Abstract
Thienopyridine-derivatives (ticlopidine, clopidogrel, and prasugrel) are the primary antiplatelet agents. Thrombotic thrombocytopenic purpura (TTP) is a rare drug-associated syndrome, with the thienopyridines being the most common drugs implicated in this syndrome. We reviewed 20 years of information on clinical, epidemiologic, and laboratory findings for thienopyridine-associated TTP. Four, 11, and 11 cases of thienopyridine-associated TTP were reported in the first year of marketing of ticlopidine (1989), clopidogrel (1998), and prasugrel (2010), respectively. As of 2011, the FDA received reports of 97 ticlopidine-, 197 clopidogrel-, and 14 prasugrel-associated TTP cases. Severe deficiency of ADAMTS-13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) was present in 80% and antibodies to 100% of these TTP patients on ticlopidine, 0% of the patients with clopidogrel-associated TTP (p < 0.05), and an unknown percentage of patients with prasugrel-associated TTP. TTP is associated with use of each of the three thienopyridines, although the mechanistic pathways may differ.
Keywords: thrombotic thrombocytopenic purpura, ticlopidine, clopidogrel, prasugrel, adverse event
Thrombotic thrombocytopenic purpura (TTP) is a life-threatening condition thought to be caused by microthrombi deposition in arterioles and capillaries of tissues.1 The thienopyridine derivatives (ticlopidine, clopidogrel, and prasugrel) are associated with large numbers of cases of TTP. An association of TTP with ticlopidine was first reported in 1991 (one year after the drug received regulatory approval for marketing), an association with clopidogrel was identified in 2000 (2 years after regulatory approval was received), and 14 cases of prasugrel-associated TTP were reported in 2009 and 2010 (following regulatory approval of this agent in 2009).2–12 Although we have reported extensive information on ticlopidine- and clopidogrel-associated TTP cases, information on prasugrel-associated TTP has not been reported previously, nor have studies of thienopyridine-associated TTP reported by other researchers been reviewed.8,13 We comprehensively reviewed data on thienopyridine-associated TTP.
Methods
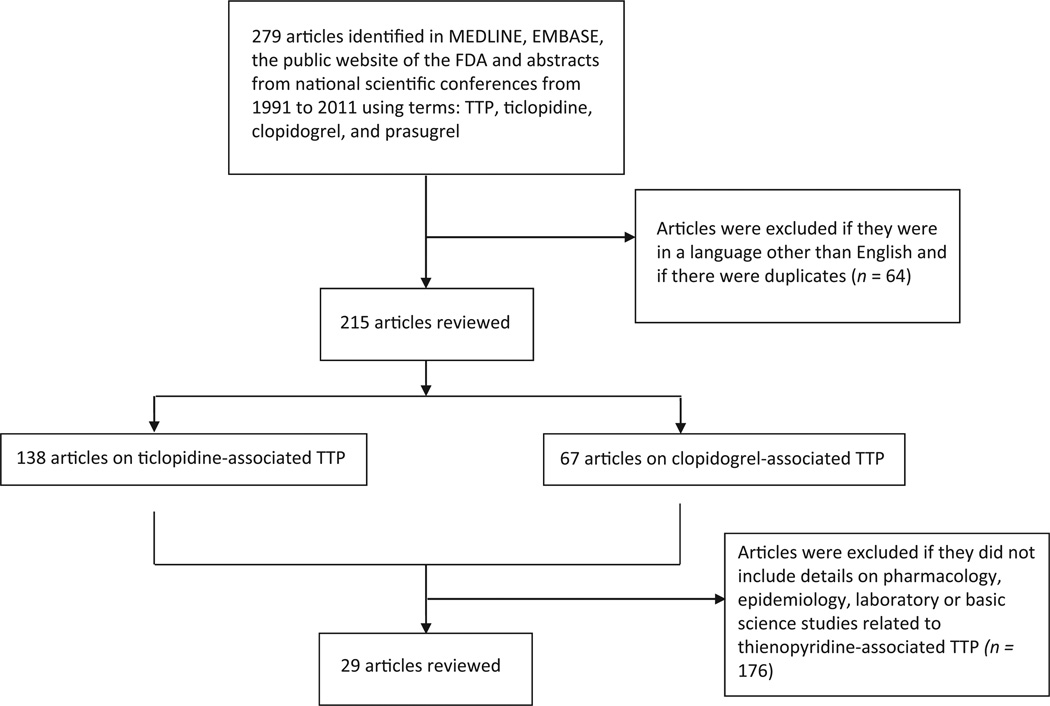
A systematic review of articles on thienopyridine-associated TTP identified in MEDLINE was conducted (search years, 1991 to 2011; MeSH terms TTP, ticlopidine, clopidogrel, and prasugrel) (Fig. 1). Articles were excluded if reported information was included in prior publications. Phase III clinical trials that reported serious adverse event rates associated with thienopyridines were also included. A total of 138, 67, and 1 articles including information on ticlopidine-, clopidogrel-, and prasugrel-associated hematologic toxicities, respectively, were identified. Of these, 29 articles included detailed information on clinical, epidemiologic laboratory studies for thienopyridine-associated TTP. Two-thirds of the publications are from our group. Information on safety, epidemiology, clinical studies, and laboratory analyses on thienopyridine-associated TTP from our group and others were reviewed (Tables 1 and 2).
Fig. 1.
Search strategy for data sources included. FDA, U.S. Food and Drug Administration; TTP, thrombotic thrombocytopenic purpura.
Table 1.
Data sources for thienopyridine-associated TTP
| Data source | Ticlopidine-associated TTP cases |
Clopidogrel-associated TTP cases |
Prasugrel-associated TTP cases |
|---|---|---|---|
| Literature review (1991–2011) | 21 publications | 8 publications | 0 publications |
| FDA Adverse Event Reporting System (1998–2011) | 97 cases, 10 reported since 2002 | 197 cases, 140 reported since 2002 | 14 cases |
| Phase III clinical trials (1989–2010) | One TTP case reported among 2,932 ticlopidine-treated patients | No TTP cases reported among 27,961 clopidogrel-treated patients | No TTP cases reported among 1,769 prasugrel-treated patients |
| FDA-approved package inserts (1989–2011) | 1996: warning added; 1998-“boxed” warning added | 2000: warning added | 2010: warning added |
| Surveys of therapeutic plasma exchange center and interventional cardiology laboratories (1998–1999) | Five TTP cases among 8,000 ticlopidine treated patients and nine TTP cases among 45,000 ticlopidine-treated patients | Nine clopidogrel-associated TTP cases identified | Not done |
| Administrative databases (1998–2001) | Two cases of ticlopidine-associated TTP identified among 15 million person-years of observation | One case of clopidogrel-associated TTP identified among 15 million person-years of observation | Not done |
| Laboratory-based study (2006–2008) | 100% of 30 ticlopidine-associated TTP cases had neutralizing antibodies to ADAMTS-13 and < 10% ADAMTS-13 activity | 0% of eight clopidogrel-associated TTP cases had neutralizing antibodies to ADAMTS-13 or < 10% ADAMTS-13 activity | Not done |
| Queries of medical personnel employed by thienopyridine manufacturers | No cases reported (1998) | One case was reported by an employee of the manufacturer of clopidogrel and one case was reported by an employee of the manufacturer of ticlopidine (1999) | To be determined (2011) |
Abbreviations: ADAMTS-13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; FDA, U.S. Food and Drug Administration; TTP, thrombotic thrombocytopenic purpura.
Table 2.
Summary of findings
| Ticlopidine (EMA approval in 1989; FDA approval in 1991) |
Clopidogrel (FDA approval in 1997) |
Prasugrel (FDA approval in 2009) |
|
|---|---|---|---|
| Systematic literature review (2001) | Ticlopidine was the leading cause of drug-induced TTP cases (12% of cases) | Clopidogrel accounted for 8% of all drug-induced TTP cases, second only to ticlopidine | No case of prasugrel-associated TTP is described in the literature |
| Adverse events reported in the FDA Adverse Event Reporting System database (1998–2011) | 1989–2001 (87 TTP cases); since 2002 (10 TTP cases); overall, ticlopidine is the fifth most frequent drug associated with TTP | 1998–2001 (57 TTP cases); since 2002, (140 TTP cases); overall, clopidogrel is the most frequent drug associated with TTP | In 2009 and 2010, 1 and 10 cases of prasugrel-associated TTP were reported to the FDA, respectively |
| Time from FDA approval to first case of TTP being reported | 2 years | 6 months | 6 months |
| Surveys of therapeutic plasma exchange directors (1998) | 98 ticlopidine-associated TTP patients; survival 60% with therapeutic plasma exchange; 20% without; onset < 2 weeks in 5% of patients | 11 clopidogrel-associated TTP cases (1 death); onset < 2 weeks in 91% of patients | Not applicable |
| Surveys of interventional cardiology centers (1998) | Five TTP cases among 8,000 ticlopidine users and nine TTP cases among 45,000 ticlopidine users; Estimated incidence: 1/1,600 to 1/5,000 ticlopidine-treated patients; onset between 2 and 12 weeks of exposure | No studies reported on this | Not applicable |
| Adverse event reports from Phase III clinical trials (1989–2010) | One TTP case in 2,932 ticlopidine-treated patients | Zero TTP cases in 27,961 clopidogrel treated patients | Zero TTP cases in 1,769 prasugrel-treated patients |
| Insurance claims files (United States) (1998–2001) | Two ticlopidine-associated TTP cases among 15 million person-years of observation | One clopidogrel-associated TTP case among 15 million person-years of observation | Not applicable |
| Insurance claims files (United States) (2001–2004) | Zero ticlopidine-associated TTP cases among 16.4 million person-years of observation | Zero clopidogrel-associated TTP cases among 16.4 million person-years of observation | Not applicable |
| Clinical findings from SERF-TTP (2003–2007) | Onset within 2 weeks, 10% (n = 93 patients); 86% survival with therapeutic plasma exchange, 46% without | Onset within 2 weeks, 74% (n = 35 patients); 72% survival with therapeutic plasma exchange, 67% without | No cases reported had onset beyond 2 weeks of drug initiation |
| Plasma samples from the SERF-TTP study (2003–2007); ADAMTS-13 deficiency | 80% (30 patients) | 0% (8 patients) | Not available |
| Plasma samples from the SERF-TTP study (2003–2007); neutralizing antibodies to ADAMTS-13 | 100% (30 patients) | 0% (8 patients) | Not available |
Abbreviations: ADAMTS-13, a disintegrin and metalloproteinase with a thrombospondin type 1motif,member 13; EMA, European Medicines Agency; FDA, U.S. Food and Drug Administration; SERF-TTP, Surveillance Epidemiology and Risk Factor for TTP; TTP, thrombotic thrombocytopenic purpura.
Adverse event case information sources included U.S. Food and Drug Administration (FDA) databases and physician surveys. The FDA database, FDA Adverse Event Reporting System (AERS), was interrogated for the years 1998 to 2011. Information on cases of ticlopidine- and clopidogrel-associated TTP was obtained from surveys of therapeutic plasma exchange centers and TTP referral practices in eight cities.8,13 Causality was assessed with the validated World Health Organization (WHO) scale. Using this scale, a causal relationship is categorized as certain if the clinical event of interest occurs in a plausible time relationship to drug administration, cannot be explained by concurrent disease or other drugs or chemicals, the event improves with dechallenge and recurs with rechallenge, the response to drug withdrawal is clinically plausible, and the event is definitive pharmacologically or phenomenologically. This analysis has not been reported in previous reviews of thienopyridine-associated TTP.
Thienopyridine-associated TTP rates were derived from one survey of directors of six therapeutic plasma exchange centers reported by our group and a second survey of nine interventional cardiology centers reported by Steinhubl et al,9,14 phase III clinical trial reports,15–22 and analyses of insurance claim files and group practice databases.23,24
As reported in our 2007 and 2009 reviews,8,13 plasma samples obtained from patients with thienopyridine-associated TTP included in the Surveillance Epidemiology and Risk Factor for TTP (SERF-TTP) project were evaluated for ADAMTS-13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) activity, as measured with standard assays.25 The inhibitory activity of the autoantibodies was determined by mixing TTP plasma samples at various dilutions with normal plasma and measuring proteinase activity of the mixture, as previously reported and described.26
Results
FDA Adverse Event Reports Database and FDA-Approved Case Information Disseminated by Product Manufacturers
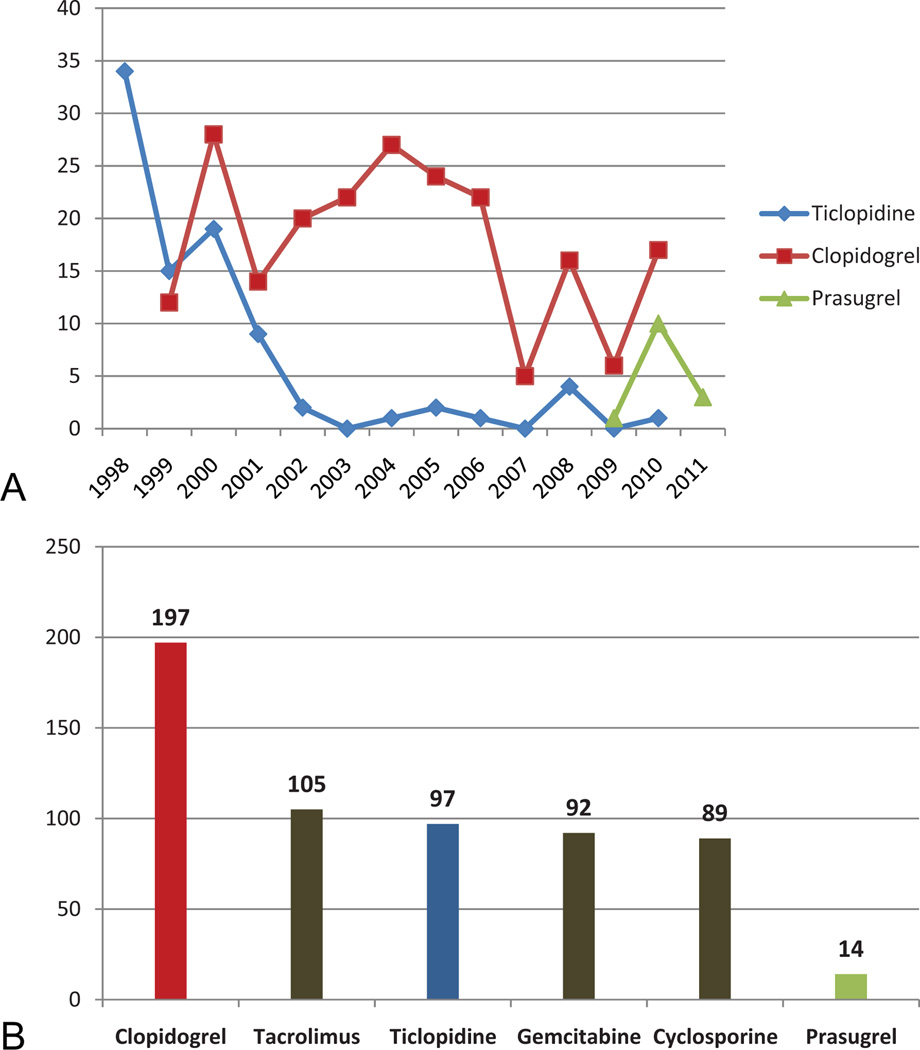
A literature review of all drugs associated with TTP, conducted in 2001, estimated that clopidogrel accounted for 8% of all drug-induced TTP cases reported to the FDA, second only to ticlopidine at the time (Table 2).27 Only one published abstract includes information of prasugrel-associated TTP cases.28 Updated review of FDA safety databases (1998–2011) identified clopidogrel as the most common drug associated with TTP (n = 197 cases) and ticlopidine as the fifth-most common drug (n = 97 cases) associated with TTP (Fig. 2). Between 1998 and 2001, 57 cases of clopidogrel-associated and 87 cases of ticlopidine-associated TTP were reported to the FDA. Since 2002, 140 cases of clopidogrel-associated TTP, 10 cases of ticlopidine-associated TTP, and 14 cases of prasugrel-associated TTP cases were reported to the FDA (Table 2). Based on the WHO criteria, more than 95% of reported ticlopidine-associated TTP cases are likely to have a causal relationship with TTP.29 For 74% of reported clopidogrel and prasugrel-associated TTP cases where onset information was available, onset occurred within 2 weeks of drug initiation. However, causal relationships in these cases are considered uncertain because of the presence of comorbid illnesses and concomitant medications.
Fig. 2.
A, Cases per year of thienopyridine-associated thrombotic thrombocytopenic purpura (TTP) reported to the U.S. Food and Drug Administration (FDA) (1998–2011). B, Drugs and number of cases reported to the FDA’s Adverse Event Reporting System in association with thrombotic thrombocytopenic purpura (1998–2011).
In the first year of marketing of each agent, the FDA received reports of 4 ticlopidine-associated-cases, 11 clopidogrel-associated cases, and 11 prasugrel-associated TTP cases. In 1989, a package insert for ticlopidine described “rare” instances of TTP in the post-marketing setting. In 1998, following our publication describing 60 cases of ticlopidine-associated TTP, this information was described as a “Boxed”warning indicating that TTP onset occurred frequently between 2 and 12 weeks after drug initiation and at an estimated rate of one TTP case per 5,000 ticlopidine-treated patients. In 2000, following our publication of 11 cases of clopidogrel-associated TTP, a “warning” was first added to the clopidogrel package insert indicating that TTP onset occurred frequently less than 2 weeks after drug initiation and at an estimated rate of 12 TTP cases per million clopidogrel-treated patients. In 2009, the initial package insert for prasugrel included a “warning” stating that cases of TTP were identified with other agents in the class because no prasugrel-associated TTP cases had been reported at that time. In 2010, after the FDA received reports of 11 prasugrel-associated TTP cases, a revised “warning” indicated that patients with prasugrel-associated TTP had been identified, with onset of the syndrome generally within 2 weeks of prasugrel initiation. No information on estimated incidence was included.
Published Cases (Ticlopidine and Clopidogrel Only)
In 1991, a therapeutic apheresis center in France reported four patients developing TTP after 3 to 8 weeks of ticlopidine.20 In two of the four patients, ticlopidine was the only medication taken prior to TTP onset; rechallenge with drugs other than ticlopidine did not produce relapse. Moreover, no relapse occurred during the 4 to 43 months of follow-up in three surviving patients.30 Steinhubl et al identified 19 patients with ticlopidine-associated TTP following coronary artery stent procedures at nine interventional cardiology centers.14 Four patients received ticlopidine for 2 weeks or less, 14 patients for 2 to 4 weeks, and 1 patient for 8 weeks. The mean time of ticlopidine treatment prior to TTP was 22 days (range, 5 to 60 days). The overall mortality rate was 21% (4 of 19), but in those patients who were not treated with plasma exchange, the mortality rate was 67% (4 of 6) compared with none of the 13 who did receive plasma exchange.
By surveying medical directors of plasma exchange centers Los Angeles, Raleigh-Durham, Boston, Baltimore, Houston, Indianapolis, Pittsburgh, and Chicago, we identified 11 patients with clopidogrel-associated TTP in 1998 and 1999.3,29 Ten of these patients had received clopidogrel for 14 days or less. Although 10 of the 11 patients responded to therapeutic plasma exchange and drug discontinuation, 2 required 20 or more exchanges, and 2 had relapses while not receiving clopidogrel. One patient died despite undergoing plasma exchange. One case report describes a patient with a drug-eluting coronary artery stent who developed TTP within days clopidogrel initiation.15 After treatment with plasma exchange and clopidogrel discontinuation, TTP resolved. The patient was subsequently re-challenged with ticlopidine and did not experience TTP relapse.
Case Series
In 1996, FDA safety personnel reported that from initial marketing of ticlopidine through 1995, the FDA had received reports of 25 ticlopidine-associated TTP patients.17 In 1998, Bennett et al reported 60 ticlopidine-associated TTP patients based on surveys of medical directors of plasma exchange centers in eight cities, reviewing FDA-adverse event reports and published case reports. Of these, 72% had received the drug for stroke prevention. More than 95% of the patients had received between 2 and 12 weeks of ticlopidine prior to TTP onset. The mortality rate was 24% (9 of 38) among patients who underwent plasma exchange and 50% (11 of 22) among patients who did not undergo this procedure (p = 0.05).2
In 1999, Bennett et al reported 42 patients with ticlopidine-associated TTP with coronary artery stents and 56 ticlopidine-associated TTP patients in stroke prevention settings.4 Compared with coronary artery stent patients, patients in stroke prevention settings were more likely to be women (62.5 vs. 28.6%; p = 0.01). Comparing patients being treated for stroke prevention versus stent placement, the time between ticlopidine initiation and TTP onset was less than 2 weeks in 5.4 and 2.4%, between 2 and 3weeks in 17.9 and 21.4%, between 3 and 4weeks in 30.4 and 38.1%, and between 4 and 12weeks in 46.4 and 38.1%, respectively. Death occurred in 60.0% of ticlopidine-associated TTP patients not receiving plasma exchange versus 21.9% of patients receiving plasma exchange in those receiving ticlopidine for stroke prevention and in 14.3% of receiving plasma exchange in those receiving ticlopidine for stent placement.
In 2007, Bennett et al compared clinical presentations for 93 ticlopidine- versus 35 clopidogrel-associated TTP cases.7 Patients with ticlopidine- and clopidogrel-induced TTP were similar with respect to age (mean, 64 vs. 58 years) and gender (male, 53 vs. 54%), but differed in duration of thienopyridine exposure prior to TTP.8 In comparison to patients with clopidogrel-associated TTP, those with ticlopidine-induced TTP were more likely to have received more than 2 weeks of a thienopyridine prior to TTP onset (90 vs. 26%; p < 0.0001) and to present with severe thrombocytopenia (platelet count < 20 × 109/L) (84 vs. 60%; p < 0.005) but less likely to have renal insufficiency (28 vs. 55%; p < 0.02).Among TTP patients who received ticlopidine, survival rates were almost two-fold greater with plasma exchange versus without (86 vs. 46%, p < 0.001). In contrast, among TTP patients who received clopidogrel, survival rates with versus without plasma exchange were similar (72 vs. 67%).
Phase III Clinical Trials
Phase III trials evaluating 2,932 patients who received ticlopidine in the setting of coronary artery stent placement, peripheral vascular disease, and stroke prevention identified one TTP case.16,18–20,22,31 The case developed in one of 902 patients who received ticlopidine in the African American Anti-Platelet Stroke Study, which evaluated aspirin versus ticlopidine therapy.21 The patient, who developed thrombocytopenia early after ticlopidine initiation, recovered after drug discontinuation and plasma exchange. No TTP cases have been identified among 27,961 clopidogrel-treated and 1,769 prasugrel-treated patients enrolled in phase III clinical trials.
Surveys
A 1998 survey of a plasma exchange center in Pittsburgh by Bennett et al identified 5 individuals with ticlopidine-associated TTP among 8,000 patients in the city who underwent coronary artery stent procedures at five interventional cardiology centers.9 All had received ticlopidine following the procedure. This corresponds to an incidence of ticlopidine-associated TTP of 1 per 1,600 ticlopidine-treated persons.5 In 1999, another survey of interventional cardiologists at nine cardiology laboratories reported nine TTP cases among 45,000 ticlopidine-treated patients undergoing coronary artery stent procedures.14
Administrative Databases—Estimating Incidence Rates of Ticlopidine- and Clopidogrel-Associated TTP
Two studies estimated rates of ticlopidine- and clopidogrel-associated TTP cases based on identification of TTP diagnoses in administrative databases merged with claims information on pharmaceutical use. The first study, reported in 2004 and funded by the manufacturer of clopidogrel, identified two cases of ticlopidine-associated TTP and one case of clopidogrel-associated TTP among 15 million person-years of observation of persons with private health insurance (1998 to 2001) data.23 That study identified 60 persons with TTP in the databases, and an estimated rate of one clopidogrel-associated TTP case per 260,000 person-years of observation. During these study years, the preferred thienopyridine was transitioning from ticlopidine to clopidogrel. The second study, reported by FDA safety personnel, did not identify any cases of ticlopidine- or clopidogrel-associated TTP among 16.4 million person-years of observation among 11 private health care plans (2001 to 2004 data).24 During these study years, clopidogrel use had increased dramatically whereas ticlopidine use declined. Incidence of TTP among patients with coronary artery stent placement is about 1 in 1,500 with ticlopidine.9 The rate of TTP with clopidogrel is between 1 in 8,500 to 1 in 26,000.31
Laboratory Studies
Tsai et al reported plasma findings from seven patients who developed TTP 2 to 7 weeks after initiation of ticlopidine therapy in 2000.11 Controls of the reported study were seven consecutive patients without thrombocytopenia who had been receiving ticlopidine for 3 to 5 weeks and 10 randomly selected hospitalized patients. Initial plasma samples from all seven patients lacked the largest vWF multimers and were severely deficient in ADAMTS-13 activity. Immunoglobulin G, isolated from plasma samples of five patients, inhibited ADAMTS-13 activity in normal human plasma. These abnormalities resolved with remission that followed plasma exchange and discontinuation of ticlopidine.11 Mauro et al reported that plasma from patients with ticlopidine without TTP or with ticlopidine-associated TTP induced apoptosis of microvascular endothelial cells.10 However, clopidogrel did not induce similar apoptosis.10
Bennett et al reported that plasma from 2 of the first 11 patients with clopidogrel-associated TTP showed severely deficient ADAMTS-13 activity and the presence of inhibitors to the ADAMTS-13.32 Both patients responded to plasma exchange; however, each suffered a spontaneous relapse without re-exposure to a thienopyridine. To date, these are the only cases of thienopyridine-associated TTP reported to have developed a spontaneous relapse, raising concern that these cases were not caused by clopidogrel.
Updated clinical and laboratory findings are available for 30 patients with ticlopidine-associated TTP and 8 patients with clopidogrel-associated TTP.7 None of these patients experienced a spontaneous relapse. These patients were similar in age (mean, 68 vs. 58 years) and gender (male, 43 vs. 62%), but more likely to present with a 2- to 12-week history of thienopyridine exposure (100 vs. 25%; p < 0.05), to have severe ADAMTS-13 deficiency (80 vs. 0%), to have neutralizing antibodies to ADAMTS-13 (100 vs. 0%), and to survive the TTP episode (87 vs. 50%; p < 0.05 for each comparison).
Discussion
Thienopyridines are prescribed widely to prevent stent thrombosis after coronary or peripheral vascular interventions and for stroke prophylaxis. Ticlopidine use is a well-recognized cause of TTP and neutropenia, and hence it has largely been replaced by clopidogrel.33 Prasugrel is the newest thienopyridine derivative and its use continues to increase following FDA approval in 2009. There have been case reports and case series published in this arena; however, this is the first comprehensive review of ticlopidine-, clopidogrel-, and prasugrel-associated TTP. In interpreting our findings, several factors should be considered.
Although clopidogrel is the most common drug associated with TTP in the FDA databases, the incidence of clopidogrel-associated TTP is only four times as frequent as that reported for idiopathic TTP, therefore raising concern over a causal relationship. Of note, since 2006, awareness of an association of clopidogrel with TTP increased after the manufacturer included descriptions of this association in the product label and publicly disseminated safety information, which may have led to decreased use and account in part for the lower annual number of case reports since then.34 Causal relationships between clopidogrel and, more recently, prasugrel and thrombotic microangiopathy have been postulated primarily based on time-course of onset (within 2 weeks of drug initiation) and absence of relapse without re-exposure.35 Moreover, these thrombotic microangiopathies differ from drug-induced microangiopathies reported with mitomycin-C, calcineurin inhibitors, and gemcitabine in that they are dose-dependent, occur after weeks of drug use, and are attributed to cumulative toxic effects on the endothelium.35
Based on updated and previously unreported analyses, ticlopidine is the only TTP-associated drug linked with neutralizing autoantibodies to ADAMTS-13 metalloproteinase. Over 90% of these cases occurred within 2 to 12 weeks of ticlopidine initiation. Clinical and laboratory findings for ticlopidine-associated TTP mirror those reported for idiopathic TTP with respect to neutralizing autoantibodies, severe ADAMTS-13 deficiency, and rapid response to therapeutic plasma exchange.36,37 The major difference is that no case of ticlopidine-associated TTP has experienced a spontaneous relapse, whereas between 30 and 50% of idiopathic immunologically mediated TTP cases experience spontaneous relapses.37–39 Although TTP occurs at an estimated rate of 1 per 1,600 to 1 per 5,000 ticlopidine-treated patients, case reports to the FDA of ticlopidine-associated TTP decreased after 1999.9,14 This change is likely related to increased awareness of an association of ticlopidine with TTP and diminished use of ticlopidine following FDA approval of clopidogrel in 1999. Basic science considerations and revision of the conceptualization of idiopathic TTP and thrombotic microangiopathies help explain our findings for ticlopidine.40 Prior pharmacologic studies implicated metabolites of drugs, rather than the parent drug, as being the cause of drug-associated TTP.36 None of the metabolites of ticlopidine, clopidogrel, and prasugrel are the same. When ticlopidine-treated patients have been noted to have TTP characterized by ADAMTS-13 deficiency and an inhibitor, there has never been documentation that the inhibitor is a drug-dependent antibody, as occurs with drug-induced immune thrombocytopenia as well as with quinine-induced hemolytic uremic syndrome.41 Potential causal mechanisms of ticlopidine-associated TTP perhaps include molecular mimicry if metabolites of ticlopidine have structural similarity to ADAMTS-13 or antigenicity, ticlopidine may act as a hapten, or metabolites of ticlopidine can form a complex with ADAMTS-13.
Package labels warn that TTP cases have occurred generally within 2 weeks of drug initiation for clopidogrel and a “Boxed” warning indicates that ticlopidine-associated TTP occurs-within 2 to 12 weeks of drug initiation. These notifications include estimates of incidence of 1 per 2,000 persons for ticlopidine and 1 per 80,000 persons for clopidogrel. For the thienopyridines, patients are advised to contact their physician immediately if signs or symptoms of TTP develop. According to FDA regulation, as soon as there is “reasonable evidence” of an association of serious hazard relative to a drug, the warnings section of product labels is revised to include descriptions of serious adverse reactions and steps to take if they occur. Special problems such as drug-associated TTP may be required by the FDA to be described as a “Boxed” warning.42
Conclusion
TTP occurs following administration of the thienopyridines, with onset generally within 2 weeks of drug initiation for clopidogrel and between 2 and 12 weeks for ticlopidine (Table 3). ADAMTS-13 deficiency is implicated in ticlopidine-associated cases, ADAMTS-13 independence is implicated in clopidogrel-associated TTP cases, and ADAMTS-13 information for prasugrel-associated TTP is unknown. Clinicians and patients should be vigilant, understanding that this syndrome could occur shortly after initiation of thienopyridines and report all cases of thienopyridine-associated TTP to MedWatch, the FDA’s safety information and adverse event reporting program.
Table 3.
Summary of differential pathophysiology
| Ticlopidine | Clopidogrel | Prasugrel | |
|---|---|---|---|
| Time to onset | 2–12 weeks | 0–2 weeks | Not measured |
| ADAMTS-13 levels | < 5% | Normal | Not measured |
| Antibodies, ADAMTS-13 | 100% | None | Not measured |
| Treatment | Emergency TPE | TPE done, but not clear if helpful | Not measured |
| Response to TPE | 90% | 50% | Not measured |
| Relapse | 0% | 0% | Not measured |
Abbreviations: ADAMTS-13, a disintegrin and metalloproteinase with a thrombospondin type 1motif,member 13; TPE, therapeutic plasma exchange.
Acknowledgments
Funding
This manuscript was supported by funding from a grant from the National Cancer Institute (1R01CA 102713–01), the Centers for Economic Excellence program of the state of South Carolina, and the Doris Levkoff Meddin Center for Medication Safety (CLB), and a grant from the NIH/NCI (1K01CA134554–01) (JMM).
Footnotes
Author Contributions
Dr. Bennett had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bennett
Acquisition of data: Jacob, Dunn, Qureshi, Raisch, Chen, Chen, Bennett
Interpretation of data: all authors
Drafting of the manuscript: all authors
Critical revision of the manuscript for important intellectual content: all authors
Administrative, technical, or material support: Bennett
Study supervision: Bennett
Disclosure
Preliminary findings of these study results were presented as a poster at the 53rd American Society of Hematology annual meeting and exposition, San Diego, December 10 to 13, 2011. They were also presented at the plenary session of the 2009 AABB held in Montreal, at an oral presentation at the 2009 American Society of Hematology in New Orleans, and at a poster session of the American Society of Hematology in Orlando, 2010.
Conflicts of Interest
Hao Johnson Chen and Fei Chen are employees and stock-holders of eHealthMe Incorporated. John Armstrong is an employee and stockholder of LeadHorse Technologies Incorporated.
References
- 1.Sadler JE, Moake JL, Miyata T, George JN. Recent advances in thrombotic thrombocytopenic purpura. Hematology (Am Soc Hematol Educ Program) 2004;(1):407–423. doi: 10.1182/asheducation-2004.1.407. [DOI] [PubMed] [Google Scholar]
- 2.Bennett CL, Weinberg PD, Rozenberg-Ben-Dror K, Yarnold PR, Kwaan HC, Green D. Thrombotic thrombocytopenic purpura associated with ticlopidine. A review of 60 cases. Ann Intern Med. 1998;128(7):541–544. doi: 10.7326/0003-4819-128-7-199804010-00004. [DOI] [PubMed] [Google Scholar]
- 3.Bennett CL, Connors JM, Carwile JM, et al. Thrombotic thrombocytopenic purpura associated with clopidogrel. N Engl J Med. 2000;342(24):1773–1777. doi: 10.1056/NEJM200006153422402. [DOI] [PubMed] [Google Scholar]
- 4.Bennett CL, Davidson CJ, Raisch DW, Weinberg PD, Bennett RH, Feldman MD. Thrombotic thrombocytopenic purpura associated with ticlopidine in the setting of coronary artery stents and stroke prevention. Arch Intern Med. 1999;159(21):2524–2528. doi: 10.1001/archinte.159.21.2524. [DOI] [PubMed] [Google Scholar]
- 5.Bennett CL, Davidson CJ, Green D, Weinberg PD, Feldman MD. Ticlopidine and TTP after coronary stenting. JAMA. 1999;282(18):1717–1719. author reply 1718–1719. [PubMed] [Google Scholar]
- 6.Zakarija A, Bandarenko N, Pandey DK, et al. Clopidogrel-associated TTP: an update of pharmacovigilance efforts conducted by independent researchers, pharmaceutical suppliers, and the Food and Drug Administration. Stroke. 2004;35(2):533–537. doi: 10.1161/01.STR.0000109253.66918.5E. [DOI] [PubMed] [Google Scholar]
- 7.Evens AM, Kwaan HC, Kaufman DB, Bennett CL. TTP/HUS occurring in a simultaneous pancreas/kidney transplant recipient after clopidogrel treatment: evidence of a nonimmunological etiology. Transplantation. 2002;74(6):885–887. doi: 10.1097/00007890-200209270-00026. [DOI] [PubMed] [Google Scholar]
- 8.Bennett CL, Kim B, Zakarija A, et al. Two mechanistic pathways for thienopyridine-associated thrombotic thrombocytopenic purpura: a report from the SERF-TTP Research Group and the RADAR Project. J Am Coll Cardiol. 2007;50(12):1138–1143. doi: 10.1016/j.jacc.2007.04.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett CL, Kiss JE, Weinberg PD, et al. Thrombotic thrombocytopenic purpura after stenting and ticlopidine. Lancet. 1998;352(9133):1036–1037. doi: 10.1016/s0140-6736(05)60079-7. [DOI] [PubMed] [Google Scholar]
- 10.Mauro M, Zlatopolskiy A, Raife TJ, Laurence J. Thienopyridine-linked thrombotic microangiopathy: association with endothelial cell apoptosis and activation of MAP kinase signalling cascades. Br J Haematol. 2004;124(2):200–210. doi: 10.1046/j.1365-2141.2003.04743.x. [DOI] [PubMed] [Google Scholar]
- 11.Tsai HM, Rice L, Sarode R, Chow TW, Moake JL. Antibody inhibitors to von Willebrand factor metalloproteinase and increased binding of von Willebrand factor to platelets in ticlopidine-associated thrombotic thrombocytopenic purpura. Ann Intern Med. 2000;132(10):794–799. doi: 10.7326/0003-4819-132-10-200005160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Indianapolis, IN: Eli Lilly and Company; 2011. Sep, [Accessed June 26, 2011]. Effient [package insert] Available at: http://pi.lilly.com/us/effient.pdf. [Google Scholar]
- 13.Zakarija A, Kwaan HC, Moake JL, et al. Ticlopidine- and clopidogrel-associated thrombotic thrombocytopenic purpura (TTP): review of clinical, laboratory, epidemiological, and pharmacovigilance findings (1989–2008) Kidney Int Suppl. 2009;112:S20–S24. doi: 10.1038/ki.2008.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinhubl SR, Tan WA, Foody JM, Topol EJ. Incidence and clinical course of thrombotic thrombocytopenic purpura due to ticlopidine following coronary stenting. EPISTENT Investigators. Evaluation of Platelet IIb/IIIa Inhibitor for Stenting. JAMA. 1999;281(9):806–810. doi: 10.1001/jama.281.9.806. [DOI] [PubMed] [Google Scholar]
- 15.Patel TN, Kreindel M, Lincoff AM. Use of ticlopidine and cilostazol after intracoronary drug-eluting stent placement in a patient with previous clopidogrel-induced thrombotic thrombocytopenic purpura: a case report. J Invasive Cardiol. 2006;18(7):E211–E213. [PubMed] [Google Scholar]
- 16.Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345(7):494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 17.Wysowksi DK, Bacsanyi J. Blood dycrasias and hematologic reactions in ticlopidine users. JAMA. 1996;276(12):952. [PubMed] [Google Scholar]
- 18.Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364(9431):331–337. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- 19.Bhatt DL, Topol EJ Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance Executive Committee. Clopidogrel added to aspirin versus aspirin alone in secondary prevention and high-risk primary prevention: rationale and design of the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial. Am Heart J. 2004;148(2):263–268. doi: 10.1016/j.ahj.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 20.Gent M, Blakely JA, Easton JD, et al. The Canadian American Ticlopidine Study (CATS) in thromboembolic stroke. Lancet. 1989;1(8649):1215–1220. doi: 10.1016/s0140-6736(89)92327-1. [DOI] [PubMed] [Google Scholar]
- 21.Gorelick PB, Richardson D, Kelly M, et al. Aspirin and ticlopidine for prevention of recurrent stroke in black patients: a randomized trial. JAMA. 2003;289(22):2947–2957. doi: 10.1001/jama.289.22.2947. [DOI] [PubMed] [Google Scholar]
- 22.Hass WK, Easton JD, Adams HP, Jr, et al. A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high-risk patients. N Engl J Med. 1989;321(8):501–507. doi: 10.1056/NEJM198908243210804. [DOI] [PubMed] [Google Scholar]
- 23.Miller DP, Kaye JA, Shea K, et al. Incidence of thrombotic thrombocytopenic purpura/hemolytic uremic syndrome. Epidemiology. 2004;15(2):208–215. doi: 10.1097/01.ede.0000113273.14807.53. [DOI] [PubMed] [Google Scholar]
- 24.Schech SD, Brinker A, Shatin D, Burgess M. New-onset and idiopathic thrombotic thrombocytopenic purpura: incidence, diagnostic validity, and potential risk factors. Am J Hematol. 2006;81(9):657–663. doi: 10.1002/ajh.20669. [DOI] [PubMed] [Google Scholar]
- 25.Tripodi A, Chantarangkul V, Böhm M, et al. Measurement of von Willebrand factor cleaving protease (ADAMTS-13): results of an international collaborative study involving 11 methods testing the same set of coded plasmas. J Thromb Haemost. 2004;2(9):1601–1609. doi: 10.1111/j.1538-7836.2004.00879.x. [DOI] [PubMed] [Google Scholar]
- 26.Tsai HM, Lian EC. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med. 1998;339(22):1585–1594. doi: 10.1056/NEJM199811263392203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Majhail NS, Lichtin AE. Clopidogrel and thrombotic thrombocytopenic purpura: no clear case for causality. Cleve Clin J Med. 2003;70(5):466–470. doi: 10.3949/ccjm.70.5.466. [DOI] [PubMed] [Google Scholar]
- 28.Qureshi ZP, Armstrong J, Bennett C. Ticlopidine-, clopidogrel-, and prasugrel-associated thrombotic thrombocytopenic purpura: a twenty year review. Presented at: 53rd Annual Meeting and Exposition of American Society of Hematology; December 11, 2011; San Diego, CA. [Google Scholar]
- 29.World Health Organization. Safety monitoring of medicinal products: guidelines for setting up and running a pharmacovigilance centre. [Accessed December 12, 2010];2010 Available at: http://apps.who.int/medicinedocs/en/d/Jh2934e/.
- 30.Page Y, Tardy B, Zeni F, Comtet C, Terrana R, Bertrand JC. Thrombotic thrombocytopenic purpura related to ticlopidine. Lancet. 1991;337(8744):774–776. doi: 10.1016/0140-6736(91)91383-6. [DOI] [PubMed] [Google Scholar]
- 31.CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) Lancet. 1996;348(9038):1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 32.Bennett CL, Connors JM, Carwile JM, et al. Thrombotic thrombocytopenic purpura associated with clopidogrel. N Engl J Med. 2000;342(24):1773–1777. doi: 10.1056/NEJM200006153422402. [DOI] [PubMed] [Google Scholar]
- 33.Sharis PJ, Cannon CP, Loscalzo J. The antiplatelet effects of ticlopidine and clopidogrel. Ann Intern Med. 1998;129(5):394–405. doi: 10.7326/0003-4819-129-5-199809010-00009. [DOI] [PubMed] [Google Scholar]
- 34.Plavix [package insert] Bridgewater, NJ: Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership; 2011. Dec, [Accessed January 5, 2012]. Available at: http://products.sanofi.us/PLAVIX/PLAVIX.html. [Google Scholar]
- 35.Zakarija A, Bennett C. Drug-induced thrombotic microangiopathy. Semin Thromb Hemost. 2005;31(6):681–690. doi: 10.1055/s-2005-925474. [DOI] [PubMed] [Google Scholar]
- 36.Furlan M, Robles R, Galbusera M, et al. von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med. 1998;339(22):1578–1584. doi: 10.1056/NEJM199811263392202. [DOI] [PubMed] [Google Scholar]
- 37.Zheng XL, Kaufman RM, Goodnough LT, Sadler JE. Effect of plasma exchange on plasma ADAMTS13 metalloprotease activity, inhibitor level, and clinical outcome in patients with idiopathic and nonidiopathic thrombotic thrombocytopenic purpura. Blood. 2004;103(11):4043–4049. doi: 10.1182/blood-2003-11-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vesely SK, George JN, Lämmle B, et al. ADAMTS13 activity in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients. Blood. 2003;102(1):60–68. doi: 10.1182/blood-2003-01-0193. [DOI] [PubMed] [Google Scholar]
- 39.Veyradier A, Obert B, Houllier A, Meyer D, Girma JP. Specific von Willebrand factor-cleaving protease in thrombotic microangiopathies: a study of 111 cases. Blood. 2001;98(6):1765–1772. doi: 10.1182/blood.v98.6.1765. [DOI] [PubMed] [Google Scholar]
- 40.Moake J. Thrombotic microangiopathies: multimers, metalloprotease, and beyond. Clin Transl Sci. 2009;2(5):366–373. doi: 10.1111/j.1752-8062.2009.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furlan M, Lämmle B. Aetiology and pathogenesis of thrombotic thrombocytopenic purpura and haemolytic uraemic syndrome: the role of von Willebrand factor-cleaving protease. Best Pract Res Clin Haematol. 2001;14(2):437–454. doi: 10.1053/beha.2001.0142. [DOI] [PubMed] [Google Scholar]
- 42.Section USC. § 201.80 (e) (21 CFR 201.80 (e)) [Accessed June 26, 2011];Electronic Code of Federal Regulations Website. Available at: http://ecfr.gpoaccess.gov/cgi/t/text/text-idx?c=ecfr&tpl=/ecfrbrowse/Title21/21cfr201_main_02.tpl.