Abstract
Most research on the effects of endurance training has focused on endurance training’s health-related benefits and metabolic effects in both children and adults. The purpose of this study was to examine the neuromuscular effects of endurance training and to investigate whether they differ in children (9.0–12.9 years) and adults (18.4–35.6 years). Maximal isometric torque, rate of torque development (RTD), rate of muscle activation (Q30), electromechanical delay (EMD), and time to peak torque and peak RTD were determined by isokinetic dynamometry and surface electromyography (EMG) in elbow and knee flexion and extension. The subjects were 12 endurance-trained and 16 untrained boys, and 15 endurance-trained and 20 untrained men. The adults displayed consistently higher peak torque, RTD, and Q30, in both absolute and normalized values, whereas the boys had longer EMD (64.7 ± 17.1 vs. 56.6 ± 15.4 ms) and time to peak RTD (98.5 ± 32.1 vs. 80.4 ± 15.0 ms for boys and men, respectively). Q30, normalized for peak EMG amplitude, was the only observed training effect (1.95 ± 1.16 vs. 1.10 ± 0.67 ms for trained and untrained men, respectively). This effect could not be shown in the boys. The findings show normalized muscle strength and rate of activation to be lower in children compared with adults, regardless of training status. Because the observed higher Q30 values were not matched by corresponding higher performance measures in the trained men, the functional and discriminatory significance of Q30 remains unclear. Endurance training does not appear to affect muscle strength or rate of force development in either men or boys.
Keywords: children, EMG, exercise, strength, swimming, training
Introduction
Previous research on the effects of endurance-type training has focused mainly on health-related benefits and physiological adaptations related to aerobic capacity. The focus on cardiovascular health and metabolic adaptations to endurance training is evident in both the adult literature (American College of Sports Medicine Position Stand 1998; Pang et al. 2006) and the pediatric literature (Armstrong et al. 2007; Janz et al. 2002). Corresponding research on neuromuscular adaptations to endurance-type training is notably lacking.
Resistance training has been shown to enhance muscle strength in both children and adults. In adults, both morphological and neurological adaptations explain the increase in strength (Aagaard 2003; Aagaard et al. 2002). In children, recent studies demonstrate that increased general physical activity (12 months to 6 years) results in enhanced total lean body mass (Baxter-Jones et al. 2008; Stenevi-Lundgren et al. 2009). However, resistance training (up to 20 weeks) has not been shown to result in muscle hypertrophy. Thus, it is presumed that strength is enhanced through neuromuscular adaptations (Behm et al. 2008; Ozmun et al. 1994; Ramsay et al. 1990; Sale 1988). Comparable data on the possible effects of endurance training on muscle performance and neuromuscular adaptations are limited. Although some studies suggest increased muscle activation and possibly increased strength in adult endurance athletes compared with untrained adults (Lattier et al. 2003; Lucía et al. 2000), there are no comparable data in children.
It has been suggested that the capacity of prepubertal boys to activate their neuromuscular systems is lower than that of adults (Belanger and McComas 1989; Grosset et al. 2008; Hatzikotoulas et al. 2008; O’Brien et al. 2009; Pääsuke et al. 2000), and that children are less capable of recruiting or utilizing their higher-threshold motor units (Asai and Aoki 1996; Falk and Dotan 2006; Falk et al. 2009b). Although not all studies have demonstrated a lower motor-unit activation in children (Streckis et al. 2007), Halin et al. (2002) proposed that children’s neuromuscular system may be more adaptive to a training stimulus, compared with that of adults.
The present study examined whether endurance training results in neuromuscular adaptations. Furthermore, because children appear to differ neuromuscularly from adults, a secondary purpose of this study was to differentiate the age-related training effects, if any.
Materials and methods
The study and its procedures were approved by the Brock University (St. Catharines, Ont.) Research Ethics Board (file 05–155). Sixty-three participants volunteered to take part in the study: 16 untrained boys (9–12 years), 20 untrained men (18–25 years), 12 endurance-trained boys (9–11 years), and 15 endurance-trained men (18–35 years). The untrained participants were involved in structured physical activity for a maximum of 2 h per week. The endurance-trained participants were highly trained athletes who trained year round in a structured swimming or triathlon program. (The adult’s group consisted of 7 triathletes and 8 swimmers, whereas the children’s group consisted of swimmers only). The boys had been training for 2.5 ± 0.9 years, and the men for 6.4 ± 4.3 years. The adult athletes specialized in middle-distance and long-distance events (200–1 500 m). No such specialization existed in the boys. Six endurance-trained men participated in their sport at a national level, 3 men competed at a regional level, and the rest were university-aged varsity swimmers. Seven boys competed at a provincial level, whereas 5 boys participated at a regional level.
All the boys were classified as prepubertal or early-pubertal, based on secondary sexual characteristics (pubic hair), as described by Tanner (1962).
Those subjects who had prior or present conditions that could affect muscle or neuromuscular function (e.g., muscular disease, use of medications, or injury to dominant hand or leg) were excluded from the study.
Procedures
All tests and measurements were performed during 2 visits to the laboratory. The subjects were instructed to refrain from excessive exercise the day preceding the testing.
On the first visit, the subjects were informed of the purpose, methods, and potential risks of the study. Before testing, an informed consent form was signed by the participant or by the child’s parents. Subsequently, anthropometric measurements (mass, height, sitting height, and skinfold thickness) were assessed, and questionnaires (medical, physical activity, pubertal stage) were filled out. Subjects then performed a shorter version of the testing protocol to become familiar with the instructions, equipment, and testing procedure. These data were not used for analysis. The initial setting on the dynamometer was individually adjusted and the positions of all dynamometer attachments to be used during the second visit were recorded. On the second visit, subjects performed only the strength-testing protocol, and electromyography (EMG) signals were acquired.
Anthropometric measurements
Height and body mass were measured using an Ellard Instrumentation stadiometer (Monroe, Wash.) and a digital scale (Zenith), respectively, with subjects in light clothing and no shoes. Height and body mass were recorded to the nearest 0.1 cm and 0.1 kg, respectively. Sitting height was also recorded to estimate the age of peak height velocity, reflecting somatic maturity (Mirwald et al. 2002). Skinfold thickness was measured in triplicate using Harpenden calipers (British Indicators, Herts, England) and the median value at each site was used. The following sites were evaluated: biceps, triceps, subscapular, and suprailiac. Adiposity (percentage of body fat) was estimated from the appropriate skinfold measurements using age-specific and maturity-specific equations (Durnin and Womersley 1974; Slaughter et al. 1988). All measurements were performed by the same investigator. The coefficient of variance was 5% and the intraobserver reliability in 10 subjects was r = 0.98.
Pubertal stage
Pubertal status was determined using secondary sex characteristics (pubic hair), as described by Tanner (1962). Pubertal stage was self-reported, using drawings (Duke et al. 1980). The self-assessment form was placed in an envelope by the subject and handed directly to the researcher, to ensure discretion.
Questionnaires
Questionnaires were completed by the subject, if needed with the help of the investigator and possibly the parent, to assess the subject’s medical history and physical activity levels, and training history for the athletes. Physical activity level was assessed using a standardized questionnaire (Godin and Shephard 1985), as well as by a personal interview. Past and present training experience was self-reported through a personal interview.
Strength-testing protocol
An isokinetic dynamometer system (Biodex III, Biodex, Shirley, N.Y.) was used to assess isometric strength (torque) of the elbow and knee flexors and extensors of the dominant arm and leg, respectively. Isometric contractions were chosen to minimize antagonist involvement, to make torque measurements attributable to agonist action as much as possible. The isokinetic dynamometer system has been found to be reliable for measuring muscle strength in children and adults (Dvir 2004). The intrasession reliability for maximal strength reliability coefficient (ICC2,1) was 0.95 and 0.93 for boys and men, respectively. A similar protocol was used in previous studies in the pediatric and adult population in our laboratory (Falk et al. 2009a, 2009b). To reduce the noise on the recorded torque channel, an EMG–analog signal access interface (Biodex) was used. This utility configures the scale factors of the analog signal outputs for torque. For each participant, the scaling factor was adjusted according to the torque values reached in the habituation session during the first visit.
For the upper limbs, subjects sat upright in a chair with the shoulder at 90° of flexion, with the upper arm resting on an arm rest adjusted for the subject’s height. The subject’s elbow was placed at 90° of flexion and the hand was in a neutral position. The torque axis was positioned in alignment with the lateral humeral epicondyle. After adjustments, subjects were secured in the chair to prevent stabilizing movements that could affect the measurements, with 2 straps secured across the chest in an X fashion and a hip strap to stabilize the trunk.
For the lower limbs, subjects sat upright in a chair with a hip angle of 120°, and the knee at 90° of flexion. The ankle was secured (using Velcro straps) to an adjustable lever arm. The torque axis was aligned with the lateral femoral epicondyle. After adjustments, subjects were secured in the chair to prevent stabilizing movements that could affect the measurements, with 2 straps secured across the chest and another strap across the thigh.
The testing protocol included a specific warm-up (~5 contractions of progressive intensity), followed by 2 sets of five 3-s maximal voluntary contractions. A 30-s rest followed each repetition. Rest between each set was 2 min. The order of the sets (flexion–extension, upper–lower limb) was counterbalanced among subjects. Additional repetitions were performed as needed, to reach at least 5 valid trials. Data were deemed unacceptable when there were execution errors, deviations in EMG baseline, or abnormal torque or EMG amplitudes. Each subject was instructed to contract “as hard and as fast as possible” from a relaxed state to ensure maximal torque and rate of torque development (RTD). Subjects were encouraged verbally to perform a maximal effort throughout each contraction. Online visual feedback of the dynamometer’s torque signal was available for the subjects on a personal computer screen. Visual feedback has been shown to be important for torque production (Kellis and Baltzopoulos 1996), especially in young children (Smits-Engelsman et al. 2003). Peak torque was recorded from the dynamometer system and stored for off-line analysis.
EMG
During each contraction, EMG signals were collected from the agonist and antagonist muscles using bipolar surface electrodes (Delsys 2.1, Delsys Inc., Boston, Mass.). In the upper limbs, electrodes were placed on the muscle belly midsections of the biceps brachii and the lateral head of the triceps brachii. In the lower limbs, electrodes were placed on the muscle belly of the vastus lateralis and biceps femoris. These were determined visually during a resisted static contraction. The electrodes were placed in line with the muscle fibres, away from the estimated motor point (Delagi and Perotto 1980). A ground electrode served as a reference electrode and was placed over the clavicle.
To reduce impedance, electrode sites were prepared by shaving the relevant area when necessary, thoroughly rubbing the skin with abrasive gel, and cleaning with alcohol, before placing the electrodes. The same investigator performed all electrode placements.
The EMG signal was band-pass filtered (20–450 Hz) using the Bagnoli-4 (Delsys) bioamplifier. All signals were sent to a 16-bit A/D converter (BNC-2110, National Instruments, Austin, Tex.) and sampled at a rate of 1 000 Hz using a computer-based oscillograph and data acquisition system (EMGworks, Delsys Inc., Boston, Mass.). Recorded data were stored for further analysis.
Data reduction and analysis
Using EGGLAB and MatLab (The MathWorks, Natick, Mass.), several variables were calculated for each type of movement tested. Mean traces of the best 5 trials of EMG agonist, EMG antagonist, and torque were created to reduce the signal-to-noise ratio. Torque and EMG traces in each set were examined visually. Inclusion for analysis was based on a clear and stable EMG baseline prior to the beginning of the contraction, and clear onset of torque and EMG activity. Any faulty trials were eliminated, and out of the remaining trials, the best 5 repetitions were used for analysis, based on the highest peak torques and RTD values. The intrasession reliability for agonist EMG amplitude reliability coefficient (ICC2,1) was 0.65 and 0.94 for boys and men, respectively.
Traces were time locked on the torque onset and averaged. The average waveform consisted of 400 ms prior to the force onset and 3 000 ms afterwards. The mean traces were used to calculate peak torque, RTD, rate of muscle activation (Q30), electromechanical delay (EMD), time to peak torque, time to peak RTD, and agonist-antagonist coactivation. Peak RTD was calculated by taking the maximum of the first derivative of the torque signal (Gabriel and Boucher 2000). Agonist and antagonist EMG amplitudes were calculated from the detected linear envelope. The peak EMG amplitudes values were calculated over 250 ms around the time of occurrence of peak torque. Q30 was measured over the first 30 ms of electromechanical activity. Q30 was defined as the area under the linear envelope of the detected EMG signal during the initial 30 ms (Gabriel and Boucher 2000; Gottlieb et al. 1989), and has been previously used to reflect rate of increase in muscle activation during a maximal task (Falk et al. 2009a, 2009b; Gabriel and Boucher 2000; Gottlieb et al. 1989). The EMG activity onset was defined as the point in time at which the signal first increased 5 SDs above the mean of the baseline and stayed above that point for more than 20 ms. The onset of torque was defined as the first point in time at which the RTD reached 5 SDs of the baseline mean for at least 10 ms. This point was confirmed visually and adjusted manually if needed. EMD was defined as the delay, in milliseconds, between the agonist EMG activity onset and the onset of torque production. The time to peak torque was calculated as the time delay (ms) between the onset of torque generation and the occurrence of peak torque. The time to peak RTD was calculated as the time delay (ms) between the onset of torque generation and the occurrence of peak RTD. Coactivation was calculated as the ratio between the antagonist’s EMG amplitude and its EMG amplitude as an agonist (i.e., for knee extension: (biceps femoris EMG amplitude in knee extension)/(biceps femoris EMG amplitude in knee flexion).
Statistical analysis
All statistical analyses were performed using SPSS, v.16 (SPSS Inc., Chicago, Ill.). The data for all groups are presented as means ± SD. The data were cleaned by checking for outliers (>2 SDs from the mean) of all dependent variables for each of the 4 contractions. In total, 4 outlying values were found (1 value of Q30 in each of the 4 contraction modes) and were not included in the analysis. A χ2 analysis was used to compare the pubertal stage distributions. Group differences in muscle performance and neuromuscular function were determined using a 2-way analysis of variance (ANOVA), with training and age as the between-subjects main effects. Post hoc comparisons (Least Significant Difference test (LSD)) were performed when a main effect or interaction was found to be statistically significant. Each contraction was analyzed separately. Subsequently, all contractions were analyzed together using a 2-way ANOVA for repeated measures to identify general patterns. The acceptable level of significance was set at p < 0.05.
Results
The physical characteristics of the subjects are displayed in Table 1. The men were older, taller, and heavier, with greater lean body mass than the children. There was no significant difference in age or height between the untrained control boys and the endurance-trained boys, or between the untrained control adults and the endurance-trained adults. There was an age-by-training interaction for body mass, reflecting the fact that among the boys, the endurance-trained boys were heavier, whereas among the adults, the pattern was reversed. There were no significant differences in relative body fat between or within the age groups. There were no significant differences in sexual maturation stage and years from age of peak height velocity between the 2 boys’ groups (Table 1).
Table 1.
Physical characteristics of the untrained and endurance-trained boys and men.
| Characteristics | Children
|
Adults
|
||
|---|---|---|---|---|
| Control (n = 16) | Endurance (n = 12) | Control (n = 20) | Endurance (n = 15) | |
| Age (y) | 10.2±1.0a | 10.7±0.7b | 22.8±4.4a | 24.5±5.9b |
| Tanner (I, II, III, IV, V) | 9,7,0,0,0 | 7,5,0,0,0 | — | — |
| Years from peak height velocity | −3.7±0.7 | −3.2±0.6 | — | — |
| Height (cm) | 141.5±8.6a | 145.9±7.2b | 180.5±7.4a | 179.2±5.7b |
| Mass (kg) | 35.7±8.0ac | 41.5±12.6bc | 80.4±12.4ad | 74.7±6.0bd |
| Body fat percentage (%) | 18.1±6.6 | 20.1±12.0 | 17.9±4.8 | 14.8±3.8 |
| Lean body mass (kg) | 28.8±4.6a | 31.9±5.2b | 65.6±8.1a | 63.5±5.1b |
Note: Values are presented as means ± SD. Similar letters indicate pairwise significant differences (p < 0.05) between groups.
There was a significant difference in training hours between groups. The adults trained 14.4 ± 5.0 h·week–1, whereas the boys trained 8.5 ± 3.6 h·week–1. Both the men and the boys participated in dry-land training, which included limited resistance exercise, in addition to their endurance-training program (2.5 ± 1.3 h·week–1 and 3.4 ± 1.5 h·week–1, respectively).
Data were collected for all 4 contraction modes (elbow flexion and extension, knee flexion and extension). Because the pattern of results was similar in all 4 types of contractions, for the purpose of simplicity, only knee extension data are presented within the text. The results of all contraction modes are summarized in Table 2.
Table 2.
Repeated measures, including all 4 types of contractions.
| Variable | Age effect | Training effect | Age × training interaction |
|---|---|---|---|
| Torque | |||
| Absolute | <0.001 | — | 0.030 |
| Per kg | <0.001 | — | — |
| RTD | |||
| Absolute | <0.001 | — | — |
| Per torque | <0.001 | — | — |
| Q30 | |||
| Absolute | <0.001 | — | — |
| Per EMGamp | <0.001 | — | 0.032 |
| Time to peak torque | 0.002 | — | — |
| Time to peak RTD | <0.001 | — | — |
| EMD | <0.001 | — | — |
| Coactivation | 0.013 | 0.031 | — |
Note: RTD, rate of torque development; Q30, rate of muscle activation; RTD, rate of torque development; EMGamp, electromyography amplitude; EMD, electromechanical delay.
In absolute terms, the men were significantly stronger than the boys (Fig. 1A). There was an age-by-training interaction, reflecting the fact that the trained boys were significantly stronger than the untrained boys, whereas no such difference was apparent in the adults. When peak torque was normalized to body mass (Fig. 1B), an age effect was still apparent, reflecting the fact that on average, normalized torque was higher in the men. However, differences between the trained and untrained boys were no longer significant.
Fig. 1.
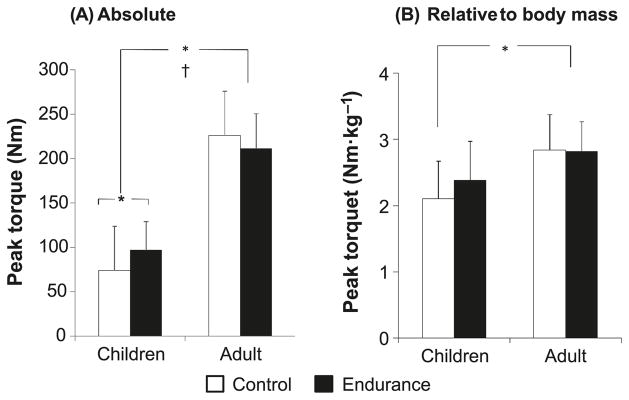
Knee extension peak torque of the endurance-trained and untrained boys and men. (A) Peak torque in absolute values. (B) Peak torque corrected to body mass. Data are presented as means ± SD. *, p < 0.05; †, age × training interaction (p < 0.05).
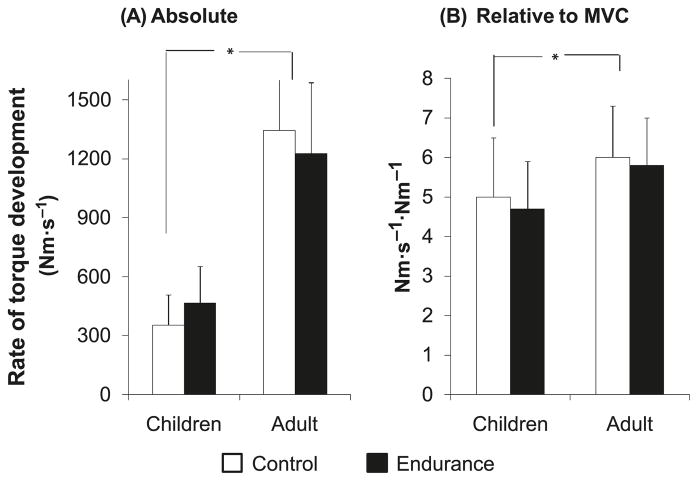
The men exhibited a more rapid absolute RTD than did the boys during knee extension (Fig. 2A). No differences were observed between trained and untrained groups within each age group. This was also the case when RTD was normalized to peak torque (Fig. 2B). No age-by-training interactions were apparent either in absolute terms or when RTD was normalized to peak torque.
Fig. 2.
Knee extension rate of torque development (RTD) of the endurance-trained and untrained boys and men. (A) RTD in absolute values. (B) RTD corrected to peak torque. Data are presented as means ± SD. *, p < 0.05. MVC, maximal voluntary contractions.
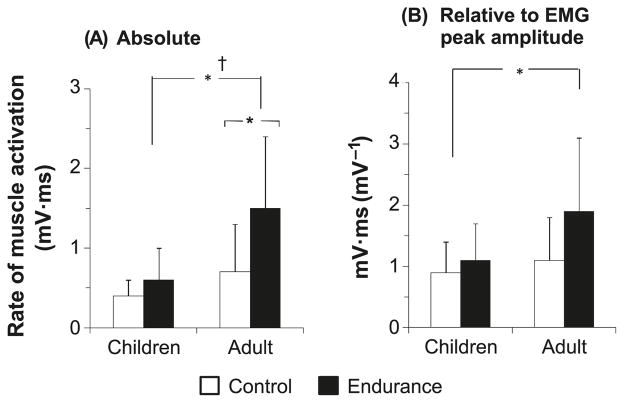
The men had significantly higher absolute Q30 compared with the boys (Fig. 3A). There was a training effect, reflecting the fact that, on average, the athletes had higher Q30 compared with the nonathletic groups. More importantly, there was an age-by-training interaction, which reflects the fact that the trained men had significantly higher Q30 compared with the age-matched untrained group, whereas the difference between the trained and untrained boys was not significant.
Fig. 3.
Knee extension rate of rise of electromyographical (EMG) activity reflecting rate of muscle activation (Q30) in the endurance-trained and untrained boys and men. (A) Q30 in absolute values. (B) Q30 corrected to peak EMG amplitude. Data are presented as means ± SD. *, p < 0.05; †, age × training interaction (p < 0.05). The p value for age × training interaction for the normalized Q30 was 0.090.
When Q30 was normalized to peak EMG amplitude (Fig. 3B), age and training effects were still significant. There was also a trend toward an age-by-training interaction (p = 0.090), reflecting the fact that the endurance-trained men had higher Q30 compared with the age-matched un-trained group. No such difference was apparent in the boys. That is, the training effect was due predominantly to the difference between the trained and untrained adults, whereas there was no training effect in the children.
There were no significant differences in time to peak torque between the 2 age and training groups (Fig. 4A). However, the time to peak RTD was significantly longer in the boys than in the men (Fig. 4B). No training effect or training-by-age interaction was evident.
Fig. 4.
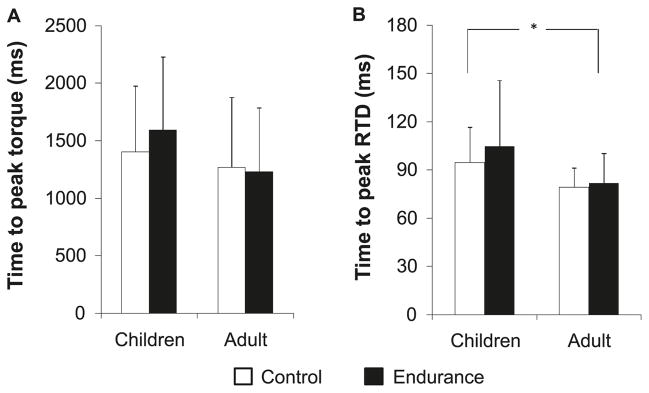
(A) Knee-extension time to peak torque of the endurance-trained and untrained boys and men. (B) Knee extension time to peak rate of torque development (RTD) of the endurance-trained and untrained boys and men. Data are presented as means ± SD. *, p < 0.05.
The men had significantly shorter EMD compared with the boys (Fig. 5). No training effect or age-by-training interactions were detected.
Fig. 5.
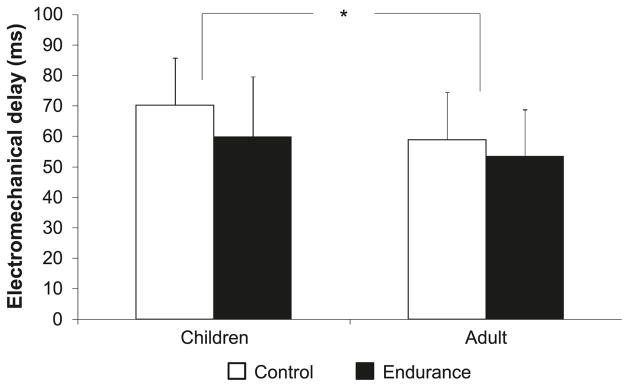
Knee-extension electromechanical delay (EMD) in endurance-trained and untrained boys and men. Data are presented as means ± SD. *, p < 0.05.
The coactivation index was low in all groups and similar in the trained and untrained boys (0.15 ± 0.17% and 0.11 ± 0.05%, respectively), as well as in the trained and untrained men (0.09 ± 0.07% and 0.13 ± 0.06%, respectively). No age effect or age-by-training interactions were found.
Repeated measures analysis
Table 2 presents the results of the ANOVA for repeated-measures analysis highlighting only the significant effects. An age effect was apparent in all variables examined, which reflects the fact that the pattern of age differences was a persistent finding across all 4 modes of contractions tested. On average, the men had higher torque, RTD, and Q30 values than the boys, whether those variables were expressed in absolute or normalized values. Furthermore, EMD, time to peak RTD, and peak torque were significantly longer in the boys than in the men. The coactivation index was lower in the men than in the boys. In addition, the training effect was apparent only in the coactivation index, which reflects the fact that on average, the athletes had a lower coactivation index than the untrained subjects. However, it should be noted that generally, the coactivation indices were very low (<0.20) in all groups.
There was an age-by-training interaction for absolute peak torque, reflecting the fact that the endurance-trained boys were significantly stronger than their age-matched untrained controls. This was not the case in the men. When peak torque was normalized for body mass, no training effect or interactions were observed. There was an age-by-training interaction when Q30 was normalized to peak EMG amplitude, reflecting the fact that the endurance-trained men had higher Q30 values than their age-matched controls, whereas no such difference was apparent between the 2 groups of boys.
Discussion
We compared maximal isometric torque, rate of torque development, and rate of muscle activation of elbow and knee flexion and extension in endurance-trained and minimally active boys and men. Our main results showed that the men were stronger and had higher RTD and Q30 than the boys, whether those results were expressed in absolute values or normalized to body mass, peak torque, or peak EMG amplitude. No training-related muscle-performance differences were observed, but the trained men had significantly higher Q30 than the untrained men. Although Q30 also tended to be higher in the trained boys, the difference was not statistically significant.
The lower peak torque observed in the boys is in agreement with previous studies of untrained children and adults (De Ste Croix et al. 1999; Lambertz et al. 2003). Our study extends previous results by demonstrating that this age-related difference also exists among endurance-trained athletes.
Overall, the children’s coactivation index was found to be similar to, or slightly higher than, that of adults. However, in both age groups and in all contraction modes, coactivation was very low and could not explain the higher size-normalized peak torque observed in our adult subjects. This is in agreement with previous studies that examined the isometric strength of untrained boys and men (Falk et al. 2009b; Morse et al. 2008; O’Brien et al. 2009), although it disagrees with others (Hatzikotoulas et al. 2008).
Age-related differences in muscle-fibre-type distribution could potentially explain the differences in normalized peak torque. However, previous studies have demonstrated no age difference in fibre-type distribution between children and adults (Dubowitz 1965). Thus, the boys’ lower peak torque should be explained, at least in part, by their lower rate of muscle activation, as reflected by the lower Q30 observed in the present study, and by a lower extent of motor unit recruitment, as suggested previously (Belanger and McComas 1989; Falk et al. 2009b; Halin et al. 2003; Pääsuke et al. 2000).
No difference was observed in peak torque between the trained and untrained men in either absolute or body-mass-normalized terms. This is consistent with previous findings (Sleivert et al. 1995), suggesting that endurance training has little or no effect on maximal strength.
In absolute terms, peak torque was significantly higher in the trained boys than in their age-matched counterparts. This was mainly due to the trained boys’ greater body mass. Indeed, normalized to body mass, the peak torque difference was statistically insignificant. Two previous studies reported greater maximal isometric knee extensor strength in young male gymnasts (power trained) compared with swimmers (endurance trained) (Bencke et al. 2002; Maffulli et al. 1994). However, no comparison was made with untrained boys. Although previous findings have demonstrated that increased general physical activity in girls (Stenevi-Lundgren et al. 2009) and cycle ergometry training in boys (Zakas 2004) can result in increased muscle strength, our findings suggest that young endurance athletes do not demonstrate a clear strength advantage over their untrained counterparts.
The boys’ lower absolute RTD is partly explained by its dependency on peak torque. Thus, normalizing RTD for peak torque can be useful in searching for more fundamental RTD-determining factors (Holtermann et al. 2007). The only 2 studies to have normalized children’s RTD to peak torque similarly reported lower RTD values in boys during elbow flexion and extension, compared with men (Asai and Aoki 1996; Falk et al. 2009b). Children’s lower RTD, then, is a persistent finding, independent of their lower maximal strength or tested muscle group. These results suggest that other factors, such as a lower rate of muscle activation or firing rate (Van Cutsem et al. 1998), are likely involved in determining children’s RTD.
Peak RTD was higher in the men than in the boys, but no training effect was evident in either age group. RTD has previously been shown to rise following heavy resistance training (Aagaard et al. 2002), and to be higher in athletes involved in primarily explosive-type training (Sleivert et al. 1995). However, no comparable difference was observed in endurance-trained athletes (Sleivert et al. 1995). Additionally, Lattier et al. (2003) found no differences in the mean rate of twitch-force development of the knee extensors between endurance-trained and sedentary men.
Shorter EMD reflects greater muscle-tendon stiffness, excitation–contraction coupling, and muscle-fibre conduction velocity (Cavanagh and Komi 1979; Halin et al. 2003). Compared with the men, our boys had longer EMD. A comparable age-related EMD difference has been reported earlier in elbow flexion (Asai and Aoki 1996; Falk et al. 2009b), elbow extension (Falk et al. 2009b), and plantar-flexion twitch contraction (Grosset et al. 2005). Although no age-related differences in muscle-tendon stiffness were reported in elbow flexion (Cornu and Goubel 2001), others have reported less stiffness in boys during dorsiflexion (Lambertz et al. 2003). Thus, the boys’ longer EMD may be attributed to their lower musculotendinous stiffness (Lambertz et al. 2003) and to lower muscle activation or muscle-fibre-conduction velocity in boys (Halin et al. 2003).
Grosset et al. (2009) found EMD to be significantly shorter after 10 weeks of endurance training in men. Although the EMD difference in the present study was not significant, there was a trend toward shorter EMD in the trained subjects (p = 0.069). Tendon stiffness was previously reported to increase after endurance training (Buchanan and Marsh 2001), and the muscle-tendon complex was found to be less compliant in long-distance runners than in untrained individuals (Kubo et al. 2000). Although it has been suggested that EMD is highly dependent on muscle-tendon stiffness (Cavanagh and Komi 1979), Grosset et al. (2009) found musculotendinous stiffness changes to account for only 20% of the variance in training-induced EMD changes. Thus, the rate of muscle activation and the type of recruited motor units could not be ruled out as likely contributors to EMD and the changes thereof.
As previously reported (Falk et al. 2009b), our boys had lower normalized Q30 values compared with the men. Also, our trained men were characterized by significantly greater Q30 values than their untrained counterparts. Although the pattern was similar in the boys, the difference did not reach statistical significance. To the best of our knowledge, this is the first study to examine Q30 in endurance-trained boys and men. Our results concur with previous studies in adults (Lattier et al. 2003; Lucía et al. 2000) that suggested that endurance training increases muscle activation and enhances motor-unit recruitment. However, we feel that because our findings could not correlate performance with Q30 differences, the relationship between Q30 and muscle performance is unclear, at least as far as endurance training is concerned. Lucia et al. (2000) suggested that the increased motor-unit activation in endurance athletes was primarily of type I fibres. This suggestion does not help in clarifying the issue because increased activation, even if only of type I motor units, should have resulted in higher peak torque and likely higher peak RTD values as well. Faster activation could have also been expected to shorten EMD and times to peak RTD and peak torque. Because none of these functional changes occurred in our trained subjects, the functional and discriminatory significance of Q30 is unclear.
It should be noted that the group of trained boys in this study consisted of only swimmers, whereas the trained men’s group also included triathletes. It may be argued that swimming may emphasize the muscles of the upper limbs, whereas cycling and running train only the lower limbs, thereby differentially affecting the training effects in each of the 2 age groups. Nevertheless, despite the greater lower-limb emphasis in the adult endurance group, no apparent training effect was detected in knee flexion or extension. This was also apparent in the upper limbs. Furthermore, the pattern of a higher rate of activation observed in the adults’, but not the boys’, trained group was also apparent in the upper limbs. Thus, it appears that the difference in subject make-up of the 2 age groups strengthens, rather than weakens, the claim that endurance training does not affect muscle force and dynamics.
Conclusion
During maximal voluntary isometric muscle contractions, the men were stronger, had higher RTD and Q30 than the boys, in both absolute and size-normalized values, and had shorter EMD and time to peak RTD. Endurance training could only be shown to have affected a higher Q30 in the men with no corresponding performance differences in any of the measured variables. Thus, Q30’s functional and discriminatory significance is unclear, at least in as much as endurance training is concerned. It thus appears that the functional effects of endurance training are mainly confined to the metabolic realm.
Acknowledgments
We thank the participants and their parents for their cooperation and enthusiasm. We also thank James Desjardin for his expertise and technical assistance in data reduction. This study was funded by the Canadian Institute of Health Research and by a student grant from the North American Society for Pediatric Exercise Medicine.
References
- Aagaard P. Training-induced changes in neural function. Exerc Sport Sci Rev. 2003;31(2):61–67. doi: 10.1097/00003677-200304000-00002. [DOI] [PubMed] [Google Scholar]
- Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol. 2002;93(4):1318–1326. doi: 10.1152/japplphysiol.00283.2002. [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30(6):975–991. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- Armstrong N, Barrett LA, Welsman JR. Cardiorespiratory training during childhood and adolescence. J Exerc Sci Physiother. 2007;3:17–25. [Google Scholar]
- Asai H, Aoki J. Force development of dynamic and static contractions in children and adults. Int J Sports Med. 1996;17(3):170–174. doi: 10.1055/s-2007-972827. [DOI] [PubMed] [Google Scholar]
- Baxter-Jones AD, Eisenmann JC, Mirwald RL, Faulkner RA, Bailey DA. The influence of physical activity on lean mass accrual during adolescence: a longitudinal analysis. J Appl Physiol. 2008;105(2):734–741. doi: 10.1152/japplphysiol.00869.2007. [DOI] [PubMed] [Google Scholar]
- Behm DG, Faigenbaum AD, Falk B, Klentrou P. Canadian Society for Exercise Physiology position paper: resistance training in children and adolescents. Appl Physiol Nutr Metab. 2008;33(3):547–561. doi: 10.1139/H08-020. [DOI] [PubMed] [Google Scholar]
- Belanger AY, McComas AJ. Contractile properties of human skeletal muscle in childhood and adolescence. Eur J Appl Physiol Occup Physiol. 1989;58(6):563–567. doi: 10.1007/BF00418500. [DOI] [PubMed] [Google Scholar]
- Bencke J, Damsgaard R, Saekmose A, Jørgensen P, Jørgensen K, Klausen K. Anaerobic power and muscle strength characteristics of 11 years old elite and non-elite boys and girls from gymnastics, team handball, tennis and swimming. Scand J Med Sci Sports. 2002;12(3):171–178. doi: 10.1034/j.1600-0838.2002.01128.x. [DOI] [PubMed] [Google Scholar]
- Buchanan CI, Marsh RL. Effects of long-term exercise on the biomechanical properties of the Achilles tendon of guinea fowl. J Appl Physiol. 2001;90(1):164–171. doi: 10.1152/jappl.2001.90.1.164. [DOI] [PubMed] [Google Scholar]
- Cavanagh PR, Komi PV. Electromechanical delay in human skeletal muscle under concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol. 1979;42(3):159–163. doi: 10.1007/BF00431022. [DOI] [PubMed] [Google Scholar]
- Cornu C, Goubel F. Musculo-tendinous and joint elastic characteristics during elbow flexion in children. Clin Biomech (Bristol, Avon) 2001;16(9):758–764. doi: 10.1016/S0268-0033(01)00076-6. [DOI] [PubMed] [Google Scholar]
- De Ste Croix MBA, Armstrong N, Welsman JR. Concentric isokinetic leg strength in pre-teen, teenage and adult males and females. Biol Sport. 1999;16:75–88. [Google Scholar]
- Delagi EF, Perotto A. Anatomic guide for the electromyographer. CC Thomas; Springfield, Ill: 1980. [Google Scholar]
- Dubowitz V. Enzyme histochemistry of skeletal muscle. J Neurol Neurosurg Psychiatry. 1965;28(6):516–524. doi: 10.1136/jnnp.28.6.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke PM, Litt IF, Gross RT. Adolescents’ self-assessment of sexual maturation. Pediatrics. 1980;66(6):918–920. [PubMed] [Google Scholar]
- Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32(1):77–97. doi: 10.1079/BJN19740060. [DOI] [PubMed] [Google Scholar]
- Dvir Z. Isokinetics: muscle testing, interpretation, and clinical applications. Churchill Livingstone; New York, N.Y: 2004. [Google Scholar]
- Falk B, Dotan R. Child-adult differences in the recovery from high-intensity exercise. Exerc Sport Sci Rev. 2006;34(3):107–112. doi: 10.1249/00003677-200607000-00004. [DOI] [PubMed] [Google Scholar]
- Falk B, Brunton L, Dotan R, Usselman C, Klentrou P, Gabriel D. Muscle strength and contractile kinetics of isometric elbow flexion in girls and women. Pediatr Exerc Sci. 2009a;21(3):354–364. doi: 10.1123/pes.21.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk B, Usselman C, Dotan R, Brunton L, Klentrou P, Shaw J, Gabriel D. Child-adult differences in muscle strength and activation pattern during isometric elbow flexion and extension. Appl Physiol Nutr Metab. 2009b;34(4):609–615. doi: 10.1139/H09-020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel DA, Boucher JP. Practicing a maximal performance task: a cooperative strategy for muscle activity. Res Q Exerc Sport. 2000;71(3):217–228. doi: 10.1080/02701367.2000.10608902. [DOI] [PubMed] [Google Scholar]
- Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10(3):141–146. [PubMed] [Google Scholar]
- Gottlieb GL, Corcos DM, Agarwal GC. Organizing principles for single-joint movements. I A speed-insensitive strategy. J Neurophysiol. 1989;62(2):342–357. doi: 10.1152/jn.1989.62.2.342. [DOI] [PubMed] [Google Scholar]
- Grosset JF, Mora I, Lambertz D, Perot C. Age-related changes in twitch properties of plantar flexor muscles in prepubertal children. Pediatr Res. 2005;58(5):966–970. doi: 10.1203/01.PDR.0000181375.61935.7D. [DOI] [PubMed] [Google Scholar]
- Grosset JF, Mora I, Lambertz D, Pérot C. Voluntary activation of the triceps surae in prepubertal children. J Electromyogr Kinesiol. 2008;18(3):455–465. doi: 10.1016/j.jelekin.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Grosset JF, Piscione J, Lambertz D, Pérot C. Paired changes in electromechanical delay and musculo-tendinous stiffness after endurance or plyometric training. Eur J Appl Physiol. 2009;105(1):131–139. doi: 10.1007/s00421-008-0882-8. [DOI] [PubMed] [Google Scholar]
- Halin R, Germain P, Buttelli O, Kapitaniak B. Differences in strength and surface electromyogram characteristics between pre-pubertal gymnasts and untrained boys during brief and maintained maximal isometric voluntary contractions. Eur J Appl Physiol. 2002;87(4–5):409–415. doi: 10.1007/s00421-002-0643-z. [DOI] [PubMed] [Google Scholar]
- Halin R, Germain P, Bercier S, Kapitaniak B, Buttelli O. Neuromuscular response of young boys versus men during sustained maximal contraction. Med Sci Sports Exerc. 2003;35(6):1042–1048. doi: 10.1249/01.MSS.0000069407.02648.47. [DOI] [PubMed] [Google Scholar]
- Hatzikotoulas K, Patikas D, Bassa E, Paraschos I, Kotzamanidis C. Differences in voluntary activation between adult and prepubertal males. In: Jurimae T, Armstrong N, Jurimae J, editors. Children and Exercise XXIV: The Proceedings of the 24th Pediatric Work Physiology Meeting; September 2007; Talinn, Estonia. . Routledge: 2008. pp. 247–251. [Google Scholar]
- Holtermann A, Roeleveld K, Vereijken B, Ettema G. The effect of rate of force development on maximal force production: acute and training-related aspects. Eur J Appl Physiol. 2007;99(6):605–613. doi: 10.1007/s00421-006-0380-9. [DOI] [PubMed] [Google Scholar]
- Janz KF, Dawson JD, Mahoney LT. Increases in physical fitness during childhood improve cardiovascular health during adolescence: the Muscatine Study. Int J Sports Med. 2002;23(Suppl 1):S15–S21. doi: 10.1055/s-2002-28456. [DOI] [PubMed] [Google Scholar]
- Kellis E, Baltzopoulos V. Resistive eccentric exercise: effects of visual feedback on maximum moment of knee extensors and flexors. J Orthop Sports Phys Ther. 1996;23(2):120–124. doi: 10.2519/jospt.1996.23.2.120. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Kawakami Y, Fukunaga T. Elastic properties of muscle-tendon complex in long-distance runners. Eur J Appl Physiol. 2000;81(3):181–187. doi: 10.1007/s004210050028. [DOI] [PubMed] [Google Scholar]
- Lambertz D, Mora I, Grosset JF, Perot C. Evaluation of musculotendinous stiffness in prepubertal children and adults, taking into account muscle activity. J Appl Physiol. 2003;95(1):64–72. doi: 10.1152/japplphysiol.00885.2002. [DOI] [PubMed] [Google Scholar]
- Lattier G, Millet GY, Maffiuletti NA, Babault N, Lepers R. Neuromuscular differences between endurance-trained, power-trained, and sedentary subjects. J Strength Cond Res. 2003;17(3):514–521. doi: 10.1519/1533-4287(2003)017<0514:NDBEPA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lucía A, Hoyos J, Pardo J, Chicharro JL. Metabolic and neuromuscular adaptations to endurance training in professional cyclists: a longitudinal study. Jpn J Physiol. 2000;50(3):381–388. doi: 10.2170/jjphysiol.50.381. [DOI] [PubMed] [Google Scholar]
- Maffulli N, King JB, Helms P. Training in élite young athletes (the Training of Young Athletes (TOYA) Study): injuries, flexibility and isometric strength. Br J Sports Med. 1994;28(2):123–136. doi: 10.1136/bjsm.28.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirwald RL, Baxter-Jones AD, Bailey DA, Beunen GP. An assessment of maturity from anthropometric measurements. Med Sci Sports Exerc. 2002;34(4):689–694. doi: 10.1097/00005768-200204000-00020. [DOI] [PubMed] [Google Scholar]
- Morse CI, Tolfrey K, Thom JM, Vassilopoulos V, Maganaris CN, Narici MV. Gastrocnemius muscle specific force in boys and men. J Appl Physiol. 2008;104(2):469–474. doi: 10.1152/japplphysiol.00697.2007. [DOI] [PubMed] [Google Scholar]
- O’Brien TD, Reeves ND, Baltzopoulos V, Jones DA, Maganaris CN. The effects of agonist and antagonist muscle activation on the knee extension moment-angle relationship in adults and children. Eur J Appl Physiol. 2009;106(6):849–856. doi: 10.1007/s00421-009-1088-4. [DOI] [PubMed] [Google Scholar]
- Ozmun JC, Mikesky AE, Surburg PR. Neuromuscular adaptations following prepubescent strength training. Med Sci Sports Exerc. 1994;26(4):510–514. [PubMed] [Google Scholar]
- Pääsuke M, Ereline J, Gapeyeva H. Twitch contraction properties of plantar flexor muscles in pre- and post-pubertal boys and men. Eur J Appl Physiol. 2000;82(5–6):459–464. doi: 10.1007/s004210000236. [DOI] [PubMed] [Google Scholar]
- Pang MY, Eng JJ, Dawson AS, Gylfadóttir S. The use of aerobic exercise training in improving aerobic capacity in individuals with stroke: a meta-analysis. Clin Rehabil. 2006;20(2):97–111. doi: 10.1191/0269215506cr926oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay JA, Blimkie CJ, Smith K, Garner S, MacDougall JD, Sale DG. Strength training effects in prepubescent boys. Med Sci Sports Exerc. 1990;22(5):605–614. doi: 10.1249/00005768-199010000-00011. [DOI] [PubMed] [Google Scholar]
- Sale DG. Neural adaptation to resistance training. Med Sci Sports Exerc. 1988;20(Suppl 5):S135–S145. doi: 10.1249/00005768-198810001-00009. [DOI] [PubMed] [Google Scholar]
- Slaughter MH, Lohman TG, Boileau RA, Horswill CA, Stillman RJ, Van Loan MD, Bemben DA. Skin-fold equations for estimation of body fatness in children and youth. Hum Biol. 1988;60(5):709–723. [PubMed] [Google Scholar]
- Sleivert GG, Backus RD, Wenger HA. Neuromuscular differences between volleyball players, middle distance runners and untrained controls. Int J Sports Med. 1995;16(6):390–398. doi: 10.1055/s-2007-973026. [DOI] [PubMed] [Google Scholar]
- Smits-Engelsman BC, Westenberg Y, Duysens J. Development of isometric force and force control in children. Brain Res Cogn Brain Res. 2003;17(1):68–74. doi: 10.1016/S0926-6410(03)00081-8. [DOI] [PubMed] [Google Scholar]
- Stenevi-Lundgren S, Daly RM, Lindén C, Gärdsell P, Karlsson MK. Effects of a daily school based physical activity intervention program on muscle development in prepubertal girls. Eur J Appl Physiol. 2009;105(4):533–541. doi: 10.1007/s00421-008-0932-2. [DOI] [PubMed] [Google Scholar]
- Streckis V, Skurvydas A, Ratkevicius A. Children are more susceptible to central fatigue than adults. Muscle Nerve. 2007;36 (3):357–363. doi: 10.1002/mus.20816. [DOI] [PubMed] [Google Scholar]
- Tanner JM. Growth at adolescence. Blackwell Scientific Publications; Oxford, England: 1962. [Google Scholar]
- Van Cutsem M, Duchateau J, Hainaut K. Changes in single motor unit behaviour contribute to the increase in contraction speed after dynamic training in humans. J Physiol. 1998;513(Pt. 1):295–305. doi: 10.1111/j.1469-7793.1998.295by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakas A. The effect of cycle ergometer strength training in prepubertal untrained males. Pediatr Exerc Sci. 2004;16(2):368–377. [Google Scholar]