Abstract
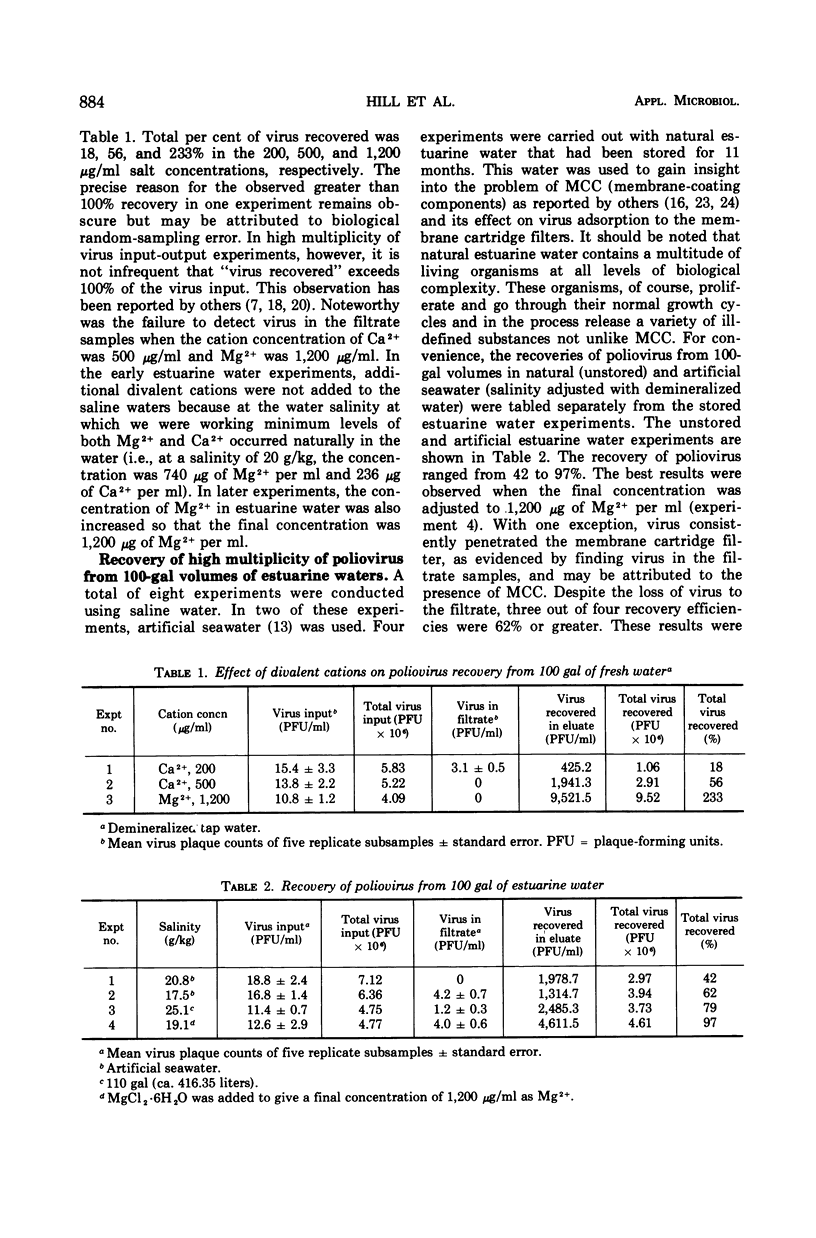
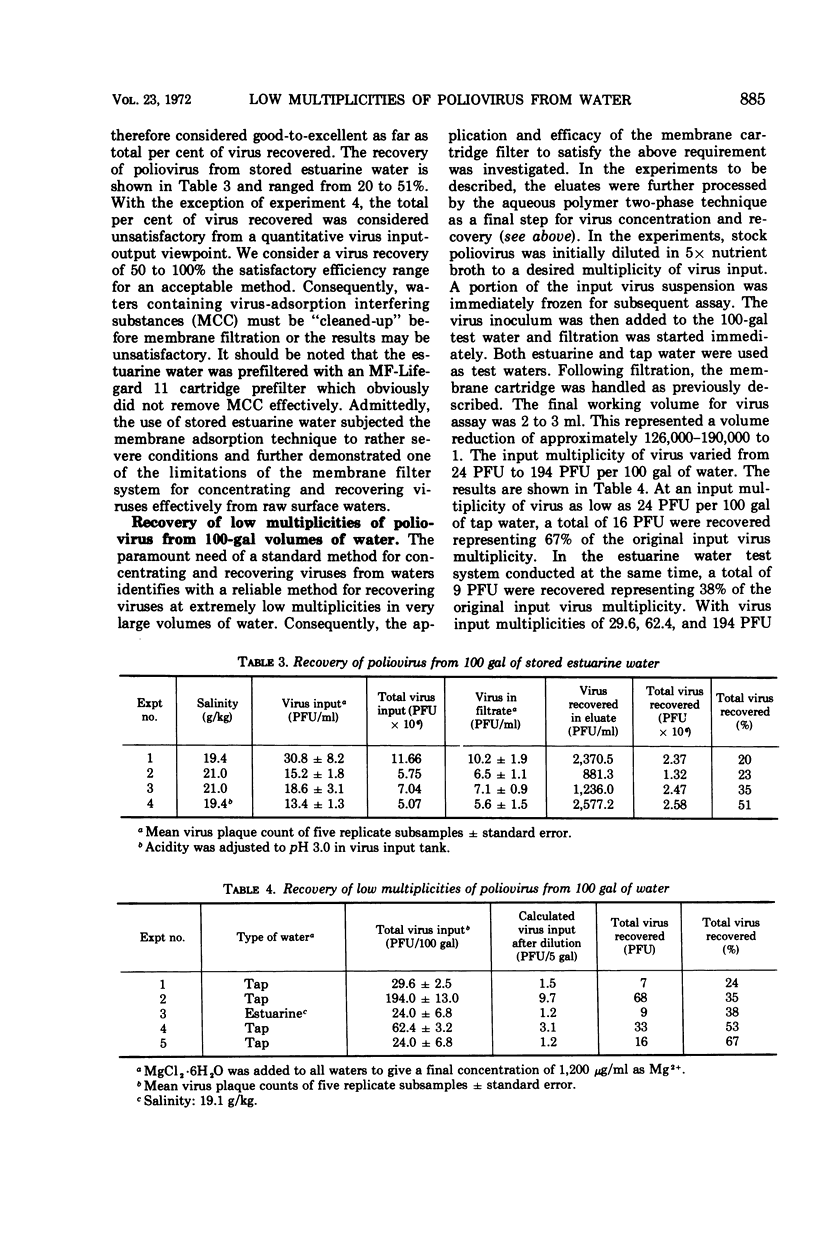
The efficiency of a Millitube MF cartridge filter, a membrane filter, for recovery of poliovirus from 100-gal volumes of both fresh (tap) and estuarine water was determined. In the high multiplicity of virus input-output experiments, recovery of 97% or greater of input virus was achieved in both types of water when the final concentration of divalent cation as Mg2+ was 1,200 μg/ml and the pH was 4.5. Virus was effectively eluted from the membrane cartridge with 5× nutrient broth in 0.05 M carbonate-bicarbonate buffer at pH 9.0. Four elutions of 250 ml each were used. In the low multiplicity of virus input-output experiments under the same cationic and pH conditions, up to 67% of the input virus was recovered when the virus was further concentrated from the eluates by the aqueous polymer two-phase separation technique. The volume reduction was 126,000-190,000 to 1. The use of the combined techniques, i.e., membrane adsorption followed by aqueous polymer two-phase separation, provided a highly sensitive, simple, and remarkably reliable sequential methodology for the quantitative recovery of poliovirus occurring at multiplicities as low as 1 to 2 plaque-forming units per 5 gal of water.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. G., Waters D. A., Nunley C. E., Gibson R. F., Schilling R. M., Denny E. C., Cline G. B., Babelay E. F., Perardi T. E. K-series centrifuges. I. Development of the K-II continuous-sample-flow-with-banding centrifuge system for vaccine purification. Anal Biochem. 1969 Dec;32(3):460–494. doi: 10.1016/s0003-2697(69)80014-x. [DOI] [PubMed] [Google Scholar]
- CLIVER D. O. FACTORS IN THE MEMBRANE FILTRATION OF ENTEROVIRUSES. Appl Microbiol. 1965 May;13:417–431. doi: 10.1128/am.13.3.417-425.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSIUNG G. D., MELNICK J. L. Plaque formation with poliomyelitis, Coxsackie, and orphan (echo) viruses in bottle cultures of monkey epithelial cells. Virology. 1955 Dec;1(5):533–535. doi: 10.1016/0042-6822(55)90041-6. [DOI] [PubMed] [Google Scholar]
- Hamblet F. E., Hill W. F., Akin E. W. Effect of plaque assay diluent upon enumeration of poliovirus type 1. Appl Microbiol. 1967 Jan;15(1):208–208. doi: 10.1128/am.15.1.208-.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. F., Jr, Hamblet F. E., Benton W. H. Inactivation of poliovirus type 1 by the Kelly-Purdy ultraviolet seawater treatment unit. Appl Microbiol. 1969 Jan;17(1):1–6. doi: 10.1128/am.17.1.1-6.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu O. C., Brashear D. A., Seraichekas H. R., Barnick J. A., Metcalf T. G. Virus in water. I. A preliminary study on a flow-through gauze sampler for recovering virus from waters. Appl Microbiol. 1971 Mar;21(3):405–410. doi: 10.1128/am.21.3.405-410.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- METCALF T. G. Use of membrane filters to facilitate the recovery of virus from aqueous suspensions. Appl Microbiol. 1961 Sep;9:376–379. doi: 10.1128/am.9.5.376-379.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. L., Ludovici P. P., Jeter W. S. Quantitative methods for the concentration of visuses in wastewater. J Water Pollut Control Fed. 1970 Feb;42(2 Suppl):R21–R28. [PubMed] [Google Scholar]
- Rao N. U., Labzoffsky N. A. A simple method for the detection of low concentration of viruses in large volumes of water by the membrane filter technique. Can J Microbiol. 1969 May;15(5):399–403. doi: 10.1139/m69-071. [DOI] [PubMed] [Google Scholar]
- Sweet B. H., McHale J. S., Hardy K. J., Klein E. Concentration of virus from water by electro-osmosis and forced-flow electrophoresis. Prep Biochem. 1971 Jan;1(1):77–89. doi: 10.1080/00327487108081931. [DOI] [PubMed] [Google Scholar]
- Wallis C., Grinstein S., Melnick J. L., Fields J. E. Concentration of viruses from sewage and excreta on insoluble polyelectrolytes. Appl Microbiol. 1969 Dec;18(6):1007–1014. doi: 10.1128/am.18.6.1007-1014.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L. Concentration of enteroviruses on membrane filters. J Virol. 1967 Jun;1(3):472–477. doi: 10.1128/jvi.1.3.472-477.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L. Concentration of viruses from sewage by adsorption on millipore membranes. Bull World Health Organ. 1967;36(2):219–225. [PMC free article] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L., Fields J. E. Concentration and purification of viruses by adsorption to and elution from insoluble polyelectrolytes. Appl Microbiol. 1971 Apr;21(4):703–709. doi: 10.1128/am.21.4.703-709.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]





