Abstract
The Aim:
To show histopathological diagnoses after colonoscopic polypectomy in the University Hospital Center (KBC) Split with recommendations on further follow-up colonoscopy depending on the endoscopic and histological findings.
Patients and Methods:
The study included 2842 patients who underwent colonoscopy in a two-year period (2008-2009), followed by a detailed analysis of 350 patients in which one or more polyps were simultaneously removed and 163 patients who were only sampled for histological analysis. Patients from the National Program for Colorectal Cancer Prevention and patients in which colonoscopy is indicated as part of daily outpatient or inpatient treatment were included as well.
Results:
During 2008 and 2009 in KBC Split, out of a total of 2842 colonoscopies, 350 patients underwent colonoscopic polypectomy, whereby 618 polyps were removed (1-8 polyps in individual patients), while in 163 patients only biopsy specimens were sampled. Out of the total of 557 polyps sent for histological analysis, 236 were hyperplastic (42%), 193 were identified as tubular adenoma (35%), 84 were tubulovillous (15%), 18 villous (3%), 9 were adenocarcinoma (2%) and other 17 (3%). In 35 (15.4%) polyps high-grade dysplasia was found. The largest number of nonpolypectomized changes confirmed the presence of adenocarcinoma (76-47%), adenomas and hyperplastic polyps were 37 (22%) and regular findings 23 (14%). Mucosal high-grade dysplasia was demonstrated in 35 (23.1%) biopsied changes.
Conclusion:
Colonoscopies with polypectomy decreased the risk of the formation of colorectal cancer in these patients almost to the level of risk in patients who have not even had a polyp during colonoscopy. Arguably the best method of prevention and early detection of colorectal cancer are already widely established national programs. The next qualitative level is constantly improving the quality of colonoscopy with clear criteria and the establishment of a body to evaluate the performers and the equipment, and making recommendations on the colonoscopy follow-up intervals depending on endoscopic and histopathological findings of patients who for any reason underwent colonoscopy.
Key words: colonoscopic polipectomy, surveillance, quality of endoscopy.
1. INTRODUCTION
Colorectal cancer (CRC) was the second leading cancer-related cause of death both in men (N=1063; 49.77/100.000) and in women (N=803; 34.89/100.000) (1) in Croatia in 2009. As much as 30-40% of patients are in the stage of a metastatic disease at the time the diagnosis is set, since even the advanced CRC can present almost none or very few non-specific symptoms. In this case, the only way of identifying the condition is colonoscopy, which makes it the diagnostic method of choice in CRC screening.1 In accordance with this, a National program of early CRC diagnostics was introduced in Croatia in 2007 (2, 3).
Today it is known for a fact that as much as 95% of carcinomas grow out of polyps (polis=many, pous=foot), neoplasms which grow due to the proliferation of the mucous membrane into the lumen of the colon and the rectum. Even though mostly benign, some of them possess a lower or higher malignancy potential and therefore need to be removed by a procedure called polypectomy. Following removal, the polyps need to be sent for histological analysis, and according to the findings (type and size of the polyp, grade of dysplasia, resection margins) colonoscopy follow-up intervals need to be recommend (4).
The aim of this study was to analyze colonoscopies performed in KBC Split within a two-year period (2008-2009), with special emphasis on polypectomies, the histological classification of polyps and complications following colonoscopic polypectomy.
Various factors which determine the current follow-up recommendations for patients who underwent colonoscopy have been analyzed as well. Of these, the findings obtained by colonoscopy are most important in epidemiological and clinical terms.
2. PATIENTS AND METHODS
The patients’ data have been gathered by a retrospective analysis of the database and the discharge letters of the Department of Internal Medicine KBC Split, as well as by the insight into the pathohistological diagnoses of the Department of Clinical Pathology KBC Split. The study included all patients who underwent colonoscopy at the Department for Gastroenterology and Hepatology of the Department of Internal Medicine KBC Split from Jan. 1st 2008 to Dec. 31st 2009, as well as those patients in which during the same period only biopsy specimens were sampled from new formations without performing polypectomy.
Patients from the National Program for Colorectal Cancer Prevention and patients in which colonoscopy is indicated as part of daily outpatient or inpatient treatment were included as well. Along with demographic data, we analyzed the number of performed colonoscopies and removed polyps, the histological structure of the polyp, complications and problems following the procedure, and finally pathohistological findings of biopsies taken from neoplasms without performing polypectomy.
3. RESULTS
Between January 1st 2008 and December 31st 2009, a total of 2842 colonoscopies were performed at the Department for Gastroenterology and Hepatology of the Department of Internal Diseases KBC Split, during which 350 patients underwent colonoscopic polypectomy and whereby 618 polyps were removed (1-8 polyps in individual patients), while in 163 patients only biopsy specimens were sampled from nonpolypectomized neoplasms (Tables 1 and 2).
Table 1.
Colonoscopies between January 1st 2008 and December 31st 2009
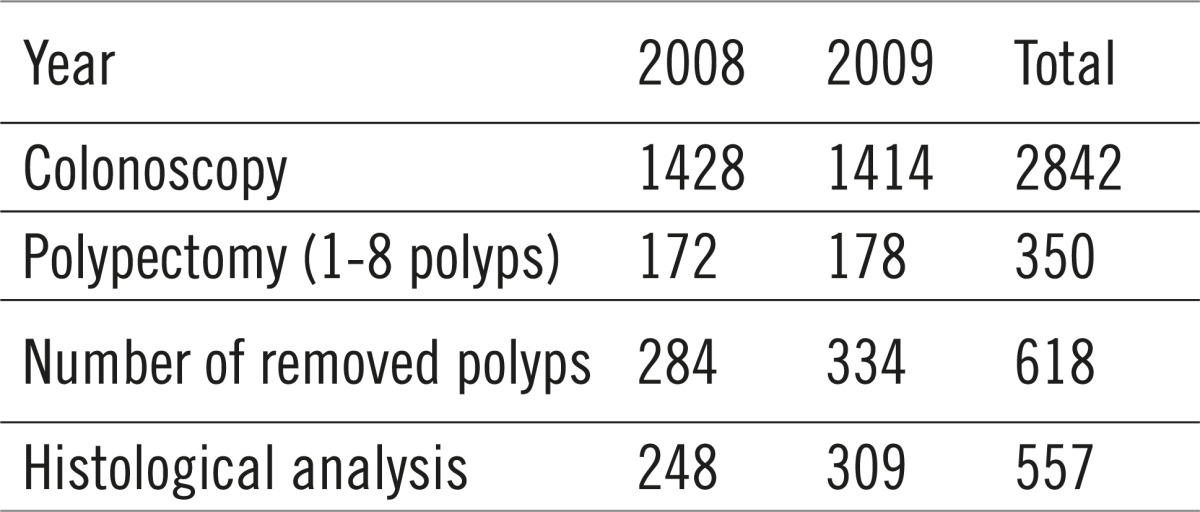 |
Table 2.
Demographic data of patients who underwent polypectomy
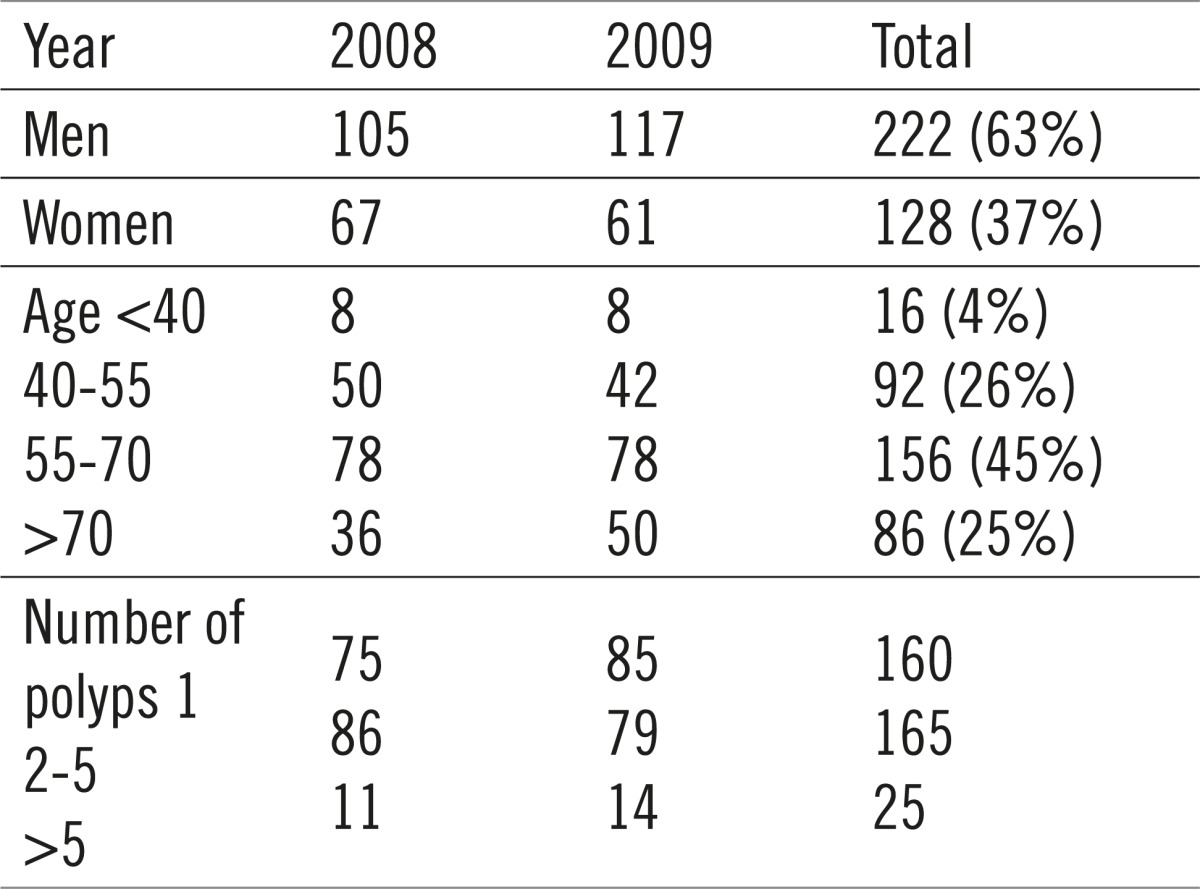 |
Out of the total number of patients which underwent polypectomy, 222 were men (63%), and 128 (37%) were women. The average age of patients was 57, with 71% of them being between 40 and 70 years old. A single polyp was found in 160 patients, 2-5 were found in 165 of them, whereas more than 5 polyps were found in 25 patients. A total of 618 polyps were removed, with 557 of them sent to histological analysis (Table 3). Polyps not sent to analysis were deemed clinically insignificant by the endoscopist, with the polypectomy itself as the undoubtedly final solution.
Table 3.
Histological structure of the polyp
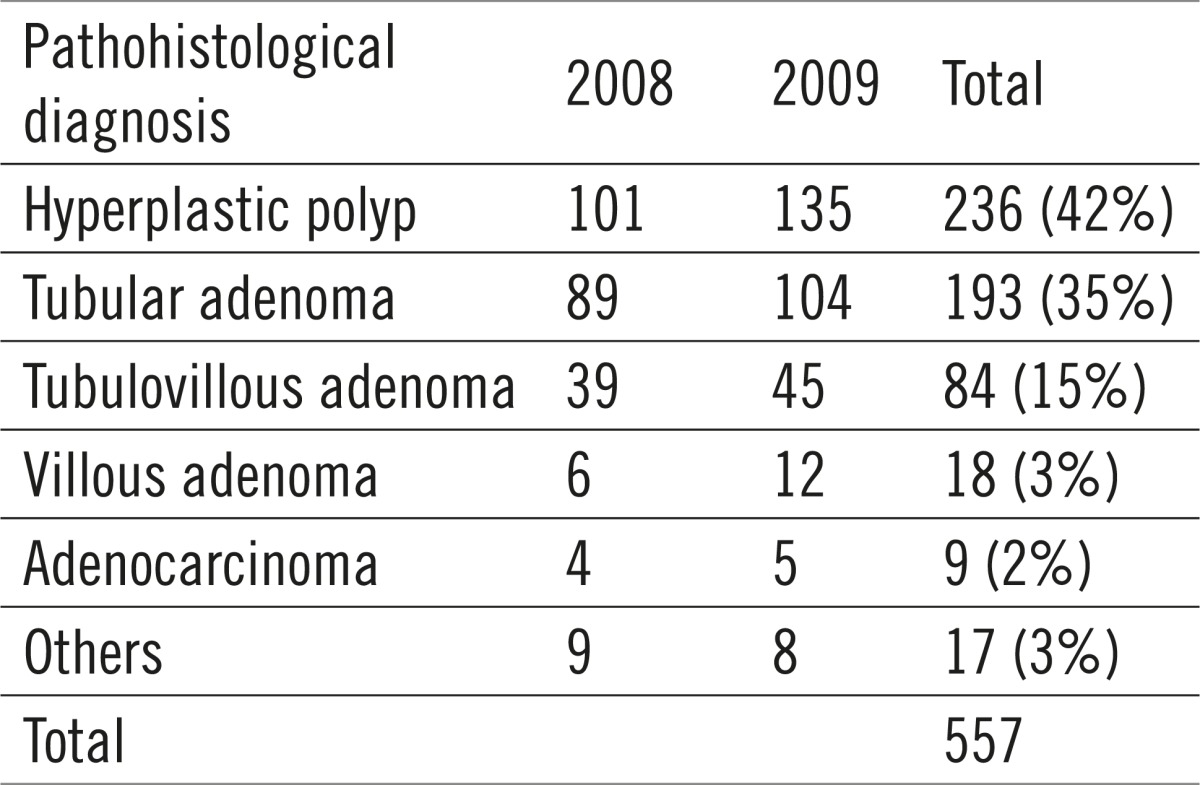 |
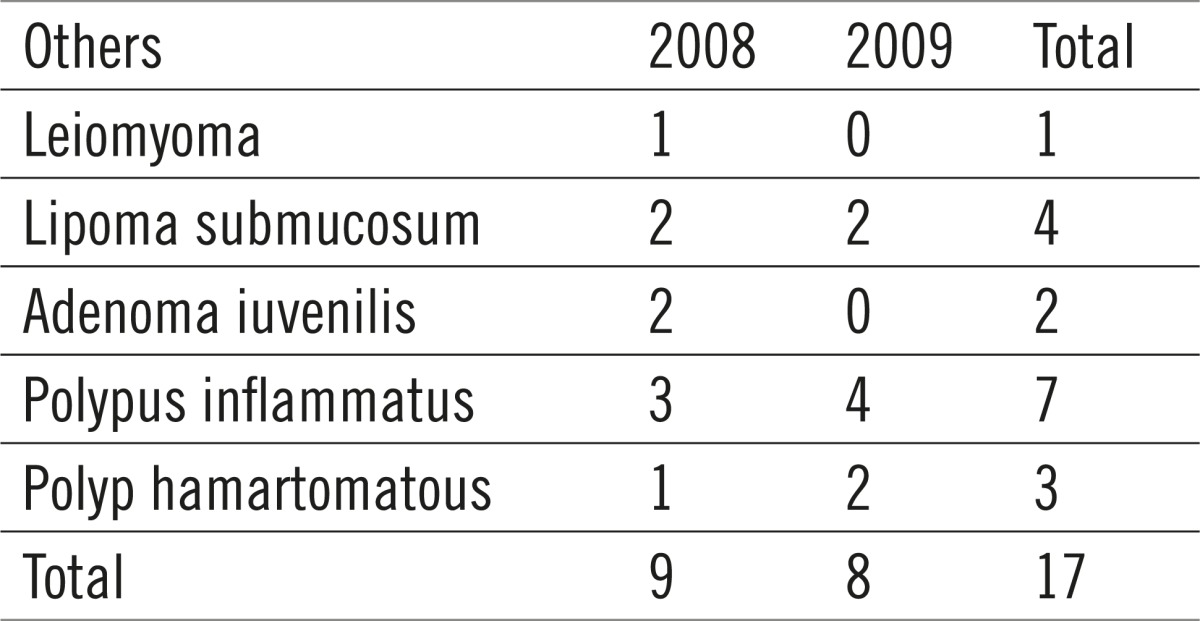
Out of the total of 557 polyps sent to histological analysis, 236 were hyperplastic (42%), 193 were identified as tubular adenoma (35%), 84 were tubulovillous (15%), 18 villous (3%), 9 were adenocarcinoma (2%) and other 17 (3%), which includes mostly the nonneoplastic mucous tumors (juvenile, inflammatory and hamartomatous polyps) and submucous lipomas and leiomyomata (Table 4).
Table 4.
Rare pathohistological diagnoses
 |
Cell dysplasia was described in 195 analyzed polyps, whereof 150 (76.9%) of low grade and 35 (23.1%) of high grade.
During and following colonoscopic polypectomy 16 patients (4.6%) developed a complication. One patient suffered bowel perforation and was successfully operated on. 9 patients who were bleeding were all hospitalized and treated conservatively. 6 patients were hospitalized for a period of 2-3 days due to subjective difficulties, such as stomach pain, flatulence and general weakness.
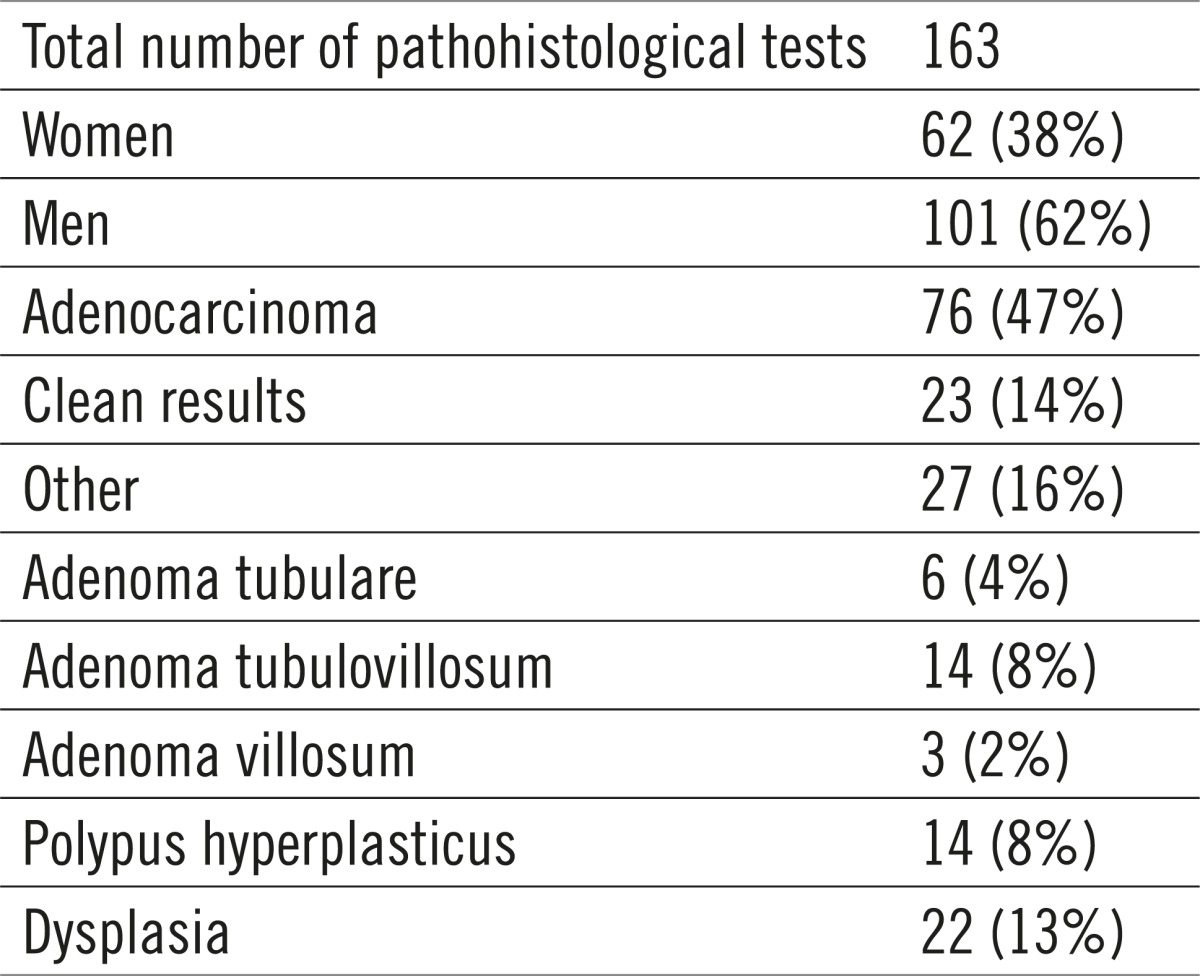
The largest number of pathohistological results confirmed the existence of adenocarcinomas, as much as 76 (47%). The other results identify the growths as adenomas and hyperplastic polyps, while there were 23 clean results. Mucosal high-grade dysplasia was proven in 22 (13%) biopsied changes. (Table 5, 6, 7).
Table 5.
Grade of cell dysplasia
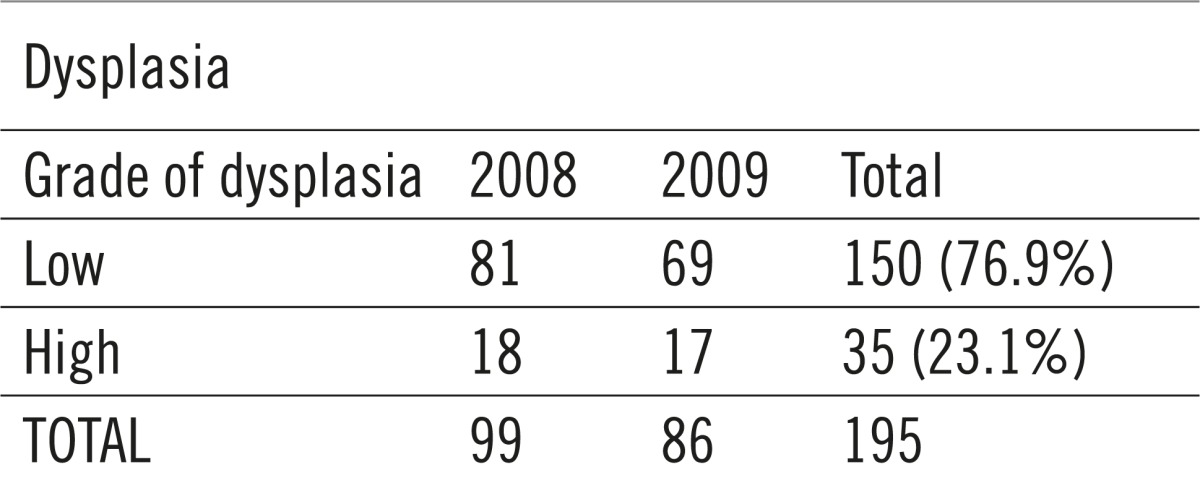 |
Table 6.
Complications following polypectomy
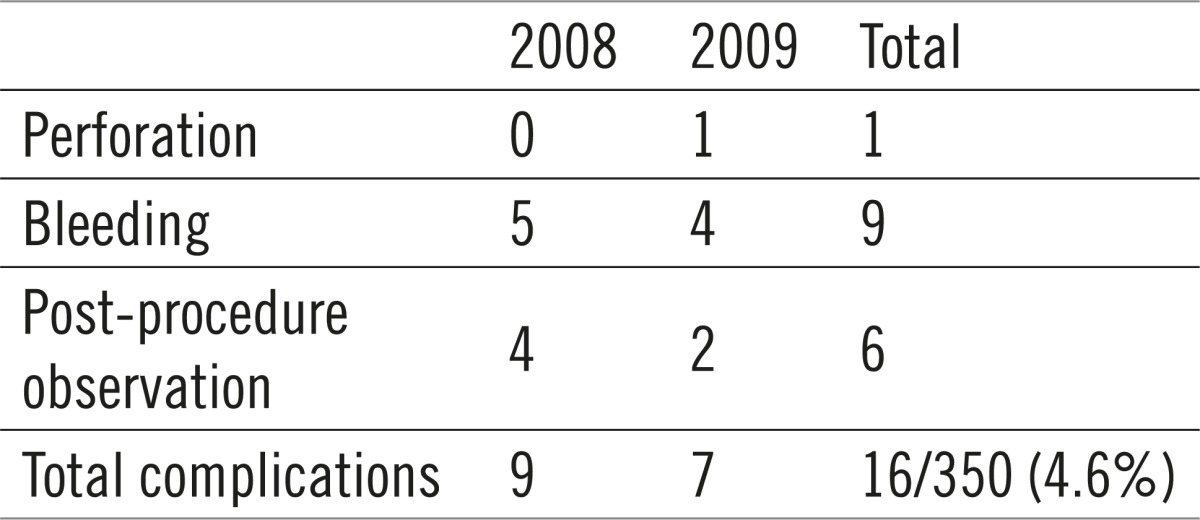 |
Table 7.
Biopsy results of nonpolypectomized neoplasms
 |
4. DISCUSSION
Hereditary and environmental factors play an important role in CRC occurrence. With the advancement of genetic research, a genetic predisposition can be accurately identified in one third of CRCs, implying that the family members of the patients also need to take genetic tests, followed by colonoscopies at precisely determined intervals (5). A diet containing large amounts of animal fats and industrially processed meats is considered to be an important environmental factor affecting polyp growth (6).
Polyps of the colon usually grow without symptoms, which clarifies why without a widely accepted screening strategy some of them are not found for too long, i.e. until they turn malignant. If the polyps are big, they can induce stomach pains and spasms. Big villous polyps can also induce persistent diarrhea. Early carcinomas are found in only 18% of patients, which is precisely why screening programs have been initiated.7
Polyps can grow at any age in both sexes, but are more common in persons over 50. In almost one third of this group, routine and screening colonoscopies show regular results. Pedunculated polyps present a much smaller problem than the large sessile polyps, which demand a polypectomy with a mucosectomy to be performed, in order to avoid an incomplete resection and prevent the neoplastic tissue from remaining in the colon wall (8).
It is an undivided opinion that screening for colon cancer decreases its fatality in two ways: by an early discovery of malignant lesions, as well as by removing polyps in their benign phase, thus preventing a possible malignant alteration of some of them. The confirmation of the first claim was obtained relatively easy, in a shorter time period and on a smaller number of patients, as patients tend to live significantly longer with CRC being discovered at an early stage. Since the finding of adenomatous polyps is a much more frequent finding than CRC, the significance of polypectomy on a later development of CRC, and the fatality it causes has been analyzed. In both cases polypectomy had a distinctly protective effect (9, 10) which was visible already after the first 10 years of monitoring, since colonoscopies with polypectomies had lowered the risk of CRC development to the risk level of patients who did not even have polyps at colonoscopy! The authors explain such a good result also by the excellent conditions in which controlled randomized studies are usually performed (most experienced endoscopists, enough time for bowel exploration, insisting on a total colonoscopy, good patient preparation, and so on), which cannot always be achieved on the conditions in actual clinical practice. Precisely owing to early CRC detection, polypectomies, but certainly also to always improving therapy options, the CRC mortality has been slowly decreasing in the last 3-4 years. An additional important factor are the possible lifestyle changes in polypectomized patients, who very often change their diets, intensify physical activity, stop smoking, and often take multivitamin preparations and NSAIDs in order to prevent adenoma reoccurrence and CRC development (11).
The risk of malignant alteration is bigger if the polyps are larger in size and villous in build, if there are more polyps and if cell atypia or dysplastic changes are present. The National Programs of CRC Prevention are therefore today a generally accepted strategy, however with growing attention redirected to further monitoring of polypectomized patients, since they are under greater risk of the growth of new polyps, and there is always a possibility of overlooked or partially resected polyps. This explains “new” polyps found in control colonoscopies of 20-50% of patients who underwent polypectomy 3-5 years before that, with even 10% of them being diagnosed also with CRC (“interval CRCs”). Risk stratification has thus been defined depending on the number, size and histological structure of the polyp (villous component, high-grade dysplasia). In the research of Lieberman et al., a polyp >10 mm increased the risk of new polyp growth after 5 years from 2.4 to 15.5% (RR, 5.01; 95% CI, 2.10 –11.96) (12). Similar results have been calculated for the risk carried by 3 or more adenomas (RR 2.52; 95% CI1.07-5.97) and high-grade dysplasia (RR 1.84; 95% CI 1.06-3.19). This is why the US Multi-Society Task Force on Colorectal Cancer has divided polypectomized patients into low (up to 2 polyps <10 mm) and high risk groups (polyp >10 mm and/or 3 or more polyps and/or more pronounced villous component and/or high-grade dysplasia) (1). In the United Kingdom an additional group of medium risk has been stratified with only one polyp >10 mm or 3-4 smaller ones (13).
CRCs discovered only 6 months to 3 years after polypectomies are usually in the proximal colon, which is explained by a poorer preparation, unreached cecum, the fact that there are more sessile polyps in this area, with the biggest problems being of course the overlooked polyps (up to 17%!) and an incomplete resection (up to 27% of new polyps are diagnosed in the same segment).
Complications which can occur during and following polypectomy are bleeding and colon perforation (14, 15). The endoscopist can stop the bleeding during the exam itself, and even smaller perforations can be successfully solved by endoscopic methods. Sometimes the patients still have to be surgically treated, which happened only once in two years in our Clinic, due to perforation.
Due to all of the above it is necessary to insist on the one hand on the quality of colonoscopy, since endoscopists with more discovered polyps and >95% cecum intubations have less cases of CRC in control colonoscopies, and on the other hand on monitoring polypectomized patients according to the guidelines of competent operative groups (16). A situation similar to a published research in the field of family medicine should not happen, where excessive monitoring of low risk patients after polypectomy was proven (20% those without polyps and even 40% those with small tubular adenomas underwent colonoscopy already after 1-3 years), whereas as much as 40% of patients with a high risk either did not undergo colonoscopy in 5 years or it was not even suggested to them (17)!
Following a regular finding or a finding of small hyperplastic polyps during a high-quality first colonoscopy, and if there is no family burden, a 10-year interval until the control colonoscopy is enough. CRC diagnosed in a member of close family who is under 60 years of age shortens that interval to two years.
High risk polyps need to be controlled in 3-year intervals. After the resection of big polyps, controls are best performed already after one year. Patients should be monitored according to guidelines up to the age of 75, then up to the age of 85 single out only those patients which carry a higher risk, whereas monitoring patients over 85 is mostly not recommended. If new symptoms characteristic for CRC occur in the interval between colonoscopies (bleeding, changes in bowel movement, pain), the early colonoscopy has to be determined by an individualized approach after taking into account all factors in the medical history and the clinical status. Sex, race, smoking and taking NSAID, as well as using modern technology such as high resolution and narrow band imaging, magnifying colonoscopy, chromoendoscopy and virtual colonoscopy, do not change the guidelines for patient monitoring after polypectomy (1).
In controlling the quality of colonoscopy, it is necessary to first of all insist on the set indication, implying most of all a suspicious pathological result obtained through another exam, bleeding, sideropenic anemia, detection and following of CRC and inflammatory bowel disease. The patients need to sign on the same day an informed consent in which they are given detailed information on the benefits of the test, alternative methods, but also on the risk of bleeding, perforations, infections, and sedation complications (18). The appropriate preparation of patients is extremely important, today mostly standardized on sodium phosphate or PEG, with clearly proven advantages of splitting dosages (19). The quality of endoscopists is usually determined by the rate of cecum intubation and polyp detection, success of polypectomies, time needed to extract the colonoscope (>6 min), and number of biopsy samples, especially in IBD (20). A lesser rate of complications, and a higher rate of promptly dealing with them endoscopically, is also a significant indicator of endoscopy quality (21). Finally, all of the above need to be described in a standardized colonoscopic medical report, which has to contain the medical history and demographic data, indications, rating of preparedness, the course of the colonoscopy and the morphological finding, interventions and unexpected events, and also the histological findings, as well as a plan of monitoring and communication with the family medicine doctor.
In conclusion it can be said that there are several levels on which the already significant preventive, diagnostic and therapeutic value of colonoscopy can be further enhanced. Preventive and diagnostic efficiency increase with the adequate detection of all risk groups and by raising the quality of colonoscopy, which especially refers to the performers and the equipment, while the therapeutic value is best increased by insisting on the removal of all changes which can be endoscopically removed, which primarily relates to polypectomies with or without mucosectomy.
Conflict of interest
None declared.
REFERENCES
- 1.Lieberman DA, et al. Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;144:844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Antoljak N. Nacionalni program rane dijagnostike raka debelog crijeva u Republici Hrvatskoj 2008-2011. HČJZ. 2011;28(7) [Google Scholar]
- 3.Štimac D, Katičić M, Kujundžić M, Ljubičić N, Poropat G, Bokun T. Značaj ranog otkrivanja raka debelog crijeva. Medicina. 2008;44(1):7–15. [Google Scholar]
- 4.Bujanda L, Cosme A, Gil I, Arenas-Mirave JI. Malignant colorectal polyps. World J Gastroenterol. 2010;16(25):3103–3011. doi: 10.3748/wjg.v16.i25.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neri E, Faggioni L, Cini L, Bartolozzi C. Colonic polyps: inheritance, susceptibility, risk evaluation, and diagnostic management. Canc Manag Res. 2011;3:17–24. doi: 10.2147/CMR.S15705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferguson LR. Recent advances in understanding of interactions between genes and diet in the etiology of colorectal cancer. World J Gastrointest Oncol. 2010;2(3):125–129. doi: 10.4251/wjgo.v2.i3.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowenfels AB, Williams JL, Holub JL, Maisonneuve P, Lieberman DA. Determinants of Polyp Size in Patients Undergoing Screening Colonoscopy. BMC Gastroenterol. 2011;11:101. doi: 10.1186/1471-230X-11-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lieberman D, Moravec M, Holub J, Michaels L, Eisen G. Polyp size and advanced histology in patients undergoing colonoscopy screening: implications for CT colonography. Gastroenterology. 2008;135:1100–1105. doi: 10.1053/j.gastro.2008.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colono-scopic polypectomy. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 10.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic Polypectomy and Long-Term Prevention of Colorectal-Cancer Deaths. N Engl J Med. 2012;366(8):687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomized and observational studies. Lancet. 2007;369:1603–1613. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 12.Lieberman DA, Weiss DG, Harford WV, et al. Five year colon surveillance after screening colonoscopy. Gastroenterology. 2007;133:1077–1085. doi: 10.1053/j.gastro.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Cairns SR, et al. British Society of Gastroenterology: Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002) Gut. 2010;59:666–690. doi: 10.1136/gut.2009.179804. [DOI] [PubMed] [Google Scholar]
- 14.Lorenzo-Zuniga V, Moreno de Vega V, Domenech E, et al. Endoscopist experience as a risk factor for colonoscopic complications. Colorectal Dis. 2010;12:273–277. doi: 10.1111/j.1463-1318.2009.02146.x. [DOI] [PubMed] [Google Scholar]
- 15.Rabeneck L, Paszat LF, Hilsden RJ, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology. 2008;135(6):1899–1906. doi: 10.1053/j.gastro.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 16.Radaelli F, Paggi S, Bortoli A, De Pretis G. Overutilization of post-polypectomy surveillance colonoscopy in clinical practice: a prospective, multicenter study. Dig Liver Dis. 2012;44(9):748–753. doi: 10.1016/j.dld.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 17.Shoen RE, et al. Utilization of surveillance colonoscopy in community practice. Gastroenterology. 2010;138:73–81. doi: 10.1053/j.gastro.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R. Jover M, Herráiz O, Alarcón, et al. Clinical practices Guidelines: quality of colonoscopy in colorectal cancer screening. Endoscopy. 2012;44(4):444–451. doi: 10.1055/s-0032-1306690. [DOI] [PubMed] [Google Scholar]
- 19.Froehlich F, Wietlisbach V, Gonvers JJ, et al. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61(3):378–384. doi: 10.1016/s0016-5107(04)02776-2. [DOI] [PubMed] [Google Scholar]
- 20.Shah HA, Paszat LF, Saskin R, et al. Factors associated with incomplete colonoscopy: a population-based study. Gastroenterology. 2007;132(7):2297–2303. doi: 10.1053/j.gastro.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 21.Rathgaber SW, Wick TM. Colonoscopy completion and complication rates in a community gastroenterology practice. Gastrointest Endosc. 2006;64(4):556–562. doi: 10.1016/j.gie.2006.03.014. [DOI] [PubMed] [Google Scholar]


