Abstract
Background:
The conduct of a randomized controlled trial (RCT) is deemed ethical only if we are in state of “equipoise” as to which treatment would be most beneficial for the patients. Individual equipoise applies to an individual clinician or a member of ethical, institutional review board (IRB), whilst collective equipoise refers to the profession as a whole. It is argued that physicians are not bound by the equipoise but their actions are directed by the confines of the expert opinion. Experts can agree or disagree in various proportions on the merit of a given treatment. Hence, the collective equipoise will be often incomplete. In turn, the opinions of content expert in the field of the proposed trial influence the IRB members’ decision regarding trial approval.
Methods:
We conducted a survey of IRB members at University of South Florida and the IRB members attending the bioethics conference organized in Clearwater, Florida, USA. The survey was made available as hard copy (paper based) and included six hypothetical scenarios outlining clinical trials targeted at measuring the collective equipoise. We defined the collective equipoise as the situation when survey participants were equally split (50:50) in their decision regarding whether a proposed clinical trial would be ethical to conduct. The opinion of 100 experts in the field expressed as proportion of experts favoring treatment A vs. B in each of the five scenarios was made available to the participants.
Results:
The response rate of our survey was 33% (71/218). Fifty percent of the IRB members would approve an RCT addressing the efficacy of two drugs for the management of headache even if 80% of experts favor one treatment over another (median: 80%; third quartile: 80%). Similarly, half of participating IRB members would approve the study when the median distribution of equipoise among experts was 70% (70 in favor of treatment A vs. 30 in favor of treatment B) for treatment of leukemia, 60% for treatment of geriatric patients and 70% for treatment of newborns. Half of IRB members would approve the study when the median distribution of equipoise among experts was 70% for treatment for leukemia in dogs and 85% for leukemia in rats (and 25% of IRB members would approve such a study even if 100% of experts favors one treatment over another). None of the demographic features of respondents affected collective equipoise.
Conclusions:
This is the first study assessing collective equipoise among ethical committee/IRB members. Our study findings show that IRB members perceived that conduct of a trial enrolling humans is unethical when the equipoise level is beyond 80% (80:20 distribution of uncertainty). IRB members require a higher level of equipoise when it comes to testing a new drug in humans than in animals. A relatively high level of equipoise is needed for IRB members to be comfortable to approve trials involving life-threatening situations, children and elderly patients.
Key words: randomized controlled trial, ethics, Ethical committees.
1. INTRODUCTION
There are three key approaches to conducting scientific research in humans: goal oriented, duty oriented and the right-based approaches. The goal oriented approach also termed as utilitarian approach ensures scientifically robust results which promise maximum benefit to the greatest number of future participants and participants enrolled in the trials (1). The duty oriented–Kantian approach refers to conduct of only research which in the best interest of patients (2, 3). The right-based approach refers to conduct of only research to which patients have provided their informed consent. The uncertainty or equipoise principle is a common factor among these three approaches to research in humans (4-6). That is, research should be conducted with a goal to resolve existing uncertainties e.g. to compare the efficacy of antibiotics use vs. watchful waiting for the management of upper respiratory tract infections among children. If there are no uncertainties, there would be no need for clinical research to inform decision-making (7).
Randomized controlled trials (RCTs) are considered as the gold standard for informing treatment decisions as RCTs are based on a deductive method. That is, if the assumptions of the test are met, a positive result obtained via a RCT implies the appropriate causal conclusion. Hence the majority of researchers and participating patients accept RCTs as a tool to resolve existing uncertainties (8) (9). However, the conduct of a RCT is deemed ethical only if we are uncertain that is in state of “equipoise” as to which treatment would be most beneficial for the patients. If we are truly in equipoise, a clinical trial, and particularly a RCT, serves the patients’ interest best, since he or she does not lose out prospectively on the benefits for participation as the treatment, that is being tested, has equal chances to be as beneficial as harmful (6).
Any new study is designed based on the existing knowledge of the treatments being tested. Existing information can range from simply not knowing to having different degrees of uncertainties including a state of equipoise when we are equally positioned in our beliefs between the benefits and harms of a certain treatment or the choice between two or more competing treatments (6, 10). The state of equipoise can be held by researchers, patients and members of the community at large including the research regulatory officials such as members of the institutional review board (IRB) who approve the trials.
Individual equipoise applies to an individual clinician or IRB member, while collective equipoise refers to the profession as a whole (10). Freedman et al argued that physicians are not bound by the equipoise but their actions are directed by the confines of the expert opinion (11). Experts can agree or disagree in various proportions on the merit of a given proposal, and in their beliefs, say if treatment A is superior to treatment B. Hence, the collective equipoise will be often incomplete. In turn, the opinions of content expert in the field (e.g. oncologists) of the proposed trial influence the individuals involved in the conduct of research in humans including IRB members’ decision regarding trial approval. Hence, it is worthwhile to investigate under which circumstances IRB members will be equally split (50:50) in their decision to approve a proposed RCT as a function of the proportion of experts favoring one treatment over another? Accordingly, we conducted a survey of IRB members to assess the degree of collective equipoise necessary for a specific type of trial to be deemed ethical and hence approved by IRB.
2. METHODS
Data collection: We conducted a survey of IRB members at University of South Florida and the IRB members attending the bioethics conference organized in Clearwater, Florida, USA. This paper represents a full description of the previously reported analysis in the abstract form only (12). The survey was distributed to 218 IRB members. The survey was made available as hard copy (paper based) and included six hypothetical scenarios outlining clinical trials targeted at measuring the collective equipoise. We defined the collective equipoise as the situation when survey participants were equally split (50:50) in their decision regarding whether a proposed clinical trial would be ethical to conduct. In each of the five scenarios the choice of the treatment was determined by chance alone (flip of a coin). The opinion of 100 experts in the field expressed as proportion of experts favoring treatment A vs. B in each of the five scenarios was made available to the participants. IRB members were requested to answer the question: “under which circumstances of the experts’ agreement/disagreement would you approve a randomized trial for each of the scenarios listed above?” The survey was modeled after the one described by who assessed collective equipoise in lay people (13). We collected data on demographics including years of service with IRB from the participants. Our study was approved by University of South Florida IRB.
Data analysis: We conducted descriptive analysis including median and interquartile range of approval of trial by participants for the six scenarios. We assessed the difference between the collective equipoise estimates of non-MD IRB members and IRB members who were physicians by employing the Mann-Whitney test (14). We conducted simple logistic regression to determine the impact of demographic variables on the collective equipoise.
3. RESULTS
The response rate of our survey was 33% (71/218). Sixty seven percent (146/218) of the participants were females and 33% (72/218) were males. Fifty eight percent (126/218) were active members of the IRB at the time of the survey. Fourteen percent (31/218) were physicians (holding a MD degree). The median age of participants was 48 years (range: 24-75 years). On average the participants served for 3 years on IRB (median: 3 years, range: 0.5-12 years).
Fifty percent of the IRB members would approve an RCT addressing the efficacy of two drugs for the management of headache even if 80% of experts favor one treatment over another (median: 80%; third quartile: 80%) (Table).
Table 1.
Results of trial testing safety and efficacy of two drugs in our sample
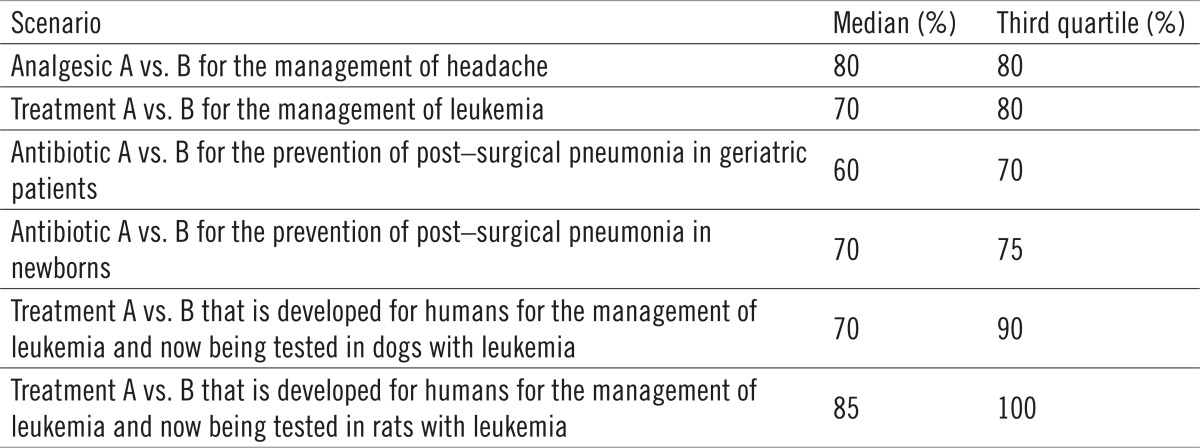 |
Even if 70% (median: 70%; third quartile: 80%) of experts favor one treatment over another 50% of IRB members would approve an RCT addressing the efficacy of two drugs for the management of leukemia. That is, 25% of IRB members would accept enrollment into a trial addressing safety and efficacy of two treatments for the management of leukemia even if 80% of the experts favor one treatment over another (Table).
Even if 60% (median: 60%; third quartile: 70%) of experts favor one treatment over another 50% of the IRB members would approve an RCT addressing the safety and efficacy of two antibiotics in the treatment of pneumonia among elderly patients. That is, 25% of the IRB members would accept enrollment into trial assessing safety and efficacy of antibiotics for the treatment of pneumonia among elderly patients even if 70% of the experts favor one treatment over another (Table).
Even if 70% (median: 70%; third quartile: 75%) of experts favor one treatment over another 50% of the IRB members would approve an RCT addressing the safety and efficacy of two antibiotics in the treatment of pneumonia among newborns recovering from surgery. That is, 25% of the IRB members would accept enrollment into trial testing safety and efficacy of the treatment of pneumonia among new born babies recovering from surgery even if 75% of the experts favor one treatment over another (Table).
Even if 70% (median: 70%; third quartile: 90%) of experts favor one treatment over another 50% of the IRB members would approve an RCT addressing the safety and efficacy of two drugs in dogs. That is, 25% of the IRB members would accept enrollment into trial testing safety and efficacy in dogs even if 90% of the experts favor one treatment over another. Even if 85% (median: 85%; third quartile: 100%) of experts favor one treatment over another 50% of the IRB members would approve an RCT addressing the safety and efficacy of two drugs in rats. That is, 25% of the IRB members would accept enrollment into trial testing safety and efficacy of two drugs in rats even if 100% of the experts favor one treatment over another (Table).
Fifty percent (93/187) of non-MD IRB members would approve an RCT assessing the efficacy of two drugs for the management of headache if 75% (median: 75%; third quartile: 90%) of experts favor one treatment over another while 50% (15/31) of MD IRB members would accept enrollment into trial even if 80% (median: 80%; third quartile: 80%) of the experts favor one treatment over another (P value= 0.01).
None of the demographic features of the participating IRB members had an impact on the collective equipoise.
4. DISCUSSION
This is the first survey assessing collective equipoise among ethical committee/IRB members. Findings of our survey indicate that IRB members require a higher level of equipoise (i.e. more uncertainty) when it comes to testing a new drug in humans than in animals. The trial enrolling humans most likely to be tolerated is the comparison of analgesics for the treatment of headache. IRB members responded with a significant variation in their acceptance ranging from 50% to 100%. A relatively high level of equipoise is needed for IRB members to be comfortable to approve a trial involving life-threatening situations, such as trial addressing efficacy and harms of two competing interventions for leukemia. Our survey results show that the highest level of equipoise is required when new drugs are tested in older populations and children who are considered to be potentially vulnerable to violation of free consent to participate in a trial. Institutional review board members participating in our survey preferred experiments on rats compared with dogs and dogs compared with humans with requirement of increasing degree of equipoise, respectively. The degree of collective equipoise was not influenced by demographic factors. However, IRB members without a MD degree required statistically significantly higher level of equipoise to approve the conduct of a trial compared to IRB members with an MD degree.
In summary, our study findings show that IRB members perceived that conduct of a trial enrolling humans is unethical when the equipoise level is beyond 80% (80:20 distribution of uncertainty). That is, when 80% or more experts prefer treatment “A” over the competing treatment, then half of the IRB members would prefer that the treatment “A” may be used in practice instead of evaluating its safety and efficacy via clinical trials. Our findings are in line with previous research conducted by Johnson et al. enrolling a convenient sample of 105 lay individuals and 8 medical students (15).
Individuals enrolled in the study by Johnson et al perceived that conduct of a trial enrolling humans in unethical when the equipoise level is beyond 70% (70:20 distribution of uncertainty) (15).
Interestingly, under the assumptions that experimental treatment is >80% successful most rational decision for patients themselves is to trust researchers (and by extension IRB members), despite the possibility that the researchers or IRB members may decide to approve the study based on the factors other than patients’ benefits: the likelihood of obtaining successful treatment appears to justify putting oneself in a vulnerable position (16).
Conflict of interest
None declared.
REFERENCES
- 1.Resnik DB. Paternalism and Utilitarianism in Research with Human Participants. Health care analysis : HCA: journal of health philosophy and policy. 2012 doi: 10.1007/s10728-012-0233-0. Epub 2012/10/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly MP, Moore TA. The judgement process in evidence-based medicine and health technology assessment. Social theory & health: STH. 2012;10(1):1–19. doi: 10.1057/sth.2011.21. Epub 2012/12/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothhaar M. Human dignity and human rights in bioethics: the Kantian approach. Medicine, health care, and philosophy. 2010;13(3):251–257. doi: 10.1007/s11019-010-9249-0. Epub 2010/04/23. [DOI] [PubMed] [Google Scholar]
- 4.Alderson P. Equipoise as a means of managing uncertainty: personal, communal and proxy. Journal of medical ethics. 1996;22(3):135–139. doi: 10.1136/jme.22.3.135. Epub 1996/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson JA. Contextualizing clinical research: the epistemological role of clinical equipoise. Theoretical medicine and bioethics. 2009;30(4):269–288. doi: 10.1007/s11017-009-9104-6. Epub 2009/05/01. [DOI] [PubMed] [Google Scholar]
- 6.Djulbegovic B. Uncertainty and equipoise: at interplay between epistemology, decision making and ethics. The American journal of the medical sciences. 2011;342(4):282–289. doi: 10.1097/MAJ.0b013e318227e0b8. Epub 2011/08/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Djulbegovic B, Hozo I. S. G. Uncertainty in clinical Medicine. In: Dov M, Gabbay, Woods J, editors. Encyclopedia of Medical Philosophy. San Dieago, North Holland: 2011. [Google Scholar]
- 8.Cartwright N. Are RCTs the Gold Standard? BioSocieties. 2007;2(1):11–20. [Google Scholar]
- 9.Cartwright N. A philosopher’s view of the long road from RCTs to effectiveness. The Lancet. 2011;377(9775):1400–1401. doi: 10.1016/s0140-6736(11)60563-1. [DOI] [PubMed] [Google Scholar]
- 10.Djulbegovic B. Articulating and responding to uncertainties in clinical research. J Med Philosophy. 2007;32:79–98. doi: 10.1080/03605310701255719. [DOI] [PubMed] [Google Scholar]
- 11.Freedman B. Equipoise and the ethics of clinical research. The New England journal of medicine. 1987;317(3):141–145. doi: 10.1056/NEJM198707163170304. Epub 1987/07/16. [DOI] [PubMed] [Google Scholar]
- 12.Djulbegovic B, Bercu BB, editors. At what level of collective equipoise does a clinical trial become ethical for the IRB members? Clearwater, FL, USA: University of South Florida Third National Symposium - Bioethical Considerations in Human Subject Research; 2002. Mar 8-10, [Google Scholar]
- 13.Johnson N, Lilford JR, Brazier W. At what level of collective equipoise does a clinical trial become ethical? J Med Ethics. 1991;17:30–34. doi: 10.1136/jme.17.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hart A. Mann-Whitney test is not just a test of medians: differences in spread can be important. BMJ. 2001;323(7309):391–393. doi: 10.1136/bmj.323.7309.391. Epub 2001/08/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson N, Lilford RJ, Brazier W. At what level of collective equipoise does a clinical trial become ethical? Journal of medical ethics. 1991;17(1):30–34. doi: 10.1136/jme.17.1.30. Epub 1991/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Djulbegovic B, Hozo I. When is it rational to participate in a clinical trial? A game theory approach incorporating trust, regret and guilt. BMC medical research methodology. 2012;12:85. doi: 10.1186/1471-2288-12-85. Epub 2012/06/26. [DOI] [PMC free article] [PubMed] [Google Scholar]


