Abstract
Drosophila wings mainly consist of two cell types, vein and intervein cells. Acquisition of either fate depends on specific expression of genes that are controlled by several signaling pathways. The nuclear mechanisms that translate signaling into regulation of gene expression are not completely understood, but they involve chromatin factors from the Trithorax (TrxG) and Enhancers of Trithorax and Polycomb (ETP) families. One of these is the ETP Corto that participates in intervein fate through interaction with the Drosophila EGF Receptor – MAP kinase ERK pathway. Precise mechanisms and molecular targets of Corto in this process are not known. We show here that Corto interacts with the Elongin transcription elongation complex. This complex, that consists of three subunits (Elongin A, B, C), increases RNA polymerase II elongation rate in vitro by suppressing transient pausing. Analysis of phenotypes induced by EloA, B, or C deregulation as well as genetic interactions suggest that the Elongin complex might participate in vein vs intervein specification, and antagonizes corto as well as several TrxG genes in this process. Chromatin immunoprecipitation experiments indicate that Elongin C and Corto bind the vein-promoting gene rhomboid in wing imaginal discs. We propose that Corto and the Elongin complex participate together in vein vs intervein fate, possibly through tissue-specific transcriptional regulation of rhomboid.
Introduction
Drosophila wings are mainly composed of two cell types, vein and intervein cells. In Drosophila melanogaster, vein cells form a stereotyped network of five longitudinal veins and two cross-veins that act as rigid supports necessary for flight. Intervein cells are much more abundant than vein cells and separate veins from each other. They are less pigmented and die shortly after adult emergence, whereas vein cells survive into adulthood. Intervein cells from the two apposed wing monolayers strongly adhere via integrins. By contrast, vein cells do not adhere to each other, and thus form fluid-conducting tubes surrounded by intervein tissue [1].
Intervein and vein cells acquire their identities during wing imaginal disc development from the third larval through the pupal stage. This process relies on several signaling pathways, including the Drosophila EGF receptor (DER) – MAP kinase ERK pathway (for a review, see [2]). While initial wing disc proliferation is induced by global activation of DER-ERK signaling, specification and differentiation of vein and intervein cells require fine-tuning of this pathway. During the third larval instar, longitudinal veins are first determined as broad regions called proveins, each expressing a specific combination of transcription factors that provides positional information. A subset of these factors induces localized expression of rhomboid (rho) in provein cells, which directs them to acquire vein fate [3]–[5]. Rho is part of a positive feedback loop on the DER-ERK pathway: Rho-mediated proteolytic processing of DER ligands leads to ERK activation in future vein cells [6]-[8] and higher DER-ERK signaling in turn increases rho expression [9]. Whereas rho is required for development of vein cells, blistered (bs), which encodes a homolog of the mammalian Serum Response Factor (SRF), is expressed in future intervein cells and controls acquisition of intervein cell fate [10], [11]. bs is repressed by DER-ERK signaling in provein territories, and represses in turn rho in future intervein cells during the pupal stage [12]. Acquisition of vein or intervein cell identity thus depends on the outcome of a fine-tuned balance between rho and bs expression, that both are regulated by the DER-ERK pathway.
Activation of the DER-ERK pathway induces diphosphorylation of ERK that can then enter the nucleus, but little is known about the nuclear mechanisms that link activated, phosphorylated ERK to regulation of gene expression. Interestingly, several studies in different organisms have connected MAP kinase signaling to chromatin factors from either the Trithorax Group (TrxG) or the Enhancers of Trithorax and Polycomb (ETP) family [13]-[16]. TrxG proteins form multimeric complexes that bind chromatin, deposit specific post-translational histone modifications, interact with the transcriptional machinery, and/or remodel nucleosomes. TrxG complexes maintain an open chromatin conformation and counteract transcriptional repression mediated by Polycomb complexes (PcG). Hence, TrxG complexes mostly maintain active gene expression (for reviews, see [17], [18]). However, TrxG complexes BAP and PBAP, Drosophila counterparts of the yeast chromatin remodeling complex SWI/SNF, can also participate in transcriptional repression [19]–[22]. ETPs are PcG and TrxG co-factors involved in both PcG silencing and TrxG activation, and might thus participate in a switch between activation and repression of transcription (for a review, see [23]). Several TrxG complexes are involved in control of vein vs intervein fate. Indeed, BAP and PBAP chromatin remodeling complexes participate in differential regulation of rho expression [20], [24], [25]. Furthermore, the Chromodomain Helicase DNA-binding protein encoded by the TrxG gene kismet (kis) [26], [27] also plays a role in vein development [28]. kis mutants present ectopic wing veins, and increase ectopic vein phenotypes of mutants affecting the BAP complex component SNR1 [22], [24], [29]. Finally, loss of the ETP Corto [30], [31] induces ectopic veins and enhances ectopic vein phenotypes of several TrxG gene mutants [24], [32], implicating corto in control of vein vs intervein fate. Interestingly, Corto associates with ERK and its scaffold protein MP1 on chromatin, potentially linking ERK signaling to transcriptional regulation mediated by TrxG and ETPs [15], [32], [33].
During a two-hybrid screen using Corto as bait [34], we isolated Elongin C (EloC). This protein is a subunit of the Elongin complex, initially purified from rat liver extracts on its ability to increase the catalytic rate of RNA polymerase II (RNA-PolII) transcription in vitro and to suppress transient RNA-PolII pausing [35], [36]. The Elongin complex is constituted of three subunits, a catalytic subunit Elongin A (EloA), responsible for transcriptional activity, and two regulatory subunits Elongin B (EloB) and EloC, that can also participate in formation of an E3 ubiquitin ligase complex [37]–[40]. Whereas EloC increases EloA activity, EloB acts as a chaperon that facilitates Elongin complex assembly and enhances its stability [41]. The Elongin complex is evolutionarily conserved since EloA, B and C homologs are found in mammals, C. elegans, D. melanogaster and S. cerevisiae [35], [42]–[46]. In Drosophila, down-regulation of EloA by RNA interference causes lethality, which suggests that the Elongin complex is essential for development [46].
Here, we confirm the interaction between Corto and the three subunits of the Elongin complex both in vitro and in vivo, and we address the role of this complex during development, particularly in control of wing tissue fates. Using genetic analyses, we first demonstrate that the Elongin complex participates in wing tissue formation, and second that it antagonizes corto and several TrxG genes during this process. We show that EloC, like EloA, binds polytene chromosomes. Furthermore, EloC largely overlaps on chromatin with the epigenetic mark H3K36me3, which is associated with transcriptional elongation. Lastly, we report chromatin immunoprecipitation experiments showing that Corto and EloC bind the vein-promoting gene rho in wing imaginal discs. All these data suggest that Corto and the Elongin complex could participate in tissue-specific transcriptional regulation of rho.
Materials and Methods
Drosophila strains and genetic crosses
Flies were grown on standard yeast-cornmeal medium at 25°C, unless stated otherwise in the text. w1118 was used as control strain. corto420, corto07128, cortoL1 and UAS::FLAG-HA-CortoCD lines were previously described [15], [31], [32]. Transgenic lines allowing Myc-EloC or FLAG-HA-EloC expression were generated by standard P-element mediated transformation, after cloning the EloC (CG9291) coding region into pUASp::Myc or pUASp::FLAG-HA vectors (Gateway™, gifts from Dr. T. Murphy, https://dgrc.cgb.indiana.edu/vectors). Lines Ubx::flp; FRT82B, ubi-nlsGFP, that induces recombination in all imaginal discs [47], and hs::flp; act>CD2>Gal4, UAS::GFP, used for flip-out experiments, were kindly provided by Drs. A. Audibert and J. Montagne, respectively. Lines EloCSH1520 and EloCSH1299 were from the Szeged Stock Center. All other lines, including drivers used to express UAS-driven transgenes ubiquitously (daugtherless::Gal4) or in wing imaginal discs (scalloped::Gal4, Beadex::Gal4, spalt::Gal4, nubbin::Gal4, rotund::Gal4, C684::Gal4, OK10::Gal4) were from the Bloomington Stock Center. Line VALIUM20 EloC (ValEloC) from the Transgenic RNAi Project (TRiP) at Harvard Medical School was used to down-regulate EloC by RNA interference (RNAi) [48]. In all crosses, wing phenotypes were analyzed in females, but arose also in males, although less frequently.
Clonal analysis
Flip-out clones of cells in which EloC was down-regulated by RNAi were obtained by crossing ValEloC and hs::flp, act>CD2>Gal4, UAS::GFP flies. First instar larvae (24-48h AEL: After Egg Laying) were heat-shocked for 60 minutes at 37°C, and development was then resumed at 25°C. Clones of cells over-expressing EloA were obtained by crossing Ubx::flp and act>CD2>Gal4, UAS::GFP; EloAG4930 flies. Clones of homozygous corto420 cells were obtained by crossing Ubx::flp; FRT82B, ubi-nlsGFP and FRT82B, corto420 flies. Third instar larval progeny from these crosses were dissected, fixed for 20 minutes in PBS with 3.7% paraformaldehyde and stained with DAPI. Wing imaginal discs were mounted in Mowiol and visualized by fluorescent microscopy (Nikon Eclipse 80i microscope).
RT-qPCR experiments
RT-qPCR experiments were carried out in a CFX96™ system (Biorad) using SsoFast EvaGreen™ Supermix (Biorad). cDNA were synthesized from total RNA extracted from 0–24h embryos or third instar larvae, as previously described [15], and quantified using the standard curve method, with Rp49, RpL12 or eIF-2α for normalization. Primers were:
EloA-F, 5′-GTGGAATCAGACTGCTGCTGTCG-3′
EloA-R, 5′-CGACCCAGCGGGATGCAACA-3′
EloB-F, 5′-CCGAGCTGAAGCGAATGATTGAG-3′
EloB-R, 5′-GTGGACACCGTCACGCCGTA-3′
EloC-F, 5′-TCGTCAAACGCGAGCACGCT-3′
EloC-R, 5′-GGCAAACTGACCCGGTCCGG-3′
Rp49-F, 5′-CCGCTTCAAGGGACAGTATC-3′
Rp49-R, 5′-GACAATCTCCTTGCGCTTCT-3′
RpL12-F, 5′-CCTCCCAAATTCGACCCAA-3′
RpL12-R, 5′-CACGCAACGCAGGTACACC-3′
eIF-2α-F, 5′- TCGCATCAACCTGATAGCAC-3′
eIF-2αR, 5′- ATCGTACTCGCTGGTCTTGG-3′
S2 cell transfection and co-immunoprecipitation
EloA (CG6755), EloB (CG4204), EloC (CG9291) and corto (CG2530) cDNA were cloned into Gateway™ Drosophila vectors (gifts from Dr. T. Murphy, https://dgrc.cgb. indiana.edu/vectors) to express either Myc- or FLAG-tagged fusion proteins under control of the actin5C promoter. These vectors were transiently transfected into S2 cells and total protein extracts were prepared as previously described [33]. For cross-linking, cells were treated with paraformaldehyde (1%) for 10 minutes at room temperature prior to protein extraction. Co-immunoprecipitation experiments were carried out as previously described, using anti-FLAG (F3165, Sigma) or anti-Myc (sc-40, Santa Cruz Biotechnology) antibodies [15].
Immunostaining of polytene chromosomes
Co-immunostainings of polytene chromosomes from da::Gal4>>Myc-EloC larval salivary glands were performed as previously described [30], using mouse anti-Myc (1:60) (sc-40, Santa Cruz Biotechnology), rabbit anti-H3K36me3 (1:60) (pAB-058-050, Diagenode) or rabbit anti-Corto (1:20) [30] antibodies. Secondary antibodies (Alexa™ Fluor 488 anti-mouse IgG and Alexa™ Fluor 594 anti-rabbit IgG, Molecular Probe) were used at 1:1000 dilution.
Chromatin immunoprecipitation (ChIP)
ChIP experiments were performed using chromatin from sd::Gal4>>UAS::FLAG-HA-EloC or sd::Gal4>>UAS::FLAG-HA-CortoCD third instar larval wing discs fixed with paraformaldehyde. The protocol, modified from Pérez-Lluch et al., was described previously [31], [49]. Immunoprecipitated and input DNAs were purified in 70 µl of water with IPure kit following the manufacturer's instructions (Diagenode). q-PCR reactions were performed on 5 µl of DNA in a CFX96™ system (Biorad) using SsoFast EvaGreen™ Supermix (Biorad). q-PCR data were normalized against input sample and expressed as percentages of input. Primers located in rhomboid (rho) or in an untranscribed region of Scr regulatory sequences not bound by Corto in embryos and S2 cells [50] and used as a negative control (NC) were:
rho-165F, 5′-TGTGGCAAGGCGGCAGATGG-3′
rho-165R, 5′ –TGTGTGGGTGGGTGGGGTGT-3′
rho+51F, 5′-AGTCAGTTGCGTGCGAGCCG-3′
rho+51R, 5′-CAGTCCGACTTTCTCAGTTTGA-3′
rho+2812F, 5′-CGTCGGATTCGGTGCTGGTC-3′
rho+2812R, 5′-GGTGGAACCAGTTGGCGTGC-3′
NC-F, 5′-GGCAGCTGTTCAAATCGGAGGCT-3′
NC-R, 5′-TCACGTCGAGGTGTTCGGCG-3′
Results
Corto interacts with the three subunits of the Elongin complex in vivo
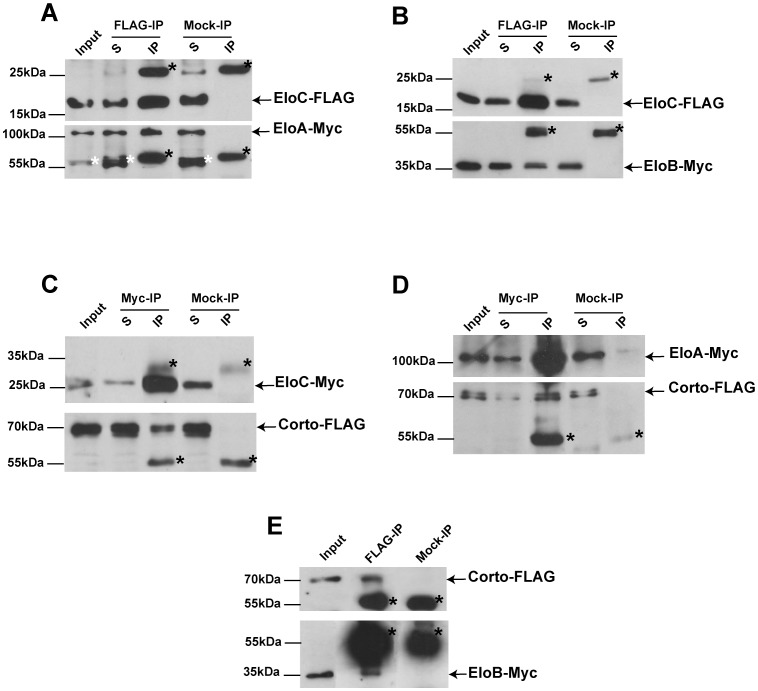
To validate the interaction between Corto and EloC detected in a two-hybrid screen [34], and to test whether Corto interacted also with the EloA catalytic and EloB regulatory subunits of the Elongin complex, we performed co-immunoprecipitation experiments with Myc- and FLAG-tagged proteins expressed in Drosophila S2 cells. We first tested co-immunoprecipitation between the three tagged Elongin proteins. These were mainly detected in nuclear extracts of S2 cells (data not shown). Using whole cell extracts, we detected a strong interaction between EloA and EloC (Figure 1A), as well as between EloB and EloC (Figure 1B). By contrast, EloA and EloB co-immunoprecipitated very weakly and only after cross-linking with paraformaldehyde (data not shown).
Figure 1. Corto interacts with the Elongin complex in vivo.
(A, B): EloA-Myc (A) and EloB-Myc (B) co-immunoprecipitate with EloC-FLAG; (C, D): Corto-FLAG co-immunoprecipitates with EloC-Myc (C) and EloA-Myc (D); (E): EloB-Myc co-immunoprecipitates with Corto-FLAG. In D and E, co-immunoprecipitations were performed after cross-linking. In E, exposure time for the lower panel was 50 times longer than for the upper panel. Immunoprecipitations were performed with anti-Myc (Myc-IP), anti-FLAG (FLAG-IP) or anti-HA (Mock-IP) antibodies. Immunoprecipitated proteins were revealed by Western blot using anti-FLAG or anti-Myc antibodies. Arrows show immunoprecipitated proteins, and black asterisks point to heavy or light IgG chains. In A, white asterisks indicate a non-specific band. S: supernatant after immunoprecipitation; IP: protein G-agarose beads. 5% of the input or supernatant and 50% of the immunoprecipitate were loaded onto the gels.
We next addressed interactions between the three Elo proteins and Corto. We observed strong co-immunoprecipitation between EloC and Corto (Figure 1C). No interaction between Corto and EloA or EloB was detected when using native protein extracts. However after paraformaldehyde cross-linking, we observed strong co-immunoprecipitation between Corto and EloA (Figure 1D), but only a very weak one between Corto and EloB (Figure 1E). These results confirm the two-hybrid interaction between Corto and EloC. In addition, they suggest that Corto interacts with the whole Elongin complex, probably via direct binding to EloC.
EloC binds polytene chromosomes
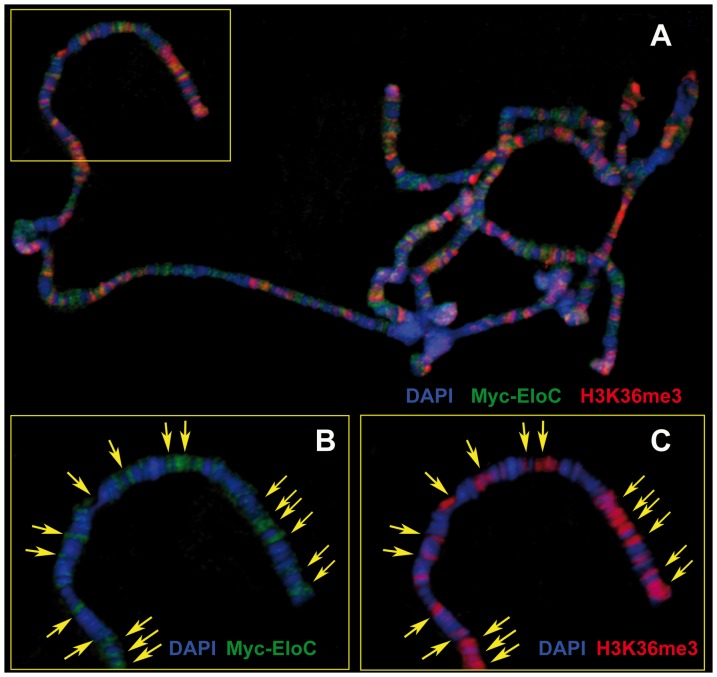
We next addressed whether EloC, like EloA and Corto, could bind polytene chromosomes. Drosophila EloA binds polytene chromosomes at many sites and extensively co-localizes with RNA-PolII phosphorylated on serine 5 (RNA-PolII-S5p, paused form) as well as on serine 2 (RNA-PolII-S2p, elongating form) [46], [51]. However, overlap between EloA and phospho-RNA-PolII was not complete, suggesting that the Elongin complex may not have a general role in transcription, but rather act as a transcriptional activator for a subset of genes. To analyze global EloC binding to chromatin, we generated a line containing a UASp-Myc-EloC transgene. Immunostainings of polytene chromosomes from larvae expressing this transgene driven by the ubiquitous daugtherless::Gal4 (da::Gal4) driver showed that Myc-EloC bound polytene chromosomes at many sites. These were preferentially located at DAPI interbands, suggesting that Myc-EloC localized to open chromatin (Figure 2A, B). When we co-immunostained polytene chromosomes from da::Gal4>>Myc-EloC larval salivary glands with antibodies against Myc and Corto, only a few common sites were observed (data not shown). Interestingly, Myc-EloC extensively co-localized with H3K36me3, an epigenetic mark that correlates with transcriptional elongation [52], [53] (Figure 2A-C). Together these data show that EloC, like EloA, binds chromatin protein and preferentially localizes to transcriptionally active sites.
Figure 2. EloC binds polytene chromosomes.
(A): Polytene chromosomes from salivary glands of da::Gal4>>Myc-EloC larva (two sets of chromosomes are shown). Myc-EloC (green) exhibits many co-localizations with H3K36me3 (red). (B, C): Close-up views of the region framed by a yellow rectangle in A. Yellow arrows on B and C indicate co-localizations between Myc-EloC and H3K36me3. DNA was stained with DAPI (blue).
EloB and EloC are essential genes
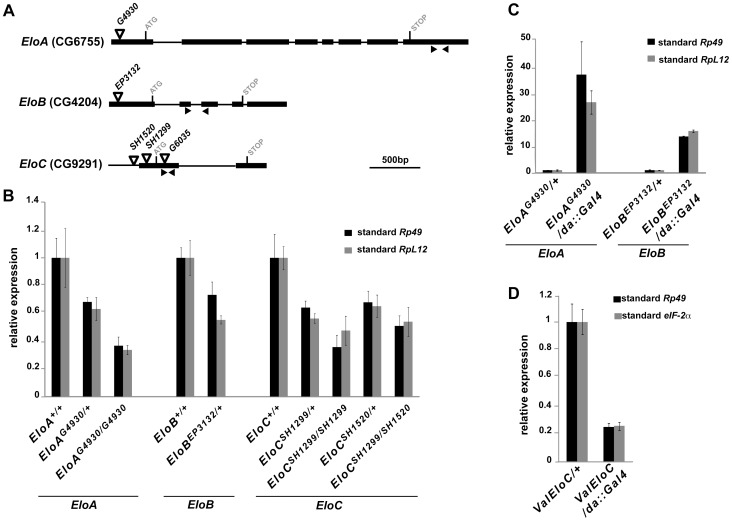
No mutations for EloA, B or C being reported so far, we analyzed transgenic lines carrying a P-element in or close to Elo genes (Figure 3A). Line EloAG4930 has a UAS-containing P-element located 58bp downstream of the EloA transcription start site (TSS), potentially allowing EloA over-expression with a Gal4 driver [54]. Line EloBEP3132 contains a similar element inserted 21bp downstream of the EloB TSS [54], [55]. Lines EloCSH1520 and EloCSH1299 have P-elements 31bp upstream and 34bp downstream of the EloC TSS, respectively [56]. To determine whether these insertions impaired Elo gene transcription, we quantified Elo mRNA levels in heterozygous or homozygous third instar larvae. As shown in Figure 3B, all four insertions decreased expression of the corresponding Elo gene (1.4 to 3-fold reduction) and behaved thus as hypomorphic, loss-of-function Elo alleles. Moreover, EloAG4930 and EloBEP3132 driven ubiquitously with da::Gal4 induced high over-expression of the corresponding gene (35- and 15-fold increase, respectively) (Figure 3C). We also tested a VALIUM20 transgenic line (ValEloC) that allows EloC down-regulation by RNA interference (RNAi) [48]. Ubiquitously driven in embryos with da::Gal4, ValEloC induced strong EloC down-regulation (Figure 3D, 5-fold reduction). Altogether, these lines allowed us to genetically address the functions of Elo genes.
Figure 3. Deregulation of EloA, EloB or EloC expression using P-element insertion lines.
(A): Structure of EloA, EloB and EloC genes showing localization of the P-elements used in this study. Exons are represented by boxes, and introns by lines. Black arrowheads show positions of primer pairs used to quantify Elo gene expression. (B): Quantification of Elo gene expression in EloAG4930, EloBEP3132, EloCSH1520 or EloCSH1299 homozygous or heterozygous larvae. (C): Quantification of Elo gene expression in da::Gal4>>EloAG4930 or da::Gal4>>EloBEP3132 larvae. (D): Quantification of EloC expression in da::Gal4>>ValEloC embryos. Relative Elo expression levels were obtained by normalization to Rp49 (black bars, B to D), RpL12 (grey bars, B, C) or eIF-2α (grey bars, D).
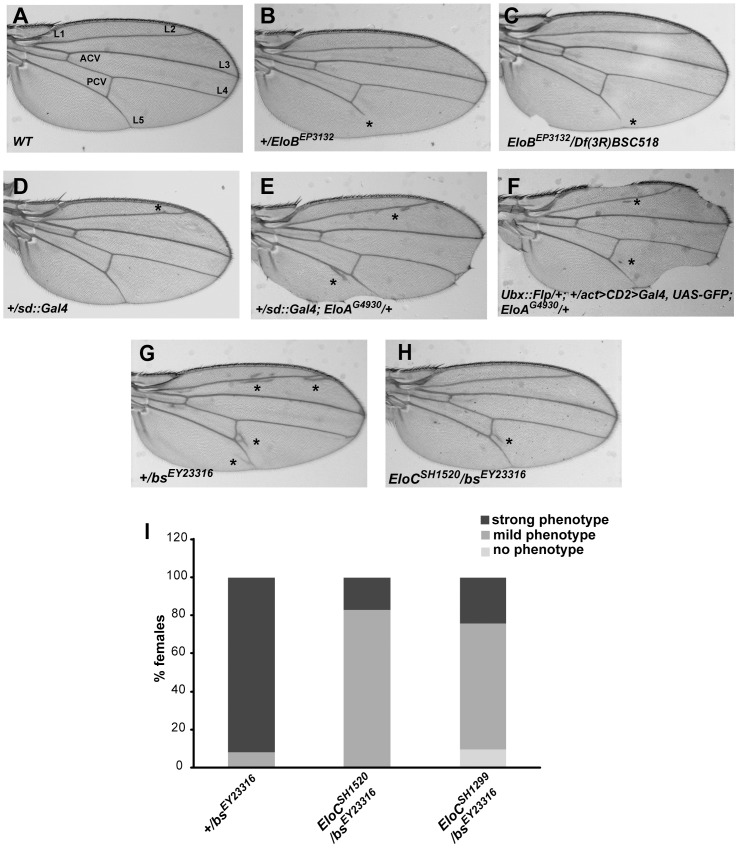
Figure 5. Elo genes control wing cell identity.
(A): Wing from control w1118 fly (L1-L5: longitudinal veins; ACV and PCV: anterior and posterior cross-veins). (B, C): Wings from +/EloBEP3132 and EloBEP3132/Df(3R)BSC518 flies exhibit truncated L5. (D): Wings from +/sd::Gal4 flies have a very faint ectopic vein phenotype and no margin phenotype. (E, F): Wings from flies over-expressing EloA exhibit ectopic vein and margin phenotypes. (G, H, I): EloCSH1520 and EloCSH1299 loss-of-function alleles diminish expressivity of the ectopic vein phenotype induced by the bsEY23316 loss-of-function allele. Strong phenotype: ectopic veins everywhere in the wing (shown in G). Mild phenotype: ectopic veins under the posterior cross-vein only (shown in H).
Down-regulation of EloA by RNAi induces lethality during the pupal stage [46], indicating that EloA is an essential gene. EloAG4930 homozygotes, on the other hand, are viable, which suggests that EloAG4930 individuals produce enough protein to correctly achieve development, despite the decreased level of EloA mRNA. Homozygous EloBEP3132 larvae died before the third larval instar. Furthermore, when associating EloBEP3132 with a deficiency uncovering EloB (Df(3R)BSC518), only one EloBEP3132/Df(3R)BSC518 adult escaper hatched among 272 balanced progeny. Together, these results indicate that EloB loss-of-function is either subviable or lethal.
To address EloC function, we used lines EloCSH1520 and EloCSH1299 as well as line EloCG6035, obtained independently of the two others. EloCG6035 line carries a P-element in EloC coding sequence 157bp downstream of the ATG [54], and could thus be a null allele of EloC. All three insertions were homozygous lethal. Lethality occurred before the third larval instar for EloCSH1520 and EloCG6035, and during this instar for EloCSH1299. Since no deficiency including EloC was available, we examined viability of heteroallelic EloC animals combining EloC alleles two by two. None of the three trans-allelic combinations gave viable adults. The rare EloCSH1520/EloCSH1299 third instar larvae died before pupariation. Furthermore, ubiquitous RNAi-mediated down-regulation of EloC (da::Gal4>>ValEloC) induced complete embryonic lethality. Altogether, these results show that EloC is an essential gene. We also addressed whether over-expression of Elo genes would affect viability. Ubiquitous over-expression of EloA, EloB or EloC was performed driving EloAG4930, EloBEP3132 or UAS::Myc-EloC with da::Gal4. None of these over-expressions affected fly viability.
In conclusion, these results showed that the EloB and EloC regulatory subunits of the Elongin complex are essential for development, like the catalytic EloA subunit.
EloC is required during wing development
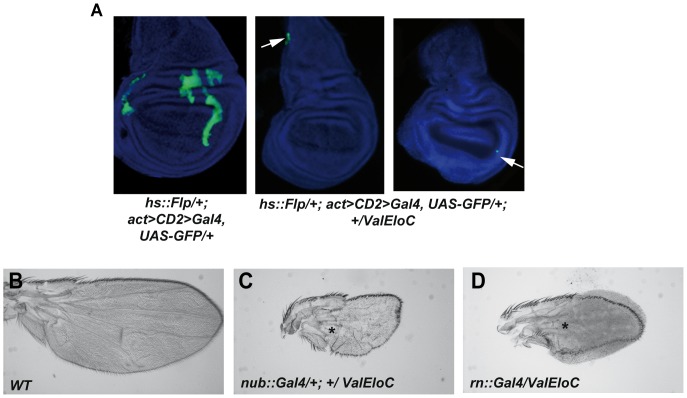
To better understand the need for EloC during wing development, we generated flip-out clones of cells expressing ValEloC, using a flipase gene under control of a heat-shock promoter (hs::flp; act>CD2>Gal4, UAS::GFP). Comparing the frequencies of late third instar wing discs with either ValEloC or control clones, we found that the former tended to be less frequent than the latter (ValEloC: 5 out of 34 discs; control: 11 out of 32 discs). ValEloC clones were much smaller than control clones, and always located at the disc periphery (Figure 4A). In addition, no adult wing phenotype was observed. This result thus shows that most of the clonal ValEloC cells induced in the wing pouch during the first larval instar either stopped dividing, died or were eliminated from the disc before the late third instar, suggesting that EloC is important for cell viability.
Figure 4. Down-regulation of EloC by RNA interference impairs both cell proliferation and cell differentiation in wing imaginal discs.
(A): Clones expressing the ValEloC transgene (GFP+ cells, shown by white arrows) are located at the periphery of the disc and are very small compared to control clones. (B, C, D): Wings from pharates in which ValEloC is driven by nub::Gal4 (C) or rn::Gal4 (D), both expressed in the wing pouch [66], [67] are small compared to wild-type pharate wings (B) and exhibit severe wing blade defects. By contrast, longitudinal veins (shown by asterisks) are formed in the proximal-most part of the wing blade where nub::Gal4 and rn::Gal4 are not expressed.
We next associated ValEloC with various wing disc drivers expressed at different developmental stages (scalloped::Gal4, spalt::Gal4, nubbin::Gal4, rotund::Gal4, C684::Gal4, OK10::Gal4). When grown at 25°C, the progeny of all these crosses died as third instar larvae or pupae, depending on the driver. At 20°C, where the UAS/Gal4 system is less active, escaper adults were obtained only with the nubbin (nub::Gal4) and rotund (rn::Gal4) drivers. Both drivers are specifically expressed in the wing pouch, and rn::Gal4 is expressed only from the third larval instar onwards. The escaper flies presented tiny misshapen wings with severe growth and differentiation defects in the area of driver expression (Figure 4B-D). Hence, even late wing pouch specific down-regulation of EloC has drastic consequences on Drosophila wing development. Altogether, these data show that decrease in EloC expression impedes cell growth and/or proliferation, stressing the importance of EloC during wing development.
The three Elongin complex subunits participate in control of wing cell identity
Among heterozygous EloBEP3132 females, 28.8% showed a truncated L5 vein (Table 1, Figure 5B). EloC heterozygous females presented the same phenotype, although less penetrant (Table 1, EloCSH1520 and EloCG6035, 1.4% and 1.1%, respectively), as did the single EloBEP3132/Df(3R)BSC518 escaper (Figure 5C). Homozygous EloAG4930 flies, on the other hand, had normal wings. To further analyze links between the Elongin complex and wing morphogenesis, we over-expressed Elo genes in wing imaginal discs. No wing phenotype was observed when over-expressing EloB or EloC, whereas EloA over-expression consistently affected wing morphogenesis. Driven by scalloped::Gal4 (sd::Gal4), EloA significantly increased ectopic veins compared to heterozygous sd::Gal4 controls (53.7% vs 33% of females) (Table 1; Figure 5D, E), and induced margin defects. Ectopic veins were also significantly enhanced when driving EloA with the wing-specific Beadex::Gal4 (Bx::Gal4) line (85.5% vs 16.5% of control females) (Table 1). Lastly, wings with EloA over-expressing clones (induced with Ubx::flp) presented both margin defects and ectopic veins (Figure 5F). Taken together, these data show that increased EloA expression caused margin and vein defects. Interestingly, the EloA over-expression phenotype (presence of ectopic veins) was opposite to the EloB and EloC loss-of-function phenotype (vein truncation).
Table 1. EloA and EloC deregulation induce wing vein defects.
| Genotype | Number of females observed | Vein phenotype observed | % females with vein phenotype |
| +/EloBEP3132 | 170 | Truncated L5 | 28.8 |
| EloCSH1520/+ | 143 | Truncated L5 | 1.4 |
| EloCG6035/+ | 175 | Truncated L5 | 1.1 |
| +/sd::Gal4 | 72 | Ectopic vein | 33 |
| +/sd::Gal4; EloAG4930/+ | 121 | Ectopic vein | 53.7 a |
| Bx::Gal4/+ | 133 | Ectopic vein | 16.5 |
| Bx::Gal4/+; +/EloAG4930 | 139 | Ectopic vein | 85.5 a |
The upper allele was brought by the mother. Numbers of +/sd::Gal4;EloAG4930/+ or Bx::Gal4/+;+/EloAG4930 females with ectopic veins were compared to numbers of +/sd::Gal4 or Bx::Gal4/+ females with ectopic veins, respectively (z-test, a p<0.001).
To further clarify the link between the subunits of the Elongin complex and specification of wing tissue fate, we combined EloC alleles with a loss-of-function allele of blistered (bs), bsEY23316. bs is required for intervein tissue formation [10], [11]. All heterozygous bsEY23316 flies presented ectopic veins, although with different expressivity (Table 2; Figure 5G). Interestingly, EloCSH1520 and EloCSH1299 alleles both strongly reduced expressivity and penetrance of the bsEY23316 ectopic vein phenotype. Indeed, significantly less flies presented the strongest phenotype (17% for EloCSH1520/bsEY23316 females and 24.2% for EloCSH1299/bsEY23316 females vs 92% for control +/bsEY23316 females), and 9.5% of EloCSH1299/bsEY23316 flies had no ectopic veins (Table 2; Figure 5H, I).
Table 2. Decreasing EloC expression suppresses ectopic veins induced by blistered loss-of-function.
| Genotype | Number of females observed | % females with no ectopic vein | % females with mild ectopic vein phenotype | % females with strong ectopic vein phenotype |
| +/bsEY23316 | 125 | 0 | 8 | 92 |
| EloCSH1520/bsEY23316 | 112 | 0 | 83 a | 17 a |
| EloCSH1299/bsEY23316 | 95 | 9.5 a | 66.3 a | 24.2 a |
The upper allele was brought by the mother. The number of EloC/bsEY23316 females with ectopic veins was compared to the number of +/bsEY23316 females with ectopic veins (z-test, a p<0.001). The mild ectopic vein phenotype corresponds to presence of ectopic veins distal to the posterior cross-vein (Figure 5H), whereas the strong ectopic vein phenotype corresponds to presence of ectopic veins everywhere in the wing (Figure 5G).
Altogether, these results suggest that the three subunits of the Elongin complex participate in specification of wing tissue fate, playing a vein-promoting role.
Elo mutations antagonize wing phenotypes of corto and TrxG mutants
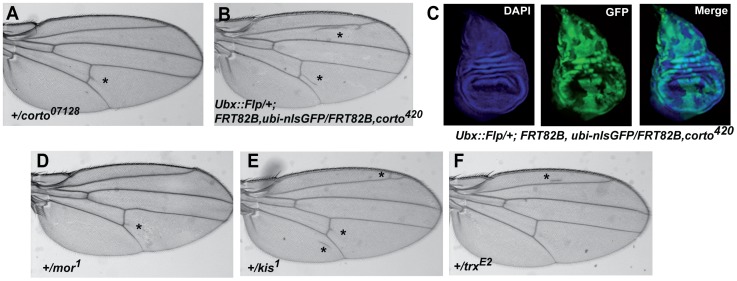
To understand the functional relationships between Elo genes and corto, we analyzed their genetic interactions. As previously reported, heterozygous corto loss-of-function flies as well as the rare heteroallelic corto escapers exhibited ectopic veins [15] (Table 3; Figure 6A). We found that ectopic veins were also observed when homozygous corto420 clones were induced in wing imaginal discs (Figure 6B). Strikingly, although clones were distributed all over the disc (GFP- cells, Figure 6C), ectopic veins preferentially formed close to longitudinal veins 2 and 5, and to the posterior cross-vein. A similar observation has been made for mutants of several other genes involved in wing tissue formation [5], [57]. When combining corto loss-of-function alleles with mutant alleles for each of the three Elo genes, ectopic veins were significantly reduced, even by the homozygous viable EloAG4930 allele (Table 3). Hence, all three Elo genes counteract corto in vein identity specification.
Table 3. Decreasing EloA, EloB or EloC expression partially suppresses ectopic veins induced by corto loss-of-function.
| Genotype | Number of females observed | % females with ectopic veins |
| +/corto07128 | 96 | 93.8 |
| corto07128/+ | 78 | 97.4 |
| corto07128/EloAG4930 | 183 | 38.3 a |
| corto07128/EloBEP3132 | 128 | 26.6 a |
| EloCSH1520/+; +/corto07128 | 156 | 14.8 a |
| EloCSH1299/+; +/corto07128 | 69 | 27.5 a |
| +/cortoL1 | 164 | 40.2 |
| cortoL1/+ | 51 | 49 |
| cortoL1/EloAG4930 | 191 | 0 a |
| cortoL1/EloBEP3132 | 129 | 0.8 a |
| EloCSH1520/+; +/cortoL1 | 119 | 0 a |
| EloCSH1299/+; +/cortoL1 | 114 | 0 a |
The upper allele was brought by the mother. The number of females with ectopic veins among flies transheterozygous for Elo and corto mutations was compared to the number of females with ectopic veins among flies with a corto mutation only (z-test, a p<0.001).
Figure 6. corto and several TrxG genes control wing cell identity.
(A, B): Ectopic vein phenotypes induced by the corto07128 loss-of-function allele (A) or by corto420 loss-of-function clones (B). (C): corto420 homozygous clones (GFP- cells) in wing imaginal discs. (D, E, F): Ectopic vein phenotypes induced by mor, kis or trx loss-of-function alleles. In A, B, D, E, F, asterisks mark ectopic veins.
We next looked for genetic interactions between Elo genes and the TrxG genes moira (mor), kismet (kis) and trithorax (trx) that are implicated in wing tissue formation and interact with corto in this process [24], [32], [58]. We confirmed that wings of flies heterozygous for mor1 or kis1 loss-of-function alleles presented ectopic veins (Table 4; Figure 6D, E) [28], [59]. Ectopic veins were also observed in flies heterozygous for the trxE2 loss-of-function allele (Table 4; Figure 6F). As observed for corto, ectopic veins were significantly reduced when combining these TrxG alleles with mutant alleles for each of the three Elo genes (Table 4). Taken together, these genetic interactions indicate that the three Elo genes promote vein cell identity in the wing disc. Furthermore, they counteract corto and several TrxG genes for vein vs intervein cell identity.
Table 4. Decreasing EloA or EloC expression partially suppresses ectopic veins induced by TrxG gene loss-of-function.
| Genotype | Number of females observed | % females with ectopic veins |
| +/mor1 | 93 | 22.5 |
| EloAG4930/mor1 | 110 | 5.5 a |
| EloCSH1520/+; +/mor1 | 132 | 1.5 a |
| EloCSH1299/+; +/mor1 | 78 | 6.4 a |
| +/kis1 | 81 | 81.5 |
| +/kis1; EloAG4930/+ | 94 | 48.8 a |
| EloCSH1520/kis1 | 80 | 17.5 a |
| EloCSH1299/kis1 | 54 | 9.2 a |
| +/trxE2 | 89 | 53.9 |
| EloAG4930/trxE2 | 96 | 10.4 a |
| EloCSH1520/+; +/trxE2 | 93 | 5.4 a |
| EloCSH1299/+; +/trxE2 | 54 | 16.7 a |
The upper allele was brought by the mother. Numbers of females with ectopic veins among flies transheterozygous for Elo and TrxG mutations were compared to numbers of females with ectopic veins among flies with a TrxG mutation only (z-test,a p<0.001).
The vein promoting gene rhomboid (rho) could be a common target of Corto and the Elongin complex
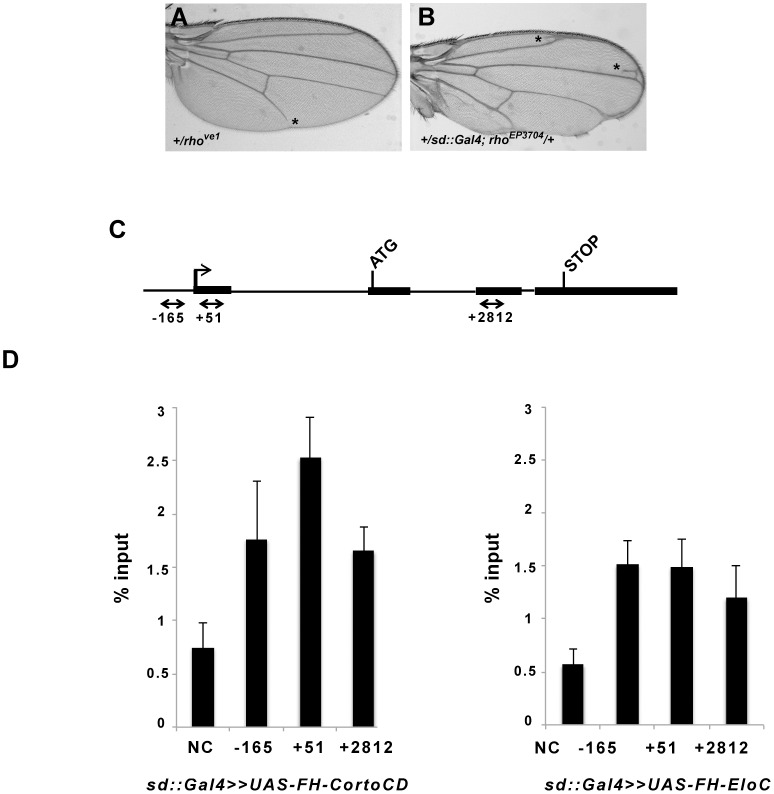
L5 vein truncation, observed in EloB and EloC heterozygous mutants, was also observed in mutants for the vein-promoting gene rhomboid (rho): 13.2% (11/83) of female heterozygotes for the hypomorphic rhove1 allele [5] and 24.2% (39/161) of female heterozygotes for the amorphic rho7M43 allele [55] presented a truncated L5 vein (Figure 7A). Furthermore, as previously described [15], over-expression of rho (sd::Gal4>>rhoEP3704) induced ectopic vein and margin phenotypes recalling the ones induced by EloA over-expression (compare Figures 7B and 5E). These observations led us to hypothesize that the Elongin complex could directly activate rho expression. On the other hand, wings of corto loss-of-function pupae exhibit ectopic expression of rho in intervein tissue [15]. Corto could thus be involved in direct repression of rho in future intervein cells. Therefore, Corto and the Elongin complex could act in opposite ways on rho regulation, an hypothesis in agreement with our genetic data showing that Elo genes antagonize the role of corto in vein vs intervein cell identity.
Figure 7. EloC and Corto bind rho in wing imaginal discs.
(A, B): Wing phenotypes induced by rho loss-of-function (A) or over-expression (B). Asterisks mark truncated L5 (in A) or ectopic veins (in B). (C): Schematic structure of rho with exons represented by boxes and introns by lines. Black arrows show primer pairs used for ChIP experiments. (D): Binding of Corto chromodomain (FH-CortoCD) and EloC (FH-EloC) on rho. For each genotype, the mean of two independent experiments is shown. Error bars correspond to standard deviations.
To address direct regulation of rho by Corto and the Elongin complex, we analyzed the binding of Corto and EloC to rho in late third instar wing imaginal discs by chromatin immunoprecipitation. We used the sd::Gal4 driver to express FLAG-HA (FH) tagged forms of either EloC (FH-EloC) or the Corto chromodomain (FH-CortoCD), that was previously shown to mimic Corto binding to polytene chromosomes [31]. Very few ectopic veins (due to the driver) were observed in wings of sd::Gal4>>FH-EloC flies or sd::gal4>>FH-CortoCD flies (data not shown), suggesting that the pattern of rho expression in these genetic contexts was similar to the one of wild-type wing imaginal discs. We found that FH-EloC as well as FH-CortoCD bound rho, the latter being slightly enriched just after the rho TSS (Figure 7C, D). In third instar larva wing imaginal discs, rho expression is restricted to the few cells that will give rise to the future veins and wing margin [5].The large majority of wing disc cells are thus future intervein cells in which rho is repressed. Hence, these results suggest that, in intervein cells, the Elongin complex and Corto are able to bind rho.
Discussion
The ETP Corto interacts with the three subunits of the elongation complex Elongin in vivo
We show here that, in Drosophila as in mammals [40], the three Elongin proteins Elo A, B, and C are mainly nuclear and interact two by two. EloC/B and EloC/A interactions may be direct, as they were observed without cross-linking treatment. By contrast, EloA/B interaction is more labile and may thus be indirect. It is possible that Drosophila EloC mediates the interaction between EloA and EloB, as previously shown in mammals [41], [60]. We also show that the ETP Corto interacts with all three Elo proteins, suggesting that Corto interacts with the Elongin complex. Hence, Corto and the Elongin Complex could share transcriptional targets. Several studies have shown that EloC binds its partners through a degenerate BC box motif, defined as (L,M)XXX(C,S)XXX(Í) [40], [44]. Two putative BC boxes (aa 357–365 and aa 542–550) are present in the C-terminal part of Corto. However, deletion of these sequences did not impair co-immunoprecipitation between Corto and EloC (data not shown), suggesting that these two proteins interact through another unidentified sequence.
The three Elongin complex subunits are essential for development
We present here the first characterization of lines allowing deregulation of EloB or EloC expression. EloB or EloC loss-of-function mutations induce early lethality (before the third larval instar), demonstrating that EloB and EloC, like EloA [46], are essential proteins. Our clonal and tissue-specific analyses of EloC mutant cells reveal that EloC is critically required all through wing development. By contrast, RNAi-mediated EloA down-regulation only induced lethality during the pupal stage [46], indicating either a less efficient reduction of EloA mRNA or a longer perdurance of maternal EloA. Alternatively, requirement of EloB and EloC in other complexes, such as an E3 ubiquitin ligase complex [37]–[40], might explain this difference.
The three subunits of the Elongin complex participate in determination of wing cell identity
EloB/C loss-of-function as well as EloA over-expression induced wing phenotypes, mostly vein phenotypes. Interestingly, these loss-of-function and over-expression phenotypes are opposite (i.e truncated L5 vein for loss-of-function, ectopic veins for over-expression). Furthermore, whereas EloA over-expression induced ectopic veins, no phenotype was observed when over-expressing EloB and EloC. This result suggests that the amount of catalytic subunit EloA might be critical for Elongin complex function. In mammals, EloA is indeed the limiting component of the Elongin complex, EloB and EloC being in large excess (100 to 1000-fold more abundant than EloA) [40], [61]. Curiously, a previous study reported that mitotic clones for a deficiency that uncovers EloA, produced ectopic wing veins [62]. As this deletion uncovers more than 10 genes that may influence vein formation, we favor the hypothesis, in agreement with all data presented above, that EloA loss- of-function leads to loss of vein tissue. Alternatively, EloB and EloC, which also belong to ubiquitin ligase complexes, might modulate vein vs intervein cell fate in this context.
Altogether, our observations suggest that the Elongin A, B, C subunits promote vein cell identity. On the opposite, Corto maintains intervein cell identity, possibly via interaction with TrxG complexes. As Corto and EloC co-localize at a few sites on polytene chromosomes, they might have common transcriptional targets. A balance between Corto and the Elongin complex might fine-tune transcription of such genes.
The vein-promoting gene rho could be a common target of Corto and the Elongin complex
In corto mutants, we previously showed that ectopic veins perfectly match with ectopic expression of rho, the first vein-promoting gene to be expressed [15]. As Elo gene mutations counteract corto mutations during formation of ectopic veins, we propose that rho could be a common target of Corto and the Elongin complex in intervein cells. In agreement with this hypothesis, immunoprecipitation using chromatin from late third instar wing imaginal discs, that can be assimilated to chromatin of intervein cells, revealed the presence of both Corto and EloC on rho. Two independent genome-wide studies on whole embryos and embryonic S2 cells have shown that poised RNA-PolII binds the rho promoter, suggesting that rho expression is controlled by “pause and release” of the transcriptional machinery [63], [64]. Interestingly, we found that Corto is slightly enriched just after the rho TSS, a position usually occupied by paused RNA-PolII (for a review, see [65]). Corto shares many sites on polytene chromosomes with paused RNA-PolII-S5p, suggesting that it is involved in transcriptional pausing [31]. On the other hand, we found that EloC co-localizes with H3K36me3, that characterizes transcriptional elongation, and the Elongin complex was shown to suppress transient RNA-PolII pausing [35], [36]. Hence, in future intervein cells, Corto and the Elongin complex could apply opposite forces on the transcriptional machinery at the rho promoter. Corto would block rho transcription whereas the Elongin complex would be ready to accompany rho elongation if release should occur. In future vein cells on the other hand, the Elongin complex could actively participate in rho transcriptional elongation, since loss of function mutants for EloB and EloC exhibit loss of vein tissue. In these cells, rho expression would be independent of Corto, since corto mutants never present truncated veins [15].
Conclusion
Our results suggest that the Elongin complex might participate in determination of vein and intervein cell identity during wing development. We propose that this complex might interact with the ETP Corto at certain target genes and fine-tune their transcription in a cell-type specific manner. One of these targets could be the vein-promoting gene rho. In intervein cells, binding of Corto to the Elongin complex could prevent transcription of rho. Corto could also recruit other chromatin factors, such as the BAP chromatin-remodeling complex that was previously shown to inhibit rho expression in intervein cells. By contrast, in vein cells, the Elongin complex could participate in rho transcriptional elongation independently of Corto.
Acknowledgments
We thank V. Ribeiro for excellent technical assistance, C. Murat for preliminary experiments and Dr. J-M. Gibert for fruitful discussions and comments on the manuscript. We thank Drs. A. Audibert, J. Montagne, T. Murphy, the Bloomington and Szeged Stock Centers as well as the TRiP at Harvard Medical School (NIH/NIGMS R01-GM084947) for fly stocks and reagents.
Funding Statement
Fundings from UPMC and CNRS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. De Celis JF (2003) Pattern formation in the Drosophila wing: the development of the veins. BioEssays 25: 443–451. [DOI] [PubMed] [Google Scholar]
- 2. Blair SS (2007) Wing vein patterning in Drosophila and the analysis of intercellular signaling. Annu Rev Cell Dev Biol 23: 293–319. [DOI] [PubMed] [Google Scholar]
- 3. Gomez-Skarmeta JL, Diez del Corral R, de la Calle-Mustienes E, Ferre-Marco D, Modolell J (1996) Araucan and caupolican, two members of the novel iroquois complex, encode homeoproteins that control proneural and vein-forming genes. Cell 85: 95–105. [DOI] [PubMed] [Google Scholar]
- 4. Crozatier M, Glise B, Khemici V, Vincent A (2003) Vein-positioning in the Drosophila wing in response to Hh; new roles of Notch signaling. Mech Dev 120: 529–535. [DOI] [PubMed] [Google Scholar]
- 5. Sturtevant MA, Roark M, Bier E (1993) The Drosophila rhomboid gene mediates the localized formation of wing veins and interacts genetically with components of the EGF-R signaling pathway. Genes Dev 7: 961–973. [DOI] [PubMed] [Google Scholar]
- 6. Guichard A, Biehs B, Sturtevant MA, Wickline L, Chacko J, et al. (1999) rhomboid and Star interact synergistically to promote EGFR/MAPK signaling during Drosophila wing vein development. Development 126: 2663–2676. [DOI] [PubMed] [Google Scholar]
- 7. Lee JR, Urban S, Garvey CF, Freeman M (2001) Regulated intracellular ligand transport and proteolysis control EGF signal activation in Drosophila . Cell 107: 161–171. [DOI] [PubMed] [Google Scholar]
- 8. Urban S, Lee JR, Freeman M (2001) Drosophila rhomboid-1 defines a family of putative intramembrane serine proteases. Cell 107: 173–182. [DOI] [PubMed] [Google Scholar]
- 9. Martin-Blanco E, Roch F, Noll E, Baonza A, Duffy JB, et al. (1999) A temporal switch in DER signaling controls the specification and differentiation of veins and interveins in the Drosophila wing. Development 126: 5739–5747. [DOI] [PubMed] [Google Scholar]
- 10. Montagne J, Groppe J, Guillemin K, Krasnow MA, Gehring WJ, et al. (1996) The Drosophila Serum Response Factor gene is required for the formation of intervein tissue of the wing and is allelic to blistered . Development 122: 2589–2597. [DOI] [PubMed] [Google Scholar]
- 11. Fristrom D, Gotwals P, Eaton S, Kornberg TB, Sturtevant M, et al. (1994) blistered: a gene required for vein/intervein formation in wings of Drosophila . Development 120: 2661–2671. [DOI] [PubMed] [Google Scholar]
- 12. Roch F, Baonza A, Martin-Blanco E, Garcia-Bellido A (1998) Genetic interactions and cell behaviour in blistered mutants during proliferation and differentiation of the Drosophila wing. Development 125: 1823–1832. [DOI] [PubMed] [Google Scholar]
- 13. Simone C, Forcales SV, Hill DA, Imbalzano AN, Latella L, et al. (2004) p38 pathway targets SWI-SNF chromatin-remodeling complex to muscle-specific loci. Nat Genet 36: 738–743. [DOI] [PubMed] [Google Scholar]
- 14. Mas G, de Nadal E, Dechant R, Rodriguez de la Concepcion ML, Logie C, et al. (2009) Recruitment of a chromatin remodelling complex by the Hog1 MAP kinase to stress genes. EMBO J 28: 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mouchel-Vielh E, Rougeot J, Decoville M, Peronnet F (2011) The MAP kinase ERK and its scaffold protein MP1 interact with the chromatin regulator Corto during Drosophila wing tissue development. BMC Dev Biol 11: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rampalli S, Li L, Mak E, Ge K, Brand M, et al. (2007) p38 MAPK signaling regulates recruitment of Ash2L-containing methyltransferase complexes to specific genes during differentiation. Nat Struct Mol Biol 14: 1150–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beck S, Faradji F, Brock H, Peronnet F (2010) Maintenance of Hox gene expression patterns. Adv Exp Med Biol 689: 41–62. [DOI] [PubMed] [Google Scholar]
- 18. Schuettengruber B, Martinez AM, Iovino N, Cavalli G (2011) Trithorax group proteins: switching genes on and keeping them active. Nat Rev Mol Cell Biol 12: 799–814. [DOI] [PubMed] [Google Scholar]
- 19. Coisy M, Roure V, Ribot M, Philips A, Muchardt C, et al. (2004) Cyclin A repression in quiescent cells is associated with chromatin remodeling of its promoter and requires Brahma/SNF2alpha. Mol Cell 15: 43–56. [DOI] [PubMed] [Google Scholar]
- 20. Rendina R, Strangi A, Avallone B, Giordano E (2010) Bap170, a subunit of the Drosophila PBAP chromatin remodeling complex, negatively regulates the EGFR signaling. Genetics 186: 167–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tamkun JW, Deuring R, Scott MP, Kissinger M, Pattatucci AM, et al. (1992) brahma: a regulator of Drosophila homeotic genes structurally related to the yeast transcriptional activator SNF2/SWI2. Cell 68: 561–572. [DOI] [PubMed] [Google Scholar]
- 22. Crosby MA, Miller C, Alon T, Watson KL, Verrijzer CP, et al. (1999) The trithorax group gene moira encodes a brahma-associated putative chromatin-remodeling factor in Drosophila melanogaster . Mol Cell Biol 19: 1159–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grimaud C, Negre N, Cavalli G (2006) From genetics to epigenetics: the tale of Polycomb group and trithorax group genes. Chromosome Res 14: 363–375. [DOI] [PubMed] [Google Scholar]
- 24. Marenda DR, Zraly CB, Dingwall AK (2004) The Drosophila Brahma (SWI/SNF) chromatin remodeling complex exhibits cell-type specific activation and repression functions. Dev Biol 267: 279–293. [DOI] [PubMed] [Google Scholar]
- 25. Terriente-Felix A, de Celis JF (2009) Osa, a subunit of the BAP chromatin-remodelling complex, participates in the regulation of gene expression in response to EGFR signalling in the Drosophila wing. Dev Biol 329: 350–361. [DOI] [PubMed] [Google Scholar]
- 26. Daubresse G, Deuring R, Moore L, Papoulas O, Zakrajsek I, et al. (1999) The Drosophila kismet gene is related to chromatin-remodeling factors and is required for both segmentation and segment identity. Development 126: 1175–1187. [DOI] [PubMed] [Google Scholar]
- 27. Therrien M, Morrison DK, Wong AM, Rubin GM (2000) A genetic screen for modifiers of a kinase suppressor of Ras-dependent rough eye phenotype in Drosophila . Genetics 156: 1231–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Terriente-Felix A, Molnar C, Gomez-Skarmeta JL, de Celis JF (2011) A conserved function of the chromatin ATPase Kismet in the regulation of hedgehog expression. Dev Biol 350: 382–392. [DOI] [PubMed] [Google Scholar]
- 29. Zraly CB, Marenda DR, Nanchal R, Cavalli G, Muchardt C, et al. (2003) SNR1 is an essential subunit in a subset of Drosophila brm complexes, targeting specific functions during development. Dev Biol 253: 291–308. [DOI] [PubMed] [Google Scholar]
- 30. Salvaing J, Lopez A, Boivin A, Deutsch JS, Peronnet F (2003) The Drosophila Corto protein interacts with Polycomb-group proteins and the GAGA factor. Nucleic Acids Res 31: 2873–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Coleno-Costes A, Jang SM, de Vanssay A, Rougeot J, Bouceba T, et al. (2012) New partners in regulation of gene expression: the Enhancer of Trithorax and Polycomb Corto interacts with methylated ribosomal protein L12 via its chromodomain. PLoS Genet 8: e1003006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lopez A, Higuet D, Rosset R, Deutsch J, Peronnet F (2001) corto genetically interacts with Pc-G and trx-G genes and maintains the anterior boundary of Ultrabithorax expression in Drosophila larvae. Mol Genet Genomics 266: 572–583. [DOI] [PubMed] [Google Scholar]
- 33. Mouchel-Vielh E, Bloyer S, Salvaing J, Randsholt NB, Peronnet F (2008) Involvement of the MP1 scaffold protein in ERK signaling regulation during Drosophila wing development. Genes Cells 13: 1099–1111. [DOI] [PubMed] [Google Scholar]
- 34. Salvaing J, Nagel AC, Mouchel-Vielh E, Bloyer S, Maier D, et al. (2008) The Enhancer of Trithorax and Polycomb Corto interacts with Cyclin G in Drosophila . PLoS One 3: e1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bradsher JN, Jackson KW, Conaway RC, Conaway JW (1993) RNA polymerase II transcription factor SIII. I. Identification, purification, and properties. J Biol Chem 268: 25587–25593. [PubMed] [Google Scholar]
- 36. Takagi Y, Conaway JW, Conaway RC (1995) A novel activity associated with RNA polymerase II elongation factor SIII. SIII directs promoter-independent transcription initiation by RNA polymerase II in the absence of initiation factors. J Biol Chem 270: 24300–24305. [DOI] [PubMed] [Google Scholar]
- 37. Brower CS, Sato S, Tomomori-Sato C, Kamura T, Pause A, et al. (2002) Mammalian mediator subunit mMED8 is an Elongin BC-interacting protein that can assemble with Cul2 and Rbx1 to reconstitute a ubiquitin ligase. Proc Natl Acad Sci U S A 99: 10353–10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kamura T, Burian D, Yan Q, Schmidt SL, Lane WS, et al. (2001) Muf1, a novel Elongin BC-interacting leucine-rich repeat protein that can assemble with Cul5 and Rbx1 to reconstitute a ubiquitin ligase. J Biol Chem 276: 29748–29753. [DOI] [PubMed] [Google Scholar]
- 39. Kamura T, Koepp DM, Conrad MN, Skowyra D, Moreland RJ, et al. (1999) Rbx1, a component of the VHL tumor suppressor complex and SCF ubiquitin ligase. Science 284: 657–661. [DOI] [PubMed] [Google Scholar]
- 40. Kamura T, Sato S, Haque D, Liu L, Kaelin WG Jr, et al. (1998) The Elongin BC complex interacts with the conserved SOCS-box motif present in members of the SOCS, ras, WD-40 repeat, and ankyrin repeat families. Genes Dev 12: 3872–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aso T, Lane WS, Conaway JW, Conaway RC (1995) Elongin (SIII): a multisubunit regulator of elongation by RNA polymerase II. Science 269: 1439–1443. [DOI] [PubMed] [Google Scholar]
- 42. Aso T, Yamazaki K, Amimoto K, Kuroiwa A, Higashi H, et al. (2000) Identification and characterization of Elongin A2, a new member of the Elongin family of transcription elongation factors, specifically expressed in the testis. J Biol Chem 275: 6546–6552. [DOI] [PubMed] [Google Scholar]
- 43. Yamazaki K, Guo L, Sugahara K, Zhang C, Enzan H, et al. (2002) Identification and biochemical characterization of a novel transcription elongation factor, Elongin A3. J Biol Chem 277: 26444–26451. [DOI] [PubMed] [Google Scholar]
- 44. Aso T, Haque D, Barstead RJ, Conaway RC, Conaway JW (1996) The inducible elongin A elongation activation domain: structure, function and interaction with the elongin BC complex. Embo J 15: 5557–5566. [PMC free article] [PubMed] [Google Scholar]
- 45. Aso T, Conrad MN (1997) Molecular cloning of DNAs encoding the regulatory subunits of elongin from Saccharomyces cerevisiae and Drosophila melanogaster . Biochem Biophys Res Commun 241: 334–340. [DOI] [PubMed] [Google Scholar]
- 46. Gerber M, Eissenberg JC, Kong S, Tenney K, Conaway JW, et al. (2004) In vivo requirement of the RNA polymerase II elongation factor elongin A for proper gene expression and development. Mol Cell Biol 24: 9911–9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Emery G, Hutterer A, Berdnik D, Mayer B, Wirtz-Peitz F, et al. (2005) Asymmetric Rab 11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell 122: 763–773. [DOI] [PubMed] [Google Scholar]
- 48. Ni JQ, Markstein M, Binari R, Pfeiffer B, Liu LP, et al. (2008) Vector and parameters for targeted transgenic RNA interference in Drosophila melanogaster . Nat Methods 5: 49–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Perez-Lluch S, Blanco E, Carbonell A, Raha D, Snyder M, et al. (2011) Genome-wide chromatin occupancy analysis reveals a role for ASH2 in transcriptional pausing. Nucleic Acids Res 39: 4628–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Salvaing J, Decoville M, Mouchel-Vielh E, Bussiere M, Daulny A, et al. (2006) Corto and DSP1 interact and bind to a maintenance element of the Scr Hox gene: understanding the role of Enhancers of Trithorax and Polycomb. BMC Biol 4: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gerber M, Tenney K, Conaway JW, Conaway RC, Eissenberg JC, et al. (2005) Regulation of heat shock gene expression by RNA polymerase II elongation factor, Elongin A. J Biol Chem. 280: 4017–4020. [DOI] [PubMed] [Google Scholar]
- 52. Pokholok DK, Harbison CT, Levine S, Cole M, Hannett NM, et al. (2005) Genome-wide map of nucleosome acetylation and methylation in yeast. Cell 122: 517–527. [DOI] [PubMed] [Google Scholar]
- 53. Joshi AA, Struhl K (2005) Eaf3 chromodomain interaction with methylated H3-K36 links histone deacetylation to Pol II elongation. Mol Cell 20: 971–978. [DOI] [PubMed] [Google Scholar]
- 54. Bellen HJ, Levis RW, Liao G, He Y, Carlson JW, et al. (2004) The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics 167: 761–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rorth P (1996) A modular misexpression screen in Drosophila detecting tissue-specific phenotypes. Proc Natl Acad Sci U S A 93: 12418–12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Oh SW, Kingsley T, Shin HH, Zheng Z, Chen HW, et al. (2003) A P-element insertion screen identified mutations in 455 novel essential genes in Drosophila . Genetics 163: 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Biehs B, Sturtevant MA, Bier E (1998) Boundaries in the Drosophila wing imaginal disc organize vein-specific genetic programs. Development 125: 4245–4257. [DOI] [PubMed] [Google Scholar]
- 58. Rozenblatt-Rosen O, Rozovskaia T, Burakov D, Sedkov Y, Tillib S, et al. (1998) The C-terminal SET domains of ALL-1 and TRITHORAX interact with the INI1 and SNR1 proteins, components of the SWI/SNF complex. Proc Natl Acad Sci U S A 95: 4152–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Curtis BJ, Zraly CB, Marenda DR, Dingwall AK (2011) Histone lysine demethylases function as co-repressors of SWI/SNF remodeling activities during Drosophila wing development. Dev Biol 350: 534–547. [DOI] [PubMed] [Google Scholar]
- 60. Takagi Y, Conaway RC, Conaway JW (1996) Characterization of elongin C functional domains required for interaction with elongin B and activation of elongin A. J Biol Chem. 271: 25562–25568. [DOI] [PubMed] [Google Scholar]
- 61. Conaway JW, Kamura T, Conaway RC (1998) The Elongin BC complex and the von Hippel-Lindau tumor suppressor protein. Biochim Biophys Acta 1377: M49–54. [DOI] [PubMed] [Google Scholar]
- 62. Chopra VS, Cande J, Hong JW, Levine M (2009) Stalled Hox promoters as chromosomal boundaries. Genes Dev 23: 1505–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Muse GW, Gilchrist DA, Nechaev S, Shah R, Parker JS, et al. (2007) RNA polymerase is poised for activation across the genome. Nat Genet 39: 1507–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zeitlinger J, Stark A, Kellis M, Hong JW, Nechaev S, et al. (2007) RNA polymerase stalling at developmental control genes in the Drosophila melanogaster embryo. Nat Genet 39: 1512–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Adelman K, Lis JT (2012) Promoter-proximal pausing of RNA polymerase II: emerging roles in metazoans. Nat Rev Genet 13: 720–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. St Pierre SE, Galindo MI, Couso JP, Thor S (2002) Control of Drosophila imaginal disc development by rotund and roughened eye: differentially expressed transcripts of the same gene encoding functionally distinct zinc finger proteins. Development 129: 1273–1281. [DOI] [PubMed] [Google Scholar]
- 67. Ng M, Diaz-Benjumea FJ, Cohen SM (1995) nubbin encodes a POU-domain protein required for proximal-distal patterning in the Drosophila wing. Development 121: 589–599. [DOI] [PubMed] [Google Scholar]