Abstract
The I locus is a 27-kb inverted repeat cluster of chalcone synthase genes CHS1-3-4 that mediates siRNA down-regulation of CHS7 and CHS8 target mRNAs during seed development leading to yellow seed coats lacking anthocyanin pigments. Here, we report small RNA sequencing of ten stages of seed development from a few days post fertilization through maturity, revealing the amplification from primary to secondary short interfering RNAs (siRNAs) occurring during development. The young seed populations had a higher proportion of siRNAs representing the CHS1-3-4 gene family members, consistent with this region as the origin of the primary siRNAs. More intriguingly, the very young seed had a higher proportion of 22-nt CHS siRNAs than did the mid-maturation seed. We infer that the primary CHS siRNAs increase during development to levels sufficient to trigger amplification of secondary CHS siRNAs from the CHS7/8 target mRNAs, enabling the total levels of 21-nt CHS siRNAs to rise dramatically. Further, we demonstrate that the soybean system exhibits tissue-specific CHS siRNA production because primary CHS siRNA levels are not sufficient to trigger secondary amplification in tissues other than the seed coat.
Introduction
Unique features of the gene silencing I (inhibitor) locus in soybean that paralleled co-suppression phenomena have been described [1] and the locus has been shown to be an example of naturally occurring RNA interference (RNAi) [2], [3]. The I locus produces tissue-specific siRNAs from an unusual arrangement of chalcone synthase (CHS) genes and these CHS siRNAs subsequently target in trans CHS mRNAs from genes located on different chromosomes [4].
The I locus was first identified as a region of duplicated and inverted CHS genes by DNA blotting and PCR mapping of 15 pairs of isogenic lines which showed that mutations of a dominant allele (I or ii) to the recessive allele (i) delete promoter sequences, paradoxically resulting in increased total CHS transcript levels and thereby pigmented (black) seed coats [1]. The inhibitory effects of this naturally occurring mutation appeared to parallel the co-suppressive phenotypes found in transgenic Petunia lines carrying multiple inserted copies of the CHS genes [5], [6]. Sequencing bacterial artificial chromosomes (BACs) from the Williams soybean variety containing the dominant ii allele revealed that five (CHS1, CHS3, CHS4, CHS5, and CHS9) of the nine CHS gene family members are located in a 230-kb region [7], [8]. CHS1, CHS3, and CHS4 are located in two 10.91-kb perfect and inverted repeat clusters separated by 5.87 kb of intervening sequence.
The relative expression profiles of CHS gene family members were examined by quantitative RT-PCR in the seed coats of two near-isogenic pairs that result from independently occurring mutations of the dominant I allele to the recessive i allele or of the dominant ii allele to the recessive i allele [3]. Decreased expression of the CHS7/8 genes results in the lack of pigmentation in the yellow seed coats (I and ii). Both small RNA blots and high throughput small RNA sequencing from three genotypes (I, ii and i alleles) of soybeans revealed that CHS siRNAs accumulated only in the yellow seed coats containing either dominant I or ii alleles and not in the pigmented seed coats with homozygous recessive i genotypes [4]. Interestingly, the CHS siRNAs were generated in a tissue-specific manner. CHS siRNAs did not accumulate in the cotyledons of the genotype with dominant I or ii alleles and yellow seed coats [4], thus allowing isoflavones to be produced in the cotyledons [9] since CHS7/8 mRNAs are not down-regulated in the cotyledons.
The generation pathway of CHS siRNAs involved in silencing the I locus was proposed [4]. A putative double stranded (ds) RNA generated from within a 27-kb inverted CHS region comprised of two clusters of the CHS1-3-4 and CHS4-3-1 genes is cleaved into primary siRNAs representing both strands that are amplified by RNA-dependent RNA polymerase (RdRP) to generate secondary CHS siRNAs from the target CHS7/8 mRNAs, which are capable of down-regulating all members of the CHS gene family. On the other hand, CHS7/8 mRNAs are highly expressed in the pigmented seed coats in which CHS siRNA production has been abolished by deletions in the CHS cluster regions in the mutant i allele.
Small RNA sequencing of co-suppressed, non-pigmented transgenic petunia flowers with introduced CHS genes has shown that CHS siRNAs are the causative factor of silencing the pigment pathway in flowers [10]. There are many commonalities between the naturally occurring soybean seed coat and the transgenic petunia systems [11]. These similarities include CHS siRNAs that are predominantly 21 nt in size and match both sense and antisense strands primarily of exon 2 of the conserved target CHS genes, likely through amplification of the original signal through RdRP.
Here, we sequenced small RNA populations from a developmental series representing ten stages of seed development from a few days post fertilization through seed maturity. Due to the sequence polymorphisms between the CHS siRNA origin and target genes, we were able to reveal the amplification from primary to secondary CHS siRNAs that occurred early in development. The primary CHS siRNAs were lower in abundance but composed of a higher proportion of 22-nt small RNAs, whereas the more abundant secondary CHS siRNAs were primarily 21 nt in size. A developmental amplification from primary to secondary siRNAs has not previously been tracked in either naturally occurring or transgenic plant systems. We also demonstrate that CHS siRNA production is specific to the seed coats as other tissues including cotyledons, roots, leaves, and stems do not produce sufficient primary siRNAs for amplification to secondary siRNAs.
Results
Differential Expression of CHS siRNAs during Seed Development
Although CHS1, 2, 3, 4, 5, 6 and 9 share 93% to 98% pairwise identity by nucleotide sequence, they are only 82% similar to CHS7 and CHS8. The dispersed nucleotide polymorphisms between these two groups of CHS genes present the opportunity to distinguish siRNAs representing the CHS7/8 mRNAs that are down-regulated in trans by CHS1/3/4 primary siRNAs originating from the I locus, which is defined by inverted repeats of CHS1, CHS3 and CHS4 genes (referred to as the CHS1-3-4 clusters). To determine the pattern of CHS siRNAs during the entire span of seed development, we constructed small RNA libraries from ten stages of seed development of the cultivar Williams (ii) as shown in Figure 1. In the early developmental periods from 4 DAF (days after flowering) through 22–24 DAF, whole seeds were used as the maternal seed coat is proportionally a larger part of the whole seed, which is too small for hand dissection. Beginning at the 5–6 mg weight range, the developmental staging is more accurate using a combination of seed fresh weight and color changes. At that time the seed are large enough to dissect the seed coat free of the cotyledons and embryonic axis. Table 1 lists details on the small RNA sequencing, ranging generally from 10 to 30 million reads from each of the ten developmental stages. Two biological repeats were made for eight of the ten developmental stages.
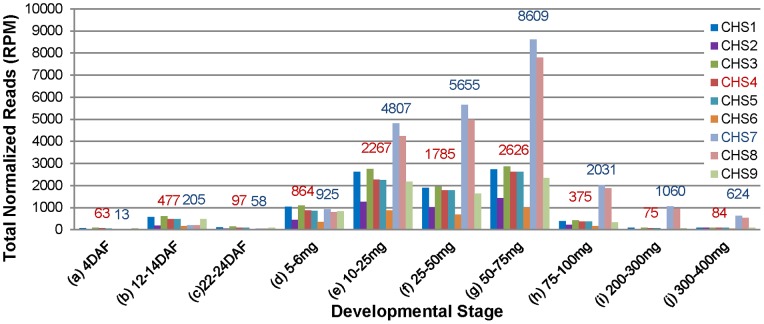
Figure 1. Total Counts of CHS siRNAs for Each CHS Gene Reveal CHS7/8 siRNAs are Dramatically Increased During Seed Coat Development.
CHS siRNAs from small RNA libraries of ten developmental stages of the cultivar Williams ii were filtered to identify those with 100% identity to individual CHS genes as indicated by the color chart. RPM, reads per million. Numbers above the bar indicate the total counts of CHS4 (red) and CHS7 (blue) siRNAs. Developmental stages are whole seed from (a) 4 DAF (Days After Flowering); (b) 12–14 DAF; and (c) 22–24 DAF; seed coats dissected from immature green seed of fresh weight (d) 5–6 mg; (e) 10–25 mg; (f) 25–50 mg; (g) 50–75 mg; (h) 75–100 mg; (i) 200–300 mg; and seed coats from (j) 300–400 mg yellow, desiccating seed.
Table 1. Summary Information on Eighteen Sequenced Small RNA Libraries from a Developmental Series of Williams (PI548631) Seed and Seed Coats Including Developmental Stage and Number of Reads.
| Tissue | Developmental stage | Replicate | Instrument model | # of Reads |
| Whole Seed | 4 days after flowering | BR1 | Illumina HiSeq 2000 | 27.0 M |
| Whole Seed | 4 days after flowering | BR2 | Illumina HiSeq 2000 | 12.1 M |
| Whole Seed | 12–14 days after flowering | BR1 | Illumina HiSeq 2000 | 35.2 M |
| Whole Seed | 12–14 days after flowering | BR2 | Illumina HiSeq 2000 | 13.1 M |
| Whole Seed | 22–24 days after flowering | BR1 | Illumina HiSeq 2000 | 30.8 M |
| Whole Seed | 22–24 days after flowering | BR2 | Illumina HiSeq 2000 | 10.3 M |
| Seed Coat | 5–6 mg fresh weight | BR1 | Illumina HiSeq 2000 | 17.8 M |
| Seed Coat | 5–6 mg fresh weight | BR2 | Illumina HiSeq 2000 | 25.3 M |
| Seed Coat | 10–25 mg fresh weight | NA | Illumina HiSeq 2000 | 12.0 M |
| Seed Coat | 25–50 mg fresh weight | NA | Illumina HiSeq 2000 | 12.0 M |
| Seed Coat | 50–75 mg fresh weight | BR1 | Illumina Genetic Analyzer | *2.9 M |
| Seed Coat | 50–75 mg fresh weight | BR2 | Illumina HiSeq 2000 | 11.5 M |
| Seed Coat | 75–100 mg fresh weight | BR1 | Illumina Genome Analyzer II | 30.1 M |
| Seed Coat | 75–100 mg fresh weight | BR2 | Illumina HiSeq 2000 | 10.5 M |
| Seed Coat | 200–300 mg fresh weight | BR1 | Illumina HiSeq 2000 | 31.3 M |
| Seed Coat | 200–300 mg fresh weight | BR2 | Illumina HiSeq 2000 | 14.8 M |
| Seed Coat | 300–400 mg fresh weight | BR1 | Illumina Genome Analyzer II | 34.0 M |
| Seed Coat | 300–400 mg fresh weight | BR2 | Illumina HiSeq 2000 | 17.1 M |
The PI number is an accession number by which information on the cultivar is searchable in the USDA GRIN (Germplasm Resources Information Network). Williams is homozygous dominant ii genotype with yellow seed coats.
This library was previously reported in [4]. BR1 and BR2 are biological repeats; NA, not applicable.
Figure 1 shows the normalized total counts in reads per million (RPM) of CHS siRNAs that map with 100% identity to each of the nine CHS genes during seed development. The normalized CHS siRNA counts were initially low in the 4 DAF seed at less than 100 RPM and also in the 12–14 DAF and 22–24 DAF seed. Then, they increased to about 900 RPM in seed coats from immature seed of 5–6 mg fresh weight, after which they increased dramatically, reaching the highest levels of 8600 for CHS7 in seed coats from 50–75 mg immature seed. After this peak, the CHS siRNAs decreased as the seed increased in size and began desiccation. An analysis of CHS siRNAs from a biological repeat (Figure S1) of eight of the ten stages shows the same pattern as Figure 1 with maximal CHS siRNAs found in the 50–75 mg stage.
Strikingly, the expression pattern of Figure 1 and Figure S1 enables us to distinguish the primary siRNAs originating from the CHS1-3-4 clusters of the I locus from those representing secondary siRNAs matching target mRNAs encoded by CHS7 and CHS8. The secondary CHS7/8 siRNAs showed an increase in the 5–6 mg seed coats and peaked at the 50–75 mg stages. The predominance of secondary CHS7/8 siRNAs remained during the rest of development in seed coats dissected from the immature green seed of 75–100 mg and 200–300 mg, as well as from 300–400 mg yellow seed coats that were undergoing desiccation. These data demonstrate that the amplification from primary to secondary CHS siRNAs occurs during seed coat development.
Primary siRNAs are Predominant before Accumulation of 21-nt Secondary siRNAs
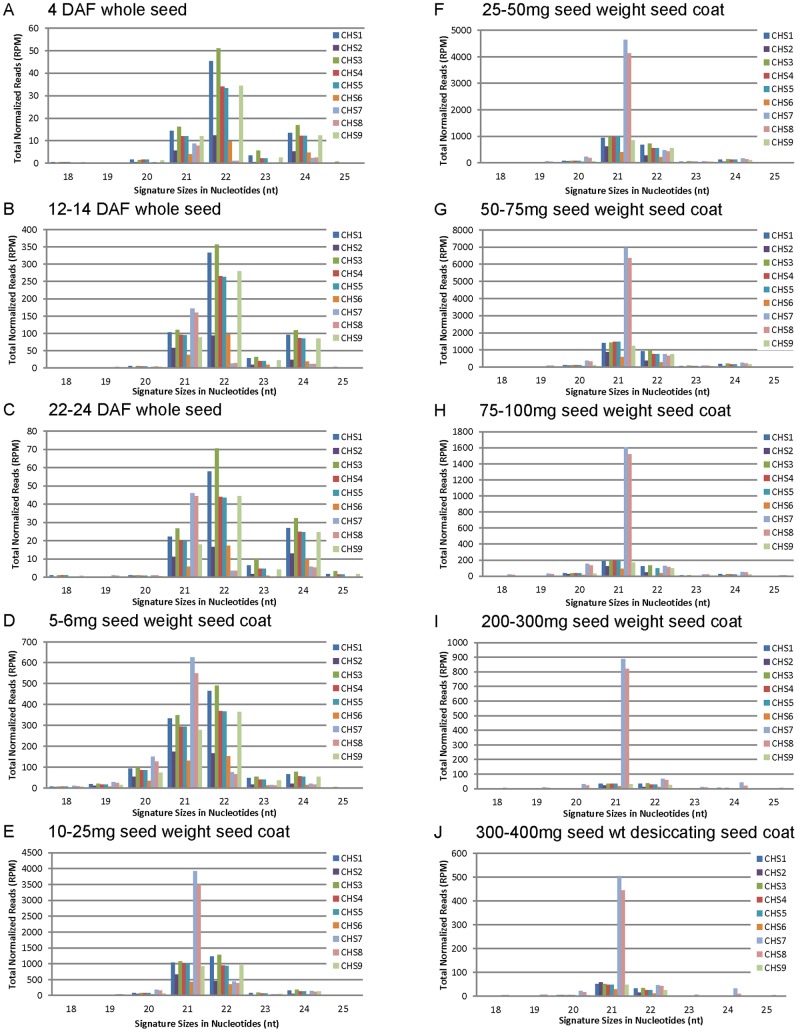
To investigate characteristics of secondary CHS siRNAs which are amplified during development, an analysis of the size distribution of CHS siRNAs was conducted. Figure 2 clearly shows that CHS7/8 siRNAs, which increased dramatically during development, were 21 nt in size. To the contrary, the CHS1/3/4 siRNAs were predominantly 22 nt in size up to the 5–6 mg stage (Figure 2). These data suggest that secondary CHS siRNAs were predominantly 21 nt in size and that the primary siRNAs consist of a slightly higher proportion of 22-nt siRNAs. However, in the early developmental stages, 21-nt primary CHS siRNAs are also actively generated along with the 22-nt size class. Similar results were found for the biological repeat (Figure S2).
Figure 2. Size Distributions of CHS siRNAs Reveal the Amplification of 21-nt Secondary siRNAs During Seed Coat Development.
CHS siRNAs from small RNA libraries of the same ten developmental stages A-J as described in Figure 1 were analyzed for their size distributions between 18 and 25 nt. The normalized total counts in reads per million (RPM) are shown for each CHS gene according to the color chart.
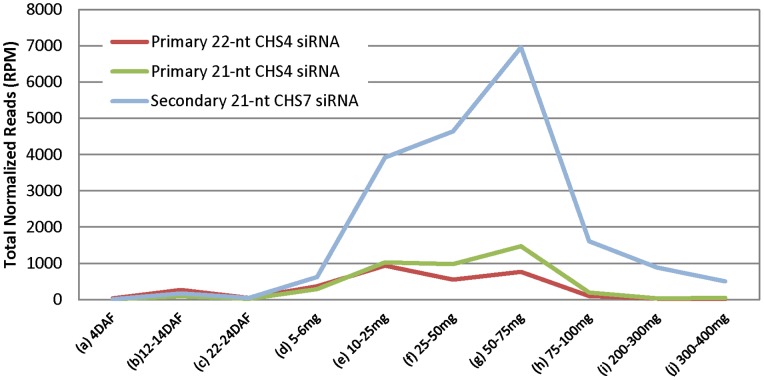
A comparison of total counts of 21-nt and 22-nt primary CHS4 siRNAs and 21-nt secondary CHS7 siRNAs that are unique to either CHS4 or CHS7 shows that 21-nt secondary siRNAs were highly amplified during seed coat development compared to those of primary CHS siRNAs (Figure 3 and Figure S3). RdRPs are involved in RNA amplification of primary siRNAs and generate secondary siRNAs [12]–[14]. In the soybean seed coat, we demonstrate that this amplification started at an early developmental stage around 5–6 mg (Figures 2 and 3) likely at the stage when CHS7/8 mRNAs begin to be expressed in the seed coats at higher levels. The CHS7/8 mRNAs are then susceptible to degradation by primary siRNAs, and amplification is guided by the primary CHS1/3/4 siRNAs that are produced initially from the triggering clusters of the CHS1-3-4 genes of the dominant ii allele.
Figure 3. Normalized Total Counts of CHS siRNAs that Match Either CHS4 or CHS7 with 100% Identity During Ten Stages of Seed Coat Development.
The 21-nt secondary CHS7 siRNAs are amplified to high levels relative to the CHS4 21-nt or 22-nt siRNAs. Developmental stages are same as described in Figures 1 and 2.
High Throughput Small RNA Sequencing Verifies Tissue Specificity of CHS siRNAs
Using mRNA blots and small RNA blots, we have previously shown that silencing of CHS gene family members occurs only in the seed coats and not in other organs [3], [4]. Using sequencing, small RNAs were detected in significant numbers only in the dissected seed coats and not the cotyledons of the developing seed [4] from the 50–75 mg weight range. Here, we investigated additional tissues such as leaf, root and germinating cotyledon with the power of deep sequencing to ascertain the tissue specificity of CHS siRNAs. As shown in Table 2, total numbers of small RNA reads were from three million to thirty million from each of the tissues from Williams cultivar or other cultivars with ii genotype, providing sufficient data to show the tissue specificity of CHS siRNAs and to examine even low levels of CHS siRNAs.
Table 2. Summary of Small RNA Libraries Constructed from Various Williams (PI548631) Tissues Including Developmental Stage and Number of Reads.
| Tissue | Replicate | Developmental stages | Instrument model | Reads |
| Seed Coat | NA | 5–6 mg fresh weight | Illumina HiSeq 2000 | 25.3 M |
| Immature Cotyledon | BR1 | 5–6 mg fresh weight | Illumina HiSeq 2000 | 31.5 M |
| Immature Cotyledon | BR2 | 5–6 mg fresh weight | Illumina HiSeq 2000 | 31.1 M |
| Immature Cotyledon | NA | 50–75 mg fresh weight | Illumina Genetic Analyzer | *3.0 M |
| Immature Cotyledon | NA | 100–300 mg fresh weight | Illumina HiSeq 2000 | 31.9 M |
| Germinated Cotyledon | NA | 10 Day Seedling | Illumina Genome Analyzer II | 16.4 M |
| Leaf | NA | 10 Day Seedling | Illumina HiSeq 2000 | 15.2 M |
| Root | NA | 10 Day Seedling | Illumina Genome Analyzer II | 17.3 M |
| Shoot Tip | NA | 10 Day Seedling | Illumina HiSeq 2000 | 15.7 M |
| Stem | NA | 10 Day Seedling | Illumina HiSeq 2000 | 22.3 M |
The PI number is an accession number by which information on the cultivar is searchable in the USDA GRIN (Germplasm Resources Information Network). Williams is homozygous dominant ii genotype with yellow seed coats.
This library was previously reported in [4].
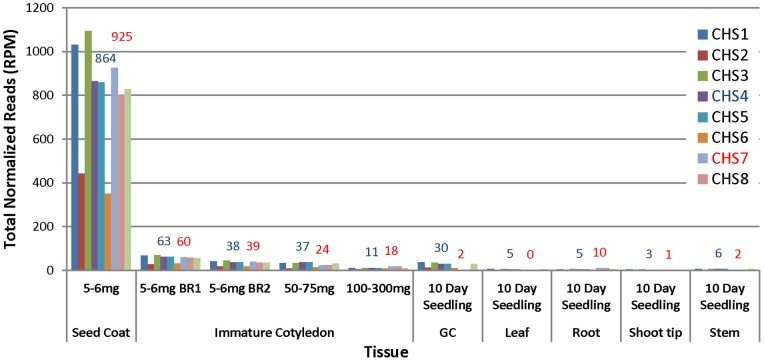
Figure 4 demonstrates the tissue specificity clearly as CHS siRNAs were limited to the seed coats in the Williams ii cultivar. The normalized total counts of CHS7 siRNAs in seed coats from 5–6 mg is 925 (RPM). In contrast, the expression levels of CHS7 siRNAs were less than 60 counts in four other tissues which is approximately a 15-fold difference (Figure 4). Most of those tissues (immature cotyledon, germinated cotyledon, unifoliate, root, shoot tip, and stem) have more CHS4 siRNAs than CHS7 siRNAs; however the counts of CHS4 siRNAs are very low compared to those of seed coat tissues (Figure 4). These data show that both primary and secondary CHS siRNAs were not significantly produced in germinated cotyledon, immature cotyledon, leaf, root, shoot tip, and stem tissues.
Figure 4. The Total Counts of CHS siRNA Family Members in Seven Different Soybean Tissues with ii Genotype Show Very Low Levels Except in the Yellow Seed Coat.
Numbers above the bar indicate the total normalized counts of CHS4 (red) and CHS7 (blue) siRNAs. BR1 and BR2 are biological repeats which were grown at different times. RPM, reads per million. GC stands for germinated cotyledon.
Seed coat tissue is close to the cotyledon physically during seed development. It is interesting that CHS siRNAs were limited to the seed coat and were not in immature cotyledons of three different stages of development, 5–6 mg, 50–75 mg and 100–300 mg fresh seed weight. The immature cotyledons contained very few CHS siRNAs in these three developmental stages (Figure 4). Because of the small size of the seed prior to the 5–6 mg stage, we used whole seed through 24 DAF; thus, it is likely also that the low levels of CHS siRNAs from these young whole seed less than 24 DAF (Figure 1) originated solely from the maternal seed coat tissue and not from the very young developing cotyledons since the cotyledons at all succeeding stages did not accumulate CHS siRNAs at significant levels as shown in Figure 4.
Discussion
The Amplification from Endogenous Primary to Secondary CHS siRNAs was Revealed by Small RNA-Seq of a Broad Range of the Developing Seed Coats
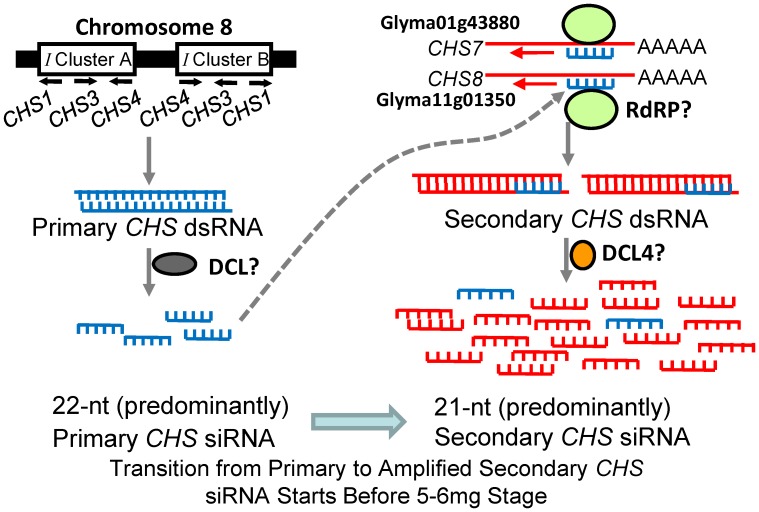
We have previously presented evidence for a model of action for the naturally occurring dominant I and ii alleles in preventing pigment formation in soybean seed coats [4] based on knowledge of RNA interference mechanisms in other organisms [12], [13]. Figure 5 summarizes the model and illustrates our data from this report on the developmental transition from primary to secondary siRNAs. The long inverted repeat of the I locus on Chromosome 8 contains cluster A (CHS1-3-4) and cluster B (CHS4-3-1) and forms the nascent CHS dsRNA although the exact size is unknown. The cleavage of CHS dsRNA by Dicer Like proteins (DCL) generates the primary CHS siRNAs, resulting in the degradation in trans of targeted CHS7 and CHS8 transcripts that originate from Chromosomes 1 and 11, respectively. After cleavage at the mRNA sites targeted by the primary CHS siRNAs, an RdRP synthesizes dsRNA from the cleaved CHS mRNA. The secondary CHS siRNAs generated from this dsRNA could target additional CHS mRNAs, amplifying the silencing response as well as spreading it over a larger region of the target. The roles for putative DCL and RdRP like functions in soybean are based on extrapolation from other systems and not by analysis of mutants of these functions in soybean.
Figure 5. Biogenesis of CHS siRNAs Preventing Pigment Formation in Developing Soybean Seed Coats of the Williams Cultivar with ii Genotype.
Partially adapted from [4]. The long inverted repeat of the ii allele of the I locus on Chromosome 8 contains cluster A (CHS1-3-4) and cluster B (CHS4-3-1) and forms the nascent CHS dsRNA although the exact size is unknown. The cleavage of CHS dsRNA by putative Dicer Like proteins (DCL) generates predominantly 22-nt primary CHS siRNAs at the earliest developmental stages, resulting in the degradation in trans of targeted transcripts from CHS7 (Glyma01g43880) and CHS8 (Glyma11g01350) located on Chromosomes 1 and 11, respectively. After cleavage at the mRNA sites targeted by the primary CHS siRNAs, a putative RdRP synthesizes dsRNA from the cleaved CHS mRNA. The 21-nt secondary CHS siRNAs likely generated by DCL4 homologs from this dsRNA can target additional CHS mRNAs, thus amplifying the silencing response as well as spreading it over a larger region of the target. The transition from predominantly 22-nt CHS siRNAs (shown in blue) representing the origin of the CHS1-3-4 siRNAs to the 21-nt secondary CHS siRNAs (shown in red) representing the target CHS7 and CHS8 mRNAs occurs at the 5–6 mg seed stage as indicated.
In this report, we show that the unique attributes of the naturally occurring soybean I locus clearly revealed the amplification of the CHS siRNA population from primary to secondary siRNAs during seed development based on the polymorphisms between the siRNAs representing the origin (CHS1-3-4) and target CHS7 and CHS8 genes. Table S1 shows that very few siRNAs match both the origin and target subgroups with 100% identity, verifying that the observed patterns (Figures 1, 2, 3 and Figures S1, S2, S3) are not due to multi-matching siRNA. The polymorphisms between the two sub-groups are widely dispersed throughout the sequence. For example, although CHS4 and CHS7 are 82% identical [4], there are no siRNAs which are longer than 20 nt matching both CHS4 and CHS7 at 100% identity as shown in the alignment of Figure S4. Allowing up to two-base mismatches between the origin and target increases the number of siRNA sequences significantly (Table S1). Thus, the targeting of CHS7 and CHS8 by the CHS1-3-4 primary siRNAs is likely primarily through mismatching rather than perfectly matched siRNAs.
A developmental transition from primary to secondary siRNAs has not previously been elucidated in either naturally occurring or transgenic plant systems. In C. elegans, deep sequencing distinguished primary and secondary siRNAs using an exogenous RNAi construct containing mismatches from the wild type C. elegans target sequence positioned at 25 base intervals [15]. Here, by examining the entire seed developmental period including the very early stages from a few days post fertilization that are labor intensive to obtain in sufficient quantity for sequencing, we demonstrated the transition from a small population of primary CHS siRNAs to the accumulation of high levels of 21-nt secondary siRNAs (Figures 1, 2, 3 and Figures S1, S2, S3). The siRNA populations in the young seed had a higher proportion representing the CHS1-3-4 gene family members, consistent with this region as the origin of the primary siRNAs. Seed at 4 DAF contained a basal level of less than 100 RPM that aligned to any CHS gene. The levels of CHS siRNAs originating from the CHS1-3-4 clusters increased thereafter to roughly 400–900 RPM very early in seed development from 12–24 DAF to 5–6 mg. By the 5–6 mg stage, the seed coats contained roughly even proportions of siRNAs representing CHS1-3-4 compared to CHS7 and CHS8 but after that period, the CHS7/8 siRNAs increased dramatically, reaching peak levels of 8,000 to 10,000 RPMs in the biological repeats of the 50–75 mg seed coats.
Intriguingly, the very young seed had a higher proportion of 22-nt CHS siRNAs than did seed from the 5–6 mg stage and older. Recent studies in Arabidopsis thaliana [16]–[18] demonstrated that 22-nt miRNAs (microRNAs) are critical for biogenesis of secondary 21-nt siRNAs as they drive amplification of secondary siRNAs via RdRP to generate ta-siRNAs (transacting siRNAs). Our data support the conclusions that the primary siRNA population has a higher proportion of 22-nt siRNAs, and that the secondary population amplified from the CHS7/8 substrates by RdRP are predominantly 21 nt in size. However, another study recently claimed that the miRNA-duplex structure is critical to the production of secondary ta-siRNAs rather than the length of miRNA [19]. Whether or not the 22-nt siRNAs are the only trigger to generate the 21-nt secondary siRNAs is unknown, since 21-nt primary CHS siRNAs were also present in the very young seed. The predominance of the 21-nt secondary siRNAs that represent CHS7/8 was maintained as the total levels of CHS siRNAs declined during the later stages of maturity.
Biogenesis of CHS siRNAs is Regulated by the Developmental Program in Soybean Seed
CHS siRNAs were found to be most abundant in the 50–75 mg stage among ten developmental stages (Figures 1 and 2). Previously, it was shown with RNA blots that CHS mRNAs are prevalent at 10–25 mg, 25–50 mg and 50–75 mg weight ranges of the seed coats of mid-maturation embryos with pigmented genotypes [3]. Also, using TaqMan real time RT-PCR, it was shown that CHS7 and CHS8 mRNAs are highest at the 50–75 mg stage of Williams 55 pigmented seed coats with the homozygous recessive i genotype. In this regard, the profile of the CHS siRNAs resembled many other genes expressed during seed coat development, including many in the isoflavone and anthocyanin pathways [20]. The most abundant levels of CHS siRNAs in the ii genotype with yellow seed coats generally coincided with the developmental time of appearance of the CHS7 and CHS8 target mRNAs in the recessive i genotype that has pigmented seed coats. Thus, in contrast with the down-regulation of the pathway by CHS siRNA-targeted destruction of CHS mRNAs in the yellow seed coats, the CHS transcripts continued to produce CHS isoforms after the 50 mg stage with the resulting accumulation of large amounts of anthocyanins leading to the pigmented seed coat.
By examining the entire seed developmental period including the small seed at 4 DAF through 24 DAF, we found that the CHS primary siRNAs representing the CHS1-3-4 origin clusters began to increase before the secondary siRNAs representing the CHS7/8 target mRNAs (summarized in Figure 5). These levels were higher than the basal level for CHS siRNAs found in non-seed tissues (Figure 4). From these results, we deduce that there was active biogenesis of the nascent CHS dsRNA that began to occur early in development between 12–24 DAF. It is not yet determined how early the CHS7 and CHS8 mRNAs begin to be expressed in the non-silencing, pigmented mutant line Williams (i, black). Although RNA-Seq data is available from the Williams (ii, yellow) variety at these early stages [21], it is not yet available from the pigmented mutant line Williams (i, black).
Primary CHS siRNA Levels are Not Sufficient to Trigger Secondary Amplification in Tissues Other than the Seed Coat
Several hypotheses have been put forward to explain the tissue-specific accumulation of CHS siRNAs [3], [4]. (1) One mechanism could involve the action of a cell or tissue-specific transcription factor or DNA binding protein that initiates sufficient production of the dsRNA progenitor molecules only in the seed coats and not in other tissues of varieties with the dominant I and ii alleles. Alternatively, (2) the dsRNA could be formed in other tissues but not cleaved properly by a DCL protein to yield enough primary siRNA to trigger secondary siRNAs, or (3) the primary CHS siRNAs might not be amplified to high levels of secondary siRNAs due to lack of an RdRP or other core components to generate secondary siRNAs in other tissues. Here, our results with high throughput sequencing of large populations of small RNAs from eight non-seed tissues revealed only very low basal levels of CHS siRNAs, generally in the range of 60 RPM. We propose that the level of the CHS primary siRNAs is too low to trigger the secondary amplification in these tissues, unlike the situation in the non-pigmented seed coats where our data clearly show a rise in the levels of the primary CHS siRNAs to nearly 1000 RPM before the secondary siRNAs overtake them in abundance.
Using RNAse protection experiments, a recent study has detected CHS dsRNA not only in the seed coat but also in the cotyledon and leaf tissues of lines with a dominant I allele [22]. Although those results were not quantitative, they suggested that the biogenesis of CHS siRNA could be regulated in a tissue-specific manner after dsRNAs are generated by the failure to amplify secondary siRNAs because of a lack of a critical component in the amplification step, such as an RdRP activity. However, this would not explain why CHS dsRNAs that are formed in non-seed coat tissues would fail to be processed and amplified when many other miRNAs and siRNAs are fully processed and amplified in other tissues such as cotyledons, leaves, and roots of soybean with the I and ii alleles [4], [23], [24] including numerous phased siRNAs (phasiRNAs) resulting from 22-nt miRNAs in soybean roots that target the NB-LRR defense gene families [25]. As shown in Figures 1, 2, 3, 4, the amplification of the secondary siRNAs starts in the 5–6 mg seed coats. Recently, RNA-Seq data of seed coat and cotyledons from the 5–6 mg stage has been reported [21]. The RNA-Seq data showed that the expression levels of Glyma models with annotations such as RdRP, DCL, and AGO (argonaute like protein), are very low and have no significant differences between seed coat and cotyledon (data not shown). For these reasons, we currently prefer hypothesis (1) that the level of the CHS siRNAs follows an increase in the nascent dsRNA to levels that are higher in the seed coats than in other tissues.
Whether the limiting factor proves to be the levels of the dsRNA or primary siRNAs in tissues other than the seed coat, it is clear that secondary amplification from the target CHS mRNAs does not occur in other tissues as seen by the lack of high levels of 21-nt CHS siRNAs that align to any of the CHS genes (Figure 4). The absence of secondary siRNAs is not from the lack of CHS mRNA substrates as can be seen from a number of studies that report reasonable levels of CHS mRNAs in cotyledons, roots, leaves, and other tissues using RNA blots [3]. In addition, using RT-PCR, most of the CHS genes including CHS7 and CHS8 were shown to be induced to high levels in pathogen-challenged leaf tissues [26] indicating that sufficient CHS mRNA substrates for secondary amplification from even a low level of primary CHS siRNAs in the leaves would be possible. It is clear that CHS is down-regulated by the CHS siRNAs only in the seed coats, though sufficient levels of CHS mRNAs exist in other tissues to serve as substrates for amplification of secondary siRNAs to high levels.
In summary, because of the unique properties of the soybean silencing system, we were able to reveal that the onset of production of the primary CHS siRNAs in the very early seed stages contained a higher proportion of 22-nt siRNAs originating from the CHS1-3-4 clusters of the ii allele. Then, the transition during development to a very large population of 21-nt secondary siRNAs representing amplification from the target CHS7 and CHS8 genes was apparent. Our results demonstrate regulation of the CHS inverted repeat genomic region is likely to rely on factors that occur during the very early seed developmental program and may reflect such changes. Further study of this system should provide more insight into the mechanisms of tissue specificity regulated by small RNAs and more broadly the blueprint of genes involved in development using the seed coat as a model.
Methods
Plant Materials, Tissue Collection, and Small RNA Isolation
The soybean line used in this study is inbred and homozygous for the indicated loci and is available from the USDA germplasm collection through GRIN (Germplasm Resources Information Network). The variety Williams (PI 548631), maturity group III, has genotype ii with a black hilum on an otherwise yellow seed coat. The genome of the closely related Williams 82 isoline was recently sequenced [27] and is used as a reference genome standard in soybean. The PI number is an accession number by which information on the cultivar is searchable in the GRIN database.
Soybean plants were grown in the greenhouse and immature seeds were harvested over the course of several weeks. The three earliest stages were harvested at the stated Days After Flowering (DAF) and the whole seeds were removed from the early pods under an Olympus SZ61 microscope (Melville, NY) and fresh-frozen in liquid nitrogen and stored at −80°C until extraction. More than 40 beans were harvested to represent the expression level of each line. For older stages, the seeds were shelled, pooled together, and then sorted by weight range. The seeds were first dissected to separate the seed coat and the cotyledon. The seed coats were frozen in liquid nitrogen for 10 minutes and stored in the freezer (−80°C) until they were lyophilized.
Total RNA was isolated by standard methods using phenol and chloroform extractions [28] and precipitated with ethanol but without lithium chloride in order to preserve small RNAs.
Small RNA Sequencing and Data Analysis
Small RNA libraries and high throughput sequencing were performed with the Genome Analyzer-II and HiSeq-2000 (Illumina, San Diego, CA) by the Keck Center (University of Illinois, Urbana, IL) using standard Illumina protocols. Some of the sequences were barcoded for sequencing within a single lane. Generally, a total of ten to eighty million reads were obtained from these deep sequencing libraries. Adapter trimming was performed using Illumina’s Flicker pipeline which finds the presence of the adapter in each read by finding the best alignment of the adapter to the read, and removing it from the read. The sizes of the small RNAs after adapter trimming ranged from 14 to 33 nucleotides with the majority in the range of 18 to 25 nucleotides. Adapter trimmed sequences were compared to obtain the number and occurrences of unique sequences. Alignments of siRNA sequences to CHS Glyma models (Phytozome, Joint Genome Institute) from the Williams 82 reference genome of Glycine max [27] and from sequenced BACs including BAC77G7a, accession EF623858, containing the I locus clusters [7], [8] were performed using the Bowtie program [29]. Alignments were made to individual CHS sequences with no mismatches allowed. Small RNA sequencing data was normalized in reads per million (RPM).
Accession Numbers
The data have been entered into Gene Expression Omnibus under series GSE43348 for the 18 samples of the developmental series small RNA shown in Table 1 and GSE49708 for the small RNA sequences from the various tissue samples shown in Table 2.
Supporting Information
The Total Counts of CHS siRNAs for Each CHS Gene Reveal Biogenesis of CHS7/8 siRNAs is Dramatically Increased during Seed Coat Development in Biological Repeats of Eight Stages. CHS siRNAs from small RNA libraries of ten developmental stages of the cultivar Williams ii were filtered to identify those with 100% identity to individual CHS genes as indicated by the color chart. Numbers above the bar indicate the total counts of CHS4 (red) and CHS7 (blue) siRNAs. Developmental stages are whole seed from (a) 4 DAF (Days After Flowering); (b) 12–14 DAF; and (c) 22–24 DAF; seed coats dissected from immature green seed of fresh weight (d) 5–6 mg; (e) 10–25 mg, no repeat data; (f) 25–50 mg, no repeat data; (g) 50–75 mg; (h) 75–100 mg; (i) 200–300 mg; and seed coats from (j) 300–400 mg yellow, desiccating seed.
(PDF)
Size Distributions of CHS siRNAs in Biological Repeats of Eight Stages of Seed Coat Development. CHS siRNAs from small RNA libraries of the same eight developmental stages as described in Supplemental Figure 1 were analyzed for their size distributions between 18 and 25 nt. The normalized total counts in reads per million (RPM) are shown for each CHS gene according to the color chart.
(PDF)
Normalized Total Counts of CHS siRNAs that Match Either CHS4 or CHS7 with 100% Identity in Biological Repeats of Eight Stages of Seed Coat Development. The 21-nt secondary CHS7 siRNAs are amplified to high levels relative to the CHS4 21-nt or 22-nt siRNAs. Developmental stages are indicated.
(PDF)
Alignment of CHS4 and CHS7 Coding Regions Shows that Polymorphisms are Widely Dispersed throughout the Two Sequences. The longest contiguous sequences, which align to both genes without mismatch, are only 20 nt. These characteristics of the CHS gene family enable us to distinguish primary CHS4 and secondary CHS7 siRNAs by filtering their sequences to obtain only those with 100% identity. Primary CHS4 siRNAs with one- or two-base mismatches to the target CHS7 transcripts enable down-regulation of the target sequence.
(PDF)
Very Few CHS siRNAs Map with 100% Identity to Both Sub-groups of CHS1/2/3/4/5/6/9 (Group 1) and CHS7/8 (Group 2) Genes. CHS siRNAs from small RNA libraries of the same ten developmental stages A–J as described in Figure 1 were analyzed for their size distributions between 18 and 25 nt. The normalized total counts in reads per million (RPM) are shown for each CHS gene. The CHS siRNAs that matched to a CHS gene within Group 1 (containing CHS1/2/3/4/5/6/9) were mapped to Group 2 targets containing CHS7 and CHS8 using Bowtie alignments and allowing no mismatches (unshaded line) or up to two mismatches (shaded line). Likewise, Group 2 CHS siRNAs that matched CHS7 and CHS8 were aligned to Group 1 targets (CHS1/2/3/4/5/6/9) allowing either zero or up to two mismatches. These data show that very few CHS siRNA were due to multi-matching siRNAs between the two groups when no mismatches are allowed but that significant numbers were multi-matching if up to 2 mismatches were allowed. Thus, imposing 100% identity enables the opportunity to distinguish CHS1/3/4 primary siRNAs originating from the CHS1-3-4 clusters at the I locus from the target CHS7/8 secondary siRNAs.
(PDF)
Acknowledgments
We are grateful to Dr. Alvaro Hernandez and staff of the University of Illinois Biotechnology Center for the sequencing services. We thank undergraduate assistants Cameron Lowe and Achira Kulasekara for help with data analysis.
Funding Statement
This research was supported by grants from the United Soybean Board, the Illinois Soybean Association, and the USDA-CSREES. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Todd JJ, Vodkin LO (1996) Duplications that suppress and deletions that restore expression from a chalcone synthase multigene family. Plant Cell 8: 687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Senda M, Masuta C, Ohnishi S, Goto K, Kasai A, et al. (2004) Patterning of virus-infected soybean seed coat is associated with suppression of endogenous silencing of chalcone synthase genes. Plant Cell 16: 807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tuteja JH, Clough SJ, Chan WC, Vodkin LO (2004) Tissue-specific gene silencing mediated by a naturally occurring chalcone synthase gene cluster in Glycine max . Plant Cell 16: 819–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tuteja JH, Zabala G, Varala K, Hudson M, Vodkin LO (2009) Endogenous, tissue-specific short interfering RNAs silence the chalcone synthase gene family in Glycine max seed coats. Plant Cell 21: 3063–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Napoli C, Lemieux C, Jorgensen R (1990) Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell 2: 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van der Krol AR, Mur LA, Beld M, Mol JNM, Stuitje AR (1990) Flavonoid genes in petunia: addition of a limited number of gene copies may lead to a suppression of gene expression. Plant Cell 2: 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clough SJ, Tuteja JH, Li M, Marek LF, Shoemaker RC, et al. (2004) Features of a 103-kb gene-rich region in soybean include an inverted perfect repeat cluster of CHS genes comprising the I locus. Genome 47: 819–831. [DOI] [PubMed] [Google Scholar]
- 8. Tuteja JH, Vodkin LO (2008) Structural features of the endogenous CHS silencing and target loci in the soybean genome. Crop Sci 48: 49–69. [Google Scholar]
- 9. Dhaubhadel S, Gijzen M, Joy P, Farhangkhoee M (2007) Transcriptome analysis reveals a critical role of CHS7 and CHS8 genes for isoflavonoid synthesis in soybean seeds. Plant Physiol 143: 326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Paoli E, Dorantes-Acosta A, Zhai J, Accerbi M, Jeong DH, et al. (2009) Distinct extremely abundant siRNAs associated with cosuppression in petunia. RNA 15: 1965–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eckardt NA (2009) Tissue-specific siRNAs that silence CHS genes in soybean. Plant Cell 21: 2983–2984. [Google Scholar]
- 12. Chapman E, Carrington JC (2007) Specialization and evolution of endogenous small RNA pathways. Nat Rev 8: 884–896. [DOI] [PubMed] [Google Scholar]
- 13. Carthew RW, Sontheimer EJ (2009) Origins and mechanisms of miRNAs and siRNAs. Cell 136: 642–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ghildiyal M, Zamore PD (2009) Small silencing RNAs: an expanding universe. Nat Rev Genet 10: 94–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pak J, Maniar JM, Mello CC, Fire A (2012) Protection from feed-forward amplification in an amplified RNAi mechanism. Cell 151: 885–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen HM, Chena LT, Patel K, Lia YH, Baulcombe DC, et al. (2010) 22-nucleotide RNAs trigger secondary siRNA biogenesis in plants. Proc Natl Acad Sci USA 34: 15269–15274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cuperus JT, Carbonell A, Fahlgren N, Garcia-Ruiz H, Burke RT, et al. (2010) Unique functionality of 22-nt miRNAs in triggering RDR6-dependent siRNA biogenesis from target transcripts in Arabidopsis . Nat Struct Mol Biol 17: 997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoshikawa M, Iki T, Tsutsui Y, Miyashita K, Poethig RS, et al. (2013) 3′ fragment of miR173-programmed RISC-cleaved RNA is protected from degradation in a complex with RISC and SGS3. Proc Natl Acad Sci 110(10): 4117–4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang CS, Todd JJ, Vodkin LO (1994) Chalcone synthase mRNA and activity are reduced in yellow soybean seed coats with dominant I alleles. Plant Physiol 105: 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jones SI, Vodkin LO (2013) Using RNA-Seq to profile soybean seed development from fertilization to maturity. PLoS ONE 8(3): e59270 doi:10.1371/journal. pone.0059270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kurauchi T, Kasai A, Tougou M, Senda M (2011) Endogenous RNA interference of chalcone synthase genes in soybean: Formation of double-stranded RNA of GmIRCHS transcripts and structure of the 5′ and 3′ ends of short interfering RNAs. J Plant Physiol 168: 1264–1270. [DOI] [PubMed] [Google Scholar]
- 23. Joshi T, Yan Z, Libault M, Jeong DH, Park S, et al. (2010) Prediction of new miRNAs and associated target genes in Glycine max . BMC Bioinformatics 11: S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zabala G, Campos E, Varala KK, Bloomfield S, Jones SI, et al. (2012) Divergent patterns of endogenous small RNA populations from seed and vegetative tissues of Glycine max . BMC Plant Biol 12: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhai J, Jeong DH, De Paoli E, Park S, Rosen BD, et al. (2011) MicroRNAs as master regulators of the plant NB-LRR defense gene family via the production of phased, trans-acting siRNAs. Genes Dev 25: 2540–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zabala G, Zou J, Tuteja JH, Gonzalez DO, Clough SJ, et al. (2006) Transcriptome changes in the phenylpropanoid pathway of Glycine max in response to Pseudomonas syringae infection. BMC Plant Biol 6: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, et al. (2010) Genome sequence of the palaeopolyploid soybean. Nature 463: 178–183. [DOI] [PubMed] [Google Scholar]
- 28. Wang CS, Vodkin LO (1994) Extraction of RNA from tissues that contain high levels of procyanidins that bind RNA. Plant Mol Biol Reporter 12: 132–145. [Google Scholar]
- 29. Langmead B, Trapnell C, Pop M, Salzberg SL (2009) Ultrafast and memory efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Total Counts of CHS siRNAs for Each CHS Gene Reveal Biogenesis of CHS7/8 siRNAs is Dramatically Increased during Seed Coat Development in Biological Repeats of Eight Stages. CHS siRNAs from small RNA libraries of ten developmental stages of the cultivar Williams ii were filtered to identify those with 100% identity to individual CHS genes as indicated by the color chart. Numbers above the bar indicate the total counts of CHS4 (red) and CHS7 (blue) siRNAs. Developmental stages are whole seed from (a) 4 DAF (Days After Flowering); (b) 12–14 DAF; and (c) 22–24 DAF; seed coats dissected from immature green seed of fresh weight (d) 5–6 mg; (e) 10–25 mg, no repeat data; (f) 25–50 mg, no repeat data; (g) 50–75 mg; (h) 75–100 mg; (i) 200–300 mg; and seed coats from (j) 300–400 mg yellow, desiccating seed.
(PDF)
Size Distributions of CHS siRNAs in Biological Repeats of Eight Stages of Seed Coat Development. CHS siRNAs from small RNA libraries of the same eight developmental stages as described in Supplemental Figure 1 were analyzed for their size distributions between 18 and 25 nt. The normalized total counts in reads per million (RPM) are shown for each CHS gene according to the color chart.
(PDF)
Normalized Total Counts of CHS siRNAs that Match Either CHS4 or CHS7 with 100% Identity in Biological Repeats of Eight Stages of Seed Coat Development. The 21-nt secondary CHS7 siRNAs are amplified to high levels relative to the CHS4 21-nt or 22-nt siRNAs. Developmental stages are indicated.
(PDF)
Alignment of CHS4 and CHS7 Coding Regions Shows that Polymorphisms are Widely Dispersed throughout the Two Sequences. The longest contiguous sequences, which align to both genes without mismatch, are only 20 nt. These characteristics of the CHS gene family enable us to distinguish primary CHS4 and secondary CHS7 siRNAs by filtering their sequences to obtain only those with 100% identity. Primary CHS4 siRNAs with one- or two-base mismatches to the target CHS7 transcripts enable down-regulation of the target sequence.
(PDF)
Very Few CHS siRNAs Map with 100% Identity to Both Sub-groups of CHS1/2/3/4/5/6/9 (Group 1) and CHS7/8 (Group 2) Genes. CHS siRNAs from small RNA libraries of the same ten developmental stages A–J as described in Figure 1 were analyzed for their size distributions between 18 and 25 nt. The normalized total counts in reads per million (RPM) are shown for each CHS gene. The CHS siRNAs that matched to a CHS gene within Group 1 (containing CHS1/2/3/4/5/6/9) were mapped to Group 2 targets containing CHS7 and CHS8 using Bowtie alignments and allowing no mismatches (unshaded line) or up to two mismatches (shaded line). Likewise, Group 2 CHS siRNAs that matched CHS7 and CHS8 were aligned to Group 1 targets (CHS1/2/3/4/5/6/9) allowing either zero or up to two mismatches. These data show that very few CHS siRNA were due to multi-matching siRNAs between the two groups when no mismatches are allowed but that significant numbers were multi-matching if up to 2 mismatches were allowed. Thus, imposing 100% identity enables the opportunity to distinguish CHS1/3/4 primary siRNAs originating from the CHS1-3-4 clusters at the I locus from the target CHS7/8 secondary siRNAs.
(PDF)