Abstract
OBJECTIVE:
To determine the impact of a multifaceted knowledge translation strategy for a new vaccination pain management guideline on public health immunizers’ attitudes, beliefs and use of pain-relieving strategies during childhood vaccination.
METHOD:
Using a randomized controlled pre-post study design, public health nurses (PHNs) at intervention sites received a multifaceted knowledge translation intervention about new pain management guidelines incorporated in the British Columbia Immunization Program Manual, including education, supplies and online support. Attitudes and beliefs of PHNs toward immunization pain and pain management, and use of pain-relieving strategies were compared for the intervention sites between the pre- and postimplementation phases.
RESULTS:
A total of 516 children were immunized by 31 PHNs pre- and postimplementation in the intervention sites. Postimplementation, satisfaction and confidence with ability to manage pain and willingness to use newly recommended strategies were significantly more positive (P<0.05) in the intervention sites, and overall use of at least one newly recommended strategy increased from 49.8% preintervention to 77.6% postimplementation (P<0.001).
CONCLUSION:
The knowledge translation intervention improved PHN immunizers’ attitudes, beliefs and practices regarding paediatric vaccination pain management. Reducing pain may result in a better immunization experience for children, caregivers and immunizers.
Keywords: Clinical practice guideline, Immunization, Knowledge translation, Pain management, Public health nursing
Abstract
OBJECTIF :
Déterminer les conséquences d’une stratégie polyvalente de transfert du savoir contenu dans un nouveau guide de gestion de la douleur causée par la vaccination sur les attitudes et les croyances de vaccinateurs de la santé publique ainsi que sur leur utilisation des stratégies de soulagement de la douleur pendant la vaccination des enfants.
MÉTHODOLOGIE :
Au moyen d’une méthodologie d’étude avantaprès aléatoire et contrôlée, des infirmières de la santé publique (ISP) ont profité d’une démarche polyvalente de transfert du savoir à leur établissement d’intervention à l’égard de nouvelles directives sur la gestion de la douleur contenues dans le British Columbia Immunization Program Manual, y compris la formation, les fournitures et le soutien virtuel. Les chercheurs ont comparé les attitudes et les croyances des ISP à l’égard de la douleur et de la gestion de la douleur de la vaccination ainsi que les stratégies de soulagement de la douleur qu’elles utilisaient aux établissements d’intervention avant et après la mise en œuvre des directives.
RÉSULTATS :
Au total, 516 enfants ont été vaccinés par 31 ISP avant et après la mise en œuvre aux établissements d’intervention. Après la mise en œuvre, la satisfaction et la confiance en la capacité de gérer la douleur et la volonté d’utiliser les stratégies nouvellement recommandées étaient significativement plus positives (P<0,05). L’utilisation globale d’au moins une stratégie nouvellement recommandée est passée de 49,8 % avant la démarche à 77,6 % après la mise en œuvre (P<0,001).
CONCLUSION :
L’intervention de transfert du savoir a amélioré les attitudes, les croyances et les pratiques des ISP vaccinatrices au sujet de la gestion de la douleur des vaccins en pédiatrie. L’atténuation de la douleur peut susciter une meilleure expérience de vaccination pour les enfants, les personnes qui s’occupent d’eux et les vaccinateurs.
Childhood immunization injections are commonly associated with anxiety and pain due to the requisite needle injection. Children in particular regard needle injections as one of the most frightening and painful health-related events (1–4). Negative experiences with needles in childhood may lead to the development of needle fears and health care avoidance behaviours in the future, including immunization noncompliance (5). Despite the potential negative consequences of immunization injection pain and the availability of effective and safe analgesic interventions, immunizers often use a procedure-focused approach, devoting only minimal attention to reducing associated pain (6). Research indicates that this is due, in part, to misconceptions about the importance of alleviating pain, and a lack of knowledge about the effectiveness and safety of pain-relieving strategies (6–9).
To overcome this knowledge-practice gap, an interdisciplinary panel from across Canada known as the Help ELiminate Pain in KIDS (HELPinKIDS) Team was convened to develop a clinical practice guideline (CPG) for pain management during vaccine injections in children, which was published in 2010 (10). The British Columbia (BC) Centre for Disease Control (BCCDC) (Vancouver, BC) adopted the HELPinKIDS CPG recommendations and, in June 2012, they were incorporated into the BC Immunization Program Manual, a resource that guides nursing practice in the provision of immuno- and chemoprophylactic agents (11).
At the time the present study was conducted, the HELPinKIDS CPG recommendations were not yet incorporated in the BC manual. The objectives of the present study were to provide initial estimates of the impact of implementing the new immunization injection pain guideline on public health immunizers’ attitudes, beliefs and pain management practices. Regarding attitudes and beliefs, we predicted that our implementation strategy would result in increased perceived importance of pain, increased willingness to use newly recommended pain-relieving strategies, and increased satisfaction and confidence with pain management. With respect to utilization, we predicted increased use of each of the newly recommended pain-relieving strategies for childhood immunization injections. These strategies included breastfeeding, sugar water, rubbing the skin near the injection site and immunizer/provider-led distraction.
METHODS
The present study was approved by the University of British Columbia Behavioural Research Ethics Board (Vancouver, BC) on February 23, 2011. Participating nurses provided informed consent before study commencement.
Participants
Two of five BC health regions volunteered to participate (one rural and one urban). Within each health region, two individual health units were recruited and randomly allocated to an intervention and a control site. A health unit could only be included if it immunized at least 100 children (0 to 18 years of age) biweekly. Participants included immunizing public health nurses (PHNs) working in participating health units.
Study procedures
The study consisted of two phases: preimplementation (baseline) and postimplementation, each two weeks in duration. To ascertain attitudes and beliefs about immunization pain and pain-relieving strategies, the same online survey was issued to PHNs before the baseline phase and after the implementation phase using SurveyMonkey. The survey contained closed-ended questions (yes/no and Likert scale) regarding attitudes and beliefs about pain and pain-relieving strategies.
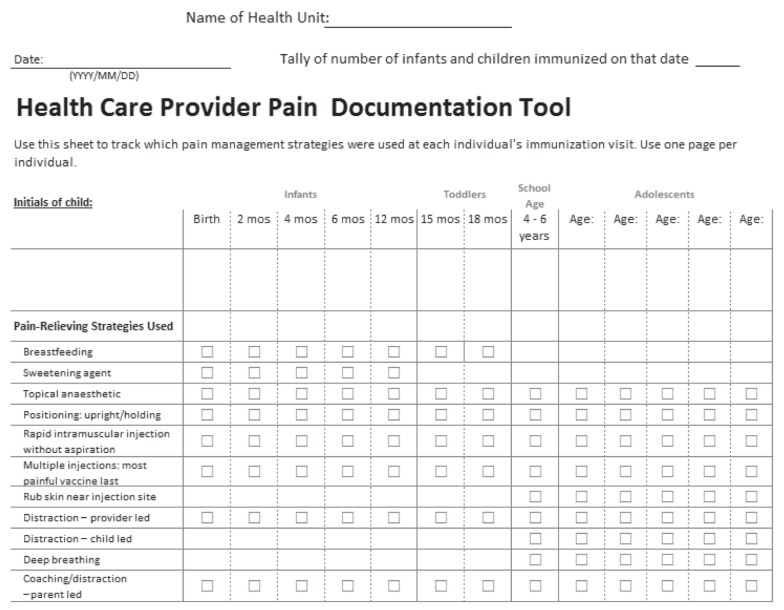
In both phases of the study, nurses documented the use of pain-relieving strategies on a documentation checklist (Appendix 1) that was modified from the HELPinKIDS CPG (10). It contained 11 age-specific pain-relieving strategies: five were new to PHNs (ie, breastfeeding, sweetening agent, topical anesthetic, distraction – provider-led, rubbing the skin near the injection site before and during injection) and the remainder were standard practice. All strategies (old and new) were documented to benchmark current practices and reduce the potential for outcome reporting bias, with the exception of topical anesthetics. Topical anesthetics were not incorporated into immunization appointments because there was no previous opportunity to educate parents about them and they could not feasibly be implemented on the day of vaccination. Certain strategies were not promoted to be given together (eg, breastfeeding and sucrose solution, child-led distraction and parent- or provider-led distraction).
Multifaceted knowledge translation intervention
A multifaceted knowledge translation intervention was used. Specifically, the BCCDC provided education and training, educational resources and support for the implementation of the guideline in the intervention sites. After the baseline phase, a 2 h in-person education session was held at each intervention site. PHNs were educated about pain-relieving strategies through a PowerPoint (Microsoft Corporation, USA) presentation and practice scenarios. The PowerPoint presentation was created by the BCCDC, using content from CPG and published literature.
Consequences of untreated immunization pain, how the strategies were developed, scientific evidence supporting the effectiveness of the strategies, and misconceptions about the strategies from immunizers and parents were presented and discussed. The session was given by a nursing manager trained by the BCCDC to deliver the content and to answer questions. Sucrose supplies were provided to intervention health units (distraction agents such as toys, books, etc, are already routinely available at all health units). Online support was provided for nurses to clarify concepts and answer questions regarding implementation of the information included in the guideline.
Sample size considerations
The primary outcome was the use of newly recommended pain-relieving strategies. Use was calculated in two ways: the percentage of vaccinated children receiving any of the newly recommended pain-relieving strategies; and the mean number of newly recommended pain-relieving strategies used. An absolute increase in use of 10% was considered to be significant (12). The sample size (400 children: 100 per phase, or 200 per site) was based on the ability to detect a doubling in use (10% versus 20%) between the baseline and postimplementation phases, or an increase by one in the mean number of pain-relieving strategies used, with SD = 2, alpha = 0.05 and power ≥80%.
Data analysis
The pattern of responses to survey questions was analyzed using descriptive statistics. For Likert scale questions, responses were grouped into dichotomous response categories (yes, no) because this was deemed more clinically relevant in the context of the present study. Responses to questions about the willingness to use the four newly recommended pain-relieving strategies were combined into a single summary score for the purposes of analysis by adding individual scores (no = 0, yes = 1) for an overall score that ranged from 0 to 4. The number (per cent) of children receiving any of the four newly recommended pain-relieving strategies (breastfeeding, sweetening agent, distraction – provider-led, rubbing the skin near the injection site) and the mean number of these strategies used per child were measured overall. The number (per cent) of children receiving each of these strategies was measured according to corresponding age group (breast-feeding and sweetening agent, <1 year of age; distraction – provider-led, all ages; rubbing the skin near the injection site, ≥4 years of age). McNemar’s test and paired t tests were used to compare paired data between baseline and postimplementation phases; χ2 and t tests were used for comparisons between unpaired data, as appropriate. All statistical analyses were performed using SPSS version 14.0 (IBM Corporation, USA); P<0.05 was considered to be statistically significant. The Bonferroni correction was applied to the P value to reduce the risk of type 1 error when appropriate.
RESULTS
Participants
A total of 53 PHNs participated in the study (31 from the intervention sites and 22 from the control sites). This represented 91% of eligible PHNs at the intervention site and 88% of eligible PHNs at the control sites.
The response rate for baseline and postimplementation surveys was 27/31 (87%) for the intervention group and 16/22 (73%) for the control group. There was no difference in the number of years of immunization experience between PHNs in the intervention and control groups (8.9 years versus 9.8 years; P=0.60). All participating PHNs were female.
The intervention sites administered 703 vaccines and used a total of 1074 pain-relieving strategies in 279 children preimplementation, and 566 vaccines and 1023 pain-relieving strategies in 237 children postimplementation. The control sites administered 519 vaccines and used a total of 896 pain-relieving strategies in 209 children preimplementation, and 451 vaccines and 928 pain-relieving strategies in 222 children postimplementation. The age distribution of immunized children is shown in Table 1.
TABLE 1.
Age distribution of immunized children at intervention and control sites
|
Preimplementation (baseline)
|
Postimplementation
|
|||||
|---|---|---|---|---|---|---|
| <1 year | 1 to <4 years | ≥4 years | <1 year | 1 to <4 years | ≥4 years | |
| Intervention sites | 187 (67) | 79 (28) | 13 (5) | 159 (67) | 50 (21) | 28 (12) |
| Control sites | 124 (59) | 41 (19) | 44 (21) | 107 (48) | 28 (12) | 87 (39) |
Data presented as n (%)
Pre- and postintervention phase PHN surveys
PHN attitudes and beliefs regarding pain and pain management are summarized for baseline and postimplementation phases in the control and intervention groups in Table 2. After implementation, confidence and satisfaction with ability to reduce pain increased (P=0.016 and P<0.001, respectively) within the intervention group. Willingness to use new strategies also increased (P<0.001). No significant differences were observed in the control sites (P≥0.19 for all analyses).
TABLE 2.
Attitudes and beliefs of public health nurses toward immunization pain and pain management strategies
| Scope of question | Attitudes and beliefs toward immunization pain and pain management strategies |
Intervention sites
|
Control sites
|
||||||
|---|---|---|---|---|---|---|---|---|---|
|
Percentage of ‘strongly agree’, ‘agree’, or ‘yes’ responses (n=27)
|
% difference post versus baseline (95% CI) |
Percentage of ‘strongly agree’, ‘agree’, or ‘yes’ responses (n=16)
|
% difference post versus baseline (95% CI) | P | |||||
| Baseline | Post | P | Baseline | Post | |||||
| Perception of immunization pain | It is important to prevent children’s pain and distress during immunization injections. | 88.9 | 92.6 | +3.7 (−13.8 to 21.5) | 1.0 | 100.0 | 100.0 | 0.0 | – |
| Self-perceived confidence | I am confident in my ability to minimize children’s pain and distress during immunization injections. | 66.7 | 92.6 | +25.9 (4.2 to 45.5) | 0.016* | 81.3 | 87.5 | +6.3 (−2.0 to 32.1) | 1.0 |
| Self-perceived satisfaction | I am satisfied with how I currently manage pain and distress in children undergoing immunization injections | 48.1 | 88.9 | +40.7 (16.1 to 59.6) | 0.001* | 93.8 | 81.3 | −12.5 (−37.3 to 12.7) | 0.50 |
| Summary score of willingness to use four newly recommended strategies, mean ± SD (0 = none, 4 = all) | Difference in mean score post versus baseline (95%CI) | P | Summary score of willingness to use four newly recommended strategies, mean ± SD (0 = none, 4 = all) | Difference in mean score post versus baseline (95% CI) | P | ||||
|
| |||||||||
| Willingness to use newly recommended pain management strategy | I would recommend breastfeeding, sucrose solution, rubbing skin near injection, and/or provider-led distraction. | 2.9±0.87 | 3.7±0.68 | +0.74 (0.36 to 1.1) | <0.001† | 3.1±0.77 | 3.3±0.86 | 0.19 (−0.10 to 0.48) | 0.19 |
P<0.05 (corrected using Bonferroni; P<0.013), comparisons between baseline versus postintervention (post) using McNemar’s test,
P<0.05 (corrected using Bonferroni; P<0.013), comparisons between baseline versus postintervention using paired t test
Use of pain-relieving strategies during immunization
Compared with baseline, intervention sites reported a significant increase in the postimplementation phase in overall use of at least one of the four new strategies recommended in the guideline (49.8% to 77.6%; +27.8% [95% CI 19.6% to 35.4%]; P<0.001); control sites did not report significant increase (84.7% to 90.1%; +5.4% [95% CI −0.01% to 11.8%]; P=0.09) (Table 3). At the intervention sites, there was a significant increase (de novo) in sucrose use, and an increase in breastfeeding in infants. In children ≥4 years of age, use of tactile stimulation increased significantly. Provider-led distraction, an intervention suitable for all ages, was significantly increased overall.
TABLE 3.
Use of new pain-relieving strategies in baseline (preimplementation) and postimplementation phases in the study groups
| Intervention type* | Study group | Baseline, % | Postimplementation, % | Difference, % (95% CI) | P |
|---|---|---|---|---|---|
| Pharmacological strategies¶ | |||||
| Breastfeeding | Intervention sites | 10.2 | 23.3 | +13.1 (5.3 to 21.1) | 0.001† |
| Control sites | 25.0 | 24.3 | −0.7 (−11.6 to 10.5) | 0.90 | |
| Sweetening agents | Intervention sites | 0.0 | 45.9 | +45.9 (38.1 to 53.7) | <0.001† |
| Control sites | 0.8 | 3.7 | +2.9 (−1.3 to 8.4) | 0.13 | |
| Physical interventions and injection techniques | |||||
| Rub skin near injection site | Intervention sites | 15.4 | 57.1 | +41.8 (9.4 to 61.5) | 0.012† |
| Control sites | 68.2 | 27.6 | −40.6 (−55.0 to 22.7) | <0.001† | |
| Psychological strategies | |||||
| Distraction – provider-led | Intervention sites | 43.7 | 58.2 | +14.5 (5.9 to 22.9) | 0.001† |
| Control sites | 78.5 | 78.8 | +0.3 (−7.4 to 8.1) | 0.51 | |
| Overall use of at least one new pain-relieving strategy | Baseline, % | Postimplementation, % | Difference, % (95% CI) | P | |
| Intervention sites | 49.8 | 77.6 | +27.8 (19.6 to 35.4) | <0.001‡ | |
| Control sites | 84.7 | 90.1 | +5.4 (−0.01 to 11.8) | 0.09 | |
| Overall mean number of new pain-relieving strategies used | Baseline, mean ± SD | Postimplementation, mean ± SD | Difference, mean (95% CI) | P | |
| Intervention sites | 0.53±0.55 | 1.1±0.77 | +0.58 (0.49 to 0.71) | <0.001§ | |
| Control sites | 1.1±0.62 | 1.1±0.51 | +0.026 (−0.081 to 0.13) | 0.63 | |
Age group for each strategy varies (breastfeeding and sweetening agent ≤1 year; rub skin near injection site ≥4 years; distraction – provider-led = any age group);
P<0.05 (corrected using Bonferroni, P<0.013), comparisons between baseline versus postintervention using χ2 test;
P<0.05, comparisons between baseline versus postintervention using χ2 test;
P<0.05, comparisons between baseline versus postintervention using t test;
Topical anesthetics were not included
The overall mean number of new strategies used in intervention sites increased (0.53 to 1.1, mean difference = +0.58 [95% CI 0.49 to 0.71]; P<0.001) but did not change in control sites (1.1 to 1.1, mean difference = +0.03 [95% CI −0.08 to 0.13]; P=0.63) (Table 3).
DISCUSSION
We found that implementation of a new CPG regarding pain management during childhood vaccine injections in a public health setting using a multifaceted knowledge translation intervention improved immunizers’ attitudes and beliefs about pain and pain management and increased the use of new analgesic interventions during routine childhood immunization injections.
The absolute increase (28%) in the utilization rate of new pain-relieving strategies observed in the present study was much greater than the 10% increase commonly accepted to be clinically significant in other studies of knowledge translation interventions (12). That the newly recommended strategies are also among the most effective strategies recommended in the HELPinKIDS CPG (considering their effect sizes and level of evidence) (10,13) suggests that these results are highly significant from a clinical perspective.
In addition, we demonstrated that implementation of the guideline led to a positive impact on immunizers’ confidence and satisfaction with the ability to reduce immunization injection pain and willingness to use the new strategies. We postulate that the improved confidence and satisfaction can sustain the increased use of these strategies because reducing distress in children reduces immunizer stress, which in turn, improves job satisfaction. That pain is important to immunizers was reported by Woodin et al (14), who showed that pain is the most concerning factor for physicians regarding multiple vaccine injections.
We hypothesize that the success of our implementation was due to the multifaceted approach that specifically addressed the key barriers to change including awareness and knowledge of relevant research evidence, organizational support, acceptance and beliefs, skills, availability of resources and attitudes about change (15–17). First, the BC Immunization Program Manual recommendations were based on the HELPinKIDS CPG, which is strictly evidence based (10). This raises PHNs’ awareness and knowledge of relevant research evidence. Second, the resources (education and supplies) needed to perform better pain management was supported by the overseeing public health organization, demonstrating organizational support. Third, interactive educational services were delivered by respected peers who could offer support, approval and feedback, and addressed the need for continuing professional competence (18), which may improve acceptance and beliefs, and attitudes about change through role modelling. A variety of other studies have demonstrated the effectiveness of educational outreach in changing clinical behaviour and practice (16,19).
Optimizing childhood immunization coverage is necessary to control vaccine-preventable disease transmission and prevent outbreaks (20,21). Concern about injection-associated pain is a well-documented barrier to immunization adherence in children (6,22–26). We hypothesize that improvements in analgesia during immunization will increase adherence to the vaccine schedule and subsequent childhood immunization coverage. Preliminary evidence supporting this hypothesis was provided by a survey study in which one in 12 children and adults alike reported noncompliance with immunization as a result of needle fear (27), and a study of adult H1N1 vaccination in which 5% of participants admitted to being vaccinated because they were guaranteed analgesia during the injection (28). Because two-thirds of all immunizations in BC are provided by PHNs, there is potential for significant improvement in pain management practices as a result of uptake of guideline recommendations.
There are several limitations to our study that warrant discussion. First, outcomes were collected using self-report and it is possible that nurses recorded either more or less strategies than they actually used. Second, our sample size was small and uneven between intervention and control sites. The high participation rate and inclusion of both urban and rural health units increased the generalizability of our results provincially, but it is unclear whether they can be generalized to other provinces. Third, sustainability of the observed improvements was not measured. We postulate that the increased use of newly recommended strategies may be self-sustainable through improved confidence and satisfaction of immunizers, but additional studies are recommended to verify this assertion. Fourth, the reliability and validity of our survey and checklist were not measured. Survey questions, however, were modified from previous pain studies evaluating attitudes, behaviours and knowledge in health care providers caring for children. Finally, it is possible that participating nurses were exposed to the HELPinKIDS CPG before the study commenced because the CPG was published and widely available. High use of at least one new pain-relieving strategy at baseline (ie, preimplementation) is evidence of this, and was mainly due to use of provider-led distraction by PHNs, despite the lack of official recommendation by the BCCDC. Thus, our estimates of the impact of our intervention may actually under-represent the true impact due to contamination.
It is important to note that we did not assess clinical outcomes, such as pain, because this was not deemed necessary. Numerous trials have already demonstrated the effectiveness of the interventions being implemented and that analgesia is improved when individual strategies are combined (10,29). These trials collectively formed the evidence base for our CPG. In the present study, our objective was to document guideline implementation. In addition, our study did not obtain child and parent measures of satisfaction. Again, it has already been demonstrated that satisfaction is improved with increased analgesic use (16). Instead, our study focused on guideline implementation within the public health context, for which there were no previous data. Public health is the predominant immunizer in Canada; therefore, the overall impact of changing attitudes, beliefs and practices of PHNs regarding the use of pain-relieving strategies has widespread implications.
Strengths of the study include the randomized design (intervention sites were randomized), standardized data documentation procedures, high participation and response rate from PHNs, inclusion of both urban and rural PHNs to maximize generalizability of results, and the use of a nonintervention control group to rule out time-dependent changes in practice patterns.
The present study demonstrated improved PHN immunizers’ attitudes, beliefs and increased use of newly recommended pain-relieving strategies during childhood immunization injections after implementation of a vaccination pain management guideline using a multifaceted knowledge translation intervention. The results of the present study may be used as a benchmark for future knowledge translation and quality improvement efforts. Customization and implementation of the guideline in the curricula of all immunizers (physicians, nurses, pharmacists) and different practice settings is recommended to facilitate additional knowledge transfer.
Appendix 1).
Health care provider pain documentation tool. Mos Months
Footnotes
DISCLOSURES: This study was funded by the British Columbia Immunization Committee. Dr Anna Taddio has received research support from Pfizer and research study supplies from Natus and Ferndale, and has consulted for Archimedes. The authors have no other competing interests to declare.
REFERENCES
- 1.Ellis J, Sharp D, Newhook K, Cohen J. Selling comfort: A survey of interventions for needle procedures in a pediatric hospital. Pain Manag Nurs. 1994;5:144–52. doi: 10.1016/j.pmn.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Megel M, Heser R, Matthews K. Parents’ assistance to children having immunizations. Issues Compr Pediatr Nurs. 2002;25:151–65. doi: 10.1080/01460860290042585. [DOI] [PubMed] [Google Scholar]
- 3.Hart D, Bossert E. Self-reported fears of hospitalized school-age children. J Pediatr Nurs. 1994;9:83–90. [PubMed] [Google Scholar]
- 4.Broome M, Hellier A. School-age children’s fears of medical experiences. Issues Compr Pediatr Nurs. 1987;10:77–86. doi: 10.3109/01460868709009015. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton J. Needle phobia: A neglected diagnosis. J Fam Pract. 1995;41:169–75. [PubMed] [Google Scholar]
- 6.Taddio A, Chambers C, Halperin S, et al. Inadequate pain management during routine childhood immunizations: The nerve of it. Clin Ther. 2009;31(Suppl 2):S152–67. doi: 10.1016/j.clinthera.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 7.Lovering S. Cultural attitudes and beliefs about pain. J Transcult Nurs. 2006;17:389–95. doi: 10.1177/1043659606291546. [DOI] [PubMed] [Google Scholar]
- 8.Lewis T, DiLillo D, Peterson L. Parental beliefs regarding developing benefits of childhood injuries. Am J Health Behav. 2004;28:S61–8. doi: 10.5993/ajhb.28.s1.7. [DOI] [PubMed] [Google Scholar]
- 9.Craig K, Lilley C, Gilbert C. Social barriers to optimal pain management in infants and children. Clin J Pain. 1996;12:232–42. doi: 10.1097/00002508-199609000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Taddio A, Appleton M, Bortolussi R, et al. Reducing the pain of childhood vaccination: An evidence-based clinical practice guideline. CMAJ. 2010;182:E843–55. doi: 10.1503/cmaj.101720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Immunization Manual Chapter 2 Section IVB. British Columbia Centre for Disease Control. 2012. <www.bccdc.ca/NR/rdonlyres/F837F34A-09A0-40E6-8B53-8602B8B7969A/0/SectionIVB_RIIP_final_June2012.pdf> (Accessed April 8, 2013)
- 12.Grimshaw J, Eccles M, Thomas R, et al. Evidence and its limitations of the effectiveness of guideline dissemination and implementation strategies 1966–1998. J Gen Intern Med. 2006;21:S14–20. doi: 10.1111/j.1525-1497.2006.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah V. Effectiveness and tolerability of pharmacologic and combined interventions for reducing injection pain during routine childhood immunizations: Systematic review and meta-analyses. Clin Ther. 2009;31(Suppl B):S104–51. doi: 10.1016/j.clinthera.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Woodin K, Rodewald L, Humiston S, Carges M, Schaffer S, Szilagyi P. Are children becoming pincushions from immunizations? Arch Pediatr Adolesc Med. 1995;149:845–9. doi: 10.1001/archpedi.1995.02170210019003. [DOI] [PubMed] [Google Scholar]
- 15.Grimshaw J, Eccles M, Walker A, Thomas R. Changing physicians’ behaviour: What works and thoughts on getting more things to work. J Contin Educ Health Prof. 2002;22:237–43. doi: 10.1002/chp.1340220408. [DOI] [PubMed] [Google Scholar]
- 16.Schechter N, Bernstein B, Zempsky W, Bright N, Willard A. Education outreach to reduce immunization pain in office settings. Pediatrics. 2010;126:1514–21. doi: 10.1542/peds.2010-1597. [DOI] [PubMed] [Google Scholar]
- 17.How to change practice. National Institute for Health and Clinical Excellence Created 2007. <www.nice.org.uk/media/AF1/73/HowToGuideChangePractice.pdf> (Accessed February 13, 2012)
- 18.Grol R. Beliefs and evidence in changing clinical practice. BMJ. 1997;315:418–21. doi: 10.1136/bmj.315.7105.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Brien M, Rogers S, Jamtvedt G, et al. Education outreach visits: Effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2007;4:CD000409. doi: 10.1002/14651858.CD000409.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diekema D. Improving childhood vaccination rates. N Engl J Med. 2012;366:391–3. doi: 10.1056/NEJMp1113008. [DOI] [PubMed] [Google Scholar]
- 21.Omer S, Salmon D, Orenstein W, deHart M, Halsey N. Vaccine refusal, mandatory immunization, and the risk of vaccine-preventable diseases. N Engl J Med. 2009;360:1981–8. doi: 10.1056/NEJMsa0806477. [DOI] [PubMed] [Google Scholar]
- 22.Mills E, Montori V, Ross C, Shea B, Wilson K, Guyatt G. Systematically reviewing qualitative studies compliments survey design: An exploratory study of barriers to paediatric immunizations. J Clin Epidemiol. 2005;58:1101–8. doi: 10.1016/j.jclinepi.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Yawn B, Xia Z, Edmonson L, Jacobson R, Jacobsen S. Barriers to immunization in a relatively affluent community. J Am Board Fam Pract. 2000;13:325–32. [PubMed] [Google Scholar]
- 24.Guerra F. Delays in immunization have potentially serious health consequences. Paediatr Drugs. 2007;9:143–8. doi: 10.2165/00148581-200709030-00002. [DOI] [PubMed] [Google Scholar]
- 25.Abbotts B, Osborn L. Immunization status and reasons for immunization delay among children using public health immunization clinics. Am J Dis Children. 1993;147:965–8. doi: 10.1001/archpedi.1993.02160330055018. [DOI] [PubMed] [Google Scholar]
- 26.Samad L, Butler N, Peckham C, Bedford H, for the Millennium Cohort Study Child Health Group Incomplete immunization uptake in infancy: Maternal reasons. Vaccine. 2006;24:6823–9. doi: 10.1016/j.vaccine.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 27.Taddio A, Ipp M, Thivakaran S, et al. Survey of the prevalence of immunization non-compliance due to needle fears in children and adults. Vaccine. 2012;30:4807–12. doi: 10.1016/j.vaccine.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Taddio A, Lord A, Hogan ME, et al. A randomized controlled trial of analgesia during vaccination in adults. Vaccine. 2010;28:5365–9. doi: 10.1016/j.vaccine.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Schechter N, Zempsky W, Cohen L, McGrath P, McMurtry C, Bright N. Pain-relieving during pediatric immunizations: Evidence-based review and recommendations. Pediatrics. 2007;119:e1184–9. doi: 10.1542/peds.2006-1107. [DOI] [PubMed] [Google Scholar]