and nuclear magnetic resonance imaging and spectroscopy, in particular. Imaging both the muscle and the motor-cortex during exercise will likely cast new light on child—adult differences in the control of motor-unit and whole muscle activation.
Acknowledgments
The authors would like to thank Phil Gardiner and Christos Kotzamanidis for their thorough and critical review of earlier drafts of this paper. Authors’ data reported in this review is largely based on original research supported in part by the Canadian Institute for Health Research and the North-American Society for Pediatric Exercise Medicine.
Contributor Information
Raffy Dotan, Faculty of Applied Health Sciences, Brock University, St. Catharines, Ontario, Canada.
Cameron Mitchell, Dept. of Kinesiology, McMaster University, Hamilton, Ontario, Canada.
Rotem Cohen, Ribstein Center for Sports Medicine and Research, Wingate Institute, Netanya, Israel.
Panagiota Klentrou, Dept. of Physical Education and Kinesiology, Faculty of Applied Health Sciences, Brock University, St. Catharines, Ontario, Canada.
David Gabriel, Dept. of Physical Education and Kinesiology, Faculty of Applied Health Sciences, Brock University, St. Catharines, Ontario, Canada.
Bareket Falk, Dept. of Physical Education and Kinesiology, Faculty of Applied Health Sciences, Brock University, St. Catharines, Ontario, Canada.
References
- 1.Andersen LL, Aagaard P. Influence of maximal muscle strength and intrinsic muscle contractile properties on contractile rate of force development. Eur J Appl Physiol. 2006;96:46–52. doi: 10.1007/s00421-005-0070-z. [DOI] [PubMed] [Google Scholar]
- 2.Armatas V, Bassa E, Patikas D, Kitsas I, Zangelidis G, Kotzamanidis C. Neuromuscular differences between men and prepubescent boys during a peak isometric knee extension intermittent fatigue test. Pediatr Exerc Sci. 2010;22:205–217. doi: 10.1123/pes.22.2.205. [DOI] [PubMed] [Google Scholar]
- 3.Arnold DL, Matthews PM, Radda GK. Metabolic recovery after exercise and the assessment of mitochondrial function in vivo in human skeletal muscle by means of 31P NMR. Magn Reson Med. 1984;1:307–315. doi: 10.1002/mrm.1910010303. [DOI] [PubMed] [Google Scholar]
- 4.Asai H, Aoki J. Force development of dynamic and static contractions in children and adults. Int J Sports Med. 1996;17:170–174. doi: 10.1055/s-2007-972827. [DOI] [PubMed] [Google Scholar]
- 5.Asmussen E. Growth in muscular strength and power. In: Rarick G, editor. Physical Activity, Human Growth and Development. London: Academic Press; 1973. pp. 60–79. [Google Scholar]
- 6.Asmussen E, Heeboll-Nielsen K. A dimensional analysis of physical performance and growth in boys. J Appl Physiol. 1955;7:593–603. doi: 10.1152/jappl.1955.7.6.593. [DOI] [PubMed] [Google Scholar]
- 7.Barrett U, Harrison D. Comparing muscle function of children and adults: Effects of scaling for muscle size. Pediatr Exerc Sci. 2002;14:369–376. [Google Scholar]
- 8.Barstow TJ, Jones AM, Nguyen PH, Casaburi R. Influence of muscle fiber type and pedal frequency on oxygen uptake kinetics of heavy exercise. J Appl Physiol. 1996;81:1642–1650. doi: 10.1152/jappl.1996.81.4.1642. [DOI] [PubMed] [Google Scholar]
- 9.Barstow TJ, Lamarra N, Whipp BJ. Modulation of muscle and pulmonary O2 uptakes by circulatory dynamics during exercise. J Appl Physiol. 1990;68:979–989. doi: 10.1152/jappl.1990.68.3.979. [DOI] [PubMed] [Google Scholar]
- 10.Bassa E, Patias D, Kotzamanidis C. Activation of antagonist knee muscles during isokinetic efforts in prepubertal and adult males. Pediatr Exerc Sci. 2005;17:65–75. [Google Scholar]
- 11.Bedu M, Fellmann N, Spielvogel H, Falgairette G, Van Praagh E, Coudert J. Force-velocity and 30-s Wingate tests in boys at high and low altitudes. J Appl Physiol. 1991;70:1031–1037. doi: 10.1152/jappl.1991.70.3.1031. [DOI] [PubMed] [Google Scholar]
- 12.Behm DG, Faigenbaum AD, Falk B, Klentrou P. Canadian Society for Exercise Physiology position paper: resistance training in children and adolescents. Appl Physiol Nutr Metab. 2008;33:547–561. doi: 10.1139/H08-020. [DOI] [PubMed] [Google Scholar]
- 13.Belanger AY, McComas AJ. Contractile properties of human skeletal muscle in childhood and adolescence. Eur J Appl Physiol Occup Physiol. 1989;58:563–567. doi: 10.1007/BF00418500. [DOI] [PubMed] [Google Scholar]
- 14.Bell RD, MacDougall JD, Billeter R, Howald H. Muscle fiber types and morphometric analysis of skeletal msucle in six-year-old children. Med Sci Sports Exerc. 1980;12:28–31. [PubMed] [Google Scholar]
- 15.Beneke R, Hutler M, Leithauser RM. Anaerobic performance and metabolism in boys and male adolescents. Eur J Appl Physiol. 2007;101:671–677. doi: 10.1007/s00421-007-0546-0. [DOI] [PubMed] [Google Scholar]
- 16.Berg A, Kim SS, Keul J. Skeletal muscle enzyme activities in healthy young subjects. Int J Sports Med. 1986;7:236–239. doi: 10.1055/s-2008-1025766. [DOI] [PubMed] [Google Scholar]
- 17.Blazevich AJ, Cannavan D, Horne S, Coleman DR, Aagaard P. Changes in muscle force-length properties affect the early rise of force in vivo. Muscle Nerve. 2009;39:512–520. doi: 10.1002/mus.21259. [DOI] [PubMed] [Google Scholar]
- 18.Blimkie CJ. Age- and sex-associated variation in strength during childhood: Anthropometric, morphologic, neurologic, biomechanical, endocrinologic, genetic, and physical activity correlates. In: Gisolfi CV, editor. Perspectives in Exercise Science and Sports Medicine, Vol. 2: Youth, Exercise and Sports. Vol. 2. Indianapolis, IN: Benchmark Press; 1989. pp. 99–163. [Google Scholar]
- 19.Blimkie CJ. Resistance training during pre- and early puberty: efficacy, trainability, mechanisms, and persistence. Can J Sport Sci. 1992;17:264–279. [PubMed] [Google Scholar]
- 20.Boisseau N, Delamarche P. Metabolic and hormonal responses to exercise in children and adolescents. Sports Med. 2000;30:405–422. doi: 10.2165/00007256-200030060-00003. [DOI] [PubMed] [Google Scholar]
- 21.Brooke MH, Engel WK. The histographic analysis of human muscle biopsies with regard to fiber types. 4. Children’s biopsies. Neurology. 1969;19:591–605. doi: 10.1212/wnl.19.6.591. [DOI] [PubMed] [Google Scholar]
- 22.Buller AJ, Eccles JC, Eccles RM. Differentiation of fast and slow muscles in the cat hind limb. J Physiol. 1960;150:399–416. doi: 10.1113/jphysiol.1960.sp006394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavanagh PR, Komi PV. Electromechanical delay in human skeletal muscle under concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol. 1979;42:159–163. doi: 10.1007/BF00431022. [DOI] [PubMed] [Google Scholar]
- 24.Claflin DR, Faulkner JA. Shortening velocity extrapolated to zero load and unloaded shortening velocity of whole rat skeletal muscle. J Physiol. 1985;359:357–363. doi: 10.1113/jphysiol.1985.sp015589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen R, Mitchell C, Dotan R, Gabriel D, Klentrou P, Falk B. Do neuromuscular adaptations occur in endurance-trained boys and men? Appl Physiol Nutr Metab. 2010;35:471–479. doi: 10.1139/H10-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies CT. Strength and mechanical properties of muscle in children and young adults. Scand J Sports Sci. 1985;7:11–15. [Google Scholar]
- 27.Davies CT, White MJ, Young K. Muscle function in children. Eur J Appl Physiol Occup Physiol. 1983;52:111–114. doi: 10.1007/BF00429036. [DOI] [PubMed] [Google Scholar]
- 28.de Haan A, Gerrits KH, de Ruiter CJ. Counterpoint: the interpolated twitch does not provide a valid measure of the voluntary activation of muscle. J Appl Physiol. 2009;107:355–357. doi: 10.1152/japplphysiol.91220.2008a. discussion 357–358. [DOI] [PubMed] [Google Scholar]
- 29.Dotan R, Mitchell C, Cohen R, Gabriel DA, Klentrou P, Falk B. The First Wingate Congress of Exercise & Sport Sciences. Wingate Institute; Israel: 2010. Child-Adult Differences in the Kinetics of Force Development; p. 114. [Google Scholar]
- 30.Dotan R, Ohana S, Bediz C, Falk B. Blood lactate disappearance dynamics in boys and men following exercise of similar and dissimilar peak-lactate concentrations. J Pediatr Endocrinol Metab. 2003;16:419–429. doi: 10.1515/jpem.2003.16.3.419. [DOI] [PubMed] [Google Scholar]
- 31.Eriksson BO, Gollnick PD, Saltin B. Muscle metabolism and enzyme activities after training in boys 11–13 years old. Acta Physiol Scand. 1973;87:485–497. doi: 10.1111/j.1748-1716.1973.tb05415.x. [DOI] [PubMed] [Google Scholar]
- 32.Eriksson BO, Persson B, Thorell JI. The effects of repeated prolonged exercise on plasma growth hormone, insulin, glucose, free fatty acids, glycerol, lactate and -hydroxybutyric acid in 13-year old boys and in adults. Acta Paediatr Scand Suppl. 1971;217:142–146. doi: 10.1111/j.1651-2227.1971.tb05715.x. [DOI] [PubMed] [Google Scholar]
- 33.Faigenbaum AD, Westcott WL, Loud RL, Long C. The effects of different resistance training protocols on muscular strength and endurance development in children. Pediatrics. 1999;104:e5. doi: 10.1542/peds.104.1.e5. [DOI] [PubMed] [Google Scholar]
- 34.Falk B, Brunton L, Dotan R, Usselman C, Klentrou P, Gabriel D. Muscle strength and contractile kinetics of isometric elbow flexion in girls and women. Pediatr Exerc Sci. 2009;21:354–364. doi: 10.1123/pes.21.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falk B, Dotan R. Child-adult differences in the recovery from high-intensity exercise. Exerc Sport Sci Rev. 2006;34:107–112. doi: 10.1249/00003677-200607000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Falk B, Tenenbaum G. The effectiveness of resistance training in children. A meta-analysis. Sports Med. 1996;22:176–186. doi: 10.2165/00007256-199622030-00004. [DOI] [PubMed] [Google Scholar]
- 37.Falk B, Usselman C, Dotan R, et al. Child-adult differences in muscle strength and activation pattern during isometric elbow flexion and extension. Appl Physiol Nutr Metab. 2009;34:609–615. doi: 10.1139/H09-020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fawkner SG, Armstrong N. Sex differences in the oxygen uptake kinetic response to heavy-intensity exercise in prepubertal children. Eur J Appl Physiol. 2004;93:210–216. doi: 10.1007/s00421-004-1201-7. [DOI] [PubMed] [Google Scholar]
- 39.Fling BW, Christie A, Kamen G. Motor unit synchronization in FDI and biceps brachii muscles of strength-trained males. J Electromyogr Kinesiol. 2009;19:800–809. doi: 10.1016/j.jelekin.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Frost G, Dowling J, Dyson K, Bar-Or O. Cocontraction in three age groups of children during treadmill locomotion. J Electromyogr Kinesiol. 1997;7:179–186. doi: 10.1016/s1050-6411(97)84626-3. [DOI] [PubMed] [Google Scholar]
- 41.Gabriel DA, Basford JR, An K. Training-related changes in the maximal rate of torque development and EMG activity. J Electromyogr Kinesiol. 2001;11:123–129. doi: 10.1016/s1050-6411(00)00041-9. [DOI] [PubMed] [Google Scholar]
- 42.Gabriel DA, Boucher JP. Practicing a maximal performance task: a cooperative strategy for muscle activity. Res Q Exerc Sport. 2000;71:217–228. doi: 10.1080/02701367.2000.10608902. [DOI] [PubMed] [Google Scholar]
- 43.Gabriel DA, Kamen G, Frost G. Neural adaptations to resistive exercise: mechanisms and recommendations for training practices. Sports Med. 2006;36:133–149. doi: 10.2165/00007256-200636020-00004. [DOI] [PubMed] [Google Scholar]
- 44.Gottlieb GL, Corcos DM, Agarwal GC. Organizing principles for single-joint movements. I. A speed-insensitive strategy. J Neurophysiol. 1989;62:342–357. doi: 10.1152/jn.1989.62.2.342. [DOI] [PubMed] [Google Scholar]
- 45.Grosset JF, Mora I, Lambertz D, Perot C. Age-related changes in twitch properties of plantar flexor muscles in prepubertal children. Pediatr Res. 2005;58:966–970. doi: 10.1203/01.PDR.0000181375.61935.7D. [DOI] [PubMed] [Google Scholar]
- 46.Grosset JF, Mora I, Lambertz D, Perot C. Changes in stretch reflexes and muscle stiffness with age in prepubescent children. J Appl Physiol. 2007;102:2352–2360. doi: 10.1152/japplphysiol.01045.2006. [DOI] [PubMed] [Google Scholar]
- 47.Grosset JF, Mora I, Lambertz D, Perot C. Voluntary activation of the triceps surae in prepubertal children. J Electromyogr Kinesiol. 2008;18:455–465. doi: 10.1016/j.jelekin.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Halin R, Germain P, Bercier S, Kapitaniak B, Buttelli O. Neuromuscular response of young boys versus men during sustained maximal contraction. Med Sci Sports Exerc. 2003;35:1042–1048. doi: 10.1249/01.MSS.0000069407.02648.47. [DOI] [PubMed] [Google Scholar]
- 49.Halin R, Germain P, Buttelli O, Kapitaniak B. Differences in strength and surface electromyogram characteristics between pre-pubertal gymnasts and untrained boys during brief and maintained maximal isometric voluntary contractions. Eur J Appl Physiol. 2002;87:409–415. doi: 10.1007/s00421-002-0643-z. [DOI] [PubMed] [Google Scholar]
- 50.Hatzikotoulas K, Patikas D, Bassa E, Hadjileontiadis L, Koutedakis Y, Kotzamanidis C. Submaximal fatigue and recovery in boys and men. Int J Sports Med. 2009;30:741–746. doi: 10.1055/s-0029-1224171. [DOI] [PubMed] [Google Scholar]
- 51.Henneman E, Somjen G, Carpenter DO. Functional Significance of Cell Size in Spinal Motoneurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- 52.Hill AV. The mechanics of active muscle. Proc R Soc Lond B Biol Sci. 1953;141:104–117. doi: 10.1098/rspb.1953.0027. [DOI] [PubMed] [Google Scholar]
- 53.Inbar O, Bar-Or O. Anaerobic characteristics in male children and adolescents. Med Sci Sports Exerc. 1986;18:264–269. doi: 10.1249/00005768-198606000-00002. [DOI] [PubMed] [Google Scholar]
- 54.Jansson E. Age-related fiber type changes in human skeletal muscle. In: Maughan RJ, Shirreffs SM, editors. Biochemistry of Exercise IX. Champaign, IL: Human Kinetics; 1996. pp. 297–307. [Google Scholar]
- 55.Kaczor JJ, Ziolkowski W, Popinigis J, Tarnopolsky MA. Anaerobic and aerobic enzyme activities in human skeletal muscle from children and adults. Pediatr Res. 2005;57:331–335. doi: 10.1203/01.PDR.0000150799.77094.DE. [DOI] [PubMed] [Google Scholar]
- 56.Kamen G, Gabriel D. Essentials of Electromyography. Champaign, IL: Human Kinetics; 2010. pp. 137–138.pp. 160–162. [Google Scholar]
- 57.Kellis E, V, Unnithan B. Co-activation of vastus lateralis and biceps femoris muscles in pubertal children and adults. Eur J Appl Physiol Occup Physiol. 1999;79:504–511. doi: 10.1007/s004210050545. [DOI] [PubMed] [Google Scholar]
- 58.Komi PV, Tesch P. EMG frequency spectrum, muscle structure, and fatigue during dynamic contractions in man. Eur J Appl Physiol Occup Physiol. 1979;42:41–50. doi: 10.1007/BF00421103. [DOI] [PubMed] [Google Scholar]
- 59.Kotzamanidou M, Michailidis I, Hatzikotoulas K, Hasani ABE, Kotzamanidis C. Differences in recovery process between adult and prepubertal males after a maximal isokinetic fatigue task. Isokinet Exerc Sci. 2005;13:261–266. [Google Scholar]
- 60.Kubo K, Kanehisa H, Kawakami Y, Fukanaga T. Growth changes in the elastic properties of human tendon structures. Int J Sports Med. 2001;22:138–143. doi: 10.1055/s-2001-11337. [DOI] [PubMed] [Google Scholar]
- 61.Kupa EJ, Roy SH, Kandarian SC, De Luca CJ. Effects of muscle fiber type and size on EMG median frequency and conduction velocity. J Appl Physiol. 1995;79:23–32. doi: 10.1152/jappl.1995.79.1.23. [DOI] [PubMed] [Google Scholar]
- 62.Lambertz D, Mora I, Grosset JF, Perot C. Evaluation of musculotendinous stiffness in prepubertal children and adults, taking into account muscle activity. J Appl Physiol. 2003;95:64–72. doi: 10.1152/japplphysiol.00885.2002. [DOI] [PubMed] [Google Scholar]
- 63.Lexell J, Sjostrom M, Nordlund AS, Taylor CC. Growth and development of human muscle: a quantitative morphological study of whole vastus lateralis from childhood to adult age. Muscle Nerve. 1992;15:404–409. doi: 10.1002/mus.880150323. [DOI] [PubMed] [Google Scholar]
- 64.Lillegard WA, Brown EW, Wilson DJ, Henderson R, Lewis E. Efficacy of strength training in prepubescent to early postpubescent males and females: effects of gender and maturity. Pediatr Rehabil. 1997;1:147–157. doi: 10.3109/17518429709167353. [DOI] [PubMed] [Google Scholar]
- 65.McComas AJ, Sica RE, Petito F. Muscle strength in boys of different ages. J Neurol Neurosurg Psychiatry. 1973;36:171–173. doi: 10.1136/jnnp.36.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mersch F, Stoboy H. Strength training and muscle hypertrophy in children. In: Oseid S, Carlson KH, editors. International Series on Sports Sciences. Children and Exercise XIII. Champaign, IL: Human Kinetics; 1989. pp. 165–192. [Google Scholar]
- 67.Mitchell C, Cohen R, Dotan R, Gabriel D, Klentrou N, Falk B. Rate of muscle activation in power- and endurance-trained boys. Int J Sports Physiol Perform. doi: 10.1123/ijspp.6.1.94. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nordander C, Willner J, Hansson GA, et al. Influence of the subcutaneous fat layer, as measured by ultrasound, skinfold calipers and BMI, on the EMG amplitude. Eur J Appl Physiol. 2003;89:514–519. doi: 10.1007/s00421-003-0819-1. [DOI] [PubMed] [Google Scholar]
- 69.O’Brien TD, Reeves ND, Baltzopoulos V, Jones DA, Maganaris CN. The effects of agonist and antagonist muscle activation on the knee extension moment-angle relationship in adults and children. Eur J Appl Physiol. 2009;106:849–856. doi: 10.1007/s00421-009-1088-4. [DOI] [PubMed] [Google Scholar]
- 70.O’Brien TD, Reeves ND, Baltzopoulos V, Jones DA, Maganaris CN. In vivo measurements of muscle specific tension in adults and children. Exp Physiol. 2010;95:202–210. doi: 10.1113/expphysiol.2009.048967. [DOI] [PubMed] [Google Scholar]
- 71.Ozmun JC, Mikesky AE, Surburg PR. Neuromuscular adaptations following prepubescent strength training. Med Sci Sports Exerc. 1994;26:510–514. [PubMed] [Google Scholar]
- 72.Paasuke M, Ereline J, Gapeyeva H. Twitch contraction properties of plantar flexor muscles in pre- and post-pubertal boys and men. Eur J Appl Physiol. 2000;82:459–464. doi: 10.1007/s004210000236. [DOI] [PubMed] [Google Scholar]
- 73.Paraschos I, Hassani A, Bassa E, Hatzikotoulas K, Patikas D, Kotzamanidis C. Fatigue differences between adults and prepubertal males. Int J Sports Med. 2007;28:958–963. doi: 10.1055/s-2007-964984. [DOI] [PubMed] [Google Scholar]
- 74.Phillips SM, Green HJ, MacDonald MJ, Hughson RL. Progressive effect of endurance training on VO2 kinetics at the onset of submaximal exercise. J Appl Physiol. 1995;79:1914–1920. doi: 10.1152/jappl.1995.79.6.1914. [DOI] [PubMed] [Google Scholar]
- 75.Ramsay JA, Blimkie CJ, Smith K, Garner S, MacDougall JD, Sale DG. Strength training effects in prepubescent boys. Med Sci Sports Exerc. 1990;22:605–614. doi: 10.1249/00005768-199010000-00011. [DOI] [PubMed] [Google Scholar]
- 76.Ratel S, Bedu M, Hennegrave A, Dore E, Duche P. Effects of age and recovery duration on peak power output during repeated cycling sprints. Int J Sports Med. 2002;23:397–402. doi: 10.1055/s-2002-33737. [DOI] [PubMed] [Google Scholar]
- 77.Ratel S, Tonson A, Le Fur Y, Cozzone P, Bendahan D. Comparative analysis of skeletal muscle oxidative capacity in children and adults: a 31P-MRS study. Appl Physiol Nutr Metab. 2008;33:720–727. doi: 10.1139/H08-039. [DOI] [PubMed] [Google Scholar]
- 78.Ratel S, Williams CA, Oliver J, Armstrong N. Effects of age and mode of exercise on power output profiles during repeated sprints. Eur J Appl Physiol. 2004;92:204–210. doi: 10.1007/s00421-004-1081-x. [DOI] [PubMed] [Google Scholar]
- 79.Riddell MC, V, Jamnik K, Iscoe KE, Timmons BW, Gledhill N. Fat oxidation rate and the exercise intensity that elicits maximal fat oxidation decreases with pubertal status in young male subjects. J Appl Physiol. 2008;105:742–748. doi: 10.1152/japplphysiol.01256.2007. [DOI] [PubMed] [Google Scholar]
- 80.Sale DG. Neural adaptation to resistance training. Med Sci Sports Exerc. 1988;20:S135–S145. doi: 10.1249/00005768-198810001-00009. [DOI] [PubMed] [Google Scholar]
- 81.Sale DG. Strength training in children. In: Gisolfi CV, Lamb DR, editors. Youth, Exercise and Sports. Vol. 2. Carmel, IN: Benchmark Press; 1989. pp. 165–222. [Google Scholar]
- 82.Sale DG, Spriet LL. Skeletal muscle function and energy metabolism. In: Bar-Or O, Lamb DR, Clarkson PM, editors. Exercise and the Female – A Life Span Approach. Vol. 19. Carmel, IN: Cooper Publishing Group; 1996. pp. 289–359. [Google Scholar]
- 83.Seger JY, Thorstensson A. Muscle strength and electromyogram in boys and girls followed through puberty. Eur J Appl Physiol. 2000;81:54–61. doi: 10.1007/PL00013797. [DOI] [PubMed] [Google Scholar]
- 84.Shield A, Zhou S. Assessing voluntary muscle activation with the twitch interpolation technique. Sports Med. 2004;34:253–267. doi: 10.2165/00007256-200434040-00005. [DOI] [PubMed] [Google Scholar]
- 85.Simon G, Berg A, Simon-Alt A, Keul J. Determination of the anaerobic threshold depending on age and performance potential. Dtsch Z Sportmed. 1981;32:7–14. [Google Scholar]
- 86.Springer C, Barstow TJ, Wasserman K, Cooper DM. Oxygen uptake and heart rate responses during hypoxic exercise in children and adults. Med Sci Sports Exerc. 1991;23:71–79. [PubMed] [Google Scholar]
- 87.Streckis V, Skurvydas A, Ratkevicius A. Children are more susceptible to central fatigue than adults. Muscle Nerve. 2007;36:357–363. doi: 10.1002/mus.20816. [DOI] [PubMed] [Google Scholar]
- 88.Tanaka H, Shindo M. Running velocity at blood lactate threshold of boys aged 6–15 years compared with untrained and trained young males. Int J Sports Med. 1985;6:90–94. doi: 10.1055/s-2008-1025820. [DOI] [PubMed] [Google Scholar]
- 89.Taylor DJ, Kemp GJ, Thompson CH, Radda GK. Ageing: effects on oxidative function of skeletal muscle in vivo. Mol Cell Biochem. 1997;174:321–324. [PubMed] [Google Scholar]
- 90.Taylor JL. Point: the interpolated twitch does/does not provide a valid measure of the voluntary activation of muscle. J Appl Physiol. 2009;107:354–355. doi: 10.1152/japplphysiol.91220.2008. [DOI] [PubMed] [Google Scholar]
- 91.Van Praagh E, Dore E. Short-term muscle power during growth and maturation. Sports Med. 2002;32:701–728. doi: 10.2165/00007256-200232110-00003. [DOI] [PubMed] [Google Scholar]
- 92.Vogler C, Bove KE. Morphology of skeletal muscle in children. An assessment of normal growth and differentiation. Arch Pathol Lab Med. 1985;109:238–242. [PubMed] [Google Scholar]
- 93.von Fukunaga T, Funato K, Ikegawa S. The effects of resistance training on muscle area and strength in prepubescent age. Ann Physiol Anthropol. 1992;11:357–364. doi: 10.2114/ahs1983.11.357. [DOI] [PubMed] [Google Scholar]
- 94.Willcocks RJ, Williams CA, Barker AR, Fulford J, Armstrong N. Age- and sex-related differences in muscle phosphocreatine and oxygenation kinetics during high-intensity exercise in adolescents and adults. NMR Biomed. 2010;23:569–577. doi: 10.1002/nbm.1495. [DOI] [PubMed] [Google Scholar]
- 95.Williams CA, Carter H, Jones AM, Doust JH. Oxygen uptake kinetics during treadmill running in boys and men. J Appl Physiol. 2001;90:1700–1706. doi: 10.1152/jappl.2001.90.5.1700. [DOI] [PubMed] [Google Scholar]
- 96.Zafeiridis A, Dalamitros A, Dipla K, Manou V, Galanis N, Kellis S. Recovery during high-intensity intermittent anaerobic exercise in boys, teens, and men. Med Sci Sports Exerc. 2005;37:505–512. doi: 10.1249/01.mss.0000155394.76722.01. [DOI] [PubMed] [Google Scholar]