Abstract
Previous studies in adults have demonstrated power athletes as having greater muscle force and muscle activation than nonathletes. Findings on endurance athletes are scarce and inconsistent. No comparable data on child athletes exist.
Purpose
This study compared peak torque (Tq), peak rate of torque development (RTD), and rate of muscle activation (EMG rise, Q30), in isometric knee extension (KE) and flexion (KF), in pre- and early-pubertal power- and endurance-trained boys vs minimally active nonathletes.
Methods
Nine gymnasts, 12 swimmers, and 18 nonathletes (7–12 y), performed fast, maximal isometric KE and KF. Values for Tq, RTD, electromechanical delay (EMD), and Q30 were calculated from averaged torque and surface EMG traces.
Results
No group differences were observed in Tq, normalized for muscle cross-sectional area. The Tq-normalized KE RTD was highest in power athletes (6.2 ± 1.9, 4.7 ± 1.2, 5.0 ± 1.5 N·m·s−1, for power, endurance, and nonathletes, respectively), whereas no group differences were observed for KF. The KE Q30 was significantly greater in power athletes, both in absolute terms and relative to peak EMG amplitude (9.8 ± 7.0, 5.9 ± 4.2, 4.4 ± 2.2 mV·ms and 1.7 ± 0.8, 1.1 ± 0.6, 0.9 ± 0.5 (mV·ms)/(mV) for power, endurance, and nonathletes, respectively), with no group differences in KF. The KE EMD tended to be shorter (P = .07) in power athletes during KE (71.0 ± 24.1, 87.8 ± 18.0, 88.4 ± 27.8 ms, for power, endurance, and nonathletes), with no group differences in KF.
Conclusions
Pre- and early-pubertal power athletes have enhanced rate of muscle activation in specifically trained muscles compared with controls or endurance athletes, suggesting that specific training can result in muscle activation-pattern changes before the onset of puberty.
Keywords: athletes, children, EMG, exercise, strength, training
Youth participation in organized sports has increased in the past decade.1 High level sports participation requires the development of muscle endurance, strength and power. While much research has focused on the effects of training and sports participation on the cardiovascular system and muscle metabolism,2,3 very little is known about training effects on children’s neuromuscular system.
Power training has been demonstrated to result in enhanced muscle strength and explosive power in adults.4 In children, power or high resistance training has been shown to result in enhanced maximal muscle strength5 but the possible effects on explosive strength are unknown. The effects of endurance training on muscle performance have been investigated to a limited extent in adults, demonstrating either no change6 or some enhancement in muscle maximal and explosive strength.7,8 No comparable data are available for children.
The training-induced enhancements in muscle performance in adults have been explained by morphological adaptations, mainly hypertrophy, and by neurological adaptations.5,9 Both, power and resistance training have been shown to result in distinct hypertrophy, as well as in neural adaptations in adults.9,10 In children on the other hand, while there are no studies which directly focused on the effects of power training, most studies examining the effects of resistance training demonstrate no hypertrophy as a result of power or high resistance training,5,11 implicating neural adaptations.12,13 However, the nature of these neural adaptations is unclear. Endurance training may affect muscle morphology in young adults by generally changing the myosin heavy-chain isoform expression from fast to slow.14 Whereas endurance training has been shown to be associated with some neural adaptations in adults,8 to our knowledge, the effects of such training on muscle morphology or neural response has not been examined in children.
Therefore, the purpose of this study was to compare muscle and neuro-motor performance in young male athletes who engage in power or endurance training. It was hypothesized that power trained boys will demonstrate greater explosive strength and rate of muscle activation compared with endurance-trained boys and nonathletes.
Materials and Methods
Subjects
All testing was reviewed and approved by the Brock University Research Ethics Board. The participants and parents/guardians were given a thorough explanation of the study’s purpose, measurement procedures, benefits and potential risks or discomforts and signed an informed consent form before testing.
Eighteen untrained boys, 12 competitive nonspecialist swimmers, and 9 gymnasts volunteered to participate in the study. All subjects were at the pre- or early-pubertal stages. Athletes were recruited from local clubs in Southern Ontario and Northern New-York. Swimmers had been training for 2.5 ± 0.9 y at a current rate of 8.5 ± 3.6 h·wk−1. Seven swimmers competed at the provincial level and five at the regional level. Four swimmers performed twice weekly dry land training, which included push-ups, sit-ups and flexibility, while three swimmers performed twice weekly resistance training for 45 min with light weights. One swimmer played soccer, one swimmer played hockey and two played baseball (2 h·wk−1) on top of their swimming program. Gymnasts trained 16.7 ± 0.5 h·wk−1 and had been training for 4.6 ± 3.3 y. Three gymnasts competed at the national level and six at the provincial or regional level. One gymnast played soccer (2 h·wk−1) on top of gymnastics. The gymnasts’ nominal training volume was larger than the swimmers’. Typically, however, swimmers actually swim 80–90% of their workout time (rest intervals excluded) most of which is continuous or short-rest interval sets. Gymnasts, on the other hand, engage in actual exercises for roughly 30% of their gym time, most of which are only 5–30 s long and characterized by explosive force and power. The control subjects were recruited from local camps and social clubs and were involved in organized sports no more than 2 h·wk−1. Subject characteristics are presented in Table 1.
Table 1.
Subject characteristics
| Variable | Control | Endurance | Power |
|---|---|---|---|
| Age (y) | 9.9 ±1.3 | 10.7 ±0.7* | 9.3 ±1.3 |
| Years from PHV | −3.9 ±0.9 | −3.2 ±0.6# | −3.7 ±0.9 |
| Height (cm) | 140 ±9 | 146 ±7* | 135 ±9 |
| Mass (kg) | 34.9 ±8.1 | 41.5 ±12.6* | 29.0 ±5.1 |
| Quadriceps CSA (cm2) | 6.04 ±1.71 | 7.13 ±1.84 | 6.25 ±1.58 |
| Body fat (%) | 17.8 ±6.3 | 20.1 ±12.0 | 13.6 ±3.2 |
| Lean body mass (kg) | 28.3 ±4.8 | 31.9 ±5.2* | 25.0 ±4.2 |
Note. Data are displayed as mean ± SD. PHV = peak height velocity; CSA = cross-sectional area.
Significantly different from power-trained group (P < .05).
Significantly different from control group (P < .05).
Procedures
Subjects made two visits to the laboratory, 2–7 d apart. On their first visit, subjects filled out medical and physical activity/training questionnaires and self-assessed their pubertal stage.15 During the first visit, anthropometric measurements were taken (see Anthropometry, below), and B-Mode ultrasound was used to assess quadriceps and hamstrings muscle depth (diameter), from which muscle cross sectional area (CSA) was estimated. The subjects were then familiarized with the testing apparatus and procedures by performing several maximal voluntary isometric contractions (MVC) for each of the test modes (flexion and extension).
On their second visit, subjects performed a light specific warm-up and 10 maximal repetitions in each mode in a counterbalanced order. Warm-up consisted of 5 isometric contractions of increasing intensity. Explicit instructions were given to contract as hard and as fast as possible. Following 2–3 min rest, maximal repetitions were performed in two sets of 5 MVCs, 30 s apart, with a 2 min of rest between sets. Subjects were asked to refrain from intense physical activity for 48 h before the experimental session.
Anthropometry
Height and sitting height were measured using a stadiometer (Length Boards, Ellard Instrumentation, Ltd., Monroe, WA) and recorded to the nearest 0.1 cm. Body mass was measured using a digital scale (Zenith) and recorded to the nearest 0.1 kg. Standing and sitting heights were used to estimate the age of peak height velocity (PHV), according to Mirwald et al.16 Triceps and subscapular skinfold thicknesses were measured in triplicate using Harpenden calipers (British Indicators, Herts, England) and the median value at each site was used for further calculation. These skinfold thickness were used to estimate adiposity (percentage of body fat), using age- and maturity-specific equations, as described by Slaughter et al.17
To estimate muscle diameter, muscle depth was measured using B-mode ultrasound (System5, GE Vingmed, Horten, Norway), with 5 MHz linear-array probe. A water-soluble transmission gel was applied over the scan head to improve the ultrasound image. The probe was placed over the bellies of the rectus femoris and biceps femoris while subjects were supine or prone, respectively, at 50% of the distance between the greater trochanter and the lateral femoral condyle. Images were captured, stored and analyzed off-line. The muscle depth was defined as the distance from the bone-muscle interface to the beginning of the muscle-subcutaneous fat interface. Muscle cross sectional area was estimated by assuming a circular shape, using muscle depth as the diameter (area = πD/2). All measurements were performed by one researcher, with intraobserver reliability (n = 10) of 0.98 and 0.97 for the rectus and biceps femoris, respectively.
Pubertal stage was self-assessed, based on secondary sexual characteristics (pubic hair).15 Children were provided with drawings of the different stages and asked to circle the most applicable drawing.
Muscle Strength
All strength testing was performed on a Biodex system 3 dynamometer. Subjects were positioned in the Biodex chair so that their back was against the backrest and thighs were supported by the chair for their entire length. The subjects were secured onto the chair to minimize movement or activation of untoward body segments and muscles. Two straps over the shoulders and crossing over the chest, a waist strap, and a strap crossing the leg being tested at mid-thigh were used to secure subjects to the chair. The knee joint was positioned at 90° and the dynamometer’s axis of rotation was aligned with lateral femoral condyle. The dynamometer attachment arm was adjusted so that it reached the subject’s ankle and was tightly fastened to the lower leg with a padded Velcro strap. All positioning were performed during rest. The subjects were instructed to contract “as fast and forcefully as possible” from a relaxed state, so as to maximize torque and rate of torque development (RTD). They were verbally encouraged throughout the testing session. In addition, all subjects had online visual feedback of their torque signal on a PC screen. Isometric contractions were chosen to minimize antagonist coactivation,18 so as to facilitate attribution of torque measurements to agonist action. The intrasession reliability for maximal strength reliability coefficient (ICC2,1) was 0.95.
Electrode Placement
The skin under the electrode was shaved to remove hair and dead skin, then treated with an abrasive gel and cleaned with isopropyl alcohol. Electrodes were placed parallel to the direction of the muscle fibers, 1 cm away from the estimated motor point, on the medial aspect of the vastus lateralis and on the belly of the biceps femoris. Electrode placement sites were determined visually during a resisted isometric contraction. The reference electrode was placed over the middle of the left clavicle. The electrodes were dabbed with conductive gel and fastened in place with an adhesive strip.
Recording Apparatus
The EMG signal was recorded for both the agonist and antagonist muscles using a Delsys (Boston, MA) Bagnoli EMG system and bipolar DE-2.1 differential surface electrodes (1 mm × 10 mm Ag electrodes, interelectrode distance 10 mm). The signal was then amplified 1000 times by a Bagnoli amplifier (frequency response range 20–450 Hz, CMRR 92 dB). An analog-to-digital (A-to-D) card was used to transfer the signal to a personal computer where it was sampled at 1000 Hz, using Delsys EMGWorks acquisition software. The torque signal from the Biodex was scaled to each subject’s maximal torque and transformed with the A-to-D.
Signal Averaging
The raw torque trace was smoothed off-line using a 10 Hz low-pass 2nd-order Butterworth filter. This was done to minimize nonphysiologic noise introduced by the Biodex. For each trial, the first derivative of the torque with respect to time (RTD) was taken and stored in a separate channel. Of the 10 trials of each test mode for each subject, the best five were averaged and analyzed (see criteria below). Trials were rejected if they did not have a stable baseline, if subjects contracted the antagonist muscle group, or if trace artifacts were visible. For the trials that were not immediately rejected, a composite score was calculated as follows: For each trial, the peak RTD and peak torque were calculated as a percentage of the highest RTD or torque, respectively, attained by that subject in all the trials. The two percentage values were added to give the composite score. The five trials with the highest composite scores were used in subsequent analysis. Torque onset was defined as the first point in time where the RTD reached above 5 standard deviations of the mean of the baseline for at least 10 ms. This point was confirmed visually and adjusted manually if needed. An average waveform was created by aligning the five highest scored trials on their force onset and then averaging them point by point. The average waveform consisted of 400 ms before the force onset and 3000 ms afterward. The agonist and antagonist raw EMG signals were rectified and low-pass filtered with a 50 Hz 2nd-order Butterworth filter. The rectified EMG signals were averaged to create average waveforms, time-locked to the torque average waveform.
Torque and EMG Variables
All EMG and torque variables were assessed based on the average waveforms described above. The onset of EMG activity was set at the point in time where the signal first increased 5 standard deviations above the mean of the baseline.19 Peak RTD was defined as the peak of the 1st derivative of the force signal.20 The area under the rectified agonist EMG curve for the first 30 ms after the onset of EMG activity was defined as the Q30 and was used to assess the rate of muscle activation.21,22 Q30 was normalized to peak EMG amplitude to account for differences in signal amplitude due to factors such as thickness of subcutaneous tissue or variations in electrode placement. The intrasession reliability for agonist EMG amplitude reliability coefficient (ICC2,1) was 0.65. Electromechanical delay (EMD) was defined as the time lapse between the onsets of EMG and torque generation and calculated in the agonist muscle. The EMG amplitude during peak torque was defined as the average EMG value of the agonist and antagonist muscles for 250 ms, centered on the time of peak force. Coactivation was calculated by dividing the average EMG value obtained for the antagonist by the value obtained when that muscle was acting as an agonist.
Statistical Analysis
All data are displayed as mean ± SD. All statistical analyses were conducted using SPSS 16.0 (SPSS Inc., Chicago, IL) with α set a P ≤ .05. A χ2 analysis was used to compare the pubertal stage distributions. Differences between means were compared using a one-way ANOVA, with training-group as the between-subject factor. An LSD post hoc test was used to assess pairwise differences when a main effect was found.
Results
Subject characteristics appear in Table 1. While all subjects were pre- or early pubertal, differences in chronological age and predicted age of peak height velocity (somatic maturity) were observed between groups. Namely, the endurance-trained boys were significantly older than the gymnasts and more mature than the control group. Furthermore, whereas no significant differences in body size were observed between controls and endurance-trained boys, the latter were significantly taller and heavier than the gymnasts. Nevertheless, no significant differences were observed between groups in muscle CSA.
Peak knee extension torque was significantly greater in endurance-trained boys compared with controls and gymnasts (25.9% and 33.1%, respectively. Peak torque: 96.9 ± 32.5, 71.8 ± 24.0, and 64.8 ± 16.9 N·m, for endurance-trained control and gymnasts, respectively; P < .05). However, when corrected for muscle CSA, no group differences were observed (5.1 ± 1.1, 4.8 ± 1.9, and 3.8 ± 0.9 N·m·cm−2, respectively, P = .20). A similar pattern was observed during knee flexion (Absolute torque: 47.5 ± 16.7, 35.3 ± 12.3 and 32.4 ± 8.2 N·m, for endurance-trained, control and gymnasts, respectively; P < .05; Relative torque: 2.5 ± 0.6, 2.2 ± 0.7, and 2.0 ± 0.5 N·m·cm−2, respectively).
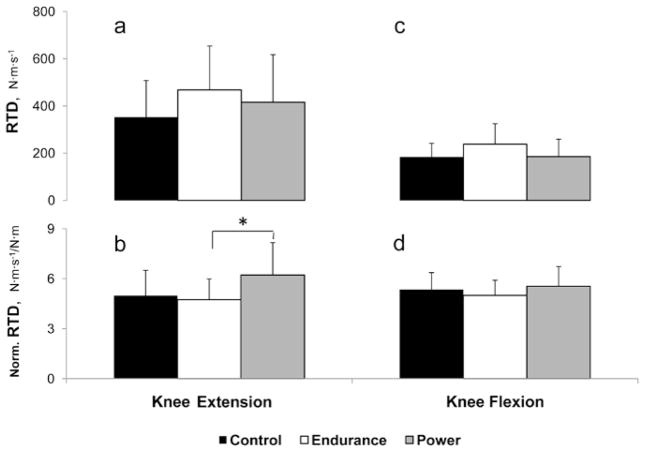
Figure 1 illustrates peak RTD during knee extension (Figures 1a and 1b) and during knee flexion (Figure 1c and 1d). No group differences were observed in absolute knee-extension peak RTD. However, when normalized for peak torque, gymnasts had significantly greater peak RTD (20% and 24% greater than control and swimmers, respectively). During knee flexion, no group differences were observed whether peak rate of torque development was expressed in absolute or normalized values.
Figure 1.
Absolute and MVC-normalized peak rate of torque development (RTD) during knee extension (a, b) and knee flexion (c, d) in control, endurance-trained and power-trained boys. *Significant difference between groups (P < .05).
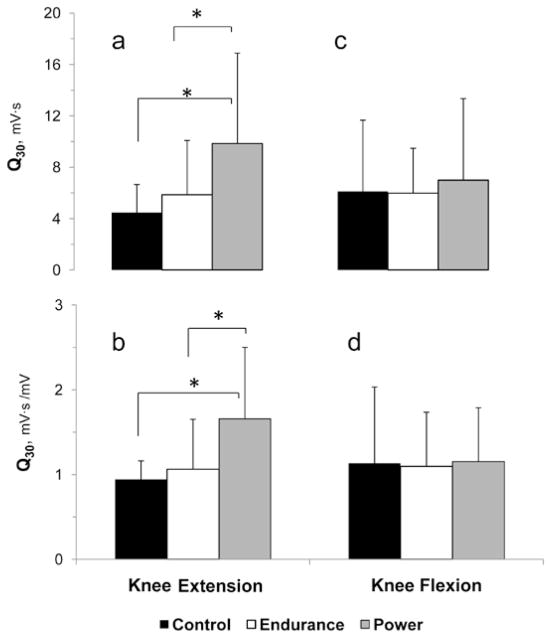
Knee extension Q30 was significantly greater in the gymnasts than in the controls and swimmers, whether expressed in absolute terms (55% and 30%, respectively) or when normalized for peak EMG amplitude (43% and 36%, respectively) (Figures 2a and 2b). No comparable Q30 differences were observed in knee flexion (Figure 2c and 2d).
Figure 2.
Absolute and amplitude-normalized rate of muscle activation (Q30) during knee extension (a, b) and during knee flexion (c, d) in control, endurance-trained and power-trained boys. *Significant difference between groups (P < .05).
EMD examination showed a tendency (P = .07) toward group differences during knee extension. Pairwise comparisons revealed significantly shorter EMD in power-athletes compared with controls (25%, P = .02) but not compared with endurance-trained athletes (P = .24) (Figure 3a). No such differences were observed during knee flexion (Figure 3b).
Figure 3.
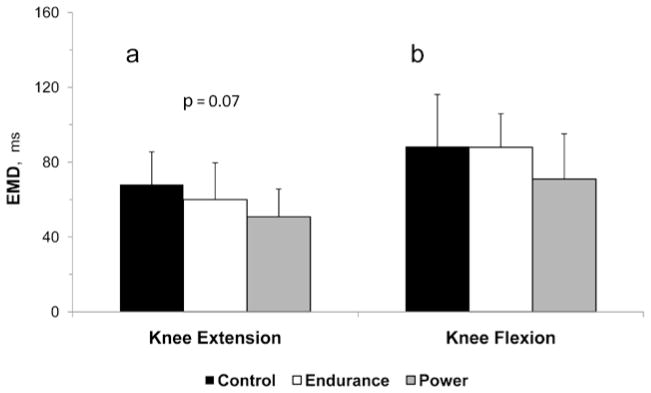
Electromechanical delay (EMD) during knee extension (a) and knee flexion (b) in control, endurance-trained and power-trained boys.
Coactivation was very low in all groups during both, knee extension and knee flexion and differences never reached statistical significance. Knee extension: 14.5 ± 12.1%, 15.5 ± 17.4% and 14.1 ± 10.4%; knee flexion: 6.4 ± 2.8%, 9.0 ± 6.7%, 8.5 ± 3.0%, for power-trained, endurance-trained, and control boys, respectively.
Discussion
The main findings of this study were the greater rate of torque development, along with greater rate of muscle activation and a tendency for lower EMD in the power-trained boys compared with endurance-trained and nonathletes. This pattern was observed during knee extension but not during knee flexion. These findings suggest that, already during preadolescence, specific training can affect not only gross muscle performance capacity such as strength, but the way muscles are activated, as well.
Due to their greater size and more advanced somatic maturity, the endurance-trained athletes had the highest absolute peak torque. When peak torque was normalized to muscle CSA, this difference disappeared. Halin et al23 examined peak elbow flexion torque in 10-year-old gymnasts and untrained boys and did not find significant group differences when torque was corrected for arm CSA. Furthermore, Maffuli et al,24 who examined young athletes using a mixed-longitudinal study, showed that male gymnasts became stronger than other athletes, including swimmers, only after age 11. Thus, it is possible that eventual differences in normalized peak torque could not be seen in the present study due to the subjects’ young age. Indeed, in the present study, group parity was maintained whether peak torque was normalized for CSA or body mass (data not shown).
A central finding was the higher knee-extension RTD in the power-trained athletes, reflecting high explosive strength. This finding is consistent with Benecke et al25 who reported greater jumping ability in young male gymnasts compared with other athletes. The gymnasts’ higher RTD is likely related to their higher rate of knee-extension muscle activation, as reflected in Q30 (Figure 2).
The rate of rise of the muscle’s EMG activity at its onset, Q30, may be affected by earlier motor-unit recruitment, higher discharge rate and higher rate of doublet discharge,4 defined as two proximate spikes, less than 5 ms apart.26 Any or all of these may have been higher in the power-trained athletes. Among adults, central drive has been shown to increase following resistance training of the knee extensors. 27 Using the interpolated twitch technique, Ramsay et al13 also suggested that resistance training increases motor-unit recruitment via enhanced central drive in prepubertal boys. Likely due to the large intersubject variability, however, training-induced differences in motor-unit recruitment did not reach statistical significance in the latter study. Furthermore, central drive was investigated during peak torque and not during the time of torque development. Presently, it is impossible to measure the direct contribution of the central drive to the rate of torque development.
Enhanced motor-unit discharge rate may have also contributed to the higher Q30 in the power-trained boys. In adults, motor-unit discharge rate has been shown to be greatest during the early stages of muscle contraction.28 Furthermore, explosive training has been shown to result in increased frequency of doublet discharge.26 Thus, it is possible that the higher Q30 in the power-trained boys may be due to greater motor unit discharge rate and higher frequency of doublet discharge. However, due to the fact that measurement of motor-unit discharge rates generally requires the use of needle EMG electrodes, to the authors’ knowledge no such data exists for children, in general, and child athletes, in particular.
Plyometric training has been previously demonstrated to result in higher stiffness of the musculotendinous complex among adults.29 Musculotendinous complex stiffness was not measured in our athletes but it is reasonable to assume that tendon stiffness was higher among our power athletes, possibly contributing to their shortened EMD and higher RTD. In view of the higher Q30 observed in the power athletes, however, both EMD and particularly RTD could also be positively affected by higher proportions of recruited Type-II motor units.30
In adults, endurance training has not been shown to affect muscle strength.31 Some investigators have even found endurance-trained athletes to be stronger than untrained counterparts.8 However, in the latter study, muscle strength was not scaled to body size. A recent study which examined the effects of different types of training on muscle strength in young adults32 suggests that endurance training actually hinders the development of lower-extremity strength and, particularly, explosive power development. The results of the present study do not support such hindrance, as endurance-trained boys were not inferior to untrained boys in terms of rate of torque development or rate of muscle activation. It is possible that with higher training duration and training volumes, endurance-type training may negatively affect explosive power even in young athletes.
In view of the cross-sectional design of this study, it may be argued that the observed differences in knee-extension RTD, Q30 and EMD between the power-trained and both the endurance or untrained boys are due to genetic predisposition and preselection bias. In other words, it is possible that the gymnasts were successful in their sport partly because of their inherent higher rate of muscle activation and resultant high RTD. However, in view of the lack of corresponding group differences in knee flexion, we suggest that heredity does not explain the observed high rate of knee-extension muscle activation and torque development in the gymnasts. Gymnastics and gymnastic training require explosive knee extensions (eg, jumping, landing), while knee flexion is underplayed. Thus, we suggest that the high rate of torque development and muscle activation, as well as the lower EMD were induced by training.
An additional possible limitation may be that some of the athletes participated in secondary sports activities, such as soccer or baseball. Of particular interest is the fact that some of the swimmers engaged in dry land training which included resistance training, albeit with light weights. Resistance training may have affected their muscle strength and power. Nevertheless, in spite of their engagement in resistance training, their RTD and Q30 were still lower than that of the gymnasts. Therefore, we feel that the swimmers’ participation in dry land training actually strengthens the conclusions of this study.
In summary, young power-trained athletes are characterized by consistently higher rate of muscle activation and shorter electromechanical delay, which may explain their higher rate of torque development. Furthermore, the observed differences in all these variables could be explained by greater utilization of the faster, Type II motor units. In view of the cross-sectional design of this study, the extent to which these differences are due to preselection bias is unclear. Nevertheless, the findings suggest that, already before the onset of puberty, specific training can result in muscle activation changes. Such changes could have implications for sports training and performance, and possibly for physical rehabilitation as well.
Acknowledgments
We would like to thank the participants and their parents for their cooperation and enthusiasm in the study. We would also like to thank James Desjardin for his expertise and technical assistance in data reduction. The study was funded by the Canadian Institute of Health Research and by a student grant of the North American Society for Pediatric Exercise Medicine.
References
- 1.Zahner L, Muehlbauer T, Schmid M, Meyer U, Puder JJ, Kriemler S. Association of sports club participation with fitness and fatness in children. Med Sci Sports Exerc. 2009;41(2):344–350. doi: 10.1249/MSS.0b013e318186d843. [DOI] [PubMed] [Google Scholar]
- 2.Rowland TW, Boyajian A. Aerobic response to endurance exercise training in children. Pediatrics. 1995;96(4 Pt 1):654–658. [PubMed] [Google Scholar]
- 3.Baquet G, van Praagh E, Berthoin S. Endurance training and aerobic fitness in young people. Sports Med. 2003;33(15):1127–1143. doi: 10.2165/00007256-200333150-00004. [DOI] [PubMed] [Google Scholar]
- 4.Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol. 2002;93(4):1318–1326. doi: 10.1152/japplphysiol.00283.2002. [DOI] [PubMed] [Google Scholar]
- 5.Behm DG, Faigenbaum AD, Falk B, Klentrou P. Canadian Society for Exercise Physiology position paper: resistance training in children and adolescents. Appl Physiol Nutr Metab. 2008;33(3):547–561. doi: 10.1139/H08-020. [DOI] [PubMed] [Google Scholar]
- 6.Grandys M, Majerczak J, Duda K, Zapart-Bukowska J, Sztefko K, Zoladz JA. The effect of endurance training on muscle strength in young, healthy men in relation to hormonal status. J Physiol Pharmacol. 2008;59(Suppl 7):89–103. [PubMed] [Google Scholar]
- 7.Izquierdo M, Hakkinen K, Gonzalez-Badillo JJ, Ibanez J, Gorostiaga EM. Effects of long-term training specificity on maximal strength and power of the upper and lower extremities in athletes from different sports. Eur J Appl Physiol. 2002;87(3):264–271. doi: 10.1007/s00421-002-0628-y. [DOI] [PubMed] [Google Scholar]
- 8.Lattier G, Millet GY, Maffiuletti NA, Babault N, Lepers R. Neuromuscular differences between endurance-trained, power-trained, and sedentary subjects. J Strength Cond Res. 2003;17(3):514–521. doi: 10.1519/1533-4287(2003)017<0514:ndbepa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Sale DG. Neural adaptation to resistance training. Med Sci Sports Exerc. 1988;20(5, Suppl):S135–S145. doi: 10.1249/00005768-198810001-00009. [DOI] [PubMed] [Google Scholar]
- 10.Folland JP, Williams AG. The adaptations to strength training : morphological and neurological contributions to increased strength. Sports Med. 2007;37(2):145–168. doi: 10.2165/00007256-200737020-00004. [DOI] [PubMed] [Google Scholar]
- 11.Falk B, Eliakim A. Resistance training, skeletal muscle and growth. Pediatr Endocrinol Rev. 2003;1(2):120–127. [PubMed] [Google Scholar]
- 12.Ozmun JC, Mikesky AE, Surburg PR. Neuromuscular adaptations following prepubescent strength training. Med Sci Sports Exerc. 1994;26(4):510–514. [PubMed] [Google Scholar]
- 13.Ramsay JA, Blimkie CJ, Smith K, Garner S, MacDougall JD, Sale DG. Strength training effects in prepubescent boys. Med Sci Sports Exerc. 1990;22(5):605–614. doi: 10.1249/00005768-199010000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Short KR, Vittone JL, Bigelow ML, et al. Changes in myosin heavy chain mRNA and protein expression in human skeletal muscle with age and endurance exercise training. J Appl Physiol. 2005;99(1):95–102. doi: 10.1152/japplphysiol.00129.2005. [DOI] [PubMed] [Google Scholar]
- 15.Tanner JM. Growth at Adolescence. 2. Oxford: Blackwell Scientific Publications; 1962. [Google Scholar]
- 16.Mirwald RL, Baxter-Jones AD, Bailey DA, Beunen GP. An assessment of maturity from anthropometric measurements. Med Sci Sports Exerc. 2002;34(4):689–694. doi: 10.1097/00005768-200204000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Slaughter MH, Lohman TG, Boileau BA. Skinfold equations for estimation of body fatness in children and youth. Hum Biol. 1988;60:709–723. [PubMed] [Google Scholar]
- 18.Calder KM, Gabriel DA. Adaptations during familiarization to resistive exercise. J Electromyogr Kinesiol. 2007;17(3):328–335. doi: 10.1016/j.jelekin.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Hodges PW, Bui BH. A comparison of computer-based methods for the determination of onset of muscle contraction using electromyography. Electroencephalogr Clin Neurophysiol. 1996;101(6):511–519. doi: 10.1016/s0013-4694(96)95190-5. [DOI] [PubMed] [Google Scholar]
- 20.Gabriel DA, Basford JR, An K. Training-related changes in the maximal rate of torque development and EMG activity. J Electromyogr Kinesiol. 2001;11(2):123–129. doi: 10.1016/s1050-6411(00)00041-9. [DOI] [PubMed] [Google Scholar]
- 21.Gottlieb GL, Corcos DM, Agarwal GC. Organizing principles for single-joint movements. I. A speed-insensitive strategy. J Neurophysiol. 1989;62(2):342–357. doi: 10.1152/jn.1989.62.2.342. [DOI] [PubMed] [Google Scholar]
- 22.Gabriel DA, Boucher JP. Practicing a maximal performance task: a cooperative strategy for muscle activity. Res Q Exerc Sport. 2000;71(3):217–228. doi: 10.1080/02701367.2000.10608902. [DOI] [PubMed] [Google Scholar]
- 23.Halin R, Germain P, Buttelli O, Kapitaniak B. Differences in strength and surface electromyogram characteristics between pre-pubertal gymnasts and untrained boys during brief and maintained maximal isometric voluntary contractions. Eur J Appl Physiol. 2002;87(4–5):409–415. doi: 10.1007/s00421-002-0643-z. [DOI] [PubMed] [Google Scholar]
- 24.Maffulli N, King JB, Helms P. Training in elite young athletes (the Training of Young Athletes (TOYA) Study): injuries, flexibility and isometric strength. Br J Sports Med. 1994;28(2):123–136. doi: 10.1136/bjsm.28.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bencke J, Damsgaard R, Saekmose A, Jorgensen P, Jorgensen K, Klausen K. Anaerobic power and muscle strength characteristics of 11 years old elite and non-elite boys and girls from gymnastics, team handball, tennis and swimming. Scand J Med Sci Sports. 2002;12(3):171–178. doi: 10.1034/j.1600-0838.2002.01128.x. [DOI] [PubMed] [Google Scholar]
- 26.Van Cutsem M, Duchateau J, Hainaut K. Changes in single motor unit behaviour contribute to the increase in contraction speed after dynamic training in humans. J Physiol. 1998;513(Pt 1):295–305. doi: 10.1111/j.1469-7793.1998.295by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knight CA, Kamen G. Adaptations in muscular activation of the knee extensor muscles with strength training in young and older adults. J Electromyogr Kinesiol. 2001;11(6):405–412. doi: 10.1016/s1050-6411(01)00023-2. [DOI] [PubMed] [Google Scholar]
- 28.Desmedt JE, Godaux E. Ballistic contractions in man: characteristic recruitment pattern of single motor units of the tibialis anterior muscle. J Physiol. 1977;264(3):673–693. doi: 10.1113/jphysiol.1977.sp011689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grosset JF, Piscione J, Lambertz D, Perot C. Paired changes in electromechanical delay and musculo-tendinous stiffness after endurance or plyometric training. Eur J Appl Physiol. 2009;105(1):131–139. doi: 10.1007/s00421-008-0882-8. [DOI] [PubMed] [Google Scholar]
- 30.Taylor AD, Humphries B, Smith P, Bronks R. Electrophoretic separation of myosin heavy chain isoforms in the human m. vastus lateralis: references to reproducibility and relationships with force, electromechanical delay, fibre conduction velocity, endurance and electromyography. Arch Physiol Biochem. 1997;105(1):10–18. doi: 10.1076/apab.105.1.10.13142. [DOI] [PubMed] [Google Scholar]
- 31.Sleivert GG, Backus RD, Wenger HA. Neuromuscular differences between volley-ball players, middle distance runners and untrained controls. Int J Sports Med. 1995;16(6):390–398. doi: 10.1055/s-2007-973026. [DOI] [PubMed] [Google Scholar]
- 32.Santtila M, Kyrolainen H, Hakkinen K. Changes in maximal and explosive strength, electromyography, and muscle thickness of lower and upper extremities induced by combined strength and endurance training in soldiers. J Strength Cond Res. 2009;23(4):1300–1308. doi: 10.1519/JSC.0b013e3181a884bc. [DOI] [PubMed] [Google Scholar]