Abstract
Background
Obstructive sleep apnea (OSA) occurs in 4% of middle-aged men and 2% of middle-aged women in the general population, and the prevalence is even higher in specific patient groups. OSA is an independent risk factor for a variety of cardiovascular diseases. Endothelial injury could be the pivotal determinant in the development of cardiovascular pathology in OSA. Endothelial damage ultimately represents a dynamic balance between the magnitude of injury and the capacity for repair. Bone marrow–derived endothelial progenitor cells (EPCs) within adult peripheral blood present a possible means of vascular maintenance that could home to sites of injury and restore endothelial integrity and normal function.
Methods
We summarized pathogenetic mechanisms of OSA and searched for available studies on numbers and functions of EPCs in patients with OSA to explore the potential links between the numbers and functions of EPCs and OSA. In particular, we tried to elucidate the molecular mechanisms of the effects of OSA on EPCs.
Conclusion
Intermittent hypoxia cycles and sleep fragmentation are major pathophysiologic characters of OSA. Intermittent hypoxia acts as a trigger of oxidative stress, systemic inflammation, and sympathetic activation. Sleep fragmentation is associated with a burst of sympathetic activation and systemic inflammation. In most studies, a reduction in circulating EPCs has emerged. The possible mechanisms underlying the decrease in the number or function of EPCs include prolonged inflammation response, oxidative stress, increased sympathetic activation, physiological adaptive responses of tissue to hypoxia, reduced EPC mobilization, EPC apoptosis, and functional impairment in untreated OSA. Continuous positive airway pressure (CPAP) therapy for OSA affects the mobilization, apoptosis, and function of EPCs through preventing intermittent hypoxia episodes, improving sleep quality, and reducing systemic inflammation, oxidative stress levels, and sympathetic overactivation. To improve CPAP adherence, the medical staff should pay attention to making the titration trial a comfortable first CPAP experience for the patients; for example, using the most appropriate ventilators or proper humidification. It is also important to give the patients education and support about CPAP use in the follow-up, especially in the early stage of the treatment.
Keywords: intermittent hypoxia, systemic inflammation, oxidative stress, sympathetic activation, continuous positive airway pressure adherence
Introduction
Obstructive sleep apnea (OSA) is a common condition characterized by repeated episodes of upper airway obstruction that result in interruptions of breathing during sleep, recurring episodes of hypoxemia, sleep fragmentation, and daytime sleepiness. OSA affects 3%–7% of adult men, 2%–5% of adult women,1–3 and up to 4% of children.3,4 At all ages, even in children, it is associated with complications in different organ systems, such as cardiovascular morbidity, hypertension, obesity, dyslipidemia, and insulin resistance.5–8 Moreover, both in children and adults, OSA causes behavioral and neuropsychological deficits in the central nervous system, including daytime sleepiness, depression,9 impaired memory,10 mood disorders, cognition deficiencies,11 and even nocturnal enuresis.12 Cognition deficiencies in patients with OSA have typically been found in attention and vigilance, memory and learning, executive functions, and simulated driving, in which endothelial dysfunctions could be the most intriguing explanation.4,13 There is evidence showing that sleep parameters can rapidly be normalized with continuous positive airway pressure (CPAP) treatment, but those deficits in cognitive performance often persist.4,13 OSA is also an independent risk factor for a variety of cardiovascular diseases such as atherosclerosis, hypertension, and coronary heart disease.14,15 The maintenance of an intact vascular endothelium is critical for preservation of the integrity of the vascular system. Endothelial injury could be the pivotal determinant in the development of cardiovascular pathology in OSA.16–22 One of the major pathophysiologic mechanisms of vascular injury is the endothelial damage from intermittent hypoxia (IH) with OSA pattern. Endothelial damage ultimately represents a dynamic balance between the magnitude of injury and the capacity for repair. The balance between the damage and repair ultimately determines the progression of cardiovascular diseases.
Vascular endothelium has a finite lifespan. Endothelial cells are shed into the circulation in both healthy and disease states, and a mechanism must exist by which these cells can be replaced.23 It conventionally has been thought that this was exclusively accomplished by the proliferation and migration of resident mature endothelial cells adjacent to regions of injury.24 However, the discovery of bone marrow–derived endothelial progenitor cells (EPCs) within adult peripheral blood presented another possible means of vascular maintenance; namely, a reservoir of circulating cells that could home to sites of injury and restore endothelial integrity and normal function. In 1997, Asahara et al described for the first time a population of putative EPCs in human peripheral blood.25 In this study, selected circulating CD34-positive cells in human peripheral blood moved into the foci of vascular injury and differentiated into vascular endothelial cells. Further studies from this group showed that a specific population of bone marrow cells, now identified as EPCs, is recruited to the foci of vascular injury and neovascular formation, and these cells differentiate into vascular endothelial cells in both physiologic and pathologic neovascular formations.26 Since then, accumulating evidence has indicated that EPCs support the integrity of the vascular endothelium and take part in repair processes throughout the cardiovascular system.25,27,28 EPCs contribute to endothelial repair and neovascularization not only by physically integrating into the endothelial layer but also by excretion of paracrine factors that can stimulate the proliferation of resident endothelial cells,29 which is of paramount importance in neovascularization.30–33
Logically, then, if the circulating progenitor pool represents an important source of endothelial cells for “repair,” a reduction in the number of progenitors might be expected to have a negative effect on endothelial function. There are several studies that have been carried out on EPCs in OSA, but the results currently available on the role of EPCs in OSA are controversial. EPCs have been reported as increased, decreased, or unchanged in OSA. In most of these studies, however, a reduction in circulating EPCs has emerged. Up until now, the mechanisms underlying the decrease in number or function of EPCs in patients with OSA have not been fully elucidated. In this review, we describe our current understanding of the effects of OSA on the number and function of EPCs and focus on the molecular mechanisms. Clarification and reinforcement of the repair mechanisms of EPCs may ameliorate endothelial damages and reduce OSA-related morbidities. In addition, pathophysiological insight will be provided for improvement of the repairing process after endothelial damage in patients with OSA.
EPCs
EPCs are premature circulating cells that are mainly derived from bone marrow and are endowed with the capacity both to be mobilized from bone marrow into the bloodstream in response to growth factors and cytokine release and to differentiate into mature endothelial cells and be involved in postnatal vasculogenesis and reendothelialization after endothelial damage.
In 1997, Asahara and colleagues demonstrated for the first time that some special purified CD34-positive hematopoietic progenitor cells from peripheral blood could differentiate, ex vivo, into an endothelial phenotype; the cells were then named EPCs.25 Since then, different markers have been used to describe in vivo circulating EPCs. EPCs are positive for CD34 or the more immature marker protein CD133. Recent studies have shown that expression of the CD34 surface antigen is shared by EPCs, hematopoietic progenitor cells, and mature endothelial cells.34 As they mature, EPCs lose the CD133 marker and acquire vascular endothelial growth factor (VEGF) receptor 2 (VEGFR2), also known as kinase insert domain-containing receptor (KDR).35 The combination of CD34+ VEGFR2+, CD34+ CD133+, CD133+ VEGFR2+, and CD34+ CD133+ VEGFR2+ has been used by different investigators to describe in vivo circulating EPCs.36 Numerous investigators have found that double-positive CD34+ VEGFR2+ cells can behave as EPCs. However, because some mature endothelial cells also coexpress CD34 and VEGFR2, better markers may be needed. The stem cell marker CD133 may be a more precise marker for defining subpopulations of cells that represent EPCs. Unlike the progenitor marker CD34, CD133 is not expressed on mature endothelial cells. A study group has shown that double-positive CD34+ CD133+ EPCs have high proliferative capacity and give rise to endothelial colonies in culture.37 CD133+ VEGFR2+ dual-positive cells have been found colonizing the luminal surfaces of left ventricular assist devices explanted from humans, suggesting these cells may play a role in endothelial repair. An intriguing hypothesis is that triple CD133+ CD34+ VEGFR2+ cells represent more primitive EPCs with high proliferative potential, which then turn into CD133− CD34+ VEGFR2+ EPCs with a more limited proliferative capacity.
Recent data have suggested that at least two EPC sub-populations can be grown from peripheral blood mononuclear cells; namely, early EPCs, which display paracrine actions, and late-outgrowth EPCs, which are characterized by high proliferative potential, promoting angiogenesis in different ways.33 Early EPCs contribute to angiogenesis in a paracrine fashion but fail to form vascular networks and to incorporate in endothelial-like structures in a newly developed angiogenesis assay. Late-outgrowth EPCs contribute to angiogenesis by directly incorporating into newly formed vascular networks but fail to stimulate angiogenesis in a paracrine fashion.33
Bone marrow–derived EPC studies in patients with OSA
The studies on EPCs in OSA have been carried out in both adult and child patients. The number and functional activity of circulating EPCs are affected not only by different cardiovascular risk factors such as hypertension, obesity, hypercholesterolemia, diabetes, and smoking, but also by different physiological conditions, such as age, sex, cigarette use, and physical inactivity.38–40 Almost all currently available studies on EPCs in OSA recruited participants who were free of any other known cardiovascular risk factors. In these studies, patients with OSA and healthy control patients are matched for age, sex, and body mass index (BMI), and in addition, patients and control subject participants were similar in blood pressure, fasting blood glucose, and total cholesterol levels.28–41
The cumulative results currently available on the role of EPCs in OSA are controversial. Data from five recent studies reported a decrease in EPCs in patients with OSA. The study by de la Peña et al41 reported that the percentage of EPCs (CD34+ VEGFR2+ cells) was significantly lower in patients with OSA who were free of any other known cardiovascular risk factor than in healthy control patients matched for age and sex. Endothelial function was not different between patients with OSA and control patients. No significant correlation between circulating EPCs and apnea-hypopnea index (AHI) was found in patients with OSA. Similarly, in 2008, Jelic et al42 reported that baseline EPC (CD34+ CD133+ cells) levels were lower in patients with OSA than in control patients. CPAP therapy increased EPC levels to those of control participants when patients adhered to CPAP for more than 4 hours daily. EPC levels remained unchanged when patients used CPAP for less than 4 hours daily or declined CPAP. Another study by Jelic et al43 in 2009 shared similar results. The authors reported that before treatment, EPC (CD34+ CD133+ VEGFR2+ cells) levels, a marker of endothelial repair capacity, were lower and endothelial microparticle levels (EMPs), a marker of endothelial apoptosis, were greater in patients with OSA than in control patients. Levels of EPCs and EMPs were inversely related. After effective treatment (CPAP >4 hours daily), EPC levels were similar in patients with OSA and control patients. Levels of EMP and EPC were unchanged in patients who declined CPAP and in a single patient who used CPAP for less than 4 hours daily. The authors concluded that OSA alone impairs endothelial repair capacity and promotes endothelial apoptosis.
Murri et al44 found that EPCs (CD34+ CD133+ VEGFR2+ cells) were lower in the patients with OSA than in the control patients. There was a significant negative correlation between EPC levels and the severity of OSA, and the EPC levels correlated negatively with the levels of oxidative stress markers, but positively with markers of protection against oxidation. After 1 month of CPAP treatment, EPC levels increased and oxidative stress variables decreased. In a study in children, performed by Kheirandish-Gozal et al,45 80 children with OSA and 20 control patients matched for BMI, age, sex, and ethnicity were recruited. Despite similar OSA severity, EPC (CD34+ CD133+ VEGFR2+ cells) counts were significantly lower among the 20 children with OSA with endothelial dysfunction when compared with either the 20 children without endothelial dysfunction or the control patients. Furthermore, EPC levels were significantly and inversely correlated with the magnitude of endothelial dysfunction, but neither EPCs nor the magnitude of endothelial dysfunction were associated with AHI. In contrast with these findings, Kizawa et al46 reported that individuals with OSA had a threefold increase in EPCs (CD34+ CD133+ cells) in their blood circulation compared with the control group. After CPAP treatment, this increase was suppressed. Martin et al18 found there were no significant differences in circulating EPCs (CD34+ CD133+ cells) between patients with OSA free of any other known cardiovascular risk factor and healthy control patients matched for age and BMI, respectively. Similarly, Yun et al47 also found that EPC levels did not differ between patients with OSA and non-OSA patients. CPAP compliance did not affect EPC levels. The levels of EMPs in patients with OSA were significantly higher than those in the non-OSA group.
There are several possible reasons for this discrepancy. First, investigators in different studies assessed circulating EPCs, using different methods. Some studies18,41–48 assessed circulating EPCs using flow cytometry alone. One study47 assessed circulating EPCs by the assay of endothelial colony-forming units. It is possible that the assay of endothelial colony-forming units48 might be more specific, and therefore more likely to detect differences between patients with OSA and control patients. Second, the EPCs studied by one group are not necessarily the same cell type as those of another. One of the major limitations in studying EPCs is the lack of unifying phenotypic markers that are employed by different investigators. Thus, different investigators employ different marker combinations for the assessment of EPCs: CD34+ VEGFR2+,41 CD34+ 133+,42,44,46 or CD34+ CD133+ VEGFR2+.43–45 Third, the recruited participants are different. For example, Kizawa et al46 recruited only male subjects and excluded the possibility of the cyclical mobilization of EPCs, whereas other studies recruited both female and male participants; several reports have demonstrated that the influence of the menstrual cycle affects the number and function of EPCs.49,50 Fourth, these studies were performed on only a relatively small number of participants. Furthermore, the numbers of circulating EPCs that can be identified from peripheral blood samples are small.51 Thus, because of the relatively rare event analysis, the sample sizes in these studies may have been too small to detect differences. Fifth, because hypoxia is critical to the changes of circulating EPCs, it is possible that participants with more profound nocturnal desaturation, such as those with lower resting lung volumes52 or longer apnea duration,53 might demonstrate altered numbers of circulating EPCs. Even though some studies41 reported no significant correlation between circulating EPCs, and AHI was found in patients with OSA, this might be explained by the narrow range of disease severity of the patients studied here (all of whom had severe OSA). Finally, these studies were done at the time of diagnosis of OSA. No one knows how long OSA had existed in those participants. It is possible there is a threshold duration of OSA that is necessary for the changes in circulating EPCs to be observed.
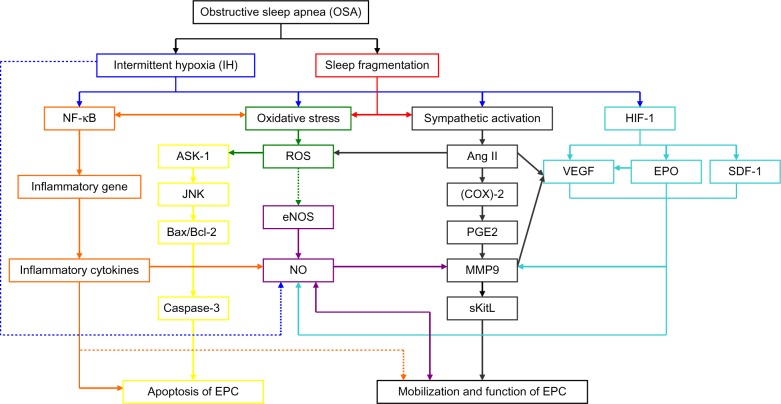
Pathogenetic mechanisms of OSA and the effects of OSA on EPCs (Figure 1)
Figure 1.
Potential molecular mechanisms through which obstructive sleep apnea has effects on endothelial progenitor cells.
Notes: Solid lines, increase or enhance; dotted lines, decrease or inhibit; blue lines, intermittent hypoxia pathologic pathway; red lines, direct effects from sleep fragmentation; orange lines, nuclear factor (NF)-κB inflammatory pathologic pathway; green lines, oxidative stress pathologic pathway; purple lines, nitric oxide (NO) pathologic pathway; yellow lines, apoptosis pathologic pathway; gray lines, angiotensin (Ang) II and matrix metalloproteinase (MMP)-9 pathologic pathway; aqua lines, hypoxia inducible factor (HIF) 1 adaptive pathologic pathway.
Abbreviations: NF-κB, nuclear factor κB; ASK-1, apoptosis signal regulating kinase 1; JNK, Jun N-terminal kinase; Bax/Bcl-2, the ratio of Bax protein to Bcl-2 protein; EPC, endothelial progenitor cell; ROS, reactive oxygen species; eNOS, endothelial nitric oxide synthase; NO, nitric oxide; Ang II, angiotensin II; (COX)-2, cyclooxygenase-2; PGE2, prostaglandin E2; MMP9, matrix metalloproteinase -9; sKitL, soluble Kit ligand; HIF-1, hypoxia inducible factor-1; VEGF, Vascular endothelial growth factor; EPO, erythropoietin; SDF-1, stroma-derived factor-1.
OSA is a common condition characterized by repeated episodes of upper airway obstruction that result in interruptions of breathing during sleep, recurring episodes of hypoxemia, sleep fragmentation, and excessive daytime sleepiness. These episodes induce cyclical alterations of arterial oxygen saturation and desaturation, which is referred to as hypoxia/reoxygenation or IH. The IH cycle is the major pathophysiologic character of OSA. IH acts as a trigger of oxidative stress, systemic inflammation, and sympathetic activation. Sleep fragmentation is associated with a burst of sympathetic activation54 and increased levels of inflammatory markers such as C-reactive protein (CRP),55 interleukin 6 (IL-6), and tumor necrosis factor-alpha (TNF-α).56 Inflammatory responses induced by IH could activate oxidative stress in OSA. In turn, increased oxidative stress will lead to activation of nuclear factor (NF)-κB, and hence increased expression of a number of downstream NF-κB target genes; for example, proinflammatory cytokines, such as TNF-α, IL-6, and IL-8, as well as adhesion molecules such as intercellular adhesion molecule 1.57
Effects of systemic inflammation on EPCs in OSA
Systemic inflammation could be caused directly by IH or caused indirectly by both oxidative stress and sympathetic activation in OSA. Increased reactive oxygen species (ROS) production57 may cause increased expression of inflammatory cytokines through activation of NF-κB, and hence increased expression of a number of downstream NF-κB target genes. In addition, increased sympathetic activity can cause an increase in inflammatory cytokines by increasing free fatty acid levels in the absence of ROS.58,59 Most studies show that patients with OSA have higher levels of circulating inflammatory markers, such as CRP and/or TNF-α and/or IL-6 and/or IL-8,60–66 with a significant fall after effective CPAP therapy.60,67,68 Patients with OSA have lower levels of circulating anti-inflammatory cytokines such as IL−10. IL-10 correlates negatively with the severity of OSA and can inhibit the production of many pro-inflammatory cytokines such as IL-6.66
Previous reports have suggested that prolonged inflammation response has been implicated with reduced EPCs mobilization, cell apoptosis, and functional impairment.69,70 Inflammatory response may affect EPCs mobilization. Increasing evidence indicates that EPC mobilization is closely correlated with variations in the levels of some inflammatory factors.71 High levels of TNF-α contribute to a reduction in EPC number.72 A positive association between CRP levels and circulating EPCs has been documented in patients with stable coronary artery disease, suggesting that a systemic inflammatory state stimulates EPC mobilization in these patients.71 In a study, circulating EPCs were diminished in IL-10 genetically deficient mice as compared with wild-type mice, which suggests that IL-10 plays a crucial role in the mobilization of EPCs.73 Inflammatory response may be implicated with EPC apoptosis, and a clinical study demonstrated that CRP is associated with apoptosis of EPCs in vitro.74 In addition, EPCs that are mobilized in response to inflammatory stimulation may be functionally impaired.69 CRP exerts direct inhibitory effects on EPC differentiation and survival, whereas EPCs exposed to CRP exhibit decreased angiogenic activity.75
Increasing evidence indicates that a transient, restricted, or low-grade inflammation induces EPC mobilization, whereas prolonged or excessive or high-grade inflammatory stimuli, such as observed in OSA, has the opposite effect.70,76 Although the mechanisms regulating this effect are still unclear, the possible mechanisms may be that prolonged exposure of bone marrow to increased proinflammatory stimulation may lead to exhaustion of the EPC pool. The association between inflammation and EPCs is largely circumstantial and observational.76 Further clinical studies are required to elucidate the exact mechanisms by which inflammation affects EPC mobilization and functional activity.
Effects of oxidative stress on EPCs in OSA
Oxidative stress is a known feature of OSA77 and is thought to be mainly caused by cyclical hypoxia/reoxygenation. Oxidative stress in OSA can also occur via activated inflammatory responses induced by hypoxia and by an increased sympathetic activity. IH and sleep fragmentation54 lead to increased sympathetic activity, which stimulates the renin-angiotensin-aldosterone system (RAAS), resulting in elevated angiotensin II (Ang II), which is known to increase oxidative stress.
Oxidative stress may play a crucial role in EPC mobilization and functional bioactivity.26 Increased superoxide generation reduces EPC levels and impairs EPC function.78 In a rat model of myocardial infarction, increased production of ROS, which is the major oxidative stress marker, is associated with reduced EPC levels.79 Some clinical studies have shown that conditions associated with increased oxidative stress have been associated with decreased EPC numbers in the peripheral circulation.80 Murri et al44 found that the levels of oxidative stress markers correlated negatively with levels of EPCs, whereas markers of protection against oxidation correlated positively with the levels of EPCs. After 1 month of CPAP treatment, oxidative stress variables decreased and EPC levels increased. In another study, incubation of EPCs with high levels of hydrogen peroxide (H2O2) induces apoptosis, profoundly reducing the numbers of EPCs.82 There is increasing evidence that oxidative stress reduces and impairs EPC functioning.76 Thum et al78 found that increased production of ROS was associated with reduced EPC levels and impaired EPCs function. Conditions associated with increased oxidative stress lead to the mobilization of functionally defective EPCs, which have a lesser capability to mobilize, migrate, and incorporate into existing vasculature.83 Therefore, it is clear that conditions associated with increased oxidative stress not only decrease the absolute numbers of circulating EPCs but also impair EPC function, with deleterious effects on vascular homeostasis.76 It is still unclear whether a direct association exists between ROS and functional bioactivity of EPCs.
Effects of IH/oxidative stress on EPCs by NO unavailability
IH in OSA reduces endothelial NO production directly. L-arginine is the substrate for NO production by endothelial nitric oxide synthase (eNOS). Biosynthesis of NO from L-arginine is an oxygen-dependent process, and hypoxia might influence NO formation in vascular beds directly.84 Hypoxia also can increase arginase II activity in endothelial cells, which degrades L-arginine. The plasma levels of L-arginine increase after a single night of CPAP therapy in patients with OSA.85 Meanwhile, increasing oxidative stress caused by repetitive episodes of hypoxia/reoxygenation reduces endothelial NO production at the transcriptional and posttranscriptional levels indirectly.86 Increased oxidative stress reduces and destabilizes eNOS messenger RNA (mRNA), in part via the Rho kinase pathway, in human venous and pulmonary artery endothelial cells82 and reduces endothelial NO production at the transcriptional level.87 Prolonged oxidative stress such as that observed in untreated OSA reduces eNOS enzymatic activity by suppressing eNOS phosphorylation.88 Tetrahydro-biopterin is a cofactor critical for NO production by eNOS.89 Increased oxidative stress limits the availability of cofactors required for NO production. When this cofactor is depleted in conditions of increased oxidative stress, eNOS, a main source of basal endothelial NO production, preferentially promotes superoxide production, which hastens NO degradation and thereby reduces NO availability.90
Mobilization of EPC from the bone marrow entails adequate NO production. Impaired recruitment of EPCs from the bone marrow is likely to be related to depressed NO production and activity in patients with OSA.91 eNOS is essential for mobilization of EPCs.91 Mice deficient in eNOS (Nos3−/−) show reduced VEGF-induced EPC mobilization. Interestingly, mice deficient in eNOS (Nos3−/−) also have reduced basal expression and activity of matrix metalloproteinase 9 (MMP-9). MMP-9 is a major target for NO, which activates MMP-9 by S-nitrosylation.92 MMP-9 is required for stem cell mobilization.93 VEGF-induced mobilization of EPCs in mice is not observed in mice deficient in MMP-9. In addition, mobilization of EPCs in response to VEGF administration is significantly inhibited by coadministration of a synthetic MMP inhibitor.94 Thus, MMP-9 activation is a decisive checkpoint for recruitment of EPCs.94 MMP-9 appears to be essential for the regulation of EPCs in response to various stimuli, but it must be activated by NO. MMP-9 degrades the extracellular matrix and transforms membrane-bounded Kit ligand (KitL, also known as stem cell factor) to soluble Kit ligand, triggering subsequent movement of cKit-positive stem cells, including EPCs, to the circulation.94–96
In addition to VEGF, oxidative stress also upregulates transcription of other angiogenic factors, such as stroma-derived factor-1 (SDF-1) or erythropoietin (EPO), in EPCs.97
Effects of increased sympathetic activation on EPCs in OSA
IH98 and sleep fragmentation54 lead to increased sympathetic activity, which stimulates RAAS axis, resulting in elevated Ang II and aldosterone in OSA. Untreated OSA is associated with an upregulation of the RAAS. Ang II derived from leukocytes, especially peripheral blood mononuclear cells, is significantly increased in patients with OSA compared with control patients.99 There is ample evidence that Ang II is involved in endothelial damage and atherogenesis via multiple mechanisms.100,101 Some of the harmful consequences of Ang II can be mediated by impairment of EPCs. A thorough analysis of Ang II-induced effects in EPCs regulation and function, and especially involved molecular mechanisms, has not been undertaken.
Effects of Ang II on EPCs by excessive generation of ROS
Accumulated evidence has shown that Ang II is implicated in a wide variety of pathologies of cardiovascular diseases.102,103 Prominent evidence among those featuring pathologies mediated by Ang II is the excessive generation of ROS.26,104 Ang II was shown to be a potent stimulus for ROS generation.
On one hand, as mentioned earlier, because ROS is thought to play an important role in the decrease in NO bioavailability, accumulation of ROS, especially resulting from the RAAS, leads to inhibition of the mobilization of EPCs from bone marrow. On the other hand, accumulation of ROS, especially resulting from the RAAS, also affects senescence and/or apoptosis of EPCs. Ang II was shown to induce the senescence of EPCs.105 Endtmann et al106 demonstrated that Ang II, through the angiotensin 1 receptor, induces oxidative stress (or ROS) and then activates the redox-sensitive apoptosis signal regulating kinase 1 (ASK-1)–dependent proapoptotic signaling pathways in early-outgrowth EPCs. Ang II enhances phosphorylation of ASK-1, activates c-Jun N-terminal kinase and p38-mitogen-activated protein kinase, and then decreases expression of antiapoptotic Bcl-2 and increases expression of proapoptotic Bax, leading to activation of caspase 3 and apoptosis of EPCs. ASK-1 is a member of the mitogen-activated protein kinase family, which activates both c-Jun N-terminal kinase and p38-mitogen-activated protein kinase pathways.107 p38-mitogen-activated protein kinase inhibition in vitro and in vivo improves the number and functional capacities of bone marrow–derived EPCs, which is associated with reduced atherosclerosis in atherosclerotic mice.108 ASK-1 constitutes a pivotal signaling pathway in stress-induced apoptosis, especially in the context of oxidative stress.109 Ang II-induced activation of ASK-1 and caspase 3, resulting in apoptosis, is mediated through the induction of oxidative stress, because both effects are inhibited by coincubation with an antioxidant.106
Effects of Ang II on EPCs by VEGF
A prominent physiological adaptive response of tissue to hypoxia, such as the IH condition under OSA, is angiogenesis, the formation of new blood vessels and increasing the blood supply.110 VEGF promotes hypoxia-induced angiogenesis in vitro and in vivo, which has been shown to be upregulated by Ang II.111 On one hand, Ang II induces expression of VEGF. Numerous reports have shown that VEGF expression is significantly increased in the plasma of patients with OSA99,112 and is induced by Ang II in peripheral blood EPCs.99 On the other hand, Ang II stimulates VEGFR2 mRNA and protein expression in human EPCs, resulting in enhanced VEGF-induced proliferation of EPCs and vascular network formation in a Matrigel assay.113
Effects of Ang II on EPCs by MMP-9
Ang II may induce the expression of the inflammatory cyclooxygenase 2 gene and influence the extracellular matrix turnover by regulating the activity of prostaglan-din E2-dependent metalloproteinase in vascular cells.114 Recently, Tazaki et al demonstrated that serum MMP-9 is increased in patients with OSA when compared with normal participants.115 They speculated that elevated serum MMP-9 might induce vascular events in patients with OSA. MMP-9 is essential for homing and differentiation of EPCs on endothelial sites where they are required.116 VEGF-induced mobilization of EPCs in mice is significantly inhibited by coadministration of a synthetic metalloproteinase inhibitor. Mobilization of EPCs in response to VEGF administration was not observed in mice deficient in MMP-9. NO appears to be essential for regulation of EPCs in response to various stimuli, but this process depends on the activation of MMP-9. Thus, MMP-9 activation is a decisive checkpoint for the recruitment of EPCs.94
In addition, EPCs have been shown to express the Ang II type 1 receptor, suggesting that direct effects of Ang II on EPCs are possible.117
Effects of HIF-1 signaling axis on EPCs
In patients with OSA, oxygen saturation may repeatedly decrease during the apneic events. A prominent physiological adaptive response of tissue to hypoxia is neovascularization and increasing the blood supply.110 Under hypoxic conditions, transcription factors such as hypoxia inducible factor 1 (HIF-1) are activated, leading to increased transcription of proangiogenic proteins including VEGF, SDF-1, and EPO,118 which mobilize EPCs, and finally, contributes to neovascularization.119
At the mRNA level, the HIF-1 gene is constitutively expressed and not significantly upregulated by hypoxia. At the transcriptional level, however, hypoxia markedly increases the levels of HIF-1 protein.120 Genes encoding vascular VEGF, SDF-1, and EPO are all under the control of HIF-1.121 There is an HIF-1 binding site in the SDF-1 gene, in the promoter of the VEGF gene, and in the enhancer of the EPO gene. All of these genes are induced by IH, both in vivo and in vitro.122
Effects of VEGF on EPCs
Numerous reports have shown that plasma levels of VEGF are elevated in patients with OSA.41,99,111,123 VEGF expression is markedly increased in patients with OSA, largely because of the effects of HIF-1 on VEGF transcription.124 Apart from HIF-1 stimulation, VEGF expression is stimulated by Ang II in peripheral blood mononuclear cells.99,111 Furthermore, a study indicated that the vascular endogenous EPO/EPO receptor system also plays an important role for upregulation of the VEGF/VEGF receptor system.
The role of VEGF in EPC mobilization has been widely studied in both mice and humans, showing that, after acute ischemic injury, plasma levels of VEGF increase rapidly, leading to a 50-fold increase in EPC percentage in peripheral blood.28 In animal models, exogenous administration of VEGF promotes mobilization of EPCs into the peripheral circulation.125 Treatment with VEGF was reported to double the number of circulating EPCs in humans.126 Gene transfer of VEGF into ischemic tissue increases circulating EPCs to levels more than two times higher than the baseline level.
However, a study reported an increase in plasma VEGF levels and a reduction in circulating EPCs in patients with OSA without any known cardiovascular risk factors compared with healthy participants of a similar age and BMI.41 Plasma levels of VEGF are elevated in patients with OSA, and VEGF could promote mobilization of EPCs into the peripheral circulation, but levels of circulating EPCs in patients with OSA are reduced. Possible explanations include that VEGF activation may constitute an adaptive mechanism to the repetition of nocturnal hypoxic events, which may potentially contribute to counterbalancing the occurrence of OSA-related cardiovascular disease. Increased VEGF concentrations in OSA may reflect a physiological effort to mobilize EPCs in these patients and can represent an early event in the natural history of the disease.41
Both experimental and clinical studies have demonstrated that VEGF significantly affects the kinetics of EPCs.125,127 VEGF stimulates VEGFR1 and VEGFR2 present on EPCs and activates MMP-9, which is essential for the homing and differentiation of EPCs.128 VEGF has been shown to strongly induce Akt phosphorylation in endothelial cells. Akt is a serine threonine protein kinase that is activated by a number of growth factors and cytokines in a phosphatidylinositol 3 kinase (PI3 K)-dependent manner. Importantly, the PI3 K/Akt pathway plays a significant role in mediating VEGF biological activity. Dimmeler et al have shown that VEGF induces EPC differentiation via the PI3 K/Akt pathway.129
Effects of SDF-1 on EPCs
Several factors have been shown to influence EPC mobilization and homing to hypoxic tissue, including chemok-ines,35 angiogenic cytokines, and pharmacologic agents. SDF-1 is one such chemokine that is considered to play an important role in EPC homing and recruitment for hypoxic neovascularization.130 SDF-1 is a chemokine of the cysteine-X-cysteine (CXC) family that binds to the chemokine receptor, cysteine-X-cysteine chemokine receptor (CXCR) 4, on target cells, which is produced within the bone marrow. The SDF-1/CXCR4 interaction is another important pathway in the mobilization of EPCs from the bone marrow. Studies have indicated that SDF-1 and its CXCR4 play a critical role in progenitor cell homing, mobilization, and differentiation.131,132 SDF-1 is capable of enhancing the recruitment and mobilization of EPCs to damaged endothelium during postnatal vasculogenesis.133 Overexpression of SDF-1 in ischemic tissues has been found to enhance EPC recruitment from peripheral blood and to induce neoangiogenesis in ischemic tissues.134 The number of circulating EPCs can be increased by SDF-1 gene transfer, using the adenovirus infection technique.94,124 Recent evidence also suggests that SDF-1 is a driving force for EPCs differentiation.132
One study reported that plasma levels of SDF-1 are positively associated with EPC number and function in response to acute ischemic events, suggesting a role of SDF-1 in EPC mobilization and differentiation in humans.135 However, data from another study showed that SDF-1 levels are inversely, rather than positively, associated with circulating EPC numbers.136 One of the possible explanations for these controversial results is that the relationship of SDF-1 with EPC homing, mobilization, and differentiation in the acute phase is different from that in a normal situation. Studies of mouse ischemia models showed that the number of EPCs in peripheral blood was lower but the level of SDF-1 was much higher at 14 days after ischemia compared with control mice,132 suggesting that EPCs are mobilized into peripheral blood from bone marrow after the onset of ischemia, but at a later stage, the numbers of mobilized EPCs in peripheral blood decrease because of their homing to the ischemic site.
Effects of EPO on EPCs
Plasma EPO increases exponentially with the degree of hypoxia in humans. Imagawa demonstrated that serum levels in patients with OSA were approximately 2-fold higher than those in normal patients.137
Of note, EPO serum levels correlate with the number and function of EPCs isolated from both bone marrow and peripheral blood, suggesting that EPO may regulate EPCs in vivo. Administration of exogenous EPO induces mobilization and proliferation of EPCs. In contrast, both VEGF concentrations and recruitment of EPCs ischemic muscle are significantly enhanced in wild-type mice but are significantly impaired in mice that lack the EPO receptor system. These results further suggest that EPO may be important for VEGF secretion, EPC mobilization, and angiogenesis in vivo.138 The authors indicate that the vascular endogenous EPO/EPO receptor system also plays an important role in angiogenesis in response to hind-limb ischemia through upregulation of VEGF/VEGF receptor system by recruiting EPCs.138
Effects of continuous CPAP therapy on EPCs
CPAP continues to be the standard, primary, and first-line therapy for patients with OSA.139 CPAP consists of an air pressure source that keeps a constant positive pressure in the airway through the respiratory cycle. The airflow is delivered through a nasal or oronasal interface and maintains patency of the upper airway. CPAP has been found to be highly efficacious, reducing OSA symptoms, including daytime sleepiness, sympathetic neural activation, and blood pressure, improves cognitive function and quality of life,140–142 and promoting mobilization of EPCs.42,43 However, there is a distinction between efficacy and effectiveness. Efficacy is the effect in the laboratory or under ideal circumstances, regardless of treatment adherence. CPAP demonstrates very good efficacy. Effectiveness is the effect in daily life, which depends on patient compliance with CPAP therapy.143
CPAP adherence is defined as more than 4 hours per night of “mask-on” time, based on data that suggest that more than 4–5 hours of CPAP usage per night results in improvement in Epworth Sleepiness Scale scores.144,145 Adequate adherence to CPAP is essential for achievement of the benefits of CPAP treatment. Observational studies have reported that patients with OSA who refused or did not adhere to CPAP therapy experienced higher rates of myocardial infarction, stroke, and death compared with CPAP adherents.146–149 In another study, CPAP produced a modest reduction in blood pressure in patients with hypertension and OSA, but continued use of CPAP for 5.3 hours per day or longer could cause significant reductions in blood pressure for patients with incompletely controlled hypertension. Stepnowsky and Dimsdale demonstrated that higher rates of compliance (ie, >4 hours of usage per night) resulted in an improvement in the respiratory disturbance index, oxygen desaturation index, and arousal index.150 Studies by Jelic et al42,43 reported that baseline EPC levels were lower in patients with OSA than in control patients. CPAP therapy increased EPC levels to those of control patients when patients adhered to CPAP for more than 4 hours daily. EPC levels remained unchanged when patients used CPAP for less than 4 hours daily or declined CPAP altogether. Although the mechanisms of how CPAP affects mobilization of EPCs are still unclear, the possible mechanisms may be through preventing hypoxia/reoxygenation or IH episodes, improving sleep quality, reducing oxidative stress levels, systemic inflammation,151–154 and excessive sympathetic activation,155 which have been implicated for impaired EPCs with reduced mobilization, increased cell apoptosis, and damaged EPC function.
In fact, although CPAP provides effective treatment for OSA, patient adherence remains challenging. CPAP adherence was low. In one study, when individuals without follow-up were assumed to be nonadherent, the overall adherence rate was only 30.4%.156 Another two retrospective surveys showed that the percentages of patients with good CPAP adherence were 56.8%157 and 54.3%,158 respectively. As reported in studies, male sex,159 higher levels of education,160 smoking,161 nocturia,161 benign prostatic hypertrophy,161 and depressive symptomatology159 were predictors of poor CPAP adherence, and increasing age,162–164 higher incomes, higher AHI values,159 and initial educational program were predictors of good CPAP adherence. Somers et al159 found that increased length of time from the initial visit to receiving the CPAP machine was associated with poorer compliance. Therefore, efforts should be made to try to minimize the length of time between the initial visit and receiving CPAP treatment to improve compliance. Wang et al165 found that only half of the patients having initial CPAP titration trial remained adherent to the CPAP treatment, and the other half of the patients either never initiated the CPAP treatment or had abandoned CPAP treatment. Wolkove et al166 similarly found that 31% of patients do not commence treatment after polysomnography (PSG) diagnosis and CPAP trial. To improve CPAP adherence, the medical staff should pay attention to making the titration trial a comfortable first CPAP experience for the patients, such as using the most appropriate ventilators or proper humidification. It is also important to give the patients education and support about CPAP use in the follow-up, especially in the early stages of the treatment.
Conclusion
We conclude that IH cycle and sleep fragmentation are major pathophysiologic characters of OSA. IH acts as a trigger of oxidative stress, systemic inflammation, and sympathetic activation. Sleep fragmentation is associated with a burst of sympathetic activation and systemic inflammation. EPCs have been reported as decreased, increased, or unchanged. However, in most studies, a reduction in circulating EPCs has emerged and EPC functions are damaged. The possible mechanisms underlying the decrease in number or function of EPCs are that prolonged inflammation response, oxidative stress, increased sympathetic activation, and physiological adaptive response of tissue to hypoxia are implicated with reduced EPC mobilization, increased cell apoptosis, and functional impairment in untreated OSA. CPAP therapy for OSA affects EPCs through preventing IH episodes, improving sleep quality, reducing systemic inflammation, oxidative stress levels, and excess sympathetic activation.
Acknowledgments
This study was supported by the grants from the National Natural Science Foundation of China (No 81270144, 30800507, 81170071).
Footnotes
Disclosure
The authors report no conflicts of interest in this work
References
- 1.Feng J, Chen BY. Prevalence and incidence of hypertension in obstructive sleep apnea patients and the relationship between obstructive sleep apnea and its confounders. Chin Med J (Engl) 2009;122(12):1464–1468. [PubMed] [Google Scholar]
- 2.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 3.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc 15. 2008;5(2):242–252. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng J, Wu Q, Zhang D, Chen BY. Hippocampal impairments are associated with intermittent hypoxia of obstructive sleep apnea. Chin Med J (Engl) 2012;125(4):696–701. [PubMed] [Google Scholar]
- 5.Lavie L. Oxidative stress – a unifying paradigm in obstructive sleep apnea and comorbidities. Prog Cardiovasc Dis. 2009;51(4):303–312. doi: 10.1016/j.pcad.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Lévy P, Bonsignore MR, Eckel J. Sleep, sleep-disordered breathing and metabolic consequences. Eur Respir J. 2009;34(1):243–260. doi: 10.1183/09031936.00166808. [DOI] [PubMed] [Google Scholar]
- 7.Sateia MJ. Neuropsychological impairment and quality of life in obstructive sleep apnea. Clin Chest Med. 2003;24(2):249–259. doi: 10.1016/s0272-5231(03)00014-5. [DOI] [PubMed] [Google Scholar]
- 8.Carotenuto M, Santoro N, Grandone A, et al. The insulin gene variable number of tandemrepeats (INS VNTR) genotype and sleep disordered breathing in childhood obesity. J Endocrinol Invest. 2009;32(9):752–755. doi: 10.1007/BF03346531. [DOI] [PubMed] [Google Scholar]
- 9.Carotenuto M, Bruni O, Santoro N, Del Giudice EM, Perrone L, Pascotto A. Waist circumference predicts the occurrence of sleep-disordered breathing in obese children and adolescents: a questionnaire-based study. Sleep Med. 2006;7(4):357–361. doi: 10.1016/j.sleep.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Carotenuto M, Esposito M, Parisi L, et al. Depressive symptoms and childhood sleep apnea syndrome. Neuropsychiatr Dis Treat. 2012:369–373. doi: 10.2147/NDT.S35974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daulatzai MA. Death by a thousand cuts in Alzheimer’s disease: hypoxia – the prodrome. Neurotox Res. 2013;24(2):216–243. doi: 10.1007/s12640-013-9379-2. [DOI] [PubMed] [Google Scholar]
- 12.Carotenuto M, Esposito M, Pascotto A. Facial patterns and primary nocturnal enuresis in children. Sleep Breath. 2011;15(2):221–227. doi: 10.1007/s11325-010-0388-6. [DOI] [PubMed] [Google Scholar]
- 13.Staats R, Stoll P, Zingler D, Virchow JC, Lommatzsch M. Regulation of brain-derived neurotrophic factor (BDNF) during sleep apnoea treatment. Thorax. 2005;60(8):688–692. doi: 10.1136/thx.2004.038208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 15.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 16.Amra B, Karbasi E, Hashemi M, Hoffmann-Castendiek B, Golshan M. Endothelial dysfunction in patients with obstructive sleep apnoea independent of metabolic syndrome. Ann Acad Med Singapore. 2009;38(5):461–464. [PubMed] [Google Scholar]
- 17.Bayram NA, Ciftci B, Keles T, et al. Endothelial function in normotensive men with obstructive sleep apnea before and 6 months after CPAP treatment. Sleep. 2009;32(10):1257–1263. doi: 10.1093/sleep/32.10.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin K, Stanchina M, Kouttab N, Harrington EO, Rounds S. Circulating endothelial cells and endothelial progenitor cells in obstructive sleep apnea. Lung. 2008;186(3):145–150. doi: 10.1007/s00408-008-9073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trzepizur W, Gagnadoux F, Abraham P, et al. Microvascular endothelial function in obstructive sleep apnea: Impact of continuous positive airway pressure and mandibular advancement. Sleep Med. 2009;10(7):746–752. doi: 10.1016/j.sleep.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Drager LF, Bortolotto LA, Figueiredo AC, Silva BC, Krieger EM, Lorenzi-Filho G. Obstructive sleep apnea, hypertension, and their interaction on arterial stiffness and heart remodeling. Chest. 2007;131(5):1379–1386. doi: 10.1378/chest.06-2703. [DOI] [PubMed] [Google Scholar]
- 21.Yim-Yeh S, Rahangdale S, Nguyen AT, et al. Vascular dysfunction in obstructive sleep apnea and type 2 diabetes mellitus. Obesity (Silver Spring) 2011;19(1):17–22. doi: 10.1038/oby.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi GF. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176(7):706–712. doi: 10.1164/rccm.200703-500OC. [DOI] [PubMed] [Google Scholar]
- 23.Dimmeler S, Zeiher AM. Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis? J Mol Med (Berl) 2004;82(10):671–677. doi: 10.1007/s00109-004-0580-x. [DOI] [PubMed] [Google Scholar]
- 24.Carmeliet P, Moons L, Stassen JM, et al. Vascular wound healing and neointima formation induced by perivascular electric injury in mice. Am J Pathol. 1997;150(2):761–776. [PMC free article] [PubMed] [Google Scholar]
- 25.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 26.Yao EH, Yu Y, Fukuda N. Oxidative stress on progenitor and stem cells in cardiovascular diseases. Curr Pharm Biotechnol. 2006;7(2):101–108. doi: 10.2174/138920106776597685. [DOI] [PubMed] [Google Scholar]
- 27.Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Transplanted adult bone marrow cells repair myocardial infarcts in mice. Ann N Y Acad Sci. 2001;938:221–229. doi: 10.1111/j.1749-6632.2001.tb03592.x. discussion 229–230. [DOI] [PubMed] [Google Scholar]
- 28.Gill M, Dias S, Hattori K, et al. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res. 2001;88(2):167–174. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- 29.Hung HS, Shyu WC, Tsai CH, Hsu SH, Lin SZ. Transplantation of endothelial progenitor cells as therapeutics for cardiovascular diseases. Cell Transplant. 2001;8(9):1003–1012. doi: 10.3727/096368909X12483162196683. [DOI] [PubMed] [Google Scholar]
- 30.Sahoo S, Klychko E, Thorne T, et al. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res. 2011;109(7):724–728. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheubel RJ, Holtz J, Friedrich I, et al. Paracrine effects of CD34 progenitor cells on angiogenic endothelial sprouting. Int J Cardiol. 2010;139(2):134–141. doi: 10.1016/j.ijcard.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Morishita T, Uzui H, Nakano A, et al. Number of endothelial progenitor cells in peripheral artery disease as a marker of severity and association with pentraxin-3, malondialdehyde-modified low-density lipoprotein and membrane type-1 matrix metalloproteinase. J Atheroscler Thromb. 2012;19(2):149–158. doi: 10.5551/jat.10074. [DOI] [PubMed] [Google Scholar]
- 33.Sieveking DP, Buckle A, Celermajer DS, Ng MK. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: insights from a novel human angiogenesis assay. J Am Coll Cardiol. 2008;51(6):660–668. doi: 10.1016/j.jacc.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 34.Zammaretti P, Zisch AH. Adult ‘endothelial progenitor cells’. Renewing vasculature. Int J Biochem Cell Biol. 2005;37(3):493–503. doi: 10.1016/j.biocel.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 35.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95(4):343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 36.Fadini GP, Baesso I, Albiero M, Sartore S, Agostini C, Avogaro A. Technical notes on endothelial progenitor cells: ways to escape from the knowledge plateau. Atherosclerosis. 2008;197(2):496–503. doi: 10.1016/j.atherosclerosis.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 37.Quirici N, Soligo D, Caneva L, Servida F, Bossolasco P, Deliliers GL. Differentiation and expansion of endothelial cells from human bone marrow CD133(+) cells. Br J Haematol. 2001;115(1):186–194. doi: 10.1046/j.1365-2141.2001.03077.x. [DOI] [PubMed] [Google Scholar]
- 38.Masuda H, Kalka C, Takahashi T, et al. Estrogen-mediated endothelial progenitor cell biology and kinetics for physiological postnatal vasculogenesis. Circ Res. 2007;101(6):598–606. doi: 10.1161/CIRCRESAHA.106.144006. [DOI] [PubMed] [Google Scholar]
- 39.Fadini GP, de Kreutzenberg SV, Coracina A, et al. Circulating CD34+ cells, metabolic syndrome, and cardiovascular risk. Eur Heart J. 2006;27(18):2247–2255. doi: 10.1093/eurheartj/ehl198. [DOI] [PubMed] [Google Scholar]
- 40.Kondo T, Hayashi M, Takeshita K, et al. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol. 2004;24(8):1442–1447. doi: 10.1161/01.ATV.0000135655.52088.c5. [DOI] [PubMed] [Google Scholar]
- 41.de la Peña M, Barceló A, Barbe F, et al. Endothelial function and circulating endothelial progenitor cells in patients with sleep apnea syndrome. Respiration. 2008;76(1):28–32. doi: 10.1159/000109643. [DOI] [PubMed] [Google Scholar]
- 42.Jelic S, Padeletti M, Kawut SM, et al. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation. 2008;117(17):2270–2278. doi: 10.1161/CIRCULATIONAHA.107.741512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jelic S, Lederer DJ, Adams T, et al. Endothelial repair capacity and apoptosis are inversely related in obstructive sleep apnea. Vasc Health Risk Manag. 2009;5:909–920. doi: 10.2147/vhrm.s8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murri M, García-Delgado R, Alcázar-Ramírez J, et al. Effect of CPAP on oxidative stress and circulating progenitor cell levels in sleep patients with apnea-hypopnea syndrome. Respir Care. 2011;56(11):1830–1836. doi: 10.4187/respcare.01081. [DOI] [PubMed] [Google Scholar]
- 45.Kheirandish-Gozal L, Bhattacharjee R, Kim J, Clair HB, Gozal D. Endothelial progenitor cells and vascular dysfunction in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2010;182(1):92–97. doi: 10.1164/rccm.200912-1845OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kizawa T, Nakamura Y, Takahashi S, Sakurai S, Yamauchi K, Inoue H. Pathogenic role of angiotensin II and oxidised LDL in obstructive sleep apnoea. Eur Respir J. 2009;34(6):1390–1398. doi: 10.1183/09031936.00009709. [DOI] [PubMed] [Google Scholar]
- 47.Yun CH, Jung KH, Chu K, et al. Increased circulating endothelial microparticles and carotid atherosclerosis in obstructive sleep apnea. J Clin Neurol. 2010;6(2):89–98. doi: 10.3988/jcn.2010.6.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348(7):593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 49.Fadini GP, de Kreutzenberg S, Albiero M, et al. Gender differences in endothelial progenitor cells and cardiovascular risk profile: the role of female estrogens. Arterioscler Thromb Vasc Biol. 2008;28(5):997–1004. doi: 10.1161/ATVBAHA.107.159558. [DOI] [PubMed] [Google Scholar]
- 50.Robb AO, Mills NL, Smith IB, et al. Influence of menstrual cycle on circulating endothelial progenitor cells. Hum Reprod. 2009;24(3):619–625. doi: 10.1093/humrep/den411. [DOI] [PubMed] [Google Scholar]
- 51.Rafii S. Circulating endothelial precursors: mystery, reality, and promise. J Clin Invest. 2000;105(1):17–19. doi: 10.1172/JCI8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stanchina ML, Malhotra A, Fogel RB, et al. The influence of lung volume on pharyngeal mechanics, collapsibility, and genioglossus muscle activation during sleep. Sleep. 2003;26(7):851–856. doi: 10.1093/sleep/26.7.851. [DOI] [PubMed] [Google Scholar]
- 53.White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005;172(11):1363–1370. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]
- 54.Blasi A, Jo JA, Valladares E, Juarez R, Baydur A, Khoo MC. Autonomic cardiovascular control following transient arousal from sleep: a time-varying closed-loop model. IEEE Trans Biomed Eng. 2006;53(1):74–82. doi: 10.1109/TBME.2005.859789. [DOI] [PubMed] [Google Scholar]
- 55.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43(4):678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 56.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166(16):1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 57.Lavie L. Obstructive sleep apnoea syndrome – an oxidative stress disorder. Sleep Med Rev. 2003;7(1):35–51. doi: 10.1053/smrv.2002.0261. [DOI] [PubMed] [Google Scholar]
- 58.Hücking K, Hamilton-Wessler M, Ellmerer M, Bergman RN. Burst-like control of lipolysis by the sympathetic nervous system in vivo. J Clin Invest. 2003;111(2):257–264. doi: 10.1172/JCI14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen MT, Satoh H, Favelyukis S, et al. JNK and tumor necrosis factor-alpha mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. J Biol Chem. 2005;280(42):35361–35371. doi: 10.1074/jbc.M504611200. [DOI] [PubMed] [Google Scholar]
- 60.Ryan S, Taylor CT, McNicholas WT. Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2006;174(7):824–830. doi: 10.1164/rccm.200601-066OC. [DOI] [PubMed] [Google Scholar]
- 61.Kobayashi K, Nishimura Y, Shimada T, et al. Effect of continuous positive airway pressure on soluble CD40 ligand in patients with obstructive sleep apnea syndrome. Chest. 2006;129(3):632–637. doi: 10.1378/chest.129.3.632. [DOI] [PubMed] [Google Scholar]
- 62.Tauman R, O’Brien LM, Gozal D. Hypoxemia and obesity modulate plasma C-reactive protein and interleukin-6 levels in sleep-disordered breathing. Sleep Breath. 2007;11(2):77–84. doi: 10.1007/s11325-006-0085-7. [DOI] [PubMed] [Google Scholar]
- 63.Gozal D, Crabtree VM, Sans Capdevila O, Witcher LA, Kheirandish-Gozal L. C-reactive protein, obstructive sleep apnea, and cognitive dysfunction in school-aged children. Am J Respir Crit Care Med. 2007;176(2):188–193. doi: 10.1164/rccm.200610-1519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khalyfa A, Capdevila OS, Buazza MO, Serpero LD, Kheirandish-Gozal L, Gozal D. Genome-wide gene expression profiling in children with non-obese obstructive sleep apnea. Sleep Med. 2009;10(1):75–86. doi: 10.1016/j.sleep.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 65.Li AM, Chan MH, Yin J, et al. C-reactive protein in children with obstructive sleep apnea and the effects of treatment. Pediatr Pulmonol. 2008;43(1):34–40. doi: 10.1002/ppul.20732. [DOI] [PubMed] [Google Scholar]
- 66.Sahlman J, Miettinen K, Peuhkurinen K, et al. Kuopio Sleep Apnoea Group The activation of the inflammatory cytokines in overweight patients with mild obstructive sleep apnoea. J Sleep Res. 2010;19(2):341–348. doi: 10.1111/j.1365-2869.2009.00787.x. [DOI] [PubMed] [Google Scholar]
- 67.Yamauchi M, Tamaki S, Tomoda K, et al. Evidence for activation of nuclear factor kappaB in obstructive sleep apnea. Sleep Breath. 2006;10(4):189–193. doi: 10.1007/s11325-006-0074-x. [DOI] [PubMed] [Google Scholar]
- 68.Minoguchi K, Yokoe T, Tanaka A, et al. Association between lipid per-oxidation and inflammation in obstructive sleep apnoea. Eur Respir J. 2006;28(2):378–385. doi: 10.1183/09031936.06.00084905. [DOI] [PubMed] [Google Scholar]
- 69.Werner N, Nickenig G. Influence of cardiovascular risk factors on endothelial progenitor cells: limitations for therapy? Arterioscler Thromb Vasc Biol. 2006;26(2):257–266. doi: 10.1161/01.ATV.0000198239.41189.5d. [DOI] [PubMed] [Google Scholar]
- 70.Andreou I, Tousoulis D, Tentolouris C, Antoniades C, Stefanadis C. Potential role of endothelial progenitor cells in the pathophysiology of heart failure: clinical implications and perspectives. Atherosclerosis. 2006;189(2):247–254. doi: 10.1016/j.atherosclerosis.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 71.George J, Goldstein E, Abashidze S, et al. Circulating endothelial progenitor cells in patients with unstable angina: association with systemic inflammation. Eur Heart J. 2004;25(12):1003–1008. doi: 10.1016/j.ehj.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 72.Chen YH, Lin SJ, Lin FY, et al. High glucose impairs early and late endothelial progenitor cells by modifying nitric oxide-related but not oxidative stress-mediated mechanisms. Diabetes. 2007;56(6):1559–1568. doi: 10.2337/db06-1103. [DOI] [PubMed] [Google Scholar]
- 73.Krishnamurthy P, Thal M, Verma S, et al. Interleukin-10 deficiency impairs bone marrow-derived endothelial progenitor cell survival and function in ischemic myocardium. Circ Res. 2011;109(11):1280–1289. doi: 10.1161/CIRCRESAHA.111.248369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sugawara J, Mitsui-Saito M, Hayashi C, et al. Decrease and senescence of endothelial progenitor cells in patients with preeclampsia. J Clin Endocrinol Metab. 2005;90(9):5329–5332. doi: 10.1210/jc.2005-0532. [DOI] [PubMed] [Google Scholar]
- 75.Suh W, Kim KL, Choi JH, et al. C-reactive protein impairs angiogenic functions and decreases the secretion of arteriogenic chemo-cytokines in human endothelial progenitor cells. Biochem Biophys Res Commun. 2004;321(1):65–71. doi: 10.1016/j.bbrc.2004.06.107. [DOI] [PubMed] [Google Scholar]
- 76.Tousoulis D, Andreou I, Antoniades C, Tentolouris C, Stefanadis C. Role of inflammation and oxidative stress in endothelial progenitor cell function and mobilization: therapeutic implications for cardiovascular diseases. Atherosclerosis. 2008;201(2):236–247. doi: 10.1016/j.atherosclerosis.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 77.Schulz R, Mahmoudi S, Hattar K, et al. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med. 2000;162(2 Pt 1):566–570. doi: 10.1164/ajrccm.162.2.9908091. [DOI] [PubMed] [Google Scholar]
- 78.Thum T, Fraccarollo D, Schultheiss M, et al. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes. 2007;56(3):666–674. doi: 10.2337/db06-0699. [DOI] [PubMed] [Google Scholar]
- 79.Thum T, Fraccarollo D, Galuppo P, et al. Bone marrow molecular alterations after myocardial infarction: Impact on endothelial progenitor cells. Cardiovasc Res. 2006;70(1):50–60. doi: 10.1016/j.cardiores.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 80.Watson T, Goon PK, Lip GY. Endothelial progenitor cells, endothelial dysfunction, inflammation, and oxidative stress in hypertension. Antioxid Redox Signal. 2008;10(6):1079–1088. doi: 10.1089/ars.2007.1998. [DOI] [PubMed] [Google Scholar]
- 81.Hung YC, Sava VM, Blagodarsky VA, Hong MY, Huang GS. Protection of tea melanin on hydrazine-induced liver injury. Life Sci. 2003;72(9):1061–1071. doi: 10.1016/s0024-3205(02)02348-2. [DOI] [PubMed] [Google Scholar]
- 82.Urbich C, Knau A, Fichtlscherer S, et al. FOXO-dependent expression of the proapoptotic protein Bim: pivotal role for apoptosis signaling in endothelial progenitor cells. FASEB J. 2005;19(8):974–976. doi: 10.1096/fj.04-2727fje. [DOI] [PubMed] [Google Scholar]
- 83.Tepper OM, Galiano RD, Capla JM, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106(22):2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 84.Leeman M, de Beyl VZ, Biarent D, Maggiorini M, Mélot C, Naeije R. Inhibition of cyclooxygenase and nitric oxide synthase in hypoxic vasoconstriction and oleic acid-induced lung injury. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1383–1390. doi: 10.1164/ajrccm.159.5.9807114. [DOI] [PubMed] [Google Scholar]
- 85.Lavie L, Hefetz A, Luboshitzky R, Lavie P. Plasma levels of nitric oxide and L-arginine in sleep apnea patients: effects of nCPAP treatment. J Mol Neurosci. 2002;1(1):57–63. doi: 10.1385/JMN:21:1:57. [DOI] [PubMed] [Google Scholar]
- 86.Liao JK, Zulueta JJ, Yu FS, Peng HB, Cote CG, Hassoun PM. Regulation of bovine endothelial constitutive nitric oxide synthase by oxygen. J Clin Invest. 1995;96(6):2661–2666. doi: 10.1172/JCI118332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Antoniades C, Shirodaria C, Warrick N, et al. 5-methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation. 2006;114(11):1193–1201. doi: 10.1161/CIRCULATIONAHA.106.612325. [DOI] [PubMed] [Google Scholar]
- 88.Tanaka T, Nakamura H, Yodoi J, Bloom ET. Redox regulation of the signaling pathways leading to eNOS phosphorylation. Free Radic Biol Med. 2005;38(9):1231–1242. doi: 10.1016/j.freeradbiomed.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 89.Channon KM. Tetrahydrobiopterin: regulator of endothelial nitric oxide synthase in vascular disease. Trends Cardiovasc Med. 2004;14(8):323–327. doi: 10.1016/j.tcm.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 90.Laursen JB, Somers M, Kurz S, et al. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103(9):1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 91.Aicher A, Heeschen C, Mildner-Rihm C, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9(11):1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 92.Gu Z, Kaul M, Yan B, et al. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297(5584):1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 93.Dimmeler S, Zeiher AM. Reactive oxygen species and vascular cell apoptosis in response to angiotensin II and pro-atherosclerotic factors. Regul Pept. 2000;90(1–3):19–25. doi: 10.1016/s0167-0115(00)00105-1. [DOI] [PubMed] [Google Scholar]
- 94.Heissig B, Hattori K, Dias S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109(5):625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Umemura T, Higashi Y. Endothelial progenitor cells: therapeutic target for cardiovascular diseases. J Pharmacol Sci. 2008;108(1):1–6. doi: 10.1254/jphs.08r01cp. [DOI] [PubMed] [Google Scholar]
- 96.Liu ZJ, Velazquez OC. Hyperoxia, endothelial progenitor cell mobilization, and diabetic wound healing. Antioxid Redox Signal. 2008;10(11):1869–1882. doi: 10.1089/ars.2008.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Janic B, Arbab AS. The role and therapeutic potential of endothelial progenitor cells in tumor neovascularization. ScientificWorldJournal. 2010;10:1088–1099. doi: 10.1100/tsw.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia – influence of chemoreceptors and sympathetic nervous system. J Hypertens. 1997;15(12 Pt 2):1593–1603. doi: 10.1097/00004872-199715120-00060. [DOI] [PubMed] [Google Scholar]
- 99.Takahashi S, Nakamura Y, Nishijima T, Sakurai S, Inoue H. Essential roles of angiotensin II in vascular endothelial growth factor expression in sleep apnea syndrome. Respir Med. 2005;99(9):1125–1131. doi: 10.1016/j.rmed.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 100.Wassmann S, Nickenig G. Pathophysiological regulation of the AT1-receptor and implications for vascular disease. J Hypertens Suppl. 2006;24(1):S15–S21. doi: 10.1097/01.hjh.0000220402.53869.72. [DOI] [PubMed] [Google Scholar]
- 101.Daugherty A, Rateri DL, Lu H, Inagami T, Cassis LA. Hypercholesterolemia stimulates angiotensin peptide synthesis and contributes to atherosclerosis through the AT1A receptor. Circulation. 2004;110(25):3849–3857. doi: 10.1161/01.CIR.0000150540.54220.C4. [DOI] [PubMed] [Google Scholar]
- 102.Steckelings UM, Rompe F, Kaschina E, Unger T. The evolving story of the RAAS in hypertension, diabetes and CV disease: moving from macrovascular to microvascular targets. Fundam Clin Pharmacol. 2009;23(6):693–703. doi: 10.1111/j.1472-8206.2009.00780.x. [DOI] [PubMed] [Google Scholar]
- 103.Vijan SG. Angiotensin-converting enzyme inhibitors (ACEIs), not angiotensin receptor blockers (ARBs), are preferred and effective mode of therapy in high cardiovascular risk patients. J Indian Med Assoc. 2009;107(3):178–182. [PubMed] [Google Scholar]
- 104.Cubbon RM, Kahn MB, Wheatcroft SB. Effects of insulin resistance on endothelial progenitor cells and vascular repair. Clin Sci (Lond) 2009;117(5):173–190. doi: 10.1042/CS20080263. [DOI] [PubMed] [Google Scholar]
- 105.Yin T, Ma X, Zhao L, Cheng K, Wang H. Angiotensin II promotes NO production, inhibits apoptosis and enhances adhesion potential of bone marrow-derived endothelial progenitor cells. Cell Res. 2008;18(7):792–799. doi: 10.1038/cr.2008.69. [DOI] [PubMed] [Google Scholar]
- 106.Endtmann C, Ebrahimian T, Czech T, et al. Angiotensin II impairs endothelial progenitor cell number and function in vitro and in vivo: implications for vascular regeneration. Hypertension. 2011;58(3):394–403. doi: 10.1161/HYPERTENSIONAHA.110.169193. [DOI] [PubMed] [Google Scholar]
- 107.Tobiume K, Matsuzawa A, Takahashi T, et al. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2(3):222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seeger FH, Sedding D, Langheinrich AC, Haendeler J, Zeiher AM, Dimmeler S. Inhibition of the p38 MAP kinase in vivo improves number and functional activity of vasculogenic cells and reduces atherosclerotic disease progression. Basic Res Cardiol. 2010;105(3):389–397. doi: 10.1007/s00395-009-0072-9. [DOI] [PubMed] [Google Scholar]
- 109.Liu H, Zhang H, Iles KE, et al. The ADP-stimulated NADPH oxidase activates the ASK-1/MKK4/JNK pathway in alveolar macrophages. Free Radic Res. 2006;40(8):865–874. doi: 10.1080/10715760600758514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Adair TH, Gay WJ, Montani JP. Growth regulation of the vascular system: evidence for a metabolic hypothesis. Am J Physiol. 1990;259(3 Pt 2):R393–R404. doi: 10.1152/ajpregu.1990.259.3.R393. [DOI] [PubMed] [Google Scholar]
- 111.Tamarat R, Silvestre JS, Kubis N, et al. Endothelial nitric oxide synthase lies downstream from angiotensin II-induced angiogenesis in ischemic hindlimb. Hypertension. 2002;39(3):830–835. doi: 10.1161/hy0302.104671. [DOI] [PubMed] [Google Scholar]
- 112.Kähler CM, Wechselberger J, Molnar C, Prior C. Serum levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea and severe night time hypoxia. Am J Respir Crit Care Med. 2003;167(1):92–93. doi: 10.1164/ajrccm.167.1.368. author reply 93. [DOI] [PubMed] [Google Scholar]
- 113.Imanishi T, Hano T, Nishio I. Angiotensin II potentiates vascular endothelial growth factor-induced proliferation and network formation of endothelial progenitor cells. Hypertens Res. 2004;27(2):101–108. doi: 10.1291/hypres.27.101. [DOI] [PubMed] [Google Scholar]
- 114.Brilla CG, Zhou G, Rupp H, Maisch B, Weber KT. Role of angiotensin II and prostaglandin E2 in regulating cardiac fibroblast collagen turnover. Am J Cardiol. 1995;76(13):8D–13D. doi: 10.1016/s0002-9149(99)80485-8. [DOI] [PubMed] [Google Scholar]
- 115.Tazaki T, Minoguchi K, Yokoe T, et al. Increased levels and activity of matrix metalloproteinase-9 in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;170(12):1354–1359. doi: 10.1164/rccm.200402-193OC. [DOI] [PubMed] [Google Scholar]
- 116.Asahara T, Takahashi T, Masuda H, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18(14):3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Haznedaroglu IC, Oztürk MA. Towards the understanding of the local hematopoietic bone marrow renin-angiotensin system. Int J Biochem Cell Biol. 2003;35(6):867–880. doi: 10.1016/s1357-2725(02)00278-9. [DOI] [PubMed] [Google Scholar]
- 118.Du R, Lu KV, Petritsch C, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13(3):206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85(3):221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 120.Huang LE, Arany Z, Livingston DM, Bunn HF. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J Biol Chem. 1996;271(50):32253–32259. doi: 10.1074/jbc.271.50.32253. [DOI] [PubMed] [Google Scholar]
- 121.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12(12):5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev. 1996;76(3):839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- 123.Cherniack EP. Vascular endothelial growth factor and sleep apnea: clutching at straws in the night. Respiration. 2007;4(1):17–18. doi: 10.1159/000096835. [DOI] [PubMed] [Google Scholar]
- 124.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359(6398):843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 125.Moore MA, Hattori K, Heissig B, et al. Mobilization of endothelial and hematopoietic stem and progenitor cells by adenovector-mediated elevation of serum levels of SDF-1, VEGF, and angiopoietin-1. Ann N Y Acad Sci. 2001;938:36–45. doi: 10.1111/j.1749-6632.2001.tb03572.x. discussion 45–47. [DOI] [PubMed] [Google Scholar]
- 126.Kalka C, Masuda H, Takahashi T, et al. Vascular endothelial growth factor(165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res. 2000;86(12):1198–1202. doi: 10.1161/01.res.86.12.1198. [DOI] [PubMed] [Google Scholar]
- 127.Khakoo AY, Finkel T. Endothelial progenitor cells. Annu Rev Med. 2005;56:79–101. doi: 10.1146/annurev.med.56.090203.104149. [DOI] [PubMed] [Google Scholar]
- 128.Déry MA, Michaud MD, Richard DE. Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem Cell Biol. 2005;37(3):535–540. doi: 10.1016/j.biocel.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 129.Dimmeler S, Aicher A, Vasa M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001 Aug;108(3):391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Urbich C, Aicher A, Heeschen C, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39(5):733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 131.Möhle R, Bautz F, Rafii S, Moore MA, Brugger W, Kanz L. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91(12):4523–4230. [PubMed] [Google Scholar]
- 132.De Falco E, Porcelli D, Torella AR, et al. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104(12):3472–3482. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 133.Garg R, Tellez A, Alviar C, Granada J, Kleiman NS, Lev EI. The effect of percutaneous coronary intervention on inflammatory response and endothelial progenitor cell recruitment. Catheter Cardiovasc Interv. 2008;72(2):205–209. doi: 10.1002/ccd.21611. [DOI] [PubMed] [Google Scholar]
- 134.Hiasa K, Ishibashi M, Ohtani K, et al. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation. 2004;109(20):2454–2461. doi: 10.1161/01.CIR.0000128213.96779.61. [DOI] [PubMed] [Google Scholar]
- 135.Xiao Q, Kiechl S, Patel S, et al. Endothelial progenitor cells, cardiovascular risk factors, cytokine levels and atherosclerosis – results from a large population-based study. PLoS One. 2007;2(10):e975. doi: 10.1371/journal.pone.0000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xiao Q, Ye S, Oberhollenzer F, et al. SDF1 gene variation is associated with circulating SDF1alpha level and endothelial progenitor cell number: the Bruneck Study. PLoS One. 2003;(12):e4061. doi: 10.1371/journal.pone.0004061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Imagawa S, Yamaguchi Y, Higuchi M, et al. Levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea--hypopnea syndrome. Blood. 2001 Aug 15;98(4):1255–1257. doi: 10.1182/blood.v98.4.1255. [DOI] [PubMed] [Google Scholar]
- 138.Nakano M, Satoh K, Fukumoto Y, et al. Important role of erythropoietin receptor to promote VEGF expression and angiogenesis in peripheral ischemia in mice. Circ Res. 2007;100(5):662–669. doi: 10.1161/01.RES.0000260179.43672.fe. [DOI] [PubMed] [Google Scholar]
- 139.Basner RC. Continuous positive airway pressure for obstructive sleep apnea. N Engl J Med. 2007;356(17):1751–1758. doi: 10.1056/NEJMct066953. [DOI] [PubMed] [Google Scholar]
- 140.Ballester E, Badia JR, Hernández L, et al. Evidence of the effectiveness of continuous positive airway pressure in the treatment of sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159(2):495–501. doi: 10.1164/ajrccm.159.2.9804061. [DOI] [PubMed] [Google Scholar]
- 141.Campos-Rodriguez F, Perez-Ronchel J, Grilo-Reina A, Lima-Alvarez J, Benitez MA, Almeida-Gonzalez C. Long-term effect of continuous positive airway pressure on BP in patients with hypertension and sleep apnea. Chest. 2007;132(6):1847–1852. doi: 10.1378/chest.07-1478. [DOI] [PubMed] [Google Scholar]
- 142.Sopkova Z, Dorkova Z, Tkacova R. Predictors of compliance with continuous positive airway pressure treatment in patients with obstructive sleep apnea and metabolic syndrome. Wien Klin Wochenschr. 2009;121(11–12):398–404. doi: 10.1007/s00508-009-1181-z. [DOI] [PubMed] [Google Scholar]
- 143.Flay BR. Efficacy and effectiveness trials (and other phases of research) in the development of health promotion programs. Prev Med. 1986;15(5):451–474. doi: 10.1016/0091-7435(86)90024-1. [DOI] [PubMed] [Google Scholar]
- 144.Stradling JR, Davies RJ. Is more NCPAP better? Sleep. 2000;23(Suppl 4):S150–S153. [PubMed] [Google Scholar]
- 145.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30(6):711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Buchner NJ, Sanner BM, Borgel J, Rump LC. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am J Respir Crit Care Med. 2007;176(12):1274–1280. doi: 10.1164/rccm.200611-1588OC. [DOI] [PubMed] [Google Scholar]
- 147.Campos-Rodriguez F, Peña-Griñan N, Reyes-Nuñez N, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest. 2005;128(2):624–633. doi: 10.1378/chest.128.2.624. [DOI] [PubMed] [Google Scholar]
- 148.Doherty LS, Kiely JL, Swan V, McNicholas WT. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest. 2005;127(6):2076–2084. doi: 10.1378/chest.127.6.2076. [DOI] [PubMed] [Google Scholar]
- 149.Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med. 2002;166(2):159–165. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- 150.Stepnowsky CJ, Dimsdale JE. Dose-response relationship between CPAP compliance and measures of sleep apnea severity. Sleep Med. 2002;3(4):329–334. doi: 10.1016/s1389-9457(02)00010-2. [DOI] [PubMed] [Google Scholar]
- 151.Mermigkis C, Bouloukaki I, Mermigkis D, et al. CRP evolution pattern in CPAP-treated obstructive sleep apnea patients. Does gender play a role? Sleep Breath. 2012;16(3):813–819. doi: 10.1007/s11325-011-0580-3. [DOI] [PubMed] [Google Scholar]
- 152.Schiza SE, Mermigkis C, Panagiotis P, et al. C-reactive protein evolution in obstructive sleep apnoea patients under CPAP therapy. Eur J Clin Invest. 2010;40(11):968–975. doi: 10.1111/j.1365-2362.2010.02348.x. [DOI] [PubMed] [Google Scholar]
- 153.Ishida K, Kato M, Kato Y, et al. Appropriate use of nasal continuous positive airway pressure decreases elevated C-reactive protein in patients with obstructive sleep apnea. Chest. 2009;136(1):125–129. doi: 10.1378/chest.08-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Burioka N, Miyata M, Fukuoka Y, Endo M, Shimizu E. Day-night variations of serum interleukin-6 in patients with severe obstructive sleep apnea syndrome before and after continuous positive airway pressure (CPAP) Chronobiol Int. 2008;25(5):827–834. doi: 10.1080/07420520802384101. [DOI] [PubMed] [Google Scholar]
- 155.Ziegler MG, Mills PJ, Loredo JS, Ancoli-Israel S, Dimsdale JE. Effect of continuous positive airway pressure and placebo treatment on sympathetic nervous activity in patients with obstructive sleep apnea. Chest. 2001;120(3):887–893. doi: 10.1378/chest.120.3.887. [DOI] [PubMed] [Google Scholar]
- 156.Joo MJ, Herdegen JJ. Sleep apnea in an urban public hospital: assessment of severity and treatment adherence. J Clin Sleep Med. 2007;3(3):285–288. [PMC free article] [PubMed] [Google Scholar]
- 157.Lindberg E, Carter N, Gislason T, Janson C. Role of snoring and daytime sleepiness in occupational accidents. Am J Respir Crit Care Med. 2001;164(11):2031–2035. doi: 10.1164/ajrccm.164.11.2102028. [DOI] [PubMed] [Google Scholar]
- 158.Masa JF, Rubio M, Findley LJ. Habitually sleepy drivers have a high frequency of automobile crashes associated with respiratory disorders during sleep. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1407–1412. doi: 10.1164/ajrccm.162.4.9907019. [DOI] [PubMed] [Google Scholar]
- 159.Somers ML, Peterson E, Sharma S, Yaremchuk K. Continuous positive airway pressure adherence for obstructive sleep apnea. ISRN Otolaryngol. 2011;2011:943586. doi: 10.5402/2011/943586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nino-Murcia G, McCann CC, Bliwise DL, Guilleminault C, Dement WC. Compliance and side effects in sleep apnea patients treated with nasal continuous positive airway pressure. West J Med. 1989;150(2):165–169. [PMC free article] [PubMed] [Google Scholar]
- 161.Weaver TE, Chasens ER. Continuous positive airway pressure treatment for sleep apnea in older adults. Sleep Med Rev. 2007;11(2):99–111. doi: 10.1016/j.smrv.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Budhiraja R, Parthasarathy S, Drake CL, et al. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30(3):320–324. [PubMed] [Google Scholar]
- 163.Simon-Tuval T, Reuveni H, Greenberg-Dotan S, Oksenberg A, Tal A, Tarasiuk A. Low socioeconomic status is a risk factor for CPAP acceptance among adult OSAS patients requiring treatment. Sleep. 2009;32(4):545–552. doi: 10.1093/sleep/32.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sin DD, Mayers I, Man GC, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population-based study. Chest. 2002;121(2):430–435. doi: 10.1378/chest.121.2.430. [DOI] [PubMed] [Google Scholar]
- 165.Wang Y, Gao W, Sun M, Chen B. Adherence to CPAP in patients with obstructive sleep apnea in a Chinese population. Respir Care. 2012;57(2):238–243. doi: 10.4187/respcare.01136. [DOI] [PubMed] [Google Scholar]
- 166.Wolkove N, Baltzan M, Kamel H, Dabrusin R, Palayew M. Long-term compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Can Respir J. 2008;15(7):365–359. doi: 10.1155/2008/534372. [DOI] [PMC free article] [PubMed] [Google Scholar]