Abstract
Heart failure (HF) in the elderly is a major public health problem, and its prevalence is rising. Outcomes of HF in the elderly have not changed in the past 2 decades despite the introduction of novel HF therapies. This may be due to the combined impact of multiple comorbidities and frailty. The majority of elderly patients with HF are frail with multiple comorbidities. These comorbidities, along with frailty, contribute to the poor outcome of HF in the elderly and pose independent management challenges. More research is needed to: better understand the interaction between frailty and multiple comorbidities and the mechanisms by which they impact HF and its management; develop prognostic tools that incorporate frailty and multiple comorbidities and provide more accurate prediction of outcomes; test available treatments in typical elderly patients; and develop and test novel interventions that directly address the adverse impact of multiple co-morbidities and frailty.
Keywords: Aging, chronic diseases, rehospitalization, outcomes
I. Introduction
According to the American Heart Association 2011 update on heart disease and stroke statistics, there are more than 5.7 million individuals living with heart failure (HF) in the United States [1]. In 2007, HF was responsible for nearly 1 million hospitalizations, 3.4 million outpatient visits, and 60,000 deaths [1]. Both incidence and prevalence of HF increase steeply with age. The annual incidence of HF in both men and women doubles with every one decade increase in age after age 65, and the prevalence of HF increases from less than 0.5% in the age group of 20-39 year old, to more than 10% in those 80 years of age and older [1]. Although age-adjusted incidence of HF has seen some decline in the past 2 decades, especially in women, the overall incidence and prevalence of HF in the elderly is still rising [2]. This may be explained partly by the improvement in life expectancy and in survival of patients with ischemic heart disease [3]. Eighty percent of patients with HF are elderly (65 years and older) whose disease differs significantly from that seen in middle-aged adults [1]. Elderly patients with HF are more likely to be women, and to have frailty, higher burden of comorbidities, and preserved left ventricular ejection fraction [4].
Mortality and rehospitalization rates in elderly patients with HF have not substantially improved in the past 2 decades despite the many advances in available therapies. According to the Framingham Heart Study, 59% of men and 45% of women age 65 to 74 die within 5 years of being diagnosed with HF, with an average one-year mortality rate of 20% [5,1]. Although there has been some overall improvement in survival of patients with HF over the past two decades [5], the survival of the more common type of HF in the elderly, heart failure with preserved ejection fraction (HFPEF), has not changed [2]. The rate of rehospitalization among elderly patients with HF has also remained high [6,7]. According to Jencks et al, one-fifth of Medicare beneficiaries hospitalized with HF are readmitted within 30 days and almost one-third are readmitted within 90 days from the day of discharge [7]. The most striking finding is that only 37% of those readmitted had HF as their primary readmission diagnosis, while others were readmitted for indications other than HF [7].
Novel therapies and other interventions have failed to show significant benefit in improving outcomes of elderly patients with HF. For instance, several medications that are proven effective in younger patients with HFREF (heart failure with reduced ejection fraction) have been tried in older patients with HFPEF and failed to show any benefit [8-10]. Other strategies in the management of HF have also been tried in this patient population, including telemonitoring and post-discharge care management, and showed no significant impact on mortality or rehospitalization rates [11].
Relative lack of progress in improving outcomes in older persons with HF may be due to the fact that most studies have not taken into account two common and key characteristics of elderly patients with HF, frailty, and multiple comorbidities. In this review, we will describe frailty and comorbidity and discuss their coexistence and interaction in the elderly person; review the available evidence about the impact of frailty and multiple comorbidities on the outcomes of elderly patients with HF; and finally discuss some of the implications these two entities have for the management of HF in elderly.
II. Frailty and Heart Failure in the Elderly
The concept of frailty in the medical literature has evolved from being synonymous to advanced age, disability, or comorbidity to being a distinct biological syndrome defined as a state of decreased reserve and resistance to stressors, resulting from cumulative declines across multiple physiologic systems, and causing vulnerability to adverse outcomes [12]. Sarcopenia, a process that is central in the pathophysiology of frailty, is defined as having lean body mass two standard deviations below the sex-specific mean in a young healthy sample [13]. Several diagnostic and staging criteria of frailty have been proposed, but the criteria proposed by Fried et al have been most widely used and proven clinically useful. According to Fried et al [12], a person is considered frail if three or more of the following criteria are present: weight loss of more that 10 lbs in one year; physical exhaustion by self report; weakness as measured by grip strength; decline in walking speed; and low physical activity. Based on Fried criteria, it is estimated that 6.9% of older community dwelling adults are frail. This prevalence increases sharply with age, from 3.2% among persons 65-70 years old to 23.1% among persons 90 years and older [12].
Several studies have shown significant associations between frailty and cardiovascular disease, including HF. In the Cardiovascular Health Study (CHS), the prevalence of HF increased from 1.8% in the non-frail, 4.6% in the intermediate group, to 14.0% in the frail group, with an adjusted odds ratio of 7.51 (95% confidence interval: 4.66-12.12) [14]. Similarly, participants of the women health initiative (WHI) who have HF were 6 to 7 folds more likely to be frail (OR = 6.16, 95% CI = 4.97-7.64) [15]. This relationship between frailty and HF may potentially be explained by the common inflammatory, metabolic, and autonomic abnormalities seen in both clinical entities. A substudy from CHS found that frail persons have significantly higher levels of C-reactive protein, factor VIII, and D-dimers even after adjusting for age and associated comorbidities [16]. Similarly, frail women form the women's health and aging studies (WHAS) were found to have higher levels of IL-6 and white blood cell counts [17]. A recent analysis of the Third National Health and Nutrition Survey (NHANES III) found that vitamin D deficiency was associated with 4 fold increase in the risk of frailty [18]. Finally several reports have shown association between frailty and autonomic dysfunction as measured by reduced heart rate variability [19]. All these inflammatory, metabolic, and autonomic abnormalities that are associated with frailty are also frequently seen in patients with HF [20,21].
Frailty is associated with worse outcome in elderly patients with HF. Among 120 patients with HF (age 75.9±6.7), Cacciatore et al found significantly higher risk of death in those with a Lachs's frailty score of 2 or 3 than in those with a score of 0 or 1 (adjusted hazard ratio1.62, 95% confidence interval: 1.08-2.45) [22]. After 9 years of follow-up, Cacciatore reported that the probability of death in patients with HF and frailty score of 3 was 100% as compared with 55% in patients with HF and frailty score of 1 (Figure: 1). Another study by Pulignano showed significantly higher rate of 1-year mortality (16.9% vs. 4.8%; P < 0.001) and higher rate of hospitalization (20.5% vs. 13.3%; P = 0.01) in elderly patients with HF who have frailty than in those who do not [23].
Cachexia is an important phenomenon in HF patients that is associated with poor outcomes. It shares some common features with frailty, though is in other ways distinct. According to the Cachexia Consensus Conference of December 2006, cachexia is defined as “a complex metabolic syndrome associated with underlying illness and characterized by weight loss of 5% in 12 months or less (or having a body mass index (BMI) < 20) in addition to satisfying at least 3 of the following 5 criteria: decreased muscle strength, fatigue, anorexia, low fat-free mass index, or abnormal biochemistry (inflammation, anemia, or low serum sodium)” [24]. Cardiac cachexia represents the terminal phase of body wasting seen in advanced stages of HF [25]. From the definition of cachexia, we appreciate how it shares several clinical features with frailty such as fatigue, muscle weakness, sarcopenia, neurohormonal activation, and inflammation. However, as discussed earlier in this review, frailty is characterized by a progressive decline in physiologic reserve, is associated with aging, and while it usually occurs with chronic illness, it can occur without an underlying chronic illness. In addition, while weight loss is essential for the diagnosis of cachexia, it is not necessarily present in frailty as the loss in muscle mass can be counterbalanced by an increase in total body fat mass, so-called ‘sarcopenic obesity’.
It has been shown that body wasting and cachexia in HF are associated with higher mortality rate, while greater body weight, even up to the moderate obesity range, appears to be associated with increased survival. Anker et.al showed that cachectic state is a strong independent risk factor of mortality in patients with HF, and when combined with low peak oxygen consumption, it identifies a subset of patients at extremely high risk of deaths [26]. In agreement with other studies in younger pateints with HFREF, among older patients with HFPEF who were participants in the Irbesartan in Heart Failure with Preserved Ejection Fraction Study (I-Preserve), higher BMI was associated with more favorable outcome, and the relationship between BMI and survival was non-linear and took a U shape [27]. While many studies have shown an association between higher BMI and better survival, there is a lack of randomized trials that examine the effect of purposeful, intentional weight loss on survival, not only in heart failure but in most other conditions [28]. In the recent first published report of the effect of intentional, controlled weight loss in a large clinical trial of elderly with chronic conditions, there was improvement in survival with intentional and controlled weight loss [29]. Whether this favorable outcome of intentional weight loss can be demonstrated in older persons with HF remains to be tested.
III. Comorbidities and Heart Failure in the Elderly
Feinstein recognized comorbid illnesses as important determinants of health outcomes. He defined comorbidity as “any distinct additional entity that has existed or may occur during the clinical course of a patient who has the index disease under study” [30]. Another definition of comorbidities was provided by Guralnik as the co-occurance of multiple diseases in one person [31].
Comorbidities are common among the elderly and far more common among those with HF. Wolff reported that 82% of the 1999 medicare beneficiaries aged ≥ 65 had one or more chronic conditions and 65% had multiple chronic conditions [32]. The burden of comorbidities in elderly patients with HF is much higher than in those without HF [3,33]. Braunstein et al studied the burden of non-cardiac comorbidities in 22,630 HF patients, 65 years and older, identified in a 5% random sample of all US Medicare beneficiaries [34]. He found that 40% of these patients had ≥ 5 comorbidities, 70% had ≥ 3 comorbidities, and only 4% had no comorbidities at all [34]. This is in strong contrast to middle aged patients with HF who usually have a lesser burden of comorbidities.
There is growing evidence indicating that comorbidities, frequently seen in elderly patient with HF, contribute greatly to their poor outcomes. Braunstein et al demonstrated that the 40% of the elderly HF population who have ≥5 comorbidities were responsible for 81% of the total inpatient hospital days experienced by all HF patients [34]. He also found that the risk of hospitalization and potentially preventable hospitalization strongly increased with the increase in the number of associated comorbidities (both p values < 0.0001). After controlling for demographic factors and other diagnoses, comorbidities that were consistently associated with notably higher risks for CHF-preventable and all-cause preventable hospitalizations, and mortality, included chronic obstructive pulmonary disease and/or bronchiectasis, renal failure, diabetes mellitus, depression, and other lower respiratory diseases (all p values were less than 0.01) [34]. Similar findings were recently reported by Muzzarelli who found that the majority of 30-day and 90-day readmissions of patients with HF from the Trial of Intensified versus standard Medical therapy in Elderly patients with Congestive Heart Failure (TIME-CHF) were for non-cardiac conditions [35].
IV. The Relationship between Frailty and Comorbidity
Frailty and comorbidity are clinical manifestations of 2 distinct aging-related processes, namely diminished functional reserve and accumulation of pathological processes. Nevertheless, frailty and comorbidity often overlap in the elderly and lead to impairment in quality of life and functional status. Wong et al recently reported that among community dwelling seniors who are frail 82% have comorbidities, 29% have disability in at least one activity of daily living (ADL), and 93% have disability in at least one instrumental activity of daily living (IADL) [36]. Similar overlap between frailty, comorbidity, and disability has been reported among CHS participants (Figure 2) [12].
Figure 2.
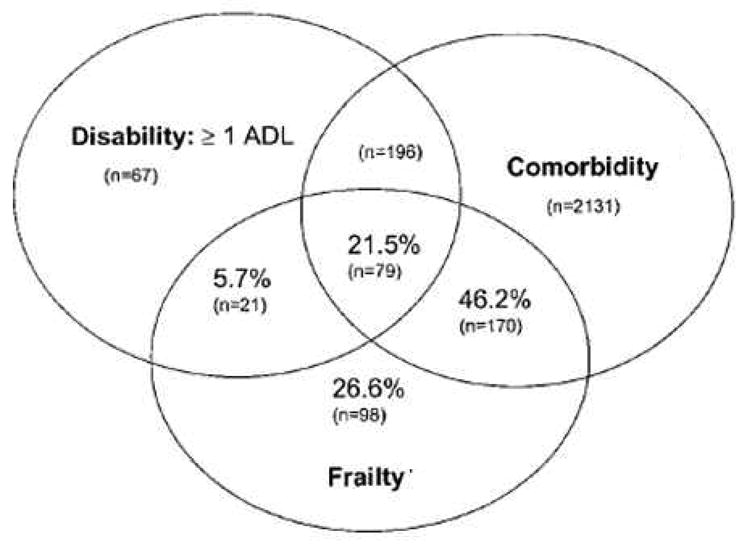
Venn diagram displaying the extent of overlap of frailty with ADL disability and comorbidity (≥2 diseases) in 2,762 subjects who have at least one of these 3 entities (reproduced with permission from Fried et al [12]).
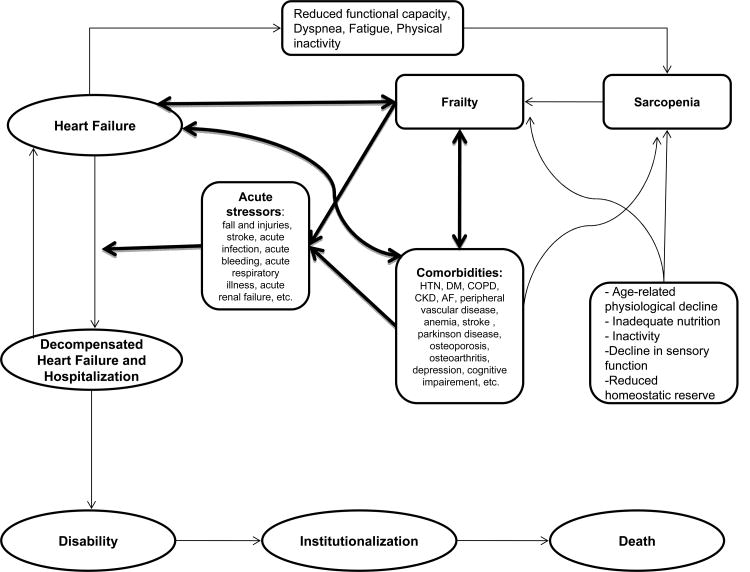
The interaction between disease processes and normal physiologic processes of aging is an important element in the development of frailty. Morley described how disease processes (HF as well as its associated comorbidities) can accelerate functional muscle loss leading to sarcopenia, the hallmark of functional frailty [13,37]. Once frailty is developed, both HF and its associated comorbidities can give rise to acute stressors which can lead to rapid functional decline resulting in disability, hospitalization, institutionalization, and eventually death (figure 3). Examples of such acute stressors are decompensated HF, acute exacerbation of chronic obstructive pulmonary disease, pneumonia, urinary tract infection, acute delirium, acute myocardial infarction, acute stroke, falls, acute injuries, and others. This ill response of a frail person to acute stressors is explained partially by the limited reserve of physiologic systems, but more importantly by the inefficient use of energy, a pathological response to the limited physiologic reserve of frailty [38]. Interventions that target already frail persons and improve the efficiency of their energy use and therefore the response to acute stressors could have potentially large impact on outcomes in elderly heart failure patients.
Figure 3.
An illustration of the complex relationships between frailty, comorbidities, and heart failure. Notice how heart failure contributes to the development of frailty, and how both frailty and comorbidities give rise to acute stressors resulting in deterioration of the clinical status of patients with heart failure.
V. Implications for Management
Frailty and multiple comorbidities adversely impact the management of HF in the elderly on many levels including recruitment and representation in clinical trials, early and accurate diagnosis of HF, treatment of HF and its associated comorbid conditions, and providing accurate prognosis to guide clinical care.
Elderly patients with HF have been clearly underrepresented in randomized clinical trials (RCTs) [39]. In fact, the more common form of HF in the elderly (HFPEF) was not formally recognized as a genuine form of HF until 2004 and to date, it has only been represented in a handful of large clinical trials [39]. In HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of exercise training) [40], despite the lack of upper age limit in the inclusion criteria and the emphasis on recruitment of older persons, the mean age of participants (59 years old) was well below the average age of persons with HF as reported in population studies. This suggests that other characteristics of the elderly, in addition to age, contribute to their exclusion from HF trials. Such characteristics include frailty and multiple comorbidities. Frail patients are more likely to be excluded from trials based on their limited mobility and access to research facilities, their cognitive impairment that limits the ability to understand and sign informed consents, and their presumed intolerance of the intervention being studied [41]. Patients with multiple comorbidities have been often excluded from clinical trials, often for the concern of being able to study the index disease in its “pure” form [39]. By excluding patients with frailty and multiple comorbidities from HF research, studies fail to capture these two key determinants of outcomes in the elderly HF patients. Despite this inherent selection bias of clinical trials toward excluding multiple comorbidities, the outcomes appear to be persuaded by them. For instance, the I-PRESERVE trial reported a majority of clinical events as non-cardiac and likely attributable to comorbidities and frailty rather than to HF [8].
This underrepresentation of elderly patients with HF in RCTs, especially those with frailty and multiple comorbidities, has resulted in a paucity of evidence in this patient population and rendered many aspects of their care largely empirical. It is uncertain whether trial results obtained in younger patients pertain directly to the management elderly persons with HF, as growing evidence has shown that older patients respond differently to current therapies than do younger patients with HF. In TIME-CHF, investigators demonstrated that HF therapy guided by N-terminal brain natriuretic peptide improved outcomes in patients aged 60 to 75 years but not in those aged 75 years or older (p value < 0.02 for interaction) [42]. Similarly, recent trials testing drugs known to be effective in younger patients with HFREF failed to show any significant impact on outcomes in older patients with HFPEF [8,9].
Frailty and multiple comorbidities delay and complicate the diagnosis of HF in the elderly. Fuat et al identified several barriers to accurate and effective diagnosis of HF in the primary care setting, many of which can be linked to the presence of frailty and comorbidities [43]. In elderly frail persons fatigue and decreased exercise endurance are common; dyspnea is a frequent symptom and often attributed to other comorbid conditions; and fluid overload can be subtle and difficult to detect by physical examination. Left ventricular ejection fraction is normal or high in more than 60% of elderly patients with HF [4]. Nevertheless, early and accurate diagnosis of HF in the primary care setting is important since it prompts the implementation of proper therapy, and may prevent unnecessary hospitalizations. The utility of newer diagnostic tools, such as tissue doppler, cardiac magnetic resonance imaging, and serologic markers, should be evaluated in this patient population.
Frailty and multiple comorbidities often limit implementation and tolerability of conventional HF therapies. Diuretics therapy in a frail person is more likely to lead to urinary incontinence, electrolytes imbalance, progression of renal dysfunction, delirium, and falls. Similarly, vasodilators therapy in a frail person is more likely to lead to orthostatic hypotension due to arterial sclerosis and autonomic dysfunction. Some comorbid conditions present relative or even absolute contraindication to the use of certain HF therapies such as the use of inhibitors of the renin-aldosterone-angiotensin axis in patients with chronic kidney disease and the use of beta blocking agents in patients with chronic obstructive pulmonary disease.
Management of certain comorbid conditions in elderly HF patients is also challenged by the presence of frailty and other comorbidities. Optimal control of systolic and diastolic hypertension, a class IA recommendation according to the ACC/AHA guidelines for the management of both HFPEF and HFREF [44], can be difficult to attain in the elderly HF patient. Orthostatic hypotension, hyperkalemia, worsening renal failure, lower extremity edema, and bronchospasm are known adverse effects of different classes of antihypertensive medications and are more often seen in the elderly frail patients. Management of atrial fibrillation (AF) in elderly patients with HF is likewise challenging, and with lower rates of success. These patients are often intolerant to rate controlling agents, leading to symptomatic bradycardia and resulting in syncope, falls, and decompensation of HF due to transient reduction in cardiac output. Rhythm maintaining agents are associated with potentially serious side-effects and proarrhythmic risk. Catheter ablation for restoration and maintenance of sinus rhythm has not been as fully evaluated in this patient population. While these patients clearly benefit from anticoagulation therapy, their risk of bleeding complications is much higher as a result of their increased risk of falling, drug and dietary interaction, cognitive impairment, and lower compliance. Atrial fibrillation also impacts the clinical course HF in the elderly by accelerating the development and progression of frailty. The loss of atrial contraction in AF can decrease exercise cardiac output resulting in reduced exercise tolerance and contributing to the development and progression of frailty [45]. In addition, the higher incidence of stroke in atrial fibrillation can result in limited mobility, disability, and cognitive impairment. Other challenges are encountered when managing other comorbidities in elderly patients with HF, such as chronic kidney disease and chronic obstructive pulmonary disease, with limited evidence to guide clinical care.
Non-adherence to medical care is common among the elderly, and is associated with poor outcomes [46]. Mocker et al found that non-persistence with medication was an independent predictor of HF rehospitalization [47]. Non-adherence to medical therapy was independently associated with higher mortality among participants in the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity (CHARM) trial [48]. Frailty and multiple comorbidities possibly contribute to non-compliance in elderly patients with HF leading to higher rate of hospitalization, rehospitalization, and ultimately institutionalization and death. Frail patients may have more limited access to healthy food, and medical follow-up. Patients with cognitive and sensory impairment are less likely to adhere to dietary restrictions, prescribed medications, weight monitoring, and timely reporting of symptoms. Patients with physical impairment and with disability are less likely to participate in physical exercises and cardiac rehabilitation programs. Patients with multiple comorbidities are often on complex medication regimes which may increase the risk of non-adherence, drug-to-drug interaction, and serious adverse effects.
Accurate estimation of prognosis of elderly patients with HF would likely empower them and their clinicians to make informed decisions about their care and would likely result in a more responsible and efficient use of health care resources. While several risk prediction models have been developed for patients with HF to estimate mortality risk and risk of hospitalization, these models did not include measures for frailty and multiple comorbidities and were derived from cohorts of younger patients often with HFREF or from participants in clinical trials with their inherent selection bias [49,50]. Reseach is needed to develop risk prediction models specific for the elderly HF patients that include measures for frailty and the burden of comorbidities.
VI. Conclusion
Frailty and multiple comorbidities are 2 distinct characteristics seen in older patients and are highly prevalent among elderly patients with HF. Frailty and multiple comorbidities often coexist and interact in this patient population adversely impacting their clinical course and their outcomes (figure 3). Elderly HF patients who are frail and have multiple comorbidities are much more likely to be hospitalized, rehospitalized, become disabled, be institutionalized, and ultimately die. Therapeutic interventions targeting specifically HF alone have failed to substantially improve outcomes in this patient population. Future research should aim at preventing the development of frailty and its progression to disability, and providing optimal management of comorbidities in order to improve outcomes in elderly patients with HF.
Figure 1.
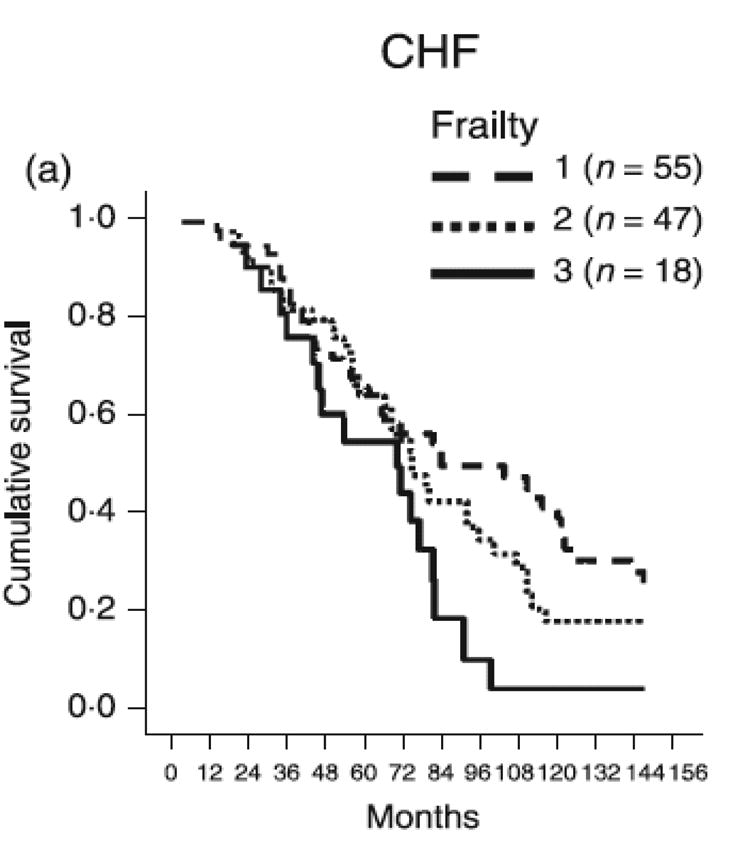
Cox regression adjusted survival curve of patients with HF stratified by Lachs Frailty score (reproduced with permission from Cacciatore et al [22]).
Acknowledgments
Funding Sources: N.I.H. 5T32HL087730: Training Grant in Quality of Care and Outcomes Research in Cardiovascular Disease and Stroke
N.I.H. R37AG18915; The Claude D. Pepper Older Americans Independence Center of Wake Forest University N.I.H. P30AG21332.
The sponsors had no role in the preparation, writing, review, or approval of this manuscript.
Dalane W Kitzman, MD: Served as consultant for and/or received grant support from Synvista (> $10K), Bristol-Meyers-Squibb (> $10K), Novartis (> $10K), Boston Scientific (> $10K), Relypsa (> $10K), Forest Laboratories, and Med
Footnotes
Conflicts of Interest Disclosure: Khalil Murad, MD: None
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart Disease and Stroke Statistics--2011 Update: A Report From the American Heart Association. Circulation. 2011 doi: 10.1161/CIR.0b013e3182009701. CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 3.Jugdutt BI. Aging and heart failure: changing demographics and implications for therapy in the elderly. Heart Fail Rev. 2010 doi: 10.1007/s10741-010-9164-8. 10.1007/s10741-010-9164-8. [DOI] [PubMed] [Google Scholar]
- 4.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288(17):2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 5.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347(18):1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 6.Krumholz H, Parent E, Tu N, Vaccarino V, Wang Y, Radford M, Hennen J. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med. 1997;157(1):99–104. [PubMed] [Google Scholar]
- 7.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 8.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359(23):2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 9.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362(9386):777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 10.Kitzman DW, Hundley WG, Brubaker PH, Morgan TM, Moore JB, Stewart KP, Little WC. A randomized double-blind trial of enalapril in older patients with heart failure and preserved ejection fraction: effects on exercise tolerance and arterial distensibility. Circ Heart Fail. 2010;3(4):477–485. doi: 10.1161/CIRCHEARTFAILURE.109.898916. CIRCHEARTFAILURE.109.898916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudhry SI, Mattera JA, Curtis JP, Spertus JA, Herrin J, Lin Z, Phillips CO, Hodshon BV, Cooper LS, Krumholz HM. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363(24):2301–2309. doi: 10.1056/NEJMoa1010029. 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 13.Morley JE. Anorexia, sarcopenia, and aging. Nutrition. 2001;17(7-8):660–663. doi: 10.1016/s0899-9007(01)00574-3. [DOI] [PubMed] [Google Scholar]
- 14.Newman AB, Gottdiener JS, McBurnie MA, Hirsch CH, Kop WJ, Tracy R, Walston JD, Fried LP. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56(3):M158–166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 15.Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, Masaki K, Murray A, Newman AB. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc. 2005;53(8):1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 16.Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, Gottdiener J, Fried LP. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162(20):2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 17.Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55(6):864–871. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- 18.Wilhelm-Leen ER, Hall YN, Deboer IH, Chertow GM. Vitamin D deficiency and frailty in older. Americans J Intern Med. 2010;268(2):171–180. doi: 10.1111/j.1365-2796.2010.02248.x. JIM2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varadhan R, Chaves PH, Lipsitz LA, Stein PK, Tian J, Windham BG, Berger RD, Fried LP. Frailty and impaired cardiac autonomic control: new insights from principal components aggregation of traditional heart rate variability indices. J Gerontol A Biol Sci Med Sci. 2009;64(6):682–687. doi: 10.1093/gerona/glp013. glp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, Liu Y, Hoffmann U, Bauer DC, Newman AB, Kritchevsky SB, Harris TB, Butler J. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol. 2010;55(19):2129–2137. doi: 10.1016/j.jacc.2009.12.045. S0735-1097(10)00865-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boxer RS, Dauser DA, Walsh SJ, Hager WD, Kenny AM. The association between vitamin D and inflammation with the 6-minute walk and frailty in patients with heart failure. J Am Geriatr Soc. 2008;56(3):454–461. doi: 10.1111/j.1532-5415.2007.01601.x. [DOI] [PubMed] [Google Scholar]
- 22.Cacciatore F, Abete P, Mazzella F, Viati L, Della Morte D, D'Ambrosio D, Gargiulo G, Testa G, Santis D, Galizia G, Ferrara N, Rengo F. Frailty predicts long-term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest. 2005;35(12):723–730. doi: 10.1111/j.1365-2362.2005.01572.x. [DOI] [PubMed] [Google Scholar]
- 23.Pulignano G, Del Sindaco D, Di Lenarda A, Tarantini L, Cioffi G, Gregori D, Tinti MD, Monzo L, Minardi G. J Cardiovasc Med. 10. Vol. 11. Hagerstown: 2010. Usefulness of frailty profile for targeting older heart failure patients in disease management programs: a cost-effectiveness, pilot study; pp. 739–747. [DOI] [PubMed] [Google Scholar]
- 24.Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD. Cachexia: a new definition. Clin Nutr. 2008;27(6):793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 25.von Haehling S, Lainscak M, Springer J, Anker SD. Cardiac cachexia: a systematic overview. Pharmacol Ther. 2009;121(3):227–252. doi: 10.1016/j.pharmthera.2008.09.009. S0163-7258(08)00198-8. [DOI] [PubMed] [Google Scholar]
- 26.Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox WJ, Poole-Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349(9058):1050–1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 27.Haass M, Kitzman DW, Anand IS, Miller A, Zile MR, Massie BM, Carson PE. Body Mass Index and Adverse Cardiovascular Outcomes in Heart Failure Patients with Preserved Ejection Fraction: Results from the I-PRESERVE Trial. Circ Heart Fail. 2011 doi: 10.1161/CIRCHEARTFAILURE.110.959890. CIRCHEARTFAILURE.110.959890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Habbu A, Lakkis NM, Dokainish H. The obesity paradox: fact or fiction? Am J Cardiol. 2006;98(7):944–948. doi: 10.1016/j.amjcard.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 29.Shea MK, Houston DK, Nicklas BJ, Messier SP, Davis CC, Miller ME, Harris TB, Kitzman DW, Kennedy K, Kritchevsky SB. The effect of randomization to weight loss on total mortality in older overweight and obese adults: the ADAPT Study. J Gerontol A Biol Sci Med Sci. 2010;65(5):519–525. doi: 10.1093/gerona/glp217. glp217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feinstein AR, Landis JR. The role of prognostic stratification in preventing the bias permitted by random allocation of treatment. J Chronic Dis. 1976;29(4):277–284. doi: 10.1016/0021-9681(76)90080-1. [DOI] [PubMed] [Google Scholar]
- 31.Guralnik JM. Assessing the impact of comorbidity in the older population. Ann Epidemiol. 1996;6(5):376–380. doi: 10.1016/s1047-2797(96)00060-9. [DOI] [PubMed] [Google Scholar]
- 32.Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162(20):2269–2276. doi: 10.1001/archinte.162.20.2269. [DOI] [PubMed] [Google Scholar]
- 33.Dahlström U. Frequent non-cardiac comorbidities in patients with chronic heart failure. Eur J Heart Fail. 2005;7(3):309–316. doi: 10.1016/j.ejheart.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Braunstein JB, Anderson GF, Gerstenblith G, Weller W, Niefeld M, Herbert R, Wu AW. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42(7):1226–1233. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 35.Muzzarelli S, Leibundgut G, Maeder MT, Rickli H, Handschin R, Gutmann M, Jeker U, Buser P, Pfisterer M, Brunner-La Rocca HP. Predictors of early readmission or death in elderly patients with heart failure. Am Heart J. 2010;160(2):308–314. doi: 10.1016/j.ahj.2010.05.007. S0002-8703(10)00413-8. [DOI] [PubMed] [Google Scholar]
- 36.Wong CH, Weiss D, Sourial N, Karunananthan S, Quail JM, Wolfson C, Bergman H. Frailty and its association with disability and comorbidity in a community-dwelling sample of seniors in Montreal: a cross-sectional study. Aging Clin Exp Res. 2010;22(1):54–62. doi: 10.1007/BF03324816. 6675. [DOI] [PubMed] [Google Scholar]
- 37.Morley JE, Haren MT, Rolland Y, Kim MJ. Frailty. Med Clin North Am. 2006;90(5):837–847. doi: 10.1016/j.mcna.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 38.Weiss CO. Frailty and chronic diseases in older adults. Clin Geriatr Med. 2010;27(1):39–52. doi: 10.1016/j.cger.2010.08.003. S0749-0690(10)00077-7. [DOI] [PubMed] [Google Scholar]
- 39.Kitzman DW, Rich MW. Age disparities in heart failure research. JAMA. 2010;304(17):1950–1951. doi: 10.1001/jama.2010.1592. 304/17/1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ridda I, Lindley R, MacIntyre RC. The challenges of clinical trials in the exclusion zone: the case of the frail elderly. Australas J Ageing. 2008;27(2):61–66. doi: 10.1111/j.1741-6612.2008.00288.x. [DOI] [PubMed] [Google Scholar]
- 42.Pfisterer M, Buser P, Rickli H, Gutmann M, Erne P, Rickenbacher P, Vuillomenet A, Jeker U, Dubach P, Beer H, Yoon SI, Suter T, Osterhues HH, Schieber MM, Hilti P, Schindler R, Brunner-La Rocca HP. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients With Congestive Heart Failure (TIME-CHF) randomized trial. JAMA. 2009;301(4):383–392. doi: 10.1001/jama.2009.2. 301/4/383. [DOI] [PubMed] [Google Scholar]
- 43.Fuat A, Hungin AP, Murphy JJ. Barriers to accurate diagnosis and effective management of heart failure in primary care: qualitative study. BMJ. 2003;326(7382):196. doi: 10.1136/bmj.326.7382.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 45.Pardaens K, Van Cleemput J, Vanhaecke J, Fagard RH. Atrial fibrillation is associated with a lower exercise capacity in male chronic heart failure patients. Heart. 1997;78(6):564–568. doi: 10.1136/hrt.78.6.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. 119/23/3028. [DOI] [PubMed] [Google Scholar]
- 47.Mockler M, O'Loughlin C, Murphy N, Ryder M, Conlon C, McDonald KM, Ledwidge MT. Causes and consequences of nonpersistence with heart failure medication. Am J Cardiol. 2009;103(6):834–838. doi: 10.1016/j.amjcard.2008.11.058. S0002-9149(08)02116-4. [DOI] [PubMed] [Google Scholar]
- 48.Granger BB, Swedberg K, Ekman I, Granger CB, Olofsson B, McMurray JJ, Yusuf S, Michelson EL, Pfeffer MA. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double-blind, randomised, controlled clinical trial. Lancet. 2005;366(9502):2005–2011. doi: 10.1016/S0140-6736(05)67760-4. [DOI] [PubMed] [Google Scholar]
- 49.Fonarow G, Heywood J, Heidenreich P, Lopatin M, Yancy C Investigators ASACa. Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2007;153(6):1021–1028. doi: 10.1016/j.ahj.2007.03.012. S0002-8703(07)00219-0. [DOI] [PubMed] [Google Scholar]
- 50.Levy W, Mozaffarian D, Linker D, Sutradhar S, Anker S, Cropp A, Anand I, Maggioni A, Burton P, Sullivan M, Pitt B, Poole-Wilson P, Mann D, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113(11):1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]