Abstract
The zebrafish has been proposed for the study of the effects of ethanol on the vertebrate brain. Behavioural tests have been successfully employed in the phenotypical characterization of these effects. However, the short scale (minute to minute) time course of ethanol induced changes of zebrafish behaviour has not been analyzed. The current study alleviates this need using a 2 × 3 chronic × acute ethanol exposure experimental design. We first expose zebrafish to ethanol chronically using a dose escalation procedure in which fish are kept in a final concentration of 0.5% vol/vol ethanol for 10 days while control fish receive identical dosing procedures but no ethanol. Subsequently, we expose zebrafish for one hour to an acute dose of ethanol (0.00, 0.50, or 1.00 % vol.vol) and monitor their behaviour throughout this. period. We quantify the mean and within-individual temporal variance of distance travelled, distance from bottom and angular velocity using video-tracking, and establish temporal trajectories of ethanol induced behavioural changes in zebrafish. For example, we find fish of the highest acute dose group previously not exposed to chronic ethanol to exhibit an inverted U shaped temporal trajectory in distance travelled (biphasic alcohol effect). We find this response to be blunted after chronic ethanol exposure (development of tolerance). We also describe an acute ethanol withdrawal induced increase in angular velocity. We conclude that temporal analysis of zebrafish behaviour is a sensitive method for the study of chronic and acute ethanol exposure induced functional changes in the vertebrate brain.
1. Introduction
Ethanol is one of the world’s most commonly abused substances and has the potential to elicit addiction. It is one of the few drugs that are legally sold, making it readily available to the public [1 – 2]. Although ethanol is known to exert effects on the central nervous system by engaging a number of complex molecular pathways, its exact mechanisms of action are still poorly understood [3 – 5]. Excessive consumption of ethanol may lead to dependence, which may be associated with the development of tolerance and can manifest behaviourally [6 – 7]. Studying the effects of ethanol on behaviour will allow one to investigate a set of mechanisms that may underlie ethanol’s actions.
The zebrafish provides a unique opportunity to investigate the effects of ethanol on behaviour. It has been a popular animal model for developmental biology and has been used to model certain neurological disorders [8 – 20]. The high nucleotide sequence homology of zebrafish genes compared to human genes offers good translational relevance [21]. The zebrafish is also a very prolific species: a single female is capable of spawning hundreds of eggs every other day. Furthermore, due to the small size and social nature of zebrafish, experimental subjects can be housed in high densities, features that are optimal for high-throughput screening (e.g. mutagenesis or drug screens) (see [22 – 23]).
The zebrafish may be an optimal laboratory vertebrate for ethanol related research, particularly because of the ethanol exposure methodology available with this species. The animal can be immersed in the desired ethanol solution, and blood ethanol concentrations in the brain plateau within 40 minutes [13, 24]. Briefly, the immersion procedure in zebrafish is devoid of motivational complications arising from self-administration procedures commonly employed with rodents. It is also arguably preferable compared to the vapour inhalation or invasive injection procedures used for rodents and other mammalian species in the laboratory.
In most ethanol related studies, zebrafish are immersed in a desired ethanol solution for a period of time, followed by behavioural observation in the presence of a response inducing stimulus [25 – 32]. Although much research has been conducted on how ethanol alters behaviour in zebrafish after a certain immersion period, no studies examined how ethanol influences behaviour as it enters the brain, i.e. the time course of ethanol effects has not been well understood. We argue that the temporal characterization of behavioural changes as ethanol enters the brain is important for the development of behavioural tests especially if one wants to conduct large scale mutagenesis screens and also for those who would like to investigate the development of behavioural tolerance.
Gerlai et al. [6] previously found that after a 60 minute ethanol exposure period, a genetically heterogeneous out bred stock of zebrafish (long-fin wild type) exhibited a significant increase in locomotor activity during the subsequent 10 minutes of observation. However, after 2 weeks of continuous exposure to 0.25% vol/vol ethanol, these zebrafish developed tolerance towards ethanol’s stimulatory effect and did not show the increased locomotor activity. A subsequent study [14] further characterized ethanol induced behavioural and neurochemical differences between two strains of zebrafish (AB and short-fin wild type). This latter study utilized the previously established chronic ethanol exposure paradigm, with the exception that here a higher chronic dose (0.50% vol/vol) was employed. Although behavioural analysis demonstrated significant alterations in shoaling and fear responses, ethanol induced locomotor changes were not quantified. Furthermore, it is unclear how behavioural tolerance may manifest as ethanol concentrations increase in the brain during an acute immersion period.
The current paper will address these questions. It will provide detailed temporal characterization of changes in zebrafish behaviour (locomotor responses and location of fish relative to the bottom of the tank) as ethanol enters the brain during an acute exposure period. The use of the previously established chronic ethanol exposure paradigm will also allow us to investigate how behavioural responses may change as a result of prior chronic exposure to ethanol (e.g. manifestation of behavioural tolerance). Finally, the current experimental approach will also provide a detailed time-course analysis of potential behavioural changes during an acute one hour long withdrawal period.
2. Methods
2.1 Animal Housing and Maintenance
Adult zebrafish obtained from our breeding facility at the University of Toronto at Mississauga Vivarium (Mississauga, Ontario, Canada) were used in the study. A total of 84 adult zebrafish (7 – 8 months old) of the AB strain were used in this experiment. This strain was chosen based on its level of homozygosity and its frequent use in behavioural brain research [11, 15, 33].
Animals were kept in groups of 20 for two weeks in 37L glass tanks (50 cm × 30 cm × 25 cm, width × depth × height) prior to testing to allow habituation to their test environment. During chronic ethanol exposure filtration was turned off and system water was changed daily to maintain good water quality and the desired ethanol concentration. Zebrafish were fed ground flake food (a mixture of 3:1 Tetramin flake (Melle, Germany) and Spirulina (Jemco Inc., Lambertville, New Jersey)) twice a day.
Fish were housed and tested under the same environmental conditions. Housing and testing tanks had white corrugated plastic sheets surrounding three sides of the tank to provide a uniform testing environment and to prevent access to external cues.
2.2 Experimental Design and Ethanol Administration
We employed a 2×3 between subject experimental design with 2 chronic ethanol doses (0.00% and 0.50% vol/vol ethanol) and three acute ethanol doses (0.00%, 0.50%, and 1.00% vol/vol). This experimental design has been utilized previously and thus makes our results comparable to previously published ones [6, 14]. A dose escalation procedure was used to achieve the 0.50% chronic exposure to prevent an increase in mortality rates associated with prolonged ethanol exposure. Briefly, zebrafish were housed in 0.125% ethanol for days 1-4, in 0.250% ethanol for days 5-8, in 0.375% ethanol for days 9-12, and finally in 0.50% ethanol for a subsequent 10 day period, a total of 22 days of chronic ethanol exposure. On day 23, zebrafish were given an acute ethanol challenge in which they were immersed in a 37L tank in one of the following concentrations: 0.00%, 0.50%, or 1.00% vol/vol ethanol for 60 minutes. Another set of zebrafish were exposed to a dosing procedure identical to that employed for the chronic exposure, but these fish were housed for 22 days in system water (0.00% ethanol) to serve as controls. These fish were also given an acute ethanol challenge on day 23. Therefore there were a total of 6 different groups designated as C0.00A0.00 (n = 14), C0.00A0.50 (n = 13), C0.00A1.00 (n = 18), C0.50A0.00 (n = 13), C0.50A0.50 (n = 16), C0.50A1.00 (n = 10), where C represents the initial chronic ethanol treatment, and A represents the subsequent acute ethanol treatment and the numbers correspond to the ethanol/water vol/vol percentage of the employed concentration. Although the chronic and acute dosing procedures employed here were identical to those used before [6, 14], here the behavioural observation started at the very beginning of the 60 min acute exposure session and continued throughout the entire session. Fish were assigned to their respective ethanol treatment group randomly and all fish were tested in a fully randomized and blind manner.
2.3 Quantification of Behaviour
Video files were transferred and analyzed using a video tracking software, Ethovision XT 8.5 (Noldus, Info Tech., Wageningen, The Netherlands). The following parameters were quantified: total distance travelled, distance from bottom and angular velocity. Ethanol has been shown to have both excitatory and depressive effects on locomotor activity depending on dose and administration regimen [16, 18] and total distance travelled may be sensitive to such effects. Total distance travelled was calculated as the distance travelled by the center point of the subject from the previous sample to the current one” [34]. Distance from bottom may also be a sensitive measure of ethanol effects as this behaviour is dependent upon level of fear as well as motor function [16, 30 – 32]. Distance from bottom was calculated as “the shortest distance between a subject’s body point and a zone” [34], in which case the zone of interest was the bottom of the tank. Angular velocity is particularly sensitive to changes in swim direction and may enable us to detect ethanol induced changes in motor function, particularly a type of behaviour termed erratic movement [9, 15, 16, 29]. Angular velocity was calculated as “the change in direction of movement of the center point or head direction line between two consecutive samples, calculated per unit time” [34]. Absolute angular velocity was calculated by taking the non-negative value and is expressed in degrees/second (o/s)”. Absolute angular velocity was reported to provide a sensitive measure of changes in direction of movement that also accounted for bidirectional turns. The average of these behavioural parameters as well as the within individual temporal variance were calculated for 1 minute intervals of the 60 minute acute exposure session. The mean and standard error of the mean of the average and within-individual temporal variance are shown on the figures.
2.4 Statistical Analysis
The data were analyzed using SPSS (Version 21). We conducted 3-way univariate repeated measures ANOVAs with factors “interval” (the repeated measure factor with 60 levels) chronic ethanol treatment (with two levels) and acute ethanol treatment (with three levels). Multiple range post hoc tests are not appropriate for repeated measures designs and therefore when significant main effects or interaction terms were found we performed the following transformation. In order to capture potential time dependent changes and to analyze potential interval effects, the 60 min session data were split into three equal periods (the first, the second, and the third (last) 20 minutes of observation). We chose these three 20 min intervals for our follow up analyses for the following reasons. Prior results suggested that ethanol took about 20 min after immersion of fish in it to reach measurable levels in the brain [13] and also to induce the first detectable significant changes at the level of neurochemistry in the brain [24]. Furthermore ethanol was shown to take another 20 min to reach a plateau level in the brain and also to induce maximal changes in neurochemistry [13, 24]. Last, after the first 40 min immersion period the level of ethanol and its effects on neurochemicals were found to remain stable [13, 24]. The average for each of the 20 min periods was calculated and analyzed separately using 2-way ANOVAs (with factors chronic and acute ethanol treatment). In case of significant main effects or interaction term, a post hoc Tukey Honestly Significant Difference (HSD) test was conducted. Also, we decided to compare all groups of our 2×3 experimental design even when no significant interaction was uncovered by ANOVA because this latter statistical procedure is known to be underpowered for the detection of interaction between main factors [35]. We accepted significance when the probability (p) of the null hypothesis was less than 0.05.
3. Results
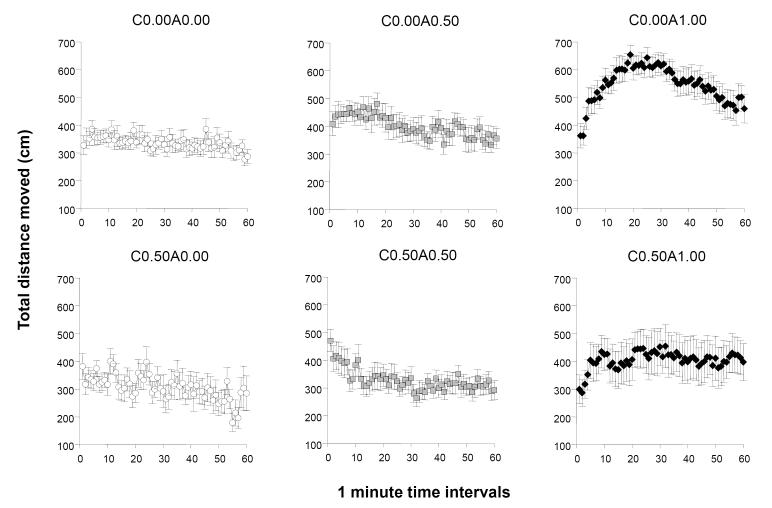
Analysis of the total distance travelled suggested that ethanol treatment had an effect on the temporal trajectory of this behaviour (Figure 1). ANOVA confirmed this observation and found a significant interval effect (F(59, 4602) = 2.999, p < 0.001), a significant chronic ethanol treatment effect (F(1, 78) = 10.312, p = 0.002) and also a significant acute ethanol treatment effect (F(2, 78) = 12.707, p<0.001). The interaction between interval and acute ethanol treatment was also found significant (F(118, 4602) = 2.435, p < 0.001) but all other interaction terms were non-significant. Perusal of figure 1 shows that the temporal changes in the distance travelled may be dependent upon the particular combination of chronic and acute ethanol treatment. Especially apparent is the inverted U shaped trajectory in the case of fish exposed to 1.00% ethanol after freshwater pre-treatment. Analysis of the 20 min interval averages showed that acute ethanol had a significant effect as early as during the first 20 min of the session and also that this effect was significant for all three 20 min intervals (F(2, 78) > 6.486, p < 0.01). The effect of chronic ethanol pre-treatment was also found significant for each 20 min interval (F1, 78) > 5.210, p < 0.05) but no significant interaction between these factors were detected for any of the 20 min intervals. Post hoc Tukey HSD tests conducted separately for the 20 min intervals demonstrated that the C0.00A1.00 group significantly (p < 0.05) differed from most of the other groups (first 20 min: C0.00A1.00 differed from C0.00A0.00, C0.50A0.00, C0.50A0.50, C0.50A1.00; second 20 min C0.00A1.00 differed from all other groups; third 20 min: C0.00A1.00 differed from all groups except C0.50A1.00), while other group differences were non-significant.
Figure 1.
The temporal trajectory of the total distance moved measured during a one hour long recording session is dependent upon the concentration of ethanol administered acutely during the session and on whether the fish were chronically pre-exposed to ethanol before. Mean ± S.E.M. are shown for 1-minute intervals of the recording session. The treatment conditions (group designations) are shown above the graphs. The values following the letter ‘C’ represent the concentration of ethanol (expressed as ethanol/water vol/vol %) employed during the chronic pre-treatment period (0.00 representing control and 0.50 the chronic ethanol exposed fish). The values following the letter ‘A’ represent the concentration of ethanol employed during the acute exposure, i.e. the behavioural recording, session (0.00 representing control, and 0.50 and 1.00 the corresponding acute ethanol exposed fish). Sample sizes (n) were as follows: C0.00A0.00 = 14; C0.00A0.50 = 13; C0.00A1.00 = 18; C0.50A0.00 = 13; C0.50A0.50 = 16; C0.50A1.00 = 10. Note the slightly elevated activity in the group of fish acutely exposed to the intermediate concentration of ethanol (C0.00A0.50) as compared to control. Also note the inverted U-shaped temporal trajectory with an initially rising activity and subsequent falling activity in the highest acute concentration group (C0.00A1.00). Last observe the attenuation of the rising and falling phase and also the blunted overall increase of activity in the fish that were chronically pre-treated with ethanol and subsequently exposed to the highest ethanol dose acutely (C0.50A1.00 vs. C0.00A1.00). For details of statistical analysis and other group differences see Results.
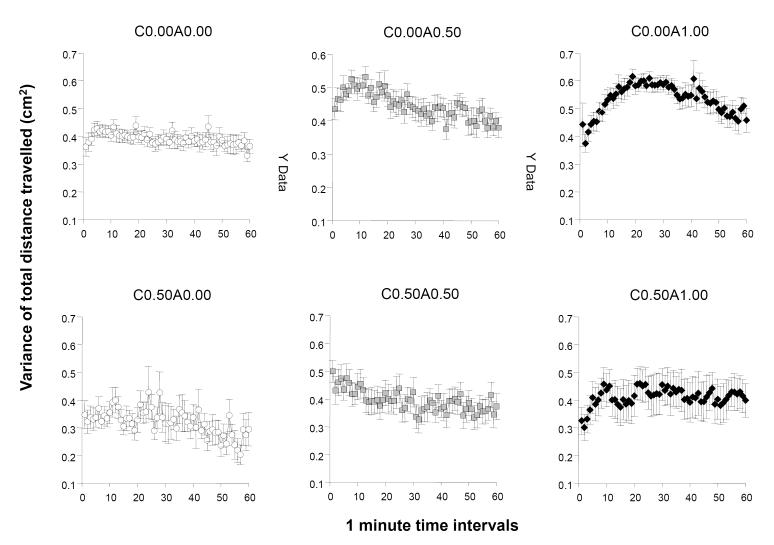
We also analyzed the variance of distance travelled in the 60 minutes of recording (figure 2). This variance represents the within individual temporal variance and quantifies how consistently each fish swam, i.e. whether their swim speed (and thus the distance travelled) varied from moment to moment (resolution). Three cases had to be excluded from the analysis due to technical error. The overall pattern of results appeared similar to that of distance travelled. ANOVA detected a significant acute ethanol treatment effect (F(2, 75) = 7.203, p = 0.001), a significant chronic ethanol treatment effect (F(1, 75) = 10.418, p < 0.01), and a significant interval effect (F(59, 4425) = 3.755, p < 0.001. All interaction terms were also significant (interval × chronic F(59, 4425) = 1.078, p = 0.012, interval × acute F(118, 4425) = 1.0959, p = 0.022, and interval × chronic × acute F(118, 4425) = 0.990, p = 0.011) except the interaction between chronic and acute ethanol treatment (F(2, 75) = 0.650, p = 0.525). Analysis of the 20 min interval averages showed that acute ethanol had a significant effect throughout all three 20 min intervals (F(2, 75) > 4.712, p < 0.05). The effect of chronic ethanol pre-treatment was also found significant for each 20 min interval (F1, 75) > 7.436, p < 0.01) but the interaction between chronic and acute ethanol treatment was not significant for any of the 20 min intervals. Tukey HSD tests revealed numerous group differences. For the first 20 min interval it found group C0.50A0.00 (the withdrawal group) to significantly differ from groups C0.00A0.50 and C0.00A1.00. For the second 20 min interval, Tukey HSD found group C0.00A1.00 to significantly (p < 0.05) differ from all other groups except for C0.50A1.00 for which the difference did not reach significance (p = 0.059). For the third 20 min interval C0.00A1.00 was found to differ significantly (p < 0.05) from C0.50A0.00 and marginally from C0.50A0.50 (p = 0.052) but other group differences were non-significant.
Figure 2.
The temporal trajectory of the within individual variance of total distance moved measured during a one hour long recording session is dependent upon the concentration of ethanol administered acutely during the session and on whether the fish were chronically pre-exposed to ethanol before. Note that this variance reflects the moment to moment changes in the speed with which the particular fish moved and thus increasing variance means less consistent swimming speed whereas decreasing variance means more steady swimming speed. Mean + S.E.M. are shown for 1-minute intervals of the recording session. The treatment conditions (group designations) are shown above the graphs. The values following the letter ‘C’ represent the concentration of ethanol (expressed as ethanol/water vol/vol %) employed during the chronic pre-treatment period (0.00 representing control and 0.50 the chronic ethanol exposed fish). The values following the letter ‘A’ represent the concentration of ethanol employed during the acute exposure, i.e. the behavioural recording, session (0.00 representing control, and 0.50 and 1.00 the corresponding acute ethanol exposed fish). Sample sizes (n) were as follows: C0.00A0.00 = 14; C0.00A0.50 = 13; C0.00A1.00 = 18; C0.50A0.00 = 13; C0.50A0.50 = 16; C0.50A1.00 = 10. Note the elevated activity in the group of fish acutely exposed to the intermediate concentration of ethanol (C0.00A0.50) as compared to control. Also note the inverted U-shaped temporal trajectory with an initially rising variance and subsequent falling variance in the highest acute concentration group (C0.00A1.00). Last observe the attenuation of the rising and falling phase and also the blunted overall increase of variance in the fish that were chronically pre-treated with ethanol and subsequently exposed to the highest ethanol dose acutely (C0.50A1.00 vs. C0.00A1.00). For details of statistical analysis and other group differences see Results.
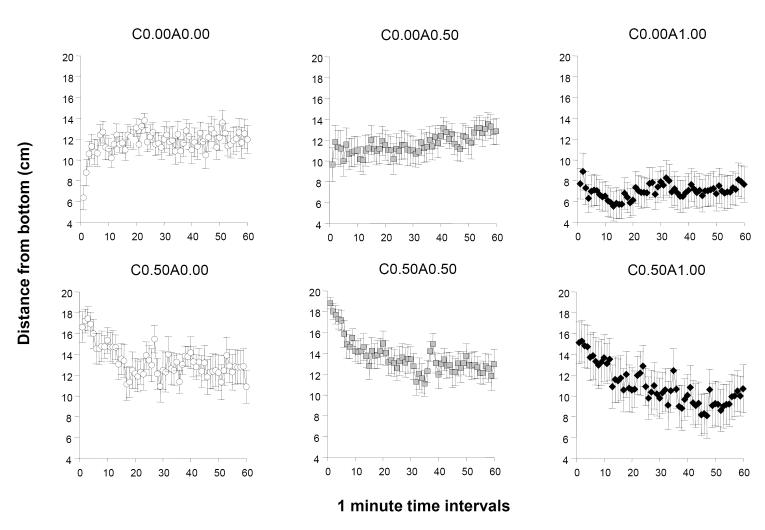
In addition to locomotor activity, we also measured the location of fish relative to the bottom of the test tank. The distance from bottom (figure 3) appeared to be affected by the different ethanol treatments. ANOVA demonstrated a significant acute ethanol treatment effect, F(2, 78) = 6.761, p < 0.01, a chronic ethanol treatment effect, F(1, 78) = 7.583, p< 0.01 and an interval effect, F(59, 4602) = 2.255, p < 0.001. Only the interaction term interval × chronic ethanol treatment was found significant (F(59, 4602) = 5.708, p < 0.001) confirming that the distance de creased with time in the chronic ethanol pre-treated groups (figure 3 lower three graphs) and it was increasing or remained steady in the fresh-water pre-treated groups (figure 3 upper three graphs). Control fish (C0.00A0.00) demonstrated an initial preference for the bottom of the tank. Paired t-tests with Bonferroni correction determined that the first minute was significant different compared to the average of the last 20 minutes, t(13) = −4.801, p < 0.001, but the effect became non-significant by the second minute, t(13) = −2.267, p > 0.05. The initial preference in the first minute was not found in alcohol treated fish (C0.00A0.50, t(12) = − 1.689, p > 0.05), (C0.00A1.00, t(17) = 0.327, p > 0.05). Analysis of the 20 min periods of the 60 min recording session showed that acute ethanol treatment had a significant effect during each of these periods (F2, 78) > 4.927, p < 0.01). Chronic ethanol treatment exerted a significant effect during the first 20 min (F(1, 78) = 22.304, p < 0.001) but by the second the effect diminished and did not reach significance (F(1, 78) = 3.823, p = 0.054) and for the subsequent third 20 min period it remained non-significant (F(1, 78) = 0.813, p > 0.35). The acute × chronic ethanol interaction was non-significant for all three 20 min periods. Tukey HSD multiple range post hoc comparisons showed that the C0.00-A1.00 group significantly (p < 0.05) differed from all other ethanol treatment groups during the first 20 min. The C0.00A1.00 group also showed a significant difference (p < 0.05) compared to C0.00A0.00, C0.50A0.00 and C0.50A0.50 for the second 20 min period, and compared to C0.00A0.50, C0.50A0.00, C0.50A0.50 for the last 20 min period. Other group differences were found non-significant.
Figure 3.
The temporal trajectory of the distance from the bottom of the tank measured during a one hour long recording session is dependent upon the concentration of ethanol administered acutely during the session and on whether the fish were chronically pre-exposed to ethanol before. Mean ± S.E.M. are shown for 1-minute intervals of the recording session. The treatment conditions (group designations) are shown above the graphs. The values following the letter ‘C’ represent the concentration of ethanol (expressed as ethanol/water vol/vol %) employed during the chronic pre-treatment period (0.00 representing control and 0.50 the chronic ethanol exposed fish). The values following the letter ‘A’ represent the concentration of ethanol employed during the acute exposure, i.e. the behavioural recording, session (0.00 representing control, and 0.50 and 1.00 the corresponding acute ethanol exposed fish). Sample sizes (n) were as follows: C0.00A0.00 = 14; C0.00A0.50 = 13; C0.00A1.00 = 18; C0.50A0.00 = 13; C0.50A0.50 = 16; C0.50A1.00 = 10. Note the rapid rise of distance from bottom at the first few minutes of the recording session in the control group (C0.00A0.00) and the lack or reversal of this response in the ethanol treated groups. Also note the robust reduction of distance from bottom in the highest acute concentration group (C0.00A1.00) and the attenuation of this effect in the fish that were chronically pre-treated with ethanol and subsequently exposed to the highest ethanol dose acutely (C0.50A1.00 vs. C0.00A1.00). For details of statistical analysis and other group differences see Results.
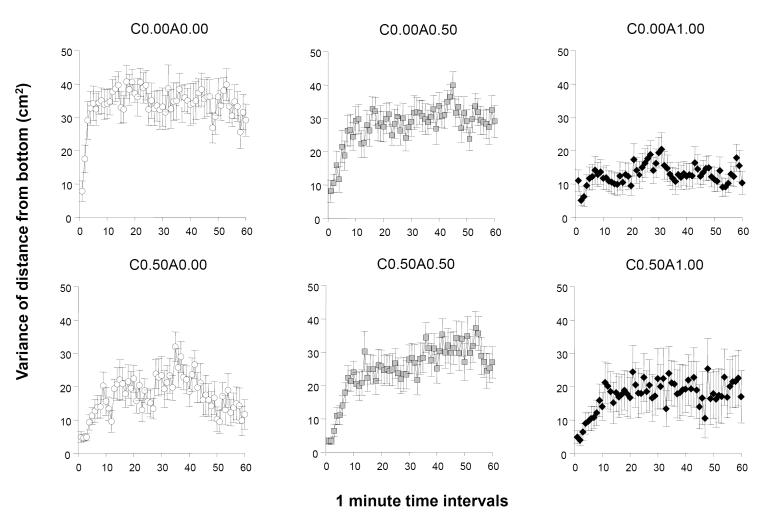
The variance of the distance from bottom showed a different pattern of results (figure 4) compared to those obtained for the parameter distance from bottom. In general it appears that most groups increased their variance as the session progressed, perhaps except the withdrawal group (C0.50A0.00), which exhibited an inverted U-shaped temporal trajectory, and the highest acute dose group (C0.00A1.00), which showed a reduced level of variance that appeared to remain throughout the test session. ANOVA detected a significant acute ethanol effect (F(2, 78) = 8.197, p = 0.001) but the chronic ethanol effect did not reach significant F(1, 78) = 3.875, p = 0.053. The effect of interval was found significant (F(59, 4602) = 7.862, p < 0.001). The interaction term interval × acute was also significant (F(118, 4602) = 1.704, p < 0.001). Other interaction terms were not detected to be significant. Analysis of the 20 min periods of the 60 min recording session revealed that acute ethanol treatment had a significant effect during each of these periods (F2, 78) > 5.373, p < 0.01). Chronic ethanol was found to exert a significant effect only for the first 20 min of the session (F(1, 78) = 8.233, p < 0.01). In addition, a significant acute × chronic ethanol treatment interaction was also detected for all periods (F(2, 78) = 3.263, p < 0.05). Tukey HSD tests showed that for the first 20 min interval the control group C0.00A0.00 was significantly (p < 0.01) different from all groups except C0.00A0.50 and that C0.00A1.00 and C0.00A0.50 also differed. For the second 20 min interval the results were different. Tukey HSD found the control group (C0.00A0.00) to differ (p < 0.01) only from C0.00A1.00 The latter group also differed (p < 0.05) from C0.00A0.50 (p < 0.05) and no other significant group differences were detected. For the third 20 min period of the recording session Tukey HSD showed a significant difference between the control group (C0.00A0.00) and C0.00A1.00 as well as C0.50A0.00. It also found C0.00A1.00 to differ (p < 0.01) from C0.00A0.50 and also from C0.50A0.50, while other group differences were non-significant.
Figure 4.
The temporal trajectory of the within-individual variance of distance from the bottom of the tank measured during a one hour long recording session is dependent upon the concentration of ethanol administered acutely during the session and on whether the fish were chronically pre-exposed to ethanol before. Note that this variance reflects the moment to moment changes in the distance the particular fish was from the bottom and thus increasing variance represents increased vertical changes whereas decreasing variance means more consistent vertical positioning of the fish. Mean ± S.E.M. are shown for 1-minute intervals of the recording session. The treatment conditions (group designations) are shown above the graphs. The values following the letter ‘C’ represent the concentration of ethanol (expressed as ethanol/water vol/vol %) employed during the chronic pre-treatment period (0.00 representing control and 0.50 the chronic ethanol exposed fish). The values following the letter ‘A’ represent the concentration of ethanol employed during the acute exposure, i.e. the behavioural recording, session (0.00 representing control, and 0.50 and 1.00 the corresponding acute ethanol exposed fish). Sample sizes (n) were as follows: C0.00A0.00 = 14; C0.00A0.50 = 13; C0.00A1.00 = 18; C0.50A0.00 = 13; C0.50A0.50 = 16; C0.50A1.00 = 10. Note the reduction of variance in the highest acute dose treated fish not previously exposed to ethanol (C0.00A1.00) as compared to control fish, for example (C0.00A0.00). For details of statistical analysis and other group differences see Results.
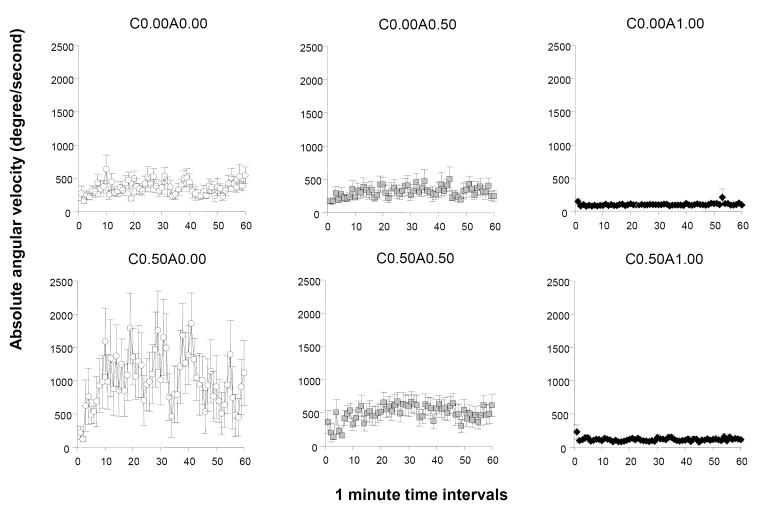
Absolute angular velocity quantifies the directional changes exhibited by zebrafish as they swim. Perusal of figure 5 suggests an apparently robust ethanol treatment effect. Particularly noteworthy is the very small values exhibited by the fish treated with the highest dose of ethanol (the C0.00A1.00 and C0.50A1.00 groups). Another robust ethanol effect is apparent in the withdrawal group (C0.50A0.00). This group of fish showed elevated absolute angular velocity values that appear also quite variable (within interval error variation as well as fluctuations across intervals). ANOVA confirmed a significant acute ethanol treatment effect (F(2, 78) = 12.490, p < 0.001), a chronic ethanol treatment effect (F(1, 78) = 8.926, p < 0.01), and an interval effect (F(59, 4602) = 2.122, p < 0.001). The interaction terms chronic × acute ethanol treatment (F(2, 78) = 4.057, p < 0.05) and interval × acute ethanol treatment (F(118, 4602) = 1.489, p < 0.01) were also found significant. Analysis of the 20 min periods of the 60 min recording session confirmed that acute ethanol treatment had a significant effect during each of these periods (F2, 78) > 8.486, p < 0.001). The effect of chronic ethanol treatment was also found significant for all three 20 min periods (F(1, 78) > 7.536, p < 0.01). The acute × chronic ethanol treatment interaction was found significant for the first two 20 min periods of the recording session (F(1, 78) > 3.180, p < 0.05) but for the last 20 min period the interaction did not reach significance (F(1, 78) = 2.720, p = 0.072).
Figure 5.
The temporal trajectory of absolute angular velocity measured during a one hour long recording session is dependent upon the concentration of ethanol administered acutely during the session and on whether the fish were chronically pre-exposed to ethanol before. Mean ± S.E.M. are shown for 1-minute intervals of the recording session. The treatment conditions (group designations) are shown above the graphs. The values following the letter ‘C’ represent the concentration of ethanol (expressed as ethanol/water vol/vol %) employed during the chronic pre-treatment period (0.00 representing control and 0.50 the chronic ethanol exposed fish). The values following the letter ‘A’ represent the concentration of ethanol employed during the acute exposure, i.e. the behavioural recording, session (0.00 representing control, and 0.50 and 1.00 the corresponding acute ethanol exposed fish). Sample sizes (n) were as follows: C0.00A0.00 = 14; C0.00A0.50 = 13; C0.00A1.00 = 18; C0.50A0.00 = 13; C0.50A0.50 = 16; C0.50A1.00 = 10. Note the apparent reduction of S.E.M in the highest acute concentration groups (C0.00A1.00 and C0.50A1.00) compared to the other groups. Also note the increased S.E.M and also the increased mean of absolute angular velocity in the ethanol withdrawal group (C0.50A0.00). For details of statistical analysis and other group comparisons see Results.
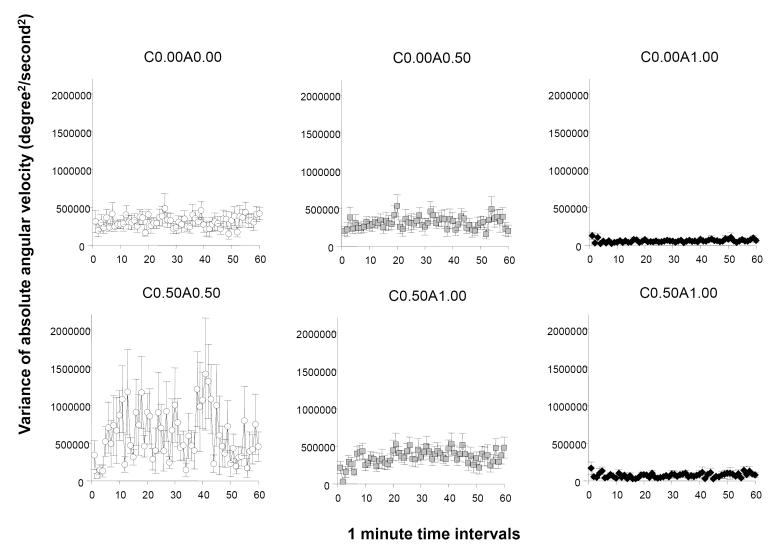
The last behavioural measure we consider for our analysis is the variance of absolute angular velocity (figure 6). The pattern of results appears very similar to what we obtained for angular velocity. ANOVA detected a significant acute ethanol treatment effect (F(2, 78) = 16.007, p < 0.001), a chronic ethanol treatment effect, F(1, 78) = 4.409, p < 0.05), and an interval effect (F(59, 4602) = 1.346, p < 0.0). The interaction term interval × chronic ethanol treatment was also significant (F(59, 4602) = 1.443, p < 0.05) but the other interaction terms did not reach significance. Similarly to the results obtained for absolute angular velocity, the analysis of the variance of absolute angular velocity conducted for the 20 min periods of the 60 min recording session confirmed that acute ethanol treatment had a significant effect during each of these periods (F2, 78) > 10.283, p < 0.001). The effect of chronic ethanol treatment was found significant for the second 20 min period (F(1, 78) > 4.054, p < 0.05). The acute × chronic ethanol treatment interaction was significant only for the first 20 min period of the recording session (F(1, 78) > 3.394, p < 0.05). Tukey HSD analyses conducted separately for each of the 20 min periods demonstrated that the withdrawal group (C0.50A0.00) was significantly (p < 0.05) different from all other groups during the first 20 min period. During the second and third 20 min periods this group (C0.50A0.00) was different (p < 0.05) from C0.00A1.00 and from C0.50A1.00. But the other groups did not significantly differ from each other.
Figure 6.
The temporal trajectory of the within-individual variance of absolute angular velocity measured during a one hour long recording session is dependent upon the concentration of ethanol administered acutely during the session and on whether the fish were chronically pre-exposed to ethanol before. Note that this variance reflects the moment to moment changes in the direction of swimming (absolute angular velocity) the particular fish performs and thus increasing variance represents increased inconsistency in directional changes whereas decreasing variance means more consistent changes in swim direction. Mean ± S.E.M. are shown for 1-minute intervals of the recording session. The treatment conditions (group designations) are shown above the graphs. The values following the letter ‘C’ represent the concentration of ethanol (expressed as ethanol/water vol/vol %) employed during the chronic pre-treatment period (0.00 representing control and 0.50 the chronic ethanol exposed fish). The values following the letter ‘A’ represent the concentration of ethanol employed during the acute exposure, i.e. the behavioural recording, session (0.00 representing control, and 0.50 and 1.00 the corresponding acute ethanol exposed fish). Sample sizes (n) were as follows: C0.00A0.00 = 14; C0.00A0.50 = 13; C0.00A1.00 = 18; C0.50A0.00 = 13; C0.50A0.50 = 16; C0.50A1.00 = 10. Note the apparent reduction of S.E.M in the highest acute concentration groups (C0.00A1.00 and C0.50A1.00) compared to the other groups. Also note the increased S.E.M and also the increased mean of within-individual variance of absolute angular velocity in the ethanol withdrawal group (C0.50A0.00). For details of statistical analysis and other group comparisons see Results.
4. Discussion
Our study provides one of the first time-course analyses of the effects of acute ethanol with as well as without prior chronic ethanol pre-exposure on the behaviour of adult zebrafish. Using three parameters of swim path we have uncovered temporal changes that were dependent upon ethanol dose and exposure regimen. Our results confirm the biphasic nature of the effects of a high dose of ethanol, demonstrate significant behavioural tolerance after chronic exposure, and also reveal significant effects of acute withdrawal from ethanol among other findings. Here we organize our discussion of the above and other interpretive questions according to the behavioural parameters employed.
Ethanol has been suggested to exert a biphasic effect on the central nervous system (CNS). Initially, ethanol acts as a stimulant, an effect often associated with enhanced euphoria and decreased inhibition in humans. Continued acute exposure to ethanol accompanied by an increase in blood ethanol concentration leads to suppression or inhibitory effects at the level of behavioural activity [36]. In zebrafish, ethanol administered acutely has been shown to increase locomotor activity at low to moderate concentrations, and decrease it at high doses [6]. Recently, Rosemberg et al. [16] demonstrated that a high concentration of ethanol (1.00% vol/vol) had a stimulatory effect after 20 minutes of exposure, while exhibiting inhibitory actions after 60 minutes. Our findings are in line with this result as they show a dose-dependent increase in locomotor activity (distance travelled) in response to acute ethanol exposure. The biphasic effect is also apparent when one observes the temporal changes seen in the highest dose of acute ethanol administered without chronic pre-treatment (C0.00A1.00 group, figure 1). The temporal change we saw in this group was inverted U shaped. This trajectory may be due to a rising excitation resulting from acute exposure induced elevation of ethanol levels in the brain after being immersed in the solution and the subsequent depression once the maximal blood/brain ethanol levels have been reached. Notably, the temporal trajectory showing a peak around 20-30 min after the start of ethanol exposure is in line with the results of temporal analysis of neurochemical responses [24] as well as with the temporal changes of ethanol levels obtained from whole brain tissue samples reported previously for zebrafish [13]. It is also notable that the overall changes seen in the distance travelled were highly similar to those found for the variance of distance travelled. This variance reflects within individual temporal variance, i.e. it quantifies how consistently or inconsistently the fish swam. The rising variance at the first phase of ethanol exposure implies that fish changed their swim speed increasingly more but subsequently slowly became more consistent again, which also coincided with overall reduction of activity.
A previous study [15] compared the effects of acute ethanol on different strains of zebrafish and found that the AB strain exhibited decreased locomotor activity with increasing doses of ethanol. At first glance, this result appears to be in contradiction with our current findings. However, in that study [15], zebrafish were tested only after the fish have been immersed in 1.00% vol/vol ethanol for 1 hour and not during this immersion period. In the current study, we found that exposure to 1.00% ethanol actually increased locomotor activity during the first half of the exposure period, which was followed by a steady decline of activity during the second half of the hour. These results thus imply that continued exposure (beyond 60 minutes) may cause a decrease in locomotor activity compared to control. In summary, our current and the previously published results demonstrate the biphasic nature of the effects of acute ethanol exposure.
Another important conclusion may be drawn from the temporal changes seen in the C0.00A1.00 group. The activity level of this group was statistically indistinguishable from control at the beginning of the exposure period. This provides evidence that the behavioural effects of acute ethanol exposure were not due to peripheral effects of ethanol including, for example, due to potential irritation of the skin or gills. Because the temporal changes of behaviour strongly correlated with the previously reported changes of ethanol levels in the brain and also with the neurochemical changes seen, we conclude that the behavioural effects of ethanol are not the result of peripheral effects but are explained by central mechanisms.
In the current study, we found prolonged exposure to ethanol (i.e. the chronic ethanol treatment) to significantly attenuate the effect of subsequent acute ethanol exposure, an effect also demonstrated previously [6, 14]. The development of behavioural tolerance is most evident in the 1.00% acute exposure group (C0.50A1.00) where the initial increase in locomotor activity within the first 20 minutes as well as its decline in the last 20 minutes of the session are both significantly blunted in the chronic condition. Such adaptation to ethanol may be due to several mechanisms including, for example, to altered metabolism of ethanol or to neuroadaptations at the cellular or biochemistry levels. It is notable, however, that because of the acute immersion procedure (the ethanol solution surrounds the fish and ethanol constantly diffuses into the fish throughout the acute exposure session), it is unlikely that changes in ethanol metabolism could have contributed to the observed behavioural tolerance.
In nature, zebrafish spend their time foraging for food near the surface of the water. In response to a predator, the animal quickly escapes to the bottom [37]. The distance from the bottom of the tank has been taken as a measure of anxiety or fear [18, 19, 25, 26]. The response has been experimentally shown in fear and anxiety paradigms, for example, in which a predator stimulus or a novel tank was used [25 – 32]. Studies have demonstrated that low concentrations of ethanol (0.3% vol/vol) exert anxiolytic effects and increase the time spent near the surface, while anxiogenic substances (e.g. caffeine and pentylenetetrazol) increase time spent at the bottom [25]. Our test tank is a novel environment which is expected to induce a moderate level of fear in zebrafish [18]. Control zebrafish exhibited a fear response in the first minute of the recording session demonstrated by an initial preference for the bottom, a response that habituated over time. Acute exposure to ethanol abolished this initial preference for the bottom confirming ethanol’s anxiolytic properties at low concentrations. Notably, a recent study [38] demonstrated that group-housed zebrafish exhibited a robust anxiety response when tested in a novel tank diving paradigm, yet failed to show the anxiolytic effect of ethanol, compared to individually housed zebrafish. Our results showed that group housed zebrafish exhibited both novelty induced fear and ethanol induced anxiolysis. We hypothesize that the use of the larger novel tank (37 l vs. 1.5 l) made our current paradigm more effective to induce fear and also made it more sensitive to quantify ethanol’s anxiolytic properties. In an open area fish may be more vulnerable to predators and the larger depth of our test tank also allowed more precise quantification of changes in the subject’s vertical position.
Zebrafish exposed to ethanol at the highest concentration (1.00% vol/vol) significantly decreased the distance from the bottom, a finding that is in line with prior results showing anxiogenic effects of high concentrations of ethanol [16, 18]. Alternatively, one may suggest that the decreased distance from bottom could also be due to sedative effects of ethanol [18]. However, our current results suggest that the latter explanation may be less likely. The increase of distance traveled induced by the highest acute dose suggest that at least during the first half of the one hour long immersion and recording session the decreased distance to the bottom could not be due to sedation. Nevertheless, it is possible that ethanol’s sedative effects did contribute to the reduced distance to bottom at least during the second half of the session. Although it is unclear whether the change in distance to bottom is the result of increased anxiety or sedation, it is clear that the effect is significantly attenuated by prior chronic ethanol exposure. The attenuated decrease of distance once again demonstrates the development of behavioural tolerance.
Although the withdrawal group did not differ from control in terms of time spent near the bottom, there was a significant difference in the variance of this parameter. The variance of distance from bottom may serve as an index of tank exploration, such that a larger variance suggests more vertical movement or exploration. The withdrawal group had a significantly lower variance compared to controls suggesting reduced tank exploration, possibly due to increased anxiety as a result of withdrawal from ethanol. The variance of distance from bottom thus may prove to be an excellent measure of tank exploration and may be utilized to quantify habituation to novelty due to its subtle sensitivity to locomotor activity.
The last measure of interest we discuss is angular velocity, a novel measure with which we hoped to assess erratic movements expected primarily in the withdrawal group [9]. Although turn angle and angular velocity has been utilized to characterize erratic movements successfully in the past [15, 16, 29], absolute angular velocity may be a more sensitive measure of the erratic swim pattern because turn angle is calculated as “the change in direction of the center point or head direction line between two consecutive samples” [34], whereas angular velocity accounts for changes per unit of time. The withdrawal group exhibited significantly higher absolute angular velocity compared to control. Withdrawal from ethanol is often accompanied by hyperactivity and anxiety-like responses [9, 20]. The increased absolute angular velocity we obtained here for zebrafish in the ethanol withdrawal group may be due to both. It may be the result of hyperexcitability leading to rapid changes in swim directions, and it may also reflect increased erratic movement, a response that was shown to be elicited by fear inducing stimuli in zebrafish [30 – 32]. The within individual temporal variance of absolute angular velocity was also found to be significantly higher in the withdrawal group suggesting that these fish performed turns and changed swim directions in a rather inconsistent manner compared to controls. The combination of a higher absolute angular velocity and variance suggest that the swim patterns of acute withdrawal fish is characterized by many short bursts of erratic swim direction episodes. It is important to note that withdrawal from ethanol did not manifest in changes in overall distance swum and also did not seem to have a measurable effect on the distance from the bottom. Thus, without the use of this novel parameter (absolute angular velocity) one may not have been able to uncover the effects of acute withdrawal from ethanol. Unrelated to withdrawal, but also noteworthy is the apparent decrease in angular velocity observed in the highest acute ethanol concentration groups (C0.00A1.00 and C0.50A1.00). This finding demonstrates that although fish exposed to a high acute dose were able to swim fast and were not sedated in a classical sense, their motor response was impaired: their swim pattern was more rigidly steady as opposed to the normal episodic turns exhibited by control fish, an abnormality perhaps representing a mild and previously undetected form of sedative effect of ethanol [16].
In the current study we utilized video-tracking-based parameters of the swim path of zebrafish to analyze the temporal trajectories of behavioural changes induced by chronic and acute ethanol exposure. Although the mechanisms underlying the observed behavioural effects are not known, our results show that detailed quantification of behaviour may uncover ethanol effects that resemble those seen in other vertebrates including humans. These results imply face validity of zebrafish as a model in ethanol research and thus suggest that zebrafish may be an appropriate tool with which the mechanisms of the actions of ethanol will be investigated.
Research highlights.
Chronic and acute ethanol combination treatment employed
Temporal changes in swim path parameters quantified
Acute and chronic ethanol effects identified
Adaptation to chronic ethanol demonstrated
Effects of withdrawal from chronic ethanol found
Acknowledgements
We would like to thank Y. Fernandes and M. Rampersad for their technical help. The presented research was supported by an NIH/NIAAA (R01AA015325-01A2) grant awarded to RG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ferreira MP, Willoughby D. Alcohol consumption: the good, the bad, and the indifferent. Appl Physiol Nutr Metab. 2008;33:12–20. doi: 10.1139/H07-175. [DOI] [PubMed] [Google Scholar]
- [2].Kessler RC, McGonagle KA, Zhao SY, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-III-R Psychiatric-disorders in the United-States - Results from the National Comorbidity Survey. Arch.Gen. Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- [3].Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hyman SE, Malenka RC. Addiction and the brain: The neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- [5].Hyman SE, Malenka RC, Nestler EJ. Neural Mechanisms of Addiction: The Role of Reward-Related Learning and Memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- [6].Gerlai R, Lee V, Blaser R. Effects of acute and chronic ethanol exposure on the behaviour of adult zebrafish (Danio rerio) Pharmacol Biochem Behav. 2006;85:752–761. doi: 10.1016/j.pbb.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Arias C, Revillo DA, Spear NE. Chronic tolerance to the locomtor stimulating effect of ethanol in preweanling rats as a fuction of social stress. Alcohol. 2012;46:245–252. doi: 10.1016/j.alcohol.2011.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lieschke G, Currie P. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- [9].Cachat J, Canavello P, Elegante M, Bartels B, Hart P, Bergner C, et al. Modeling withdrawal syndrome in zebrafish. Behav Brain Res. 2010;208:371–376. doi: 10.1016/j.bbr.2009.12.004. [DOI] [PubMed] [Google Scholar]
- [10].Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, et al. Understanding behavioural and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res. 2009;205:38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fernandes Y, Gerlai R. Long-Term Behavioural Changes in Response to Early Developmental Exposure to Ethanol in Zebrafish. Alcohol Clin Exp Res. 2009;33:601–609. doi: 10.1111/j.1530-0277.2008.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Blaser RE, Koid A, Poliner RM. Context-dependent sensitization to ethanol in zebrafish (Danio rerio) Pharmacol Biochem Behav. 2010;95:278–284. doi: 10.1016/j.pbb.2010.02.002. [DOI] [PubMed] [Google Scholar]
- [13].Dlugos CA, Rabin RA. Ethanol effects on three strains of zebrafish: model system for genetic investigations. Pharmacol Biochem Behav. 2003;74:471–80. doi: 10.1016/s0091-3057(02)01026-2. [DOI] [PubMed] [Google Scholar]
- [14].Gerlai R, Chatterjee D, Pereira T, Sawashima T, Krishnannair R. Acute and chronic alcohol dose: population differences in behaviour and neurochemistry of zebrafish. Genes Brain Behav. 2009;8:586–599. doi: 10.1111/j.1601-183X.2009.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gerlai R, Ahmad F, Prajapati S. Differences in Acute Alcohol-Induced Behavioural Responses Among Zebrafish Populations. Alcohol Clin Exp Res. 2008;32:1763–1773. doi: 10.1111/j.1530-0277.2008.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rosemberg DB, Braga MM, Rico EP, Loss CM, Cordova SD, Mssulini BHM, et al. Behavioural effects of taurine pretreatment in zebrafish acutely exposed to ethanol. Neuropharmacol. 2012;63:613–623. doi: 10.1016/j.neuropharm.2012.05.009. [DOI] [PubMed] [Google Scholar]
- [18].Gerlai R, Lahav M, Guo S, Rosenthall A. Drinks like a fish: zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol Biochem Behav. 2000;67:773–782. doi: 10.1016/s0091-3057(00)00422-6. [DOI] [PubMed] [Google Scholar]
- [19].Levin ED, Bencan Z, Ceruitti DT. Anxiolytic effects of nicotine in zebrafish. Phsyiol. Behav. 2007;90:54–58. doi: 10.1016/j.physbeh.2006.08.026. [DOI] [PubMed] [Google Scholar]
- [20].Mathur P, Guo S. Differences of acute versus chronic ethanol exposure on anxiety-like behavioural response in zebrafish. Behav Brain Res. 2011;219:234–239. doi: 10.1016/j.bbr.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Crollius H, Weissenbach J. Fish genomics and biology. Genome Res. 2005;15:1675–1682. doi: 10.1101/gr.3735805. [DOI] [PubMed] [Google Scholar]
- [22].Sison M, Gerlai R. Associative Learning in Zebrafish (Danio rerio) in the plus maze. Behav Brain Res. 2010;207:99–104. doi: 10.1016/j.bbr.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sison M, Gerlai R. Associative learning performance is impaired in zebrafish (Danio rerio) by the NMDA-R antagonist MK-801. Neurobiol Learn Mem. 2011;2:230–237. doi: 10.1016/j.nlm.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chatterjee D, Gerlai R. High precision liquid chromatography analysis of dopaminergic and serotoninergic responses to acute alcohol exposure in zebrafish. Behav Brain Res. 2009;200:208–213. doi: 10.1016/j.bbr.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wong K, Elegante M, Bartels B, Elkhayat S, Tien D, Roy S, et al. Analyzing habituation responses to novelty in zebrafish (Danio rerio) Behav Brain Res. 2010;208:450–457. doi: 10.1016/j.bbr.2009.12.023. [DOI] [PubMed] [Google Scholar]
- [26].Cachat J, Stewart A, Grossman L, Gaikwad S, Kadri F, Chung KM, et al. Measuring behavioural and endocrine responses to novelty stress in zebrafish. Nat Protoc. 2010;5:1786–1799. doi: 10.1038/nprot.2010.140. [DOI] [PubMed] [Google Scholar]
- [27].Ahmed O, Seguin D, Gerlai R. An automated predator avoidance task in zebrafish. Behav Brain Res. 2011;216:166–171. doi: 10.1016/j.bbr.2010.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Speedie N, Gerlai R. Alarm substance induced behavioural responses in zebrafish (Daniorerio) Behav Brain Res. 2008;188:168–177. doi: 10.1016/j.bbr.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Blaser R, Chadwick L, McGinnis G. Behavioral measures of anxiety in zebrafish (Danio rerio) Behav Brain Res. 2010;208:56–62. doi: 10.1016/j.bbr.2009.11.009. [DOI] [PubMed] [Google Scholar]
- [30].Luca RM, Gerlai R. In search of optimal fear inducing stimuli: Differential behavioural responses to computer animated images in zebrafish. Behav Brain Res. 2012;226:66–76. doi: 10.1016/j.bbr.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Luca RM, Gerlai R. Animated bird silhouette above the tank: Acute alcohol diminishes fear responses in zebrafish. Behav Brain Res. 2012;229:194–201. doi: 10.1016/j.bbr.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Parra N, Adrian JC, Gerlai R. The synthetic substance hypoxanthein 3-N-oxide elicits alarm reactions in zebrafish (Danio rerio) Behav Brain Res. 2009;205:336–34. doi: 10.1016/j.bbr.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ramsay JM, Feist GW, Varga Z, Westerfield M, Kent ML, Schreck MK. Whole-body cortisol response of zebrafish to acute net handling stress. Aquaculture. 2009;297:157–162. doi: 10.1016/j.aquaculture.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ethovision XT: Reference Manual. Version 7.0 Wageningen; The Netherlands: 2009. Noldus Information Technology. [Google Scholar]
- [35].Wahlsten D. Insensitivity of the analysis of variance to heredity × environment interaction. Behav Brain Sci. 1990;13:109–61. [Google Scholar]
- [36].Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and Validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- [37].Engeszer RE, PattersonL B, Rao AA, Parichy DM. Zebrafish in the wild: a review of nature history and new notes from the field. Zebrafish. 2007;4:21–40. doi: 10.1089/zeb.2006.9997. [DOI] [PubMed] [Google Scholar]
- [38].Parker MO, Millington ME, Combe FJ, Brennan CH. Housing conditions differentially affect physiological and behavioural stress responses of zebrafish, as well as the response to anxiolytic drugs. PLOS One. 2012;7:e34992. doi: 10.1371/journal.pone.0034992. [DOI] [PMC free article] [PubMed] [Google Scholar]