Abstract
Background
The objectives of this study were to assess the tuberculosis (TB) burden and to provide an insight into the type of circulating M. tuberculosis species in urban slums of Bangladesh. We also aimed to test the feasibility of a larger transmission study in this setting.
Methods
This cross-sectional study was conducted in an urban slum of Dhaka city. The household members were actively screened to assess the presence of TB-related signs and symptoms; cough ≥3 weeks and body mass index (BMI) <17 kg/m2. Sputum specimens from suspects were collected for acid fast bacilli (AFB) microscopy, culture and drug susceptibility testing. Genotyping of M. tuberculosis was done using spoligotyping and variable number tandem repeats of mycobacterial interspersed repetitive units typing.
Results
Among 9,877 adult screened for pulmonary TB (PTB), 25 were positive for AFB on microscopy and/or culture and the prevalence of new PTB cases was estimated to be 253/100,000. Only one child TB case was diagnosed among 5,147 child screened. Out of 26 cases, 21(81%) had cough for several duration and 5(19%) did not present with cough at the time of screening. One multidrug resistant case was found. Fifty two percent of all TB cases had BMI <17 kg/m2 (p = <0.001). Among the 20 analyzed isolates, 13 different spoligotype patterns were identified in which 5 clusters contained 12 strains and 8 strains had unique pattern.
Conclusions
The study revealed high prevalence of TB in urban slums. Screening using low BMI can be beneficial among risk group population. It is important to conduct larger study to validate clinical variables like cough <3 weeks and low BMI to define TB suspect and also to investigate the transmission of TB in slum settings.
Introduction
The global burden of tuberculosis (TB) in 2010 is estimated to be 8.8 million in the form of incident cases, 12 million in the form of prevalent cases [1]. The incidence is estimated indirectly through notification of passively detected patients. The real burden of TB could be markedly higher if active case finding would be employed on larger scale than is now often done.
In many countries, differences in TB incidence between urban and rural areas have been described [2]. TB in urban areas often results specifically among certain urban risk groups, such as slum dwellers who are exposed to poor environmental conditions (overcrowding, poor living conditions) [3]. TB is still believed to be a disease that disproportionately affects the poor and marginalized [4].
The National Tuberculosis Control Programme (NTP) of Bangladesh first adopted the directly observed treatment short course (DOTS) strategy in 1993. The programme rapidly expanded in the following years to almost all areas of the country reaching 100% coverage in 2006 [5]. There are still some gaps in the DOTS services provided for the urban slum dwellers. DOTS strategy is entirely based on passive case finding which is often influenced to a great extent by the treatment seeking behavior of the patients suffering from active TB, social stigmatization, access to health service and even diagnostic delay at health facility [6]. This in turn results in decreased TB case detection with underestimated number of actual TB cases prevailing in the community. Bangladesh had comparatively higher percentage (81%) of notified cases of pulmonary TB (PTB) that were sputum smear-positive (SS+) among the 22 high burden countries with TB [1]. Delay in the diagnosis of these open TB cases can results in transmission of TB among the contacts of the active TB cases and more likely to fuel its transmission in the community apart from increased morbidity and mortality [7].
The prevalence of SS+ TB was found to be higher in the rural population (86.0/100 000) compared to urban (51.1/100 000) in the recently completed (2007–2009) nationwide TB prevalence survey of Bangladesh [8]. A lesser number of cases in urban areas was also notified in the NTP in 2010 [9]. In 2010, the NTP notified a total of 158,709 of all forms of TB cases (103/100 000 population) nationwide, of these 13% were reported from urban areas. However, there are still some pockets in urban area where TB notification rate believed to be higher. One of these is high burden settings like urban slums.
There are still major gaps in our epidemiological knowledge regarding the transmission dynamic of TB despite the fact that TB is endemic and highly prevalent in Bangladesh. Results from one of our previous studies performed in a rural community of Matlab showed that TB in rural Bangladesh is caused primarily by reactivation of latent infections, with the recent emergence of Beijing strain clusters [10]. Urban areas in Bangladesh are densely populated and about one third of the populations are slum dwellers, creating conditions where a high transmission can occur.
Typing of M. tuberculosis strains is of potential importance for the epidemiological studies of TB. The most frequently used genotyping methods for M. tuberculosis are restriction fragment-length polymorphism (RFLP), which targets the insertion sequence (IS) 6110 transposable element, and spoligotyping [11]. An alternative polymerase chain reaction (PCR)-based technique targets 12 loci containing variable number tandem repeats (VNTRs) of genetic elements named mycobacterial interspersed repetitive units (MIRUs) [12], [13]. This method has a discriminatory power close to that of IS6110 RFLP analysis [14], [15]. These techniques are well implemented both in urban and rural settings, in several studies related to transmission dynamics of TB across the globe [10], [16].
The aims of this study were to investigate the burden of active TB in an urban slum of Dhaka city with increased number of case detection based on active symptom screening and also to provide an insight into the type of circulating M. tuberculosis species. We also aimed to test the feasibility of a larger study to investigate TB transmission in this setting.
Methods
Study setting
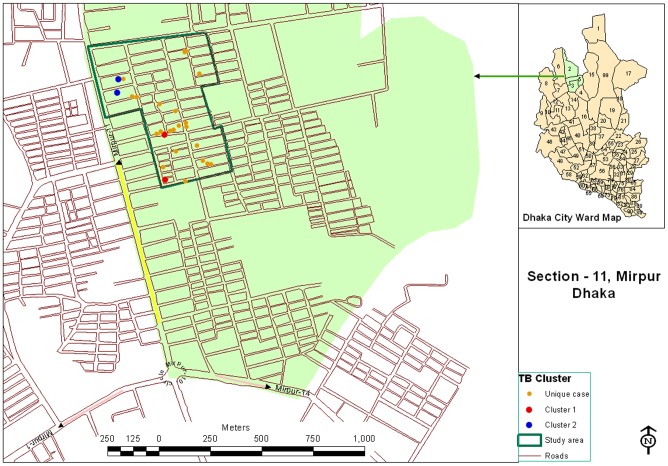
The study was conducted at a densely populated low income urban slum (Mirpur slum) in Dhaka, Bangladesh (Figure 1). Mirpur is one of the 41 thanas of Dhaka City with a population of about one million in an area of 59 square kilometers. Mirpur Thana is divided into 14 sections and we conducted the activities of this project in section 11. The population of section 11 is approximately 50,000. There are several camps in Section 11 and these are inhabited by poor and lower middle class families, residential and sanitary conditions are typical of any congested urban settlement.
Figure 1. Map of the section-11, Mirpur area showing the location of unique and clustered strains isolated from tuberculosis cases.
Ethics and consent
The study protocol was reviewed and approved by the Research Review Committee and the Ethical Review Committee of International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b). The participants were enrolled into the study only after receiving informed written consent from themselves or from their parents/guardians if they were children.
Study procedure
This cross-sectional house-to-house survey was conducted from August 2009 to December 2010. Individuals not present at the household during the first house visit were attempted to be included on at least one subsequent visit. The team consisting of two trained field assistants (FAs) and one research physician visited the study area on a daily basis and screened them for TB symptoms using simple standardized questionnaires. The socio-demographic characteristics, history regarding TB symptoms and other relevant information were collected from the consenting participants. Individuals having productive cough for three or more weeks with or without other clinical presentation were identified as suspects for PTB. We also considered a body mass index (BMI) <17 kg/m2 as a single inclusion criteria for the study as one of our previous study showed more likelihood of developing TB among individuals with BMI<17 [17]. In case of children, we considered a child to be a TB suspect if he/she had cough ≥2 weeks and presence of TB patient in the family [18]. Three sputum specimens from each PTB suspect were collected by the FAs after further clinical evaluation of the suspects by the research physician. The first sputum specimen was obtained immediately after identifying the subject as suspect. The second specimen was overnight sputum collected on the next morning and the third was spot during collection of the second sputum specimen. PTB suspects who did not provide consent to provide sputum specimens in the study were re-invited at least once. The specimens were immediately brought to icddr,b Tuberculosis Laboratory in a cool box for acid-fast bacilli (AFB) microscopy, culture and drug susceptibility testing (DST). We also arranged chest X-ray and Mantoux test for child TB suspects in an adjacent private diagnostic facility. Modified Kenneth-Jones' criteria was used to diagnose child TB [19], [20].
Laboratory investigations
Concentrated sputum smears were examined for AFB using the Ziehl-Neelsen staining under light microscope. Sputum specimens were decontaminated following the Petroffs' NaOH method [21]. Conventional culture using Löwenstein-Jension solid media were used for TB culture using the method as described previously [22].
The standard proportion method was followed for DST of M. tuberculosis isolates to isoniazid (0.2 mg/l), rifampicin (40 mg/l), ethambutol (2 mg/l) and streptomycin (4 mg/l) [23]. An isolate was considered resistant to a given drug when any growth of 1% or more above the control was observed in each drug- containing quadrant plate.
Spoligotyping was performed as previously described by Kamerbeek et al. [24] with minor modifications. The direct repeat (DR) region was amplified by PCR with oligonucleotide primers derived from the DR sequence. Mycobacterial genomic DNA was extracted from cultured cells as described previously [25], [26]. The amplified product was hybridized to a set of 43 immobilized oligonucleotides, each corresponding to one of the unique spacer DNA sequences within the DR locus. Detection of hybridizing DNA was by the chemiluminescent ECL method (Amersham) [26], [27] and by exposure to X-ray film (Hyperfilm ECL: Amersham) in accordance with the instructions of the manufacturer.
PCR and calculation of MIRU copy number per locus were carried out as described previously [13], [28], [29]. All isolates were typed using 11 loci (2, 4, 10, 16, 23, 24, 26, 27, 31, 39 and 40) VNTR-MIRU typing. Most of the strains failed to give a PCR product with primers for the MIRU 20 locus, as seen in our previous study [29]; therefore, the results of MIRU 20 locus were excluded from this study.
Case definitions for TB disease
The diagnosis of TB was made according to the case definition given by the NTP depending on the site and bacteriological status [30]. SS+ PTB was defined as a positive sputum smear confirmed with a second positive smear or culture of M. tuberculosis or chest radiological X-ray abnormalities consistent with active TB; and smear negative PTB was defined by two positive cultures of M. tuberculosis while three sputum specimens negative for AFB [30].
Epidemiological investigation
A cluster was defined as two or more isolates from different patients with identical spoligotype or MIRU patterns, whereas nonclustered patterns were referred to as unique [10]. Clustered patients were investigated to further establish or strengthen potential epidemiological connections in place, time, and person among cluster members. Participants were being considered to share a strong epidemiological link if they would have had been in the same workplace, household, village or area at overlapping times (even a known single exposure to patients).
Statistical analysis
Data were entered and analyzed using the Statistical Package for Social Sciences (SPSS) version 17.0. Univariate analyses were performed to examine the association between demographic and clinical variables of TB cases. P<0.05 was considered as evidence of significant difference. To identify the independent risk factors for TB and non TB, adjusted odds ratio (AOR) and 95% CI were calculated by logistic regression analysis.
Results
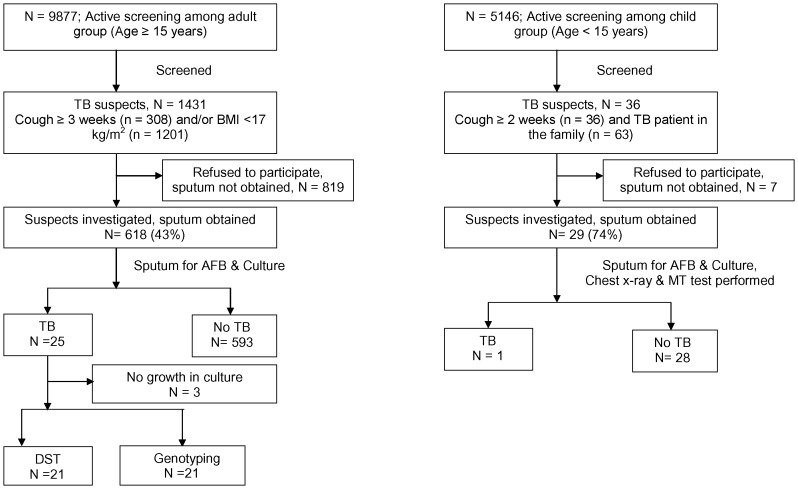
A total of 3716 households were visited and 16,706 eligible participants belonged to those households; of them 15,024 (90%) consented subjects were screened during 17 month period. Of these 9,877 (66%) were aged 15 years or more (adults) and 5,147 (34%) were aged below 15 years (children) (Table 1). Out of 1431(14% of 9,877) adult PTB suspects, sputum specimens were collected from 618 (43%) suspects. Majority (87%) of these suspects who were unable to provide sputum specimen were initially identified as a suspect because of their low BMI even in the absence of cough for any duration. Among the child population, 36 child suspects were identified and sputum smear microscopy and relevant tests were performed in 29 child suspects (Figure 2). We have detected 26 (4% of 647 suspects) TB cases during this time span. Out of 26 cases, 16 (62%) were male and 10 (38%) were female, with a ratio of 1.6∶1. Only one child TB case was diagnosed. Out of the 25 adult TB cases identified in our study, 19 (76%) were smear positive; and the remaining 6 (24%) cases were smear negative but showed growth in culture. The estimated number of new pulmonary TB cases (AFB and/or culture) was 253/100,000 population and the estimated number of new SS+ TB cases was 192/100,000 populations; among the subjects aged ≥15 years, who participated in the study. All 26 identified PTB cases were brought under treatment by DOTS programme.
Table 1. Characteristics of overall study population.
| Variables | Study population | ||||
| All screened (n = 15024) | TB (n = 26) | Non-TB (n = 14998) | |||
| Category | Sub categories | number (%) | number (%) | number (%) | p Value |
| Demographics | |||||
| Sex | Male | 6757 (45.0) | 16 (61.5) | 6741 (44.9) | 0.09 |
| Female | 8267 (55.0) | 10 (38.5) | 8257 (55.1) | ||
| Age | 0–14 yrs | 5151 (34.3) | 1 (3.8) | 5150 (34.3) | <0.001 |
| 15–24 yrs | 3739 (24.9) | 7 (26.9) | 3732 (24.9) | ||
| 25–34 yrs | 2601 (17.3) | 4 (15.4) | 2597 (17.3) | ||
| 35–44 yrs | 1665 (11.1) | 4 (15.4) | 1661 (11.1) | ||
| 45–54 yrs | 926 (6.2) | 6 (23.1) | 920 (6.1) | ||
| 55–64 yrs | 553 (3.7) | 2 (7.7) | 551 (3.7) | ||
| 65+ yrs | 389 (2.6) | 2 (7.7) | 387 (2.6) | ||
| Occupation * | Self-employed | 1681(17.0) | 10 (40.0) | 1671 (17.0) | 0.02 |
| Business | 481(4.9) | 2 (8.0) | 479 (4.9) | ||
| Service | 2490 (25.2) | 5 (20.0) | 2485 (25.2) | ||
| Unemployed | 1982 (20.1) | 5 (20.0) | 1977 (20.1) | ||
| Housewife | 3236 (32.8) | 3 (12.0) | 3233 (32.8) | ||
| Smoking | No | 14346 (95.5) | 19 (73.1) | 14327 (95.5) | <0.001 |
| Yes | 678 (4.5) | 7 (26.9) | 671 (4.5) | ||
| Symptoms | |||||
| Cough | No | 14488 (96.4) | 5 (19.2) | 14483 (96.6) | <0.001 |
| Yes | 537 (3.6) | 21 (80.8) | 516 (3.4) | ||
| <2 weeks | 157 (29.2) | 1(4.8) | 156 (30.2) | 0.02 | |
| ≥2 weeks <3 | 47 (8.8) | 3 (14.3) | 44 (8.5) | ||
| ≥3 weeks | 333 (62.0) | 17 (81.0) | 316 (61.2) | ||
| Haemoptysis | No | 14989 (99.8) | 24 (92.3) | 14965 (99.8) | <0.001 |
| Yes | 35 (0.2) | 2 (7.7) | 33 (0.2) | ||
| Evening rise of temperature | No | 14898 (99.2) | 14 (53.8) | 14884 (99.2) | <0.001 |
| Yes | 126 (0.8) | 12 (46.2) | 114 (0.8) | ||
| Chest pain | No | 14847 (98.8) | 19 (73.1) | 14828 (98.9) | <0.001 |
| Yes | 177 (1.2) | 7 (26.9) | 170 (1.1) | ||
| Shortness of breathe | No | 14884 (99.1) | 11 (42.3) | 14873 (99.2) | <0.001 |
| Yes | 140 (0.9) | 15 (57.7) | 125 (0.8) | ||
| Risk factors | |||||
| Previously diagnosed as TB | No | 14784 (98.4) | 17 (65.4) | 14767 (98.5) | <0.001 |
| Yes | 240 (1.6) | 9 (34.6) | 231(1.5) | ||
| Exposure to TB patient | No | 13991 (93.1) | 25 (96.2) | 13966 (93.1) | >0.1 |
| Yes | 1033 (6.9) | 01 (3.8) | 1032 (6.9) | ||
| BMI * | BMI (≥17.0) | 8676 (87.8) | 12 (48.0) | 8664 (87.9) | <0.001 |
| BMI (<17.0) | 1201 (12.2) | 13 (52.0) | 1188 (12.1) | ||
All value are n (%). p values are comparing TB patients (n = 26) against no. no-TB (n = 14998).
Occupation and BMI were calculated among the adult group only;
Fisher exact test are shown in boldface font.
Figure 2. Screening profile of individuals suspected of having tuberculosis.
A number of clinical variables were assessed for an association with TB in this study. Out of 26 diagnosed cases; 21(81%) had cough and 5(19%) did not present with any cough at the time of active screening, they were suspected on the basis of their poor nutritional status (Table 1). Malnutrition, as defined by low BMI (<17 kg/m2), was also associated with TB; this can either be a risk factor or result of TB. Fifty two percent of all TB cases had BMI <17 kg/m2 (p = <0.001) (Table 1). Thirty five percent of the identified TB cases had a previous history of TB (p = <0.001). Other clinical variables which were associated with TB were fever, haemoptysis, chest pain and shortness of breath (not statistically significant). No significant association was observed between TB and diabetes mellitus, exposure to TB patients, and alcohol consumption. After adjusting for confounding factors, a risk factor analysis showed that a higher likelihood of developing active TB was associated with smoking, previous history of anti-TB treatment and low BMI (Table 2).
Table 2. Odds ratios (ORs) for TB by socio demographic characteristics and potential risk factors.
| Variables | Unadjusted | Adjusted | |||
| Category | Sub categories | OR (95% CI) | P Value | AOR (95% CI) | P Value |
| Socio demographic | |||||
| Sex | Female | 1.00 | |||
| Male | 1.960 (0.9 – 4.3) | 0.09 | |||
| Age | 0–14 yrs | 1.00 | |||
| 15–24 yrs | 9.7 (1.2–78.5) | 0.03 | |||
| 25–34 yrs | 7.9 (0.9 –71.0) | 0.06 | |||
| 35–44 yrs | 12.4 (1.4–111.0) | 0.02 | |||
| 45–54 yrs | 33.6 (4.0–279.3) | 0.001 | |||
| 55–64 yrs | 18.7 (1.7–206.5) | 0.01 | |||
| 65+ yrs | 26.6 (2.4–294.2) | 0.007 | |||
| Occupation* | Self-employed | 1.0 | |||
| Business | 6.4 (1.8 – 23.4) | 0.005 | |||
| Service | 4.5 (0.8 – 26.9) | 0.10 | |||
| Unemployed | 2.2 (0.5 – 9.1) | 0.29 | |||
| Housewife | 2.7 (0.7 – 11.4) | 0.17 | |||
| Smoking | No | 1.0 | 1.0 | ||
| Yes | 7.9 (3.3 – 18.8) | <0.001 | 3.7 (1.5– 9.3) | 0.005 | |
| Risk factors | |||||
| Previously diagnosed as TB | No | 1.0 | 1.0 | ||
| Yes | 33.8 (14.9 –76.7) | <0.001 | 15.7 (6.6–37.3) | <0.001 | |
| BMI* | BMI (≥17.0) | 1.0 | 1.0 | ||
| BMI (<17.0) | 7.9 (3.6–17.4) | <0.001 | 5.3 (2.3–12.0) | <0.001 | |
OR = odds ratio; AOR = adjusted odds ratio; CI = confidence interval; adjusted odds ratio are not presented for variables with P values more than 0.1; * Occupation and BMI were calculated among the adult group only.
Out of 26 cases, DST was done on 20 strains of M. tuberculosis available. Five cases were not available (one was unable to produce valid culture result, two started anti-TB treatment before collection of specimens for culture, one specimen was missing for culture and the child TB case was negative on culture) for DST and another one was excluded from the analysis as it was non tuberculous mycobacterial (NTM) strain. One (5%) strain was resistant to streptomycin alone and 1 (5%) was resistant to all four drugs. The remaining 18 (90%) strains were susceptible to first line drugs. The DR-TB case was notified to the NTP for subsequent management.
Twenty isolates from M. tuberculosis cultures were spoligotyped. This analysis revealed 13 different patterns, 12 of which were grouped into 5 clusters and remaining 8 isolates were unique. About 60% of the isolates were clustered by spoligotyping (Table 3). Twenty five percent of the isolates were of East African Indian (EAI) type [31], which corresponds to the ancestral TbD1+ M. tuberculosis type [32], also named Indo-Oceanic cluster [33]. There was a single cluster, EAI5 among the EAI type strains. The other strains of the family were EAI6_BGD1 and EAI-1-SOM types. EAI5 was also the most predominant of the EAI type consisting of 3 isolates. Other clusters included the Beijing family and T-family strains consisted of two isolates in each cluster (Table 3). Using MIRU typing, only 4/20 (20%) strains could be grouped into two clusters. Remaining sixteen isolates (80%) exhibited unique (non-clustered) patterns.
Table 3. Clusters and unique M. tuberculosis strains determined by various typing methods and distribution of spoligotyping-defined phylogenetic clades.
| Strain | Spoligo pattern | Phylogenetic clade | VNTR-MIRU type Allele profile | |
| 1 | MP132901 | □□□□□□□□□□□□□□□□□□□□□□□□□□□□□□□□□□▪▪▪▪▪▪▪▪▪ | Beijing | 22435173512 |
| 2 | MP348502 | □□□□□□□□□□□□□□□□□□□□□□□□□□□□□□□□□□▪▪▪▪▪▪▪▪▪ | Beijing | 22333273733 |
| 3 | MP283202 | ▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪□□□□▪▪▪▪▪▪▪ | T1 | 22436153321 |
| 4 | MP391501 | ▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪□□□□▪▪▪▪▪▪▪ | T1 | 26536221531 |
| 5 | MP26440 | ▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪□□□□▪□▪▪□▪▪▪▪▪▪ | EAI5 | 24536221533 |
| 6 | MP272401 | ▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪□□□□▪□▪▪□▪▪▪▪▪▪ | EAI5 | 24536221431 |
| 7 | MP327701 | ▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪□□□□▪□▪▪□▪▪▪▪▪▪ | EAI5 | 25433321232 |
| 8 | MP29002 | ▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪□□□□□□▪▪□▪▪▪▪▪▪ | ND | 24536221532 |
| 9 | MP307404 | ▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪□□□□□□▪▪□▪▪▪▪▪▪ | ND | 24536221532 |
| 10 | MP322803 | ▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪□□□□▪□□□□□□□□▪▪□▪▪▪▪▪▪ | ND | 24443321233 |
| 11 | MP338904 | ▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪□□□□▪□□□□□□□□▪▪□▪▪▪▪▪▪ | ND | 24443321233 |
| 12 | MP329301 | ▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪□□□□▪□□□□□□□□▪▪□▪▪▪▪▪▪ | ND | 22333253533 |
| 13 | MP43301 | ▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪□□▪▪▪▪▪▪▪▪▪ | MANU2 | 22645173532 |
| 14 | MP26204 | ▪▪▪□□▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪□□▪▪▪▪▪▪▪▪▪ | ND | 22745173533 |
| 15 | MP82703 | ▪▪▪□□□□□□□□□▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪□□□□▪▪▪▪▪▪▪ | T1 | 22535153324 |
| 16 | MP79701 | ▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪□▪▪□▪▪▪▪▪▪▪ | ND | 24433221533 |
| 17 | MP48404 | ▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪□▪▪▪▪▪□□□□▪□▪▪□▪▪▪▪▪▪ | EAI6_BGD1 | 24536221731 |
| 18 | MP252901 | ▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪□□□□▪□▪▪▪▪▪□▪▪▪ | EAI1_SOM | 21535223512 |
| 19 | MP245201 | ▪▪▪▪▪▪▪▪▪▪□▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪□□□□□□▪▪▪▪▪□▪▪▪ | ND | 25536223512 |
| 20 | MP338504 | ▪▪▪▪▪▪▪▪▪▪▪▪□▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪□□□□▪□▪▪▪▪▪□□□□ | ND | 35531323213 |
Discussion
There is lack of data on the prevalence of TB in urban slums of Bangladesh. According to the recently completed national TB prevalence survey in Bangladesh, the prevalence of new SS+ TB was estimated to be 79.4 per 100,000 and the prevalence rate for urban area was 51.1 per 100,000 [34]. Whereas our study, conducted in an urban slum area, revealed high prevalence of TB which is more than two times higher than overall prevalence and nearly four times higher than the prevalence in urban settings. This study has provided insight into the prevalence of TB, increased the case detection and identified some of the contributing factors for increased rate of SS+ PTB in a densely populated urban slum in Bangladesh.
This study also showed that active case finding for TB in urban slum setting is effective, given the high rate of participation, the feasibility of timely transportation of collected specimens to the central laboratory (no contamination), as well as laboratory testing which were used to investigate the TB burden along with molecular epidemiology of TB in settings like an urban slums.
The study results of our study indicate that clinical variables like cough <3 weeks and low BMI should be considered for suspecting TB cases and these issues should be addressed in the current NTP guidelines and awareness campaign which usually addresses only those with cough for at least three weeks. This could eventually detect those masked TB cases without cough or cough for less than three weeks duration. According to existing NTP guidelines in Bangladesh a patient is not suspected to have TB unless the patient has been coughing for three or more weeks. The gap of this current criteria poses the risk that we might be missing quite a large number of TB cases. Our study indicates that pulmonary TB with a history of cough is not significantly less frequent, when duration of cough is shorter than three weeks. This observation was also done in the prevalence study where the majority of TB cases identified did not have a history of cough [8]. One of the recent studies also presents the data with relevance of duration of cough and TB in line with our findings [35]. Several studies in the past showed an association between malnutrition and TB [36], [37], [38] and in one of our previous studies in prison we confirmed that in the prison population in Bangladesh [17]. The presence of low BMI in such a population is an important screening indicator of the disease in this population. However, more research with larger population should be performed to support these findings among different population group of Bangladesh.
In our study, among the detected cases the male-female ratio was 1.6∶1. In recently completed prevalence survey, TB in males was three times higher than females [34]. Among the adult population a higher male: female ratio has also been found in data from the NTP report [5]. However, we cannot comment whether TB is more common in men or we have missed the women with TB due to their perceptions of TB, denial about the suspicion of having TB related with social stigma or their ability to produce quality sputum specimen. It is evident that, women experienced longer delays in help seeking for TB at different stages of the disease ccompared with men [39]. There is definite need of study addressing gender differences in TB diagnosis and treatment to see the gender variations in treatment seeking behaviour in the community.
One of the objectives of our study was to characterize those strains that caused TB in an urban slum of Bangladesh and to investigate the extent of transmission. However, it is difficult to draw a conclusion from our study results as we have smaller numbers of detected cases and the study period was short to observe the transmission pattern. A relatively higher proportion (20%) of our M. tuberculosis isolates were clustered during this short study period compared to 11% cluster in our previous study in the rural community [10]. There was no apparent epidemiological link among the clustered cases. However, there is every possibility of recent transmission of TB among the infected persons considering the fact that this study was performed in an urban slum which was overcrowded and congested. Interestingly, the clustering was found among the younger age group (mean age 29; range: 19–40 yrs) which also favours recent transmission. It might not be possible to find the index case but considering the TB incubation period ranging from few months to few years there is possibility of recent transmission. It is important to determine whether TB disease has resulted from recent exogenous infection/reinfection or endogenous reactivation of a long-term latent infection to have an effective TB control measure strategy [40]. The results showed that it is feasible to conduct this transmission study in urban slum settings and being a high burden country, Bangladesh warrants these transmission dynamic studies in larger scale.
One of the limitations of our study was the purposive selection of Mirpur urban slum. This was selected as the area had been used as field sites for different studies conducted by icddr,b and we have a harmonious relationship with the population of the study area. We have not taken the HIV status into consideration as the prevalence of HIV in Bangladesh is low (less than 1%). Another reason for not taking the HIV status into consideration was the requirement of voluntary counseling prior asking the patient about the HIV status, which was difficult in active screening based field study. However, we believe that the interpretation of results in our study has not been influenced by the HIV status. It is important to conduct a prospective study with a larger sample size in the urban slum settings to estimate the prevalence of TB and its transmission in these high risk group. The current study identified areas in which design and data collection can be strengthened. It will be interesting to understand and know how the programmatic factors like screening only for chronic cough (less sensitivity), diagnosis by sputum smear microscopy (<70% sensitivity), others (services, human resource, quality etc.) and non-programmatic factors like care seeking, private sector, socioeconomic status etc. affecting detection of SS+ TB cases in urban improvised areas. This system bypass or non use of or non-detection by DOTS is important and should be addressed particularly with the growing threat of HIV infection and drug resistant TB in the country.
Acknowledgments
We are thankful to the NTP of Bangladesh for their support. Our sincere gratitude to all the study participants. In addition, we wish to thank the field and laboratory staff for their excellent work on this study.
Funding Statement
This work was supported by grants from the KNCV Tuberculosis Foundation, The Netherlands. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO (2011) Global tuberculosis control: WHO report 2011: World Health Organization.
- 2. Hayward A, Darton T, Van-Tam J, Watson J, Coker R, et al. (2003) Epidemiology and control of tuberculosis in Western European cities. The International Journal of Tuberculosis and Lung Disease 7: 751–757. [PubMed] [Google Scholar]
- 3. Braden CR, Templeton GL, Cave MD, Valway S, Onorato IM, et al. (1997) Interpretation of restriction fragment length polymorphism analysis of Mycobacterium tuberculosis isolates from a state with a large rural population. Journal of Infectious Diseases 175: 1446. [DOI] [PubMed] [Google Scholar]
- 4. Hossain S, Quaiyum MA, Zaman K, Banu S, Husain MA, et al. (2012) Socio Economic Position in TB Prevalence and Access to Services: Results from a Population Prevalence Survey and a Facility-Based Survey in Bangladesh. PloS one 7: e44980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hossain S, Quaiyum MA, Zaman K, Banu S, Husain MA, et al. (2012) Socio Economic Position in TB Prevalence and Access to Services: Results from a Population Prevalence Survey and a Facility-Based Survey in Bangladesh. PLoS One 7: e44980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ford CM, Bayer AM, Gilman RH, Onifade D, Acosta C, et al. (2009) Factors associated with delayed tuberculosis test-seeking behavior in the Peruvian Amazon. The American journal of tropical medicine and hygiene 81: 1097–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Asch S, Leake B, Anderson R, Gelberg L (1998) Why do symptomatic patients delay obtaining care for tuberculosis? American journal of respiratory and critical care medicine 157: 1244–1248. [DOI] [PubMed] [Google Scholar]
- 8. Zaman K, Hossain S, Banu S, Quaiyum M, Barua P, et al. (2012) Prevalence of smear-positive tuberculosis in persons aged 15 years in Bangladesh: results from a national survey, 2007-2009. Epidemiology and Infection 140: 1018–1027. [DOI] [PubMed] [Google Scholar]
- 9.(DGHS) DGoHS (2011) ‘Tuberculosis Control in Bangladesh’ Annual Report 2011. National Tuberculosis Control Programme (NTP).
- 10. Banu S, Uddin M, Islam M, Zaman K, Ahmed T, et al. (2012) Molecular epidemiology of tuberculosis in rural Matlab, Bangladesh. The International Journal of Tuberculosis and Lung Disease 16: 319–326. [DOI] [PubMed] [Google Scholar]
- 11. Van Embden J, Cave M, Crawford J, Dale J, Eisenach K, et al. (1993) Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. Journal of Clinical Microbiology 31: 406–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Supply P, Magdalena J, Himpens S, Locht C (1997) Identification of novel intergenic repetitive units in a mycobacterial two-component system operon. Molecular microbiology 26: 991–1003. [DOI] [PubMed] [Google Scholar]
- 13. Supply P, Mazars E, Lesjean S, Vincent V, Gicquel B, et al. (2000) Variable human minisatellite-like regions in the Mycobacterium tuberculosis genome. Molecular microbiology 36: 762–771. [DOI] [PubMed] [Google Scholar]
- 14. Hawkey PM, Smith EG, Evans JT, Monk P, Bryan G, et al. (2003) Mycobacterial interspersed repetitive unit typing of Mycobacterium tuberculosis compared to IS6110-based restriction fragment length polymorphism analysis for investigation of apparently clustered cases of tuberculosis. Journal of Clinical Microbiology 41: 3514–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mazars E, Lesjean S, Banuls A-L, Gilbert M, Vincent V, et al. (2001) High-resolution minisatellite-based typing as a portable approach to global analysis of Mycobacterium tuberculosis molecular epidemiology. Proceedings of the National Academy of Sciences 98: 1901–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tessema B, Beer J, Merker M, Emmrich F, Sack U, et al. (2013) Molecular epidemiology and transmission dynamics of Mycobacterium tuberculosis in Northwest Ethiopia: new phylogenetic lineages found in Northwest Ethiopia. BMC infectious diseases 13: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Banu S, Hossain A, Uddin MKM, Uddin MR, Ahmed T, et al. (2010) Pulmonary tuberculosis and drug resistance in Dhaka central jail, the largest prison in Bangladesh. PloS one 5: e10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Graham SM, Ahmed T, Amanullah F, Browning R, Cardenas V, et al. (2012) Evaluation of tuberculosis diagnostics in children: 1. Proposed clinical case definitions for classification of intrathoracic tuberculosis disease. Consensus from an expert panel. Journal of Infectious Diseases 205: S199–S208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stegen G, Jones K, Kaplan P (1969) Criteria for guidance in the diagnosis of tuberculosis. Pediatrics 43: 260–263. [PubMed] [Google Scholar]
- 20. Mehnaz A, Arif F (2005) Applicability of scoring chart in the early detection of tuberculosis in children. Journal of the College of Physicians and Surgeons—Pakistan: JCPSP 15: 543. [PubMed] [Google Scholar]
- 21. Petroff S (1915) A new and rapid method for the isolation and culture of tubercle bacilli directly from the sputum and faeces. J Exp Med 21: 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Banu S, Mahmud AM, Rahman MT, Hossain A, Uddin MKM, et al. (2012) Multidrug-Resistant Tuberculosis in Admitted Patients at a Tertiary Referral Hospital of Bangladesh. PloS one 7: e40545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Canetti G, Fox W, Khomenko Aa, Mahler H, Menon N, et al. (1969) Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bulletin of the World Health Organization 41: 21. [PMC free article] [PubMed] [Google Scholar]
- 24. Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, et al. (1997) Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol 35: 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kox LF, Rhienthong D, Miranda AM, Udomsantisuk N, Ellis K, et al. (1994) A more reliable PCR for detection of Mycobacterium tuberculosis in clinical samples. J Clin Microbiol 32: 672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van Soolingen D, De Haas P, Hermans P, Groenen P, Van Embden J (1993) Comparison of various repetitive DNA elements as genetic markers for strain differentiation and epidemiology of Mycobacterium tuberculosis. Journal of clinical microbiology 31: 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brosch R, Gordon SV, Marmiesse M, Brodin P, Buchrieser C, et al. (2002) A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc Natl Acad Sci U S A 99: 3684–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mazars E, Lesjean S, Banuls AL, Gilbert M, Vincent V, et al. (2001) High-resolution minisatellite-based typing as a portable approach to global analysis of Mycobacterium tuberculosis molecular epidemiology. Proceedings of the national academy of Sciences 98: 1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Banu S, Gordon SV, Palmer S, Islam R, Ahmed S, et al. (2004) Genotypic analysis of Mycobacterium tuberculosis in Bangladesh and prevalence of the Beijing strain. Journal of clinical microbiology 42: 674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.(2009) National Guidelines and Operational Manual for Tuberculosis Control: National Tuberculosis Control Programme, Bangladesh.
- 31. Sola C, Filliol I, Legrand E, Mokrousov I, Rastogi N (2001) Mycobacterium tuberculosis phylogeny reconstruction based on combined numerical analysis with IS 1081, IS 6110, VNTR, and DR-based spoligotyping suggests the existence of two new phylogeographical clades. Journal of molecular evolution 53: 680–689. [DOI] [PubMed] [Google Scholar]
- 32. Brosch R, Gordon SV, Marmiesse M, Brodin P, Buchrieser C, et al. (2002) A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proceedings of the national academy of Sciences 99: 3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gagneux S, Small PM (2007) Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. The Lancet infectious diseases 7: 328–337. [DOI] [PubMed] [Google Scholar]
- 34. Zaman K, Hossain S, Banu S, Quaiyum M, Barua P, et al. (2011) Prevalence of smear-positive tuberculosis in persons aged⩾ 15 years in Bangladesh: results from a national survey, 2007–2009. Epidemiology and Infection 1: 1–10. [DOI] [PubMed] [Google Scholar]
- 35. Ngadaya ES, Mfinanga GS, Wandwalo ER, Morkve O (2009) Detection of pulmonary tuberculosis among patients with cough attending outpatient departments in Dar Es Salaam, Tanzania: does duration of cough matter? BMC health services research 9: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karyadi E, Schultink W, Nelwan RHH, Gross R, Amin Z, et al. (2000) Poor micronutrient status of active pulmonary tuberculosis patients in Indonesia. The Journal of nutrition 130: 2953–2958. [DOI] [PubMed] [Google Scholar]
- 37. Onwubalili J (1988) Malnutrition among tuberculosis patients in Harrow, England. European journal of clinical nutrition 42: 363. [PubMed] [Google Scholar]
- 38. Saha K, Rao K (1989) Undernutrition in lepromatous leprosy. V. Severe nutritional deficit in lepromatous patients co-infected with pulmonary tuberculosis. European journal of clinical nutrition 43: 117. [PubMed] [Google Scholar]
- 39. Karim F, Islam MA, Chowdhury A, Johansson E, Diwan VK (2007) Gender differences in delays in diagnosis and treatment of tuberculosis. Health policy and planning 22: 329–334. [DOI] [PubMed] [Google Scholar]
- 40. Cohen T, Colijn C, Finklea B, Murray M (2007) Exogenous re-infection and the dynamics of tuberculosis epidemics: local effects in a network model of transmission. J R Soc Interface 4: 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]