Abstract
This article provides an overview of the obstetric and gynecological manifestations of Crohn’s disease (CD). High incidence of the new onset of the disease in young women in their reproductive years demands special concern from physicians involved in their treatment. Pregnant women with CD are considered high-risk patients, regardless of disease activity index, due to associated complications. Predominately described complications are premature birth, low birth weight, and congenital anomalies. To minimize the risk for adverse pregnancy/birth outcomes, it is recommended that remission be achieved before conception. Treatment of CD in pregnant women is similar to that among the nonpregnant population, and there is no valid reason to terminate it, since most of the drugs are proven to be safe. Women with CD who wish to conceive or are already pregnant need to be properly advised according to the newest guidelines on the subject, given by the European Crohn’s and Colitis Organization. Gynecological manifestations are another special feature of CD. They are important in that they may facilitate early recognition of the underlying disease, which usually stays unrecognized for years before intestinal manifestation; in this way, the underlying manifestations are often mistreated.
Keywords: adverse pregnancy outcome, Crohn’s disease, gynecological complications, immunosuppressive therapy
Introduction
Crohn’s disease (CD) is a chronic inflammatory disease characterized by the tendency of chronic or relapsing activation of the immune system within the gastrointestinal (GI) tract. Although CD primarily affects the alimentary tract from the mouth to the anus, there are numerous extraintestinal manifestations (EIMs).
The disease usually begins within the alimentary tract, with a propensity for developing in the distal small bowel and proximal large bowel. Due to its subtle clinical presentation, a long delay between the onset of the disease and its diagnosis is common. Early features usually include diarrhea, fever, weight loss, and abdominal pain.
The incidence rates of CD vary between 0.1–20.2 per 100,000 person-years, most likely due to genetic and environmental factors.1 The highest reported incidence rate of inflammatory bowel disease (IBD) to date was found on the Faroe Islands (81.5 per 100,000 person-years).2
Higher incidence rates have been noted in more northern latitudes, although the north–south gradient is now less than previously observed. Recent years have seen changes in the classical geographical distribution of the disease, and we are witnessing rising incidence and prevalence rates in traditionally low-incident regions such as Asia, South America, and southern and eastern Europe.2 Now, it is worthwhile to investigate the west–east gradient regarding IBD occurrence in Europe.2
A specific feature of CD is that it holds the possibility for the development of EIMs of the disease.3 They occur in almost one-third (21%–41%) of all patients with CD, depending on the patient population and the criteria used to identify them. Commonly, the number of these manifestations increases with the duration of the disease. In a cohort of 480 patients followed from the time of diagnosis, it was observed that the cumulative probability of inflammatory EIMs varied from 22% at diagnosis to 40% at 10 years after diagnosis.4
Among others, fistulization is a frequent complication of longstanding disease. Special attention has to be paid to women due to the possibility that fistulization may occur in the genitourinary tract, which can cause vulvovaginal, perianal, perineal, or urological symptoms. Due to the predilection of CD for young women of reproductive age, it can cause substantial disturbances in reproductive function. Thus, it is important that gynecologists understand the mechanisms and possible complications of CD. This review provides an overview of the obstetric and gynecological manifestations of CD.
Obstetrics
CD affects women in their peak reproductive years (Figure 1).5 Approximately 50% of patients are under 35 years of age at the time of the initial diagnosis, and 25% of them conceive for the first time during that period.6
Figure 1.
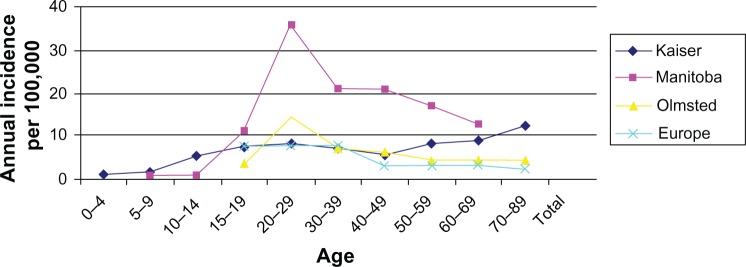
Incidence of Crohn’s disease in women by age.
Note: Copyright © 2008 Nature Publishing Group. Reproduced with permission from Herrinton LJ, Liu L, Lewis JD, et al. Incidence and prevalence of inflammatory bowel disease in a Northern California managed care organization, 1996–2002. Am J Gastroenterol. 2008;103(8):1998–2006.5
Newer studies showed that women with inactive CD have similar fertility rates as the general population.7 However, fertility is reduced in women with active CD and during the postsurgery period.8 Infertility caused by surgical treatment may be due to adhesions in the pelvis after the surgery, as well as by fallopian tube occlusion.9 Apart from the effects of the disease itself (anemia, fatigue, and flatulence), libido and sexual activity can be influenced and reduced by a distorted body image.10 Increased voluntary childlessness is also noticed in this group of patients.11 The main reason behind this seems to be misconception and fear that an adverse pregnancy outcome is likely to occur, and this belief is often not justified.12 A knowledge assessment tool was designed to assess the knowledge of women with CD about the possible outcomes of their pregnancies. This measure is named the Crohn’s and Colitis Pregnancy Knowledge Score (CCPKnow). The findings from studies using this measure showed that nearly half of the women demonstrated poor results on the test, and more than one-third of them believed that IBD treatment during pregnancy is harmful to their unborn child.13,14 These negative findings lead to the conclusion that patients need to be better informed to avoid unnecessary complications, especially regarding treatment of the disease.
The risk for an adverse birth outcome depends on many factors that should be taken into consideration among pregnant women with CD. Some of these factors are general, such as parity, mother’s age at the time of delivery, gestational age, and smoking during pregnancy, and some are directly connected to the mother’s underlying disease like the disease activity index, anti-inflammatory drug therapy used during pregnancy, and disease duration.15
It is recommended that pregnant women with CD are routinely considered as a high-risk group of patients, regardless of disease activity.16,17 Women with quiescent CD at the time of conception have the same risk for adverse pregnancy outcomes as the general population.6 It is considered that active disease at the time of conception, or relapse during pregnancy, increases the risk for adverse pregnancy outcomes.18 If pregnancy occurs at a time of active disease, two-thirds of patients may have persistent activity during pregnancy and, of these, two-thirds may deteriorate;19 this is known as “the rule of thirds.”6 This statement specifically refers to pregnant women with active CD in comparison with pregnant women with ulcerative colitis (UC).6,20,21 The primary aim of preconception counseling should be to achieve remission of disease activity for at least 3 months before conception, especially if we keep in mind that the disease state during conception usually tends to remain throughout the pregnancy.22 The first and, so far, only prospective study (by Bortoli et al23) showed that there was no significant difference in pregnancy outcomes between pregnant women with IBD and healthy controls. It is important to emphasize that 88% of pregnant women in this study had quiescent disease.
Pregnancy outcomes
The mechanisms behind the pathophysiology of adverse pregnancy outcomes are not yet completely determined. They appear to be mainly associated with the nature of the disease itself, the disease activity index, anti-inflammatory drug therapy during pregnancy and, in some cases, with the child’s genetic susceptibility to CD.15,24 Some of the assumed pathophysiological mechanisms include increased intestinal permeability, vascular abnormalities, infectious agents, abnormal immunological response, production of autoantibodies, and proinflammatory molecules, which can all lead to malnutrition that is not so rare in patients with CD.15 There is a hypothesis that increased levels of prostaglandins during flares of inflammation can provoke premature contractions of the smooth muscles in the uterus, causing premature birth.25
Premature birth, low birth weight (LBW), and congenital anomalies (CA) are the most common complications observed by the majority of studies.26,27 Stillbirth and a need for Cesarean section seem to be less common. Premature birth is defined as birth before 37 weeks of gestation, and LBW is defined as weight under 2,500 g.25 In most studies, the exact definition of LBW remains unclear because it can be seen in premature newborns, newborns with intrauterine growth retardation born at term, and newborns with LBW for genetic reasons. CA are described in terms that do not distinguish between minor and major anomalies. A Danish study found no increased risk for overall CA in pregnant women with CD, but instead found an increased risk for specific anomalies like limb deficiency, urinary tract defects, and multiple malformations.15 These findings refer to birth outcomes in pregnant women with UC rather than those with CD.28
Other rare complications include stillbirth, and some other conditions which can influence decision about the mode of delivery.25 The choice of the mode of delivery is closely connected to the clinical appearance of the disease. Studies conducted in the past have shown an increased rate of Cesarean section in pregnant women with CD, because it was considered that vaginal delivery and episiotomy could be the main reasons for relapse or de novo involvement of perianal disease.29 The progression of perianal disease was found to be less frequent after vaginal delivery when compared to Cesarean section, which diminishes Cesarean section assumed protective role.30 The general opinion is that decisions about Cesarean section should be based on obstetric indications. There are only two gastroenterological indications for Cesarean section: active perianal disease and the presence of an ileoanal pouch.7 In the first case, vaginal delivery and episiotomy can aggravate the existing condition, causing anal sphincter dysfunction.31
The effects of pregnancy on CD can be observed during the pregnancy itself and during the postpartum period.32 Disease activity is the same in pregnant women with CD as in nonpregnant women, which means that pregnancy does not represent an independent risk factor for increased flare rates.33 A retrospective study even suggests that the disease activity index slightly decreases during pregnancy, as compared to the years preceding and following the pregnancy.34 This phenomenon could be explained by reduced tobacco consumption during pregnancy, or due to more aggressive drug treatments used in accordance to the recommendations established to maintain remission in pregnant women.34,35 Pregnancy does not impact on disease phenotype or resection rates.35 A comparison of the influence of pregnancy on the overall course of IBD and the need for surgical treatment showed a significant difference between multiparous and nulliparous women. Multiparous women had a lower rate of relapse at 3 years postpartum.35,36 The need for surgical treatment was reduced, and the interval between surgical interventions was prolonged.37 Some theories suggest that this phenomenon can be explained by the disparity between the mother’s and fetus’s class 2 human leukocyte antigen.38 Paternal antigens in the fetus induce a protective downregulation in the maternal immune system that results in a more benign form of the disease after pregnancy than was evident before the pregnancy.
Pharmacotherapy
The pharmacotherapy used during pregnancy is important to treat active disease, to maintain remission, and to treat flare-ups during pregnancy. Anti-inflammatory and immunomodulatory therapies for CD have possible teratogenic and mutagenic effects. Teratogenic effects affect the development of a normal embryo and fetus, and these effects are dependent on dosage; conversely, mutagenic effects are most likely caused by mutations that occur during deoxyribonucleic acid synthesis in sperm, and these mutations are subsequently transferred by the father.15 This is the reason why detailed studies have been performed on the safety of drug therapy in pregnant women.
Some of the most relevant groups of drugs used during pregnancy include aminosalicylates, corticosteroids, azathioprine/6-mercaptopurine (AZA/6-MP), and antibodies against tumor necrosis factor (anti-TNF). Antibiotics are used predominantly in the management of perianal disease; methotrexate is contraindicated during pregnancy.7 Among aminosalicylates, studies conducted in the past considered sulphasalazine to be teratogenic, but newer population-based studies have shown lower rates of adverse outcomes in pregnant women with IBD than those in the general population after sulphasalazine therapy.39–42 The same findings and conclusions can be applied to mesalazine use during pregnancy.43–45
Corticosteroids cross the placental barrier and are considered to have possible adverse effects on fetal development. In a meta-analysis of ten cohort and case control studies, there were no increased risks of overall congenital abnormalities, but the researchers found a higher risk of oral clefts; the study was conducted on non-IBD women.46 One prospective study did not find an increased risk of overall congenital abnormalities, nor did the authors find an increased risk of oral clefts in pregnant women taking corticosteroids.47 The European Crohn’s and Colitis Organization’s (ECCO) guidelines regarding therapy during pregnancy mention that although corticosteroids cross the placental barrier, they are converted into less active metabolites, so in fetal blood, they can be found only in low concentrations.17 This is the reason why their usage is not prohibited during pregnancy. The effects of AZA and its metabolite, 6-MP, are mostly observed in transplant and rheumatology cohorts.17 They are placed in category D of drugs for use during pregnancy (there is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience – or from studies conducted on humans – but the potential benefits may warrant use of the drug in pregnant women despite the potential risks associated with this treatment), as categorized by the United States Food and Drug Administration.48 A Danish study found an increased risk of preterm birth, perinatal mortality, and increased risk of CA in women who were on therapy with AZA/6-MP during pregnancy.49 The CA associated with thiopurine therapy in pregnant women with CD included congenital cataracts, occipital encephalocele, and CA of the sternocleidomastoid and skin. The study was conducted by comparing a small number of pregnant women with IBD on thiopurine therapy with the general population, so the role of the disease itself remains unclear. A recent retrospective study by Casanova et al50 focused on the safety of thiopurine and anti-TNF therapy during pregnancy. The authors were searching for different obstetric complications in the mother and the newborn that they could connect to the therapy, and described them in terms of unfavorable Global Pregnancy Outcome. The conclusion was that treatment with thiopurines is the only predictor of favorable Global Pregnancy Outcome, as compared to pregnant women who were not exposed to thiopurine therapy, and to those receiving anti-TNF treatments. Other studies did not confirm the increased risk of adverse birth outcomes, so the ECCO guidelines consider these treatments to be safe forms of therapy in pregnant women with IBD.51–54
TNF-α is a proinflammatory cytokine that plays an important role in IBD and during the pregnancy.51–53 Anti-TNF drugs are effective for achieving and maintaining remission in moderate or severe IBD that is refractory to conventional therapy. However, these drugs cross the placental barrier, and the question that imposes is how they affect the fetus and its development. Among the anti-TNF agents, the effect of the infliximab (IFX) is the most studied. It is important to know that IFX crosses the placenta in the second and third trimesters. If it is used after the second trimester, it remains in the newborn’s system for up to 6 months after delivery, which increases the risk of newborn infections.54,55 Two large studies conducted on pregnant women on IFX therapy showed no increase in adverse outcomes such as congenital malformation, miscarriage, or neonatal complications when compared with IFX-naïve pregnant women.56,57 Adalimumab levels can also be detected in the cord and in the infant after delivery, and this is why the recommendation for the discontinuation of therapy with anti-TNF agents is the same for both of these drugs. Certolizumab pegol is a polyethylene glycosylated Fab’ fragment of a humanized TNF inhibitor monoclonal antibody. It crosses the placenta in the first trimester during organogenesis, but its levels in the cord and in the infant after delivery are low.55 The latest systematic literature reviews agree on the opinion that IFX and adalimumab therapy is acceptable during conception, and that the therapy should be stopped at least by the end of the second trimester.54,58
To conclude, there are a growing number of studies and evidence to date that support the standpoint that there is no need to terminate therapy in pregnant women with IBD.59 Most of the drugs have been proven to be safe, and it is considered that the alternative of higher disease activity can cause much greater problems than the therapy itself.7,33
Gynecology
CD is linked to specific complications in women’s genitourinary tracts. These complications require special attention and collaboration between gynecologists and gastroenterologists because, in most cases, they are difficult to manage and affect a woman’s reproductive and psychosocial health. Some of these complications include vulvovaginal disease, perianal disease, and rectovaginal and enterovesical fistulas.
Clinical manifestations
One of the manifestations of CD in the genitourinary tract is described as non-caseating granulomatous inflammation of the skin. More than half of the registered lesions are presented in the vulvar region.22,60 A variety of clinical manifestations is described in that area such as unilateral edema, erythema, papule and nodule lesions, ulcerations, vulvar pruritus, and pain.61 The classic lesion is known as “knife-like”, and it is predominately located in the skin-fold region.62 The most frequent lesion seen in women with CD is genital swelling with or without erythema, which occurs in 60% of cases, and is accompanied by perianal disease, especially in children.61,62 There is a difference in the time of presentation between adults and children. In adults, it usually occurs after the initial diagnosis, and in children, it occurs at the same time or even prior to the initial diagnosis of CD. Approximately 25% of vulvar lesions are recognized before GI symptoms appear.60 It is important to emphasize the significance of these statements because differential diagnoses of vulvovaginal lesions can represent the critical point for early recognition and intervention in non-recognized CD patients.
CD is often complicated by perianal manifestations.63 It is considered that the incidence of these manifestations increases the more distal the involvement of the GI tract is, and the incidence rate ranges from 12% in patients with ileal disease to 92% in those with rectal and colonic disease. Up to 40% of patients will develop perianal manifestations before the other GI symptoms appear;64 perianal fistulas can precede the diagnosis of CD by several years.65 Women are more likely to seek help from their gynecologists than from a gastroenterologist because they experience symptoms of perianal or perineal discomfort.64 The most common findings in the perianal region are abscesses and fistulas, and the less common ones are fissures and anal ulcers.66,67 The medical approach should recognize perianal fistulizing CD as a distinct disease phenotype from luminal fistulizing disease, because of the aggressive and chronic behavior of the perianal lesions.68,69
Treatment
Women with CD can develop specific complications due to the anatomy of their genitourinary tracts, including the presence of rectovaginal and enterovesical fistulas. Data suggest that 10% of women could develop rectovaginal fistulas, which represents an important risk factor for the development of mucinous adenocarcinomas.70–72
The treatment of special gynecological manifestations usually includes a combination of medical and surgical treatments, depending on the type and severity of the clinical appearance. If recognized in time, vulvovaginal disease is treated with systemic therapy for CD, and antibiotics are added in case of secondary infection. Surgery should be considered only in rare cases of failure of medical therapy.22 The treatment of perianal fistulas depends on the symptoms and complexity of fistulas. Abscesses must always be ruled out, because they require immediate drainage. ECCO guidelines recommend antibiotics, metronidazole or ciprofloxacin, as first-line conventional therapy in combination with second-line AZA/6-MP. Anti-TNF agents are still a third-line therapy, which are used in combination with antibiotics, thiopurine therapy, and surgical treatment. The sequence of each therapy is not strictly determined.17 The treatment of rectovaginal fistulas with medical treatment alone often fails, which is why the recommendation is to combine medical and surgical treatments to achieve better rates of success.22
Crohn’s disease and the menstrual cycle
The other important physiological condition that physicians have to bear in mind is the menstrual cycle. The significance of the menstrual cycle in female patients with CD lies in recognizing alterations in GI symptoms that are influenced by hormonal changes, and distinguishing them from true exacerbations of the disease. Lim et al73 conducted the first prospective study that investigated the connection between the menstrual cycle and GI symptoms in women with IBD. The authors divided GI symptoms into non-IBD-specific and IBD-specific categories. Non-IBD-specific GI symptoms included nausea, abdominal discomfort/pain, flatulence, tenesmus, diarrhea, and constipation, and they seemed to be similar to those present in the general healthy population during the premenstrual/menstrual phase. IBD-specific symptoms referred to nocturnal diarrhea, hematochezia, and fecal incontinence. Systemic menstrual symptoms included headache, anxiety, depression, acne, edema, urinary frequency, and mastalgia.
The results of this study also showed increased frequency and severity of premenstrual symptoms in women with IBD compared with the general healthy population.73 The findings especially referred to nausea, abdominal pain, flatulence, and tenesmus, while the incidence of other symptoms was not significantly increased. In addition, IBD patients had a higher number of stools, loose stools, and more severe abdominal pain across all of the menstrual phases. The difference in incidence in IBD-specific symptoms observed among different menstrual phases was found not to be significantly altered, leading to the conclusion that changes in hormone status during the menstrual cycle do not influence the pathological mechanism of the disease itself. The exact pathway through which gonadal hormones affect GI changes remains unclear, and is mostly based on presumptions. It is known that serotonin is ubiquitous in the GI tract and that it plays a key role in motor sensory function.74,75 Since estrogen and progesterone influence the serotonergic system in the brain by increasing its availability, some studies have used this connection to explain disturbances in the GI tract during the premenstrual and menstrual phases.76 Prostaglandin also interferes with the physiology of the GI system,77,78 and it is thought to be the main cause of looser stools, as it inhibits transepithelial ion transport in the small intestine.79 Most of these studies were conducted predominately in patients with UC and IBS, which is why it is not possible to draw conclusions about the exact way in which hormonal changes during the premenstrual and menstrual phases affect women with CD.
The reversed situation – how CD affects women’s menstrual cycles – has also been investigated. Sides et al22 found that the onset of the disease before or during pregnancy could result with a delayed menarche. In adult women, the main problem seems to be irregular menses; oligomenorrhea is the most common abnormality.80 All of these problems are secondary to the underlying disease and appear as a result of poor nutrition, low body fat index, chronic inflammation, and the physiological stress of the disease.22
Conclusion
This article provides an overview of the specific features of CD in women. CD is a chronic inflammatory disease with a rising prevalence rate observed throughout the world, and it features a high incidence rate in women in their peak reproductive years.
Fertility rates in women with inactive CD are similar to those of the general population. It is important to properly advise patients according to the newest findings, to reduce increased rates of voluntary childlessness and to prevent unnecessary complications in pregnancy. Women with quiescent disease at the time of conception have a similar risk for experiencing adverse pregnancy outcomes as the healthy population, while active disease at conception or relapse during pregnancy increases the possibility of adverse pregnancy outcomes. Preconception counseling should involve both obstetricians and gastroenterologists, and the aim should be to achieve remission at least 3 months before conception. The type of pharmacotherapy used for treating active disease and for maintaining remission in pregnant women with CD does not differ from standard therapy in other groups of patients. The fear that drugs would lead to adverse effects during the course of pregnancy has led to detailed studies that have been performed regarding the safety of drug therapy among pregnant women. So far, most of these drugs have been shown to be safe, and the newest ECCO guidelines advise that there is no need to terminate therapy in pregnant women. The opinion is that the alternative of higher disease activity can cause much greater problems than the therapy itself. Physicians have to be aware of the recommendations and limitations for each type of drug because, despite the guidelines, pregnant women with CD have to be treated as a high-risk group of patients that needs special attention regardless of disease activity.
Abnormalities of the menstrual cycle have been observed in these patients, and it was noticed that some symptoms of CD are closely connected to hormonal changes; specifically, during the menstrual cycle, these symptoms can be emphasized. It is important to distinguish between these abnormalities and true flares of the disease. An awareness of these special features of CD in women can lead to a reduced number of complications and their adequate treatment.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54.e42. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Burisch J, Munkholm P. Inflammatory bowel disease epidemiology. Curr Opin Gastroenterol. 2013 May 20; doi: 10.1097/MOG.0b013e32836229fb. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Veloso FT. Extraintestinal manifestations of inflammatory bowel disease: do they influence treatment and outcome? World J Gastroenterol. 2011;17:2702–2707. doi: 10.3748/wjg.v17.i22.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veloso FT, Ferreira JT, Barros L, Almeida S. Clinical outcome of Crohn’s disease: analysis according to the Vienna classification and clinical activity. Inflamm Bowel Dis. 2001;7(4):306–313. doi: 10.1097/00054725-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Herrinton LJ, Liu L, Lewis JD, Griffin PM, Allison J. Incidence and Prevalence of Inflammatory Bowel Disease in a Northern California Managed Care Organization 1996–2002. Am J Gastroenterol. 2008;103(8):1998–2006. doi: 10.1111/j.1572-0241.2008.01960.x. [DOI] [PubMed] [Google Scholar]
- 6.Beaulieu DB, Kane S. Inflammatory bowel disease in pregnancy. World J Gastroenterol. 2011;17(22):2696–2701. doi: 10.3748/wjg.v17.i22.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahedavan U. Fertility and pregnancy in the patient with inflammatory bowel disease. Gut. 2006;55(8):1198–1206. doi: 10.1136/gut.2005.078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hudson M, Flett G, Sinclair TS, Brunt PW, Templeton A, Mowat NA. Fertility and pregnancy in inflammatory bowel disease. Int J Gynaecol Obstet. 1997;58(2):229–237. doi: 10.1016/s0020-7292(97)00088-x. [DOI] [PubMed] [Google Scholar]
- 9.Selinger SP, Leong RW, Lal S. Pregnancy related issues in inflammatory bowel disease: Evidence base and patients’ perspective. World J Gastroenterol. 2012;18(21):2600–2608. doi: 10.3748/wjg.v18.i21.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubinsky M, Abraham B, Mahadevan U. Management of the pregnant IBD patient. Inflamm Bowel Dis. 2008;14(12):1736–1750. doi: 10.1002/ibd.20532. [DOI] [PubMed] [Google Scholar]
- 11.Marri SR, Ahn C, Buchman AL. Voluntary childlessness is increased in women with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13(5):591–599. doi: 10.1002/ibd.20082. [DOI] [PubMed] [Google Scholar]
- 12.Mountifield R, Bampton P, Prosser R, Muller K, Andrews JM. Fear and fertility in inflammatory bowel disease: a mismatch of perception and reality affects family planning decisions. Inflamm Bowel Dis. 2009;15(5):720–725. doi: 10.1002/ibd.20839. [DOI] [PubMed] [Google Scholar]
- 13.Selinger CP, Eaden J, Selby W, et al. Patients’ knowledge of pregnancy-related issues in inflammatory bowel disease and validation of a novel assessment tool (‘CCPKnow’) (Abstract) Aliment Pharmacol Ther. 2012;36(1):57–63. doi: 10.1111/j.1365-2036.2012.05130.x. [DOI] [PubMed] [Google Scholar]
- 14.Selinger CP, Eaden J, Selby W, et al. Inflammatory bowel disease and pregnancy: lack of knowledge is associated with negative views. (Abstract) J Crohns Colitis. 2013;7(6):e206–13. doi: 10.1016/j.crohns.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Nørgård B, Pedersen L, Christensen LA, Sørensen HT. Therapeutic Drug Use in Women with Crohn’s Disease and Birth Outcomes: A Danish nationwide cohort study. Am J Gastroenterol. 2007;102:1406–1413. doi: 10.1111/j.1572-0241.2007.01216.x. [DOI] [PubMed] [Google Scholar]
- 16.Cornish J, Tan E, Teare J, et al. A meta-analysis on the influence of inflammatory bowel disease on pregnancy. Gut. 2007;56(6):830–837. doi: 10.1136/gut.2006.108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Assche G, Dignass A, Reinisch W, et al. European Crohn’s and Colitis Organisation (ECCO) The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Special situations. J Crohns Colitis. 2010;4(1):63–101. doi: 10.1016/j.crohns.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Baird DD, Narendranathan M, Sandler RS. Increased risk of preterm birth for women with inflammatory bowel disease. Gastroenterology. 1990;99(4):987–994. doi: 10.1016/0016-5085(90)90617-a. [DOI] [PubMed] [Google Scholar]
- 19.Alstead EM. Inflammatory bowel disease in pregnancy. Postgrad Med J. 2002;78(915):23–26. doi: 10.1136/pmj.78.915.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dominitz JA, Young JC, Boyko EJ. Outcomes of infants born to mothers with inflammatory bowel disease: a population-based cohort study. Am J Gastroenterol. 2002;97(3):641–648. doi: 10.1111/j.1572-0241.2002.05543.x. [DOI] [PubMed] [Google Scholar]
- 21.Bortoli A, Saibeni S, Tatarella M, et al. Study Group for Inflammatory Bowel Diseases GSMII Pregnancy before and after the diagnosis of inflammatory bowel diseases: retrospective case-control study. J Gastroenterol Hepatol. 2007;22(4):542–549. doi: 10.1111/j.1440-1746.2006.04754.x. [DOI] [PubMed] [Google Scholar]
- 22.Sides C, Trinidad MC, Heitlinger L, Anasti J. Chron disease and the gynecologic patient. Obstet Gynecol Surv. 2013;68(1):51–61. doi: 10.1097/OGX.0b013e31827b1658. [DOI] [PubMed] [Google Scholar]
- 23.Bortoli A, Pedersen N, Duricova D, et al. European Crohn-Colitis Organisation (ECCO) Study Group of Epidemiologic Committee (EpiCom) Pregnancy outcome in inflammatory bowel disease: prospective European case-control ECCO-EpiCom study, 2003–2006. Aliment Pharmacol Ther. 2011;34(7):724–734. doi: 10.1111/j.1365-2036.2011.04794.x. [DOI] [PubMed] [Google Scholar]
- 24.Fonager K, Sørensen HT, Olsen J, Dahlerup JF, Rasmussen SN. Pregnancy outcome for women with Crohn’s disease: a follow-up study based on linkage between national registries. Am J Gastroenterol. 1998;93(12):2426–2430. doi: 10.1111/j.1572-0241.1998.00698.x. [DOI] [PubMed] [Google Scholar]
- 25.Reddy D, Murphy SJ, Kane SV, Present DH, Kornbluth AA. Relapses of inflammatory bowel disease during pregnancy: in-hospital management and birth outcomes. Am J Gastroenterol. 2008;103(5):1203–1209. doi: 10.1111/j.1572-0241.2007.01756.x. [DOI] [PubMed] [Google Scholar]
- 26.Kornfeld D, Cnattingius S, Ekbom A. Pregnancy outcomes in women with inflammatory bowel disease—a population-based cohort study. Am J Obstet Gynecol. 1997;177(4):942–946. doi: 10.1016/s0002-9378(97)70298-9. [DOI] [PubMed] [Google Scholar]
- 27.Nørgård B, Fonager K, Sørensen HT, Olsen J. Birth outcomes of women with ulcerative colitis: a nationwide Danish cohort study. Am J Gastroenterol. 2000;95(11):3165–3170. doi: 10.1111/j.1572-0241.2000.03290.x. [DOI] [PubMed] [Google Scholar]
- 28.Nørgård B, Puho E, Pedersen L, Czeizel AE, Sørensen HT. Risk of congenital abnormalities in children born to women with ulcerative colitis: a population-based, case-control study. Am J Gastroenterol. 2003;98(9):2006–2010. doi: 10.1111/j.1572-0241.2003.07578.x. [DOI] [PubMed] [Google Scholar]
- 29.Ilnyckyj A, Blanchard JF, Rawsthorne P, Bernstein CN. Perianal Crohn’s disease and pregnancy: role of the mode of delivery. Am J Gastroenterol. 1999;94(11):3274–3278. doi: 10.1111/j.1572-0241.1999.01537.x. [DOI] [PubMed] [Google Scholar]
- 30.Smink M, Lotgering FK, Albers L, de Jong DJ. Effect of childbirth on the course of Crohn’s disease; results from a retrospective cohort study in the Netherlands. BMC Gastroenterol. 2011;11:6. doi: 10.1186/1471-230X-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandt LJ, Estabrook SG, Reinus JF. Results of a survey to evaluate whether vaginal delivery and episiotomy lead to perineal involvement in women with Crohn’s disease. Am J Gastroenterol. 1995;90(11):1918–1922. [PubMed] [Google Scholar]
- 32.Kwan LY, Mahedavan U. Inflammatory bowel disease and pregnancy: an update. Expert Rev Clin Immunol. 2010;6(4):643–657. doi: 10.1586/eci.10.35. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen OH, Andreasson B, Bondesen S, Jacobsen O, Jarnum S. Pregnancy in Crohn’s disease. Scand J Gastroenterol. 1984;19(6):724–732. [PubMed] [Google Scholar]
- 34.Agret F, Cosnes J, Hassani Z, et al. Impact of pregnancy on the clinical activity of Crohn’s disease. Aliment Pharmacol Ther. 2005;21(5):509–513. doi: 10.1111/j.1365-2036.2005.02384.x. [DOI] [PubMed] [Google Scholar]
- 35.Riis L, Vind I, Politi P, et al. Does pregnancy change the disease course? A study in a European cohort of patients with inflammatory bowel disease. Am J Gastroenterol. 2006;101(7):1539–1545. doi: 10.1111/j.1572-0241.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 36.Castiglione F, Pignata S, Morace F, et al. Effect of pregnancy on the clinical course of a cohort of women with inflammatory bowel disease. Ital J Gastroenterol. 1996;28(4):199–204. [PubMed] [Google Scholar]
- 37.Nwokolo CU, Tan WC, Andrews HA, Allan RN. Surgical resections in parous patients with distal ileal and colonic Crohn’s disease. Gut. 1994;35(2):220–223. doi: 10.1136/gut.35.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kane S, Kisiel J, Shih L, Hanauer S. HLA disparity determines disease activity through pregnancy in women with inflammatory bowel disease. Am J Gastroenterol. 2004;99(8):1523–1526. doi: 10.1111/j.1572-0241.2004.30472.x. [DOI] [PubMed] [Google Scholar]
- 39.Craxi A, Pagliarello F. Possible embryotoxicity of sulfasalazine. Arch Intern Med. 1980;140(12):1674. doi: 10.1001/archinte.140.12.1674c. [DOI] [PubMed] [Google Scholar]
- 40.Hoo JJ, Hadro TA, Von Behren P. Possible teratogenicity of sulfasalazine. N Engl J Med. 1988;318(17):1128. doi: 10.1056/NEJM198804283181714. [DOI] [PubMed] [Google Scholar]
- 41.Newman NM, Correy JF. Possible teratogenicity of sulphasalazine. Med J Aust. 1983;1(11):528–529. doi: 10.5694/j.1326-5377.1983.tb136199.x. [DOI] [PubMed] [Google Scholar]
- 42.Nørgård B, Hundborg HH, Jacobsen BA, Nielsen GL, Fonager K. Disease activity in pregnant women with Crohn’s disease and birth outcomes: a regional Danish cohort study. Am J Gastroenterol. 2007;102(9):1947–1954. doi: 10.1111/j.1572-0241.2007.01355.x. [DOI] [PubMed] [Google Scholar]
- 43.Marteau P, Tennenbaum R, Elefant E, Lémann M, Cosnes J. Foetal outcome in women with inflammatory bowel disease treated during pregnancy with oral mesalazine microgranules. Aliment Pharmacol Ther. 1998;12(11):1101–1108. doi: 10.1046/j.1365-2036.1998.00417.x. [DOI] [PubMed] [Google Scholar]
- 44.Diav-Citrin O, Park YH, Veerasuntharam G, et al. The safety of mesalamine in human pregnancy: a prospective controlled cohort study. Gastroenterology. 1998;114(1):23–28. doi: 10.1016/s0016-5085(98)70628-6. [DOI] [PubMed] [Google Scholar]
- 45.Nørgård B, Fonager K, Pedersen L, Jacobsen BA, Sørensen HT. Birth outcome in women exposed to 5-aminosalicylic acid during pregnancy: a Danish cohort study. Gut. 2003;52(2):243–247. doi: 10.1136/gut.52.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids:prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62(6):385–392. doi: 10.1002/1096-9926(200012)62:6<385::AID-TERA5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 47.Gur C, Diav-Citrin O, Shechtman S, Arnon J, Ornoy A. Pregnancy outcome after first trimester exposure to corticosteroids: a prospective controlled study. Reprod Toxicol. 2004;18(1):93–101. doi: 10.1016/j.reprotox.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Administration FDA.Regulations 19804437434–37467.Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/016324s034s035lbl.pdfAccesed May 6, 2013 [Google Scholar]
- 49.Nørgård B, Pedersen L, Fonager K, Rasmussen SN, Sørensen HT. Azathioprine, mercaptopurine and birth outcome: a population-based cohort study. Aliment Pharmacol Ther. 2003;17(6):827–834. doi: 10.1046/j.1365-2036.2003.01537.x. [DOI] [PubMed] [Google Scholar]
- 50.Casanova MJ, Chaparro M, Domènech E, et al. Safety of thiopurines and anti-TNF-α drugs during pregnancy in patients with inflammatory bowel disease. (Abstract) Am J Gastroenterol. 2013;108(3):433–440. doi: 10.1038/ajg.2012.430. [DOI] [PubMed] [Google Scholar]
- 51.Coelho J, Beaugerie L, Colombel JF, et al. CESAME Pregnancy Study Group (France) Pregnancy outcome in patients with inflammatory bowel disease treated with thiopurines: cohort from the CESAME Study. Gut. 2011;60(2):198–203. doi: 10.1136/gut.2010.222893. [DOI] [PubMed] [Google Scholar]
- 52.Francella A, Dyan A, Bodian C, Rubin P, Chapman M, Present DH. The safety of 6-mercaptopurine for childbearing patients with inflammatory bowel disease: a retrospective cohort study. Gastroenterology. 2003;124(1):9–17. doi: 10.1053/gast.2003.50014. [DOI] [PubMed] [Google Scholar]
- 53.Khan ZH, Mayberry JF, Spiers N, Wicks AC. Retrospective case series analysis of patients with inflammatory bowel disease on azathioprine. A district general hospital experience. Digestion. 2000;62:249–254. doi: 10.1159/000007823. [DOI] [PubMed] [Google Scholar]
- 54.Marchioni RM, Lichtenstein GR. Tumor necrosis factor-α inhibitor therapy and fetal risk: A systematic literature review. World J Gastro-enterol. 2013;19(17):2591–2602. doi: 10.3748/wjg.v19.i17.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11(3):286–292. doi: 10.1016/j.cgh.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mahadevan U, Kane S, Sandborn WJ, et al. Intentional infliximab use during pregnancy for induction or maintenance of remission in Crohn’s disease. Aliment Pharmacol Ther. 2005;21(6):733–738. doi: 10.1111/j.1365-2036.2005.02405.x. [DOI] [PubMed] [Google Scholar]
- 57.Katz JA, Antoni C, Keenan GF, Smith DE, Jacobs SJ, Lichtenstein GR. Outcome of pregnancy in women receiving infliximab for the treatment of Crohn’s disease and rheumatoid arthritis. Am J Gastroenterol. 2004;99(12):2385–2392. doi: 10.1111/j.1572-0241.2004.30186.x. [DOI] [PubMed] [Google Scholar]
- 58.Gisbert JP, Chaparro M. Safety of Anti-TNF Agents During Pregnancy and Breastfeeding in Women With Inflammatory Bowel Disease. Am J Gastroenterol. 2013 Jun 11; doi: 10.1038/ajg.2013.171. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 59.Habal FM, Ravindran NC. Management of inflammatory bowel disease in the pregnant patient. World J Gastroenterol. 2008;14(9):1326–1332. doi: 10.3748/wjg.14.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn’s disease: a review. J Eur Acad Dermatol Venereol. 2008;22(9):1033–1043. doi: 10.1111/j.1468-3083.2008.02741.x. [DOI] [PubMed] [Google Scholar]
- 61.Andreani SM, Ratnasingham K, Dang HH, Gravante G, Giordano P. Crohn’s disease of the vulva. Int J Surg. 2010;8(1):2–5. doi: 10.1016/j.ijsu.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 62.Leu S, Sun PK, Collyer J, et al. Clinical spectrum of vulva metastatic Crohn’s disease. Dig Dis Sci. 2009;54(7):1565–1571. doi: 10.1007/s10620-008-0448-y. [DOI] [PubMed] [Google Scholar]
- 63.Safar B, Sands D. Perianal Crohn’s Disease. Clin Colon Rectal Surg. 2007;20(4):282–293. doi: 10.1055/s-2007-991027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hellers G, Bergstrand O, Ewerth S, Holmström B. Occurrence and outcome after primary treatment of`anal fistulae in Cohn’s disease. Gut. 1980;21(6):525–527. doi: 10.1136/gut.21.6.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nielsen OH, Rogler G, Hahnloser D, Thomsen OØ. Diagnosis and management of fistulizing Crohn’s disease. Nat Clin Pract Gastroenterol Hepatol. 2009;6(2):92–106. doi: 10.1038/ncpgasthep1340. [DOI] [PubMed] [Google Scholar]
- 66.Keighley MR, Allan RN. Current status and influence of operation on perianal Crohn’s disease. Int J Colorectal Dis. 1986;1(2):104–107. doi: 10.1007/BF01648416. [DOI] [PubMed] [Google Scholar]
- 67.Kane SV, Sable K, Hanauer SB. The menstrual cycle and its effect on inflammatory bowel disease and irritable bowel syndrome: a prevalence study. Am J Gastroenterol. 1998;93(10):1867–1872. doi: 10.1111/j.1572-0241.1998.540_i.x. [DOI] [PubMed] [Google Scholar]
- 68.Tang LY, Rawsthorne P, Bernstein CN. Are perineal and luminal fistulas associated in Crohn’s disease? A population based study. Clin Gastroenterol Hepatol. 2006;4(9):1130–1134. doi: 10.1016/j.cgh.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 69.Ruffolo C, Citton M, Scarpa M, et al. Perianal Crohn’s disease: Is there something new? World J Gastroenterol. 2011;17(15):1939–1946. doi: 10.3748/wjg.v17.i15.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deveaux PG, Kimberling J, Galandiuk S. Crohn’s disease: presentation and severity compared between black patients and white patients. Dis Colon Rectum. 2005;48(7):1404–1409. doi: 10.1007/s10350-005-0034-8. [DOI] [PubMed] [Google Scholar]
- 71.Kane S. Urogenital complications of Crohn’s disease. Am J Gastroenterol. 2006;101(12 Suppl):S640–3. doi: 10.1111/j.1572-0241.2006.00976.x. [DOI] [PubMed] [Google Scholar]
- 72.Chu M, Crist H, Zaino RJ. Adenocarcinoma arising in a rectovaginal fistula in Chron disease. Int J Gynecol Pathol. 2010;29(5):497–500. doi: 10.1097/PGP.0b013e3181d0cb2c. [DOI] [PubMed] [Google Scholar]
- 73.Lim SM, Nam CM, Kim YN, et al. The effect of the menstrual cycle on inflammatory bowel disease: a prospective study. Gut Liver. 2013;7(1):51–57. doi: 10.5009/gnl.2013.7.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Borman RA, Tilford NS, Harmer DW, et al. 5-HT(2B) receptors play a key role in mediating the excitatory effects of 5-HT in human colon in vitro. Br J Pharmacol. 2002;135(5):1144–1151. doi: 10.1038/sj.bjp.0704571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Houghton LA, Brown H, Atkinson W, et al. 5-hydroxytryptamine signalling in irritable bowel syndrome with diarrhoea: effects of gender and menstrual status. Aliment Pharmacol Ther. 2009;30(9):919–929. doi: 10.1111/j.1365-2036.2009.04121.x. [DOI] [PubMed] [Google Scholar]
- 76.Bethea CL, Pecins-Thompson M, Schutzer WE, Gundlah C, Lu ZN. Ovarian steroids and serotonin neural function. Mol Neurobiol. 1998;18(2):87–123. doi: 10.1007/BF02914268. [DOI] [PubMed] [Google Scholar]
- 77.Heitkemper MM, Chang L. Do fluctuations in ovarian hormones affect gastrointestinal symptoms in women with irritable bowel syndrome? Gend Med. 2009;6(Suppl 2):152–167. doi: 10.1016/j.genm.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okoro NI, Kane SV. Gender-related issues in the female inflammatory bowel disease patient. Expert Rev Gastroenterol Hepatol. 2009;3(2):145–154. doi: 10.1586/egh.09.1. [DOI] [PubMed] [Google Scholar]
- 79.Eberhart CE, Dubois RN. Eicosanoids and the gastrointestinal tract. Gastroenterology. 1995;109(1):285–301. doi: 10.1016/0016-5085(95)90296-1. [DOI] [PubMed] [Google Scholar]
- 80.Weber AM, Ziegler C, Belinson JL, Mitchinson AR, Widrich T, Fazio V. Gynecologic history of women with inflammatory bowel disease. Obstet Gynecol. 1995;86(5):843–847. doi: 10.1016/0029-7844(95)00286-Z. [DOI] [PubMed] [Google Scholar]


