Abstract
Objective
In this study, we investigate the role of adiponectin in the interaction between leukocytes and endothelium in the secondary inflammatory reaction of cerebral ischemia.
Methods
Adiponectin knock-out mice group (APN-KO) (n = 8) and wild-type mice group (WT) (n = 8) were prepared. Each group was sub-divided into 2 groups by reperfusion time. One-hour middle cerebral artery occlusion and reperfusion were induced using the intraluminal filament technique. At 6 and 12 hours after the occlusion, the mice were placed on a stereotactic frame to perform craniotomy in the left parietal area. After craniotomy, a straight pial venule was selected as a target vessel. With the fluorescence intravital microscope, the number of rolling leukocytes and leukocytes that adhered to endothelium were counted and documented at 6 and 12 hours after the reperfusion.
Results
At 6 and 12 hours after the reperfusion, more rolling leukocyte and leukocyte adhesion were observed in the APN-KO mice than in the WT mice. The difference in leukocyte numbers between the APN-KO and WT mice was found to be statistically significant (p = 0.029) by Mann-Whitney U-test.
Conclusion
We found that adiponectin inhibits the interaction between the endothelium and leukocytes in cerebral ischemia-reperfusion. Therefore adiponectin might prevent the secondary insult caused by the inflammation reaction.
Keywords: Adiponectin, Cerebral ischemia, Secondary inflammatory reaction, MCAO-R
INTRODUCTION
The morbidity and mortality rates of ischemic stroke are high all over the world. Research on stroke mechanisms reveals secondary injury develops through the inflammatory reaction induced by the leukocytes that adhere to the micro vessels during cerebral ischemia-reperfusion.9) This mechanism was verified in animal experiments using middle cerebral artery occlusion and reperfusion (MCAO-R) models.5),12) Experiments using the MCAO-R model, found reduced leukocyte infiltration and enhanced neurologic functions in mice that were injected with adhesion-molecule-specific monoclonal antibodies (mAbs), or in mice whose adhesion molecules were removed through genetic manipulation, compared with wild-type mice.13),21),25)
Studies on adiponectin are active, and many favorable effects of adiponectin on the vascular system reported. Adiponectin activates angiogenesis in ischemic damages,19) and inhibits atherosclerosis.15) In addition, a negative association between the initial infarction volume and the adiponectin level in ischemic stroke was confirmed.6) In serial measurement of adiponectin level in ischemic cerebrovascular disease patients, a gradual decrease was observed.24)
In this study, the effects of adiponectin on the interaction between leukocytes and endothelia were investigated through experiments based on the hypothesis that adiponectin might inhibit the secondary inflammatory reaction in cerebral ischemia.
MATERIALS AND METHODS
Animal preparation
All experiments were conducted according to the guidelines issued by the Institutional Animal Care and Use Committee and complied with the National Institutes of Health (NIH) guide for the care and use of laboratory animals. For the experiments of this study, 10 to 12-week-old adiponectin-deficient mice (APN-KO) (n = 8) and wild-type mice (WT) (n = 8) were prepared. Each group was sub-divided into 2 groups by reperfusion time; 6h APN-KO, 12h APN-KO, 6h WT and 12h WT. General anesthesia was given to mice using 2% isoflurane and 70% nitrous oxide, and 30% oxygen was supplied during the experiments via a face mask. The mice's body temperatures were maintained at 36.5-37.5℃ during the experiments, as measured by a rectal thermometer.
Model of middle cerebral artery occlusion and reperfusion
Focal cerebral ischemia was induced using the intraluminal filament technique.11) After the left common carotid artery was dissected and completely exposed, the external carotid artery was tied by 4-0 silk. Thereafter, silicon-coated 8-0 monofilament was headed to the middle cerebral artery through the common carotid artery. The inserted monofilament was tightened with a 6-0 silk suture to prevent bleeding while advancing the monofilament and removing the monofilament during reperfusion. One hour after the occlusion, the filaments were removed for reperfusion (Fig. 1).
Fig. 1.
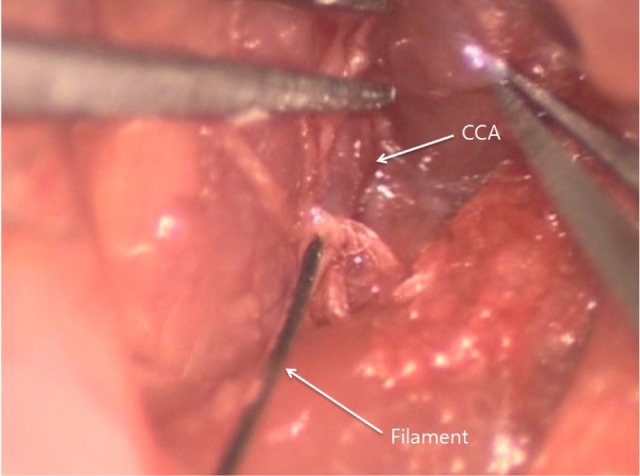
Representative image of intraluminal filament technique. Silicon-coated 8-0 monofilament is directed to the middle cerebral artery through the common carotid artery.
Cranial-window preparation
After preparation of the focal cerebral ischemia and reperfusion model, the mice were placed on a stereotactic frame to perform 4×6 mm-sized craniotomy in the left parietal area using a dental drill. Extreme caution was required to avoid damage to the dura mater and the tissues beneath it. After the craniotomy, the dura mater was opened using 26-gauge needles to expose the brain cortex of the MCA territory and the artificial cerebrospinal fluid was arranged to flow over the surface of the brain (Fig. 2).
Fig. 2.
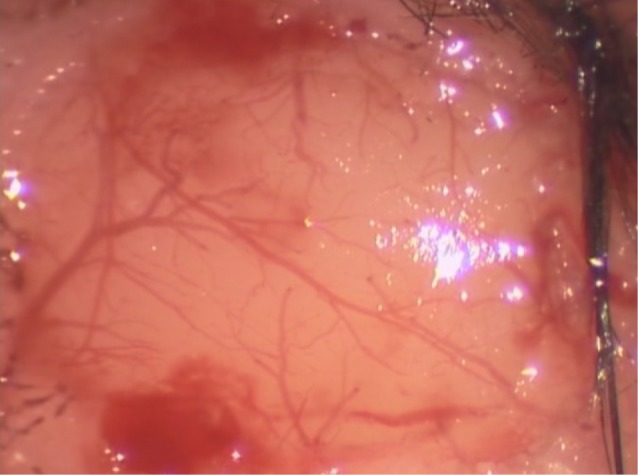
Representative image of cranial window preparation. After performing 4×6 mm-sized craniotomy in the left parietal area using a dental drill.
Intravital microscopy
As a target vessel, a straight pial venule (100 µm long and 30-40 µm in diameter) was selected. To observe the microcirculation, a fluorescence intravital microscope was used at 780× specimen-to-monitor magnification. Using 1 ml fluorescein isothiocyanate-albumin that was intravenously injected shortly before data collection, a bright contrast was obtained, and the length and diameter of the microvessel were measured. Using 1 ml of the 0.1% rhodamine that was intravenously injected 10 minutes before data collection, the leukocytes and platelets were selectively stained.
Data were collected at 6 and 12 hours after 1-hour occlusion. At each data selection, the target venule was recorded for 30 seconds through a microscope, and the number of rolling leukocytes and leukocytes that adhered to endothelium were counted and documented (Fig. 3).
Fig. 3.
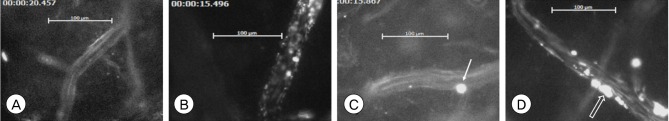
Representative images of rhodamine-labeled leukocytes in venules. (A) 6hWT, (B) 6hKO, (C) 12hWT, (D) 12hKO (Single arrow: rolling leukocyte, blank arrow: leukocyte aggregate). 6hWT: Wild-type mice at 6 hours of reperfusion, 6hKO: Adiponectin-deficient mice at 6 hours of reperfusion, 12hWT: Wild-type mice at 12 hours of reperfusion, 12hKO: Adiponectin-deficient mice at 12 hours of reperfusion.
Statistical analysis
The statistical significance of differences between the groups of APN-KO and WT mice in the measurements taken 6 and 12 hours after the reperfusion was assessed by the Mann-Whitney U-test. Null hypotheses of no difference were rejected if p values were less than 0.05.
RESULTS
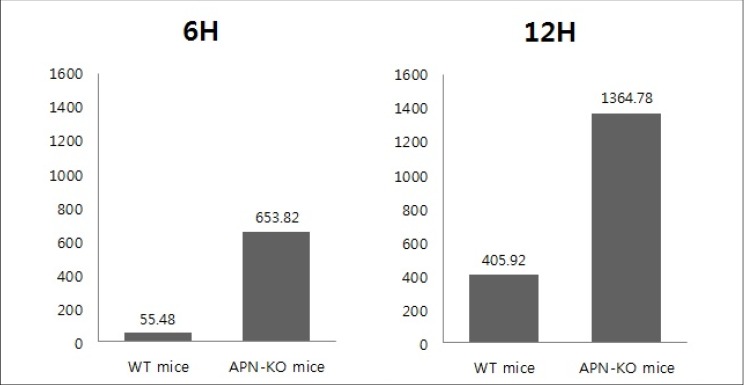
The behaviors of the leukocytes in the target vessel of APN-KO and WT mice was observed and recorded for 30 seconds at 6 and 12 hours after the occlusion, using an intravital fluorescence microscope. The number of rolling and adhesion leukocytes of each experimental group was counted and recorded and converted to per mm2 · sec (Table 1). In 6hWT mice, a mean of 55.48 per mm2 · sec of rolling leukocytes were observed while a mean of 653.82 per mm2 · sec of rolling leukocytes were observed in 6hAPN-KO mice. In 12hWT mice, a mean of 405.92 per mm2 · sec of rolling leukocytes was observed in all 4 groups. In the 12hAPN-KO mice, rolling leukocytes were observed in all 4 groups, and in particular, leukocyte adhesion was observed in experimental group III, with a mean of 1364.78 per mm2 · sec of total leukocytes.
Table 1.
Comparison of the experimental data between the APN-KO and WT mice
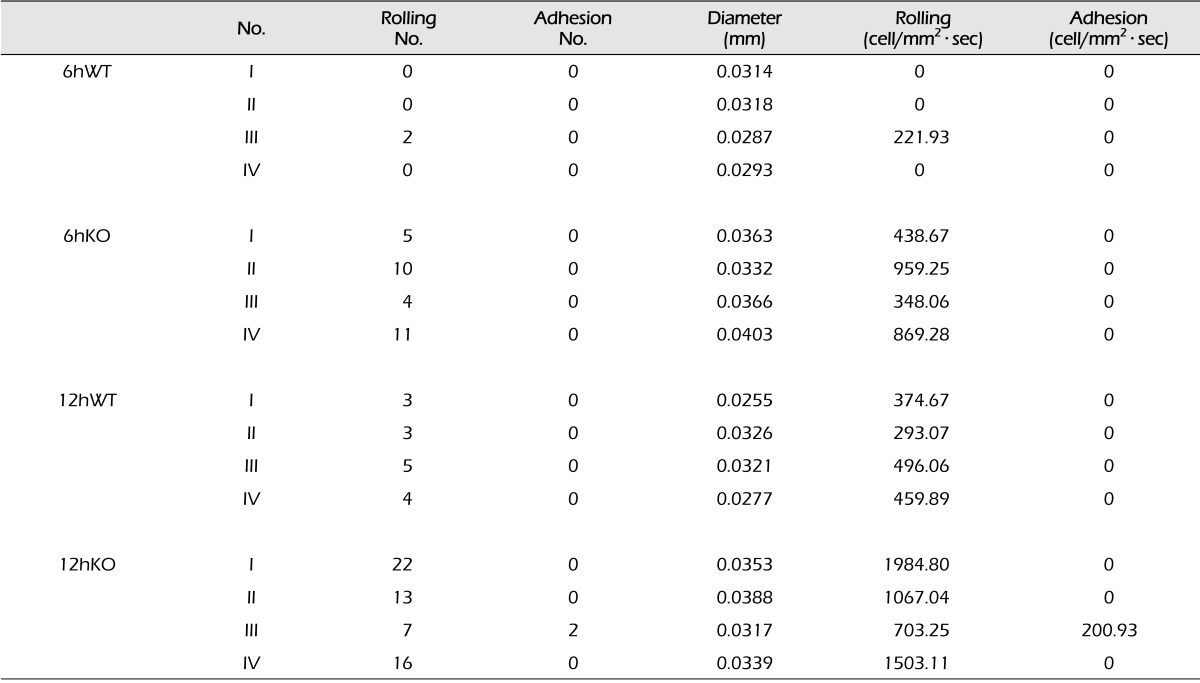
6hWT: Wild-type mice at 6 hours of reperfusion, 6hKO: Adiponectin-deficient mice at 6 hours of reperfusion, 12hWT: Wild-type mice at 12 hours of reperfusion, 12hKO: Adiponectin-deficient mice at 12 hours of reperfusion. APN-KO= adiponectin-deficient mice; WT= wild-type mice.
Mann-Whitney U-test was used to test for statistical significance of the outcomes. Leukocyte numbers between the APN-KO and WT mice 6 hours after the occlusion differed significantly with p = 0.029. The difference at 12 hours after the occlusion was also statistically significant with p = 0.029 (Fig. 4).
Fig. 4.
Comparison of the experimental groups at 6 hours and 12 hours after ischemia. The difference in leukocyte number between the APN-KO and WT mice at 6, 12 hours after the occlusion was statistically significant (Mann-Whitney U-test, p value = 0.029).
DISCUSSION
Adiponectin is an adipocyte-derived bioactive protein that initially attracted attention in the treatment of metabolic syndrome.14) Clinically, the plasma adiponectin level is negatively associated with dyslipidemia, hypertension, and the C-reactive protein level,17) and hypoadiponectinemia (defined as plasma adiponectin level < 4 µg/mL) increases the risk of type 2 DM, hypertension, and coronary heart disease.16),17) Studies of the role of adiponectin in the cardiovascular system have been conducted using APN-KO mice. These studies found that APN-KO mice showed exaggerated myocardial remodeling under pressure-overload conditions, and severe cardiac injuries were reported during ischemia-reperfusion.20),22) By contrast, when adiponectin was injected into the APN-KO mice, pathological cardiac hypertrophy and ischemia-induced myocardial damages were inhibited.18) These protective effects have driven research on the effects of adiponectin on the cerebrovascular system.
The most recent studies find that plasma adiponectin level is closely related with the cerebrovascular system. Chen at al. described hypoadiponectinemia as an independent and significant risk factor for cerebrovascular disease while Efstathiou et al. reported an increased risk of mortality for hypoadiponectinemia patients who suffered ischemic insults.4),6) In addition, advanced intracranial atherosclerosis patients were observed to have had significantly low plasma adiponectin levels.1)
In this study, the effects of adiponectin on the early stage of inflammation reaction in cerebral ischemia-reperfusion were investigated. At both 6 and 12 hours after the occlusion, the APN-KO mice group showed significantly more rolling and adhesion leukocytes than the WT mice group did. Although firm adhesion of the leukocytes to the endothelium was observed only in experimental group III of the 12hAPN-KO mice, the rolling leukocytes would form a firm adhesion on the endothelium through the media of leukocyte adhesion molecule CD11b/CD18 (integrin) and its endothelial ligand ICAM-1. The activated leukocytes that adhered to the endothelium release toxic mediators to damage the surrounding vasculatures or parenchymal cells, or induce a change in blood rheology and accelerated thrombosis by inducing platelet aggregation.7),8),10)
Inhibition of the endothelial adhesion-accumulation of the leukocytes after cerebral ischemia-reperfusion improves electrophysiological and neurological function, and reduces cerebral edema and cerebral infarction size.3),5),23) Therefore, adiponectin that restrains the leukocyte-endothelium interaction is considered to inhibit the secondary inflammatory reaction and is neuroprotective in cerebral ischemia-reperfusion.
The limitation of this study is the limited number of its experimental groups. In addition, the patterns beyond 12 hours after ischemia were not observed. Although the leukocyte accumulation in cerebral ischemia-reperfusion steadily increased to reach a peak after 24-48 in other studies,2),10) more advanced studies with a larger sample size may be needed in the future.
CONCLUSION
In this study, the effects of adiponectin on the early inflammatory reactions in cerebral ischemia-reperfusion were investigated. As a result, statistically more rolling and adhesion leukocytes were observed in the APN-KO mice than in the WT mice in the experiments with the MCAO-R model. In conclusion, adiponectin was observed to inhibit the interaction between the endothelium and leukocytes in cerebral ischemia-reperfusion, and accordingly, adiponectin might prevent the secondary insult caused by the inflammation reaction.
References
- 1.Bang OY, Saver JL, Ovbiagele B, Choi YJ, Yoon SR, Lee KH. Adiponectin levels in patients with intracranial atherosclerosis. Neurology. 2007 May;68(22):1931–1937. doi: 10.1212/01.wnl.0000263186.20988.9f. [DOI] [PubMed] [Google Scholar]
- 2.Barone FC, Hillegass LM, Price WJ, White RF, Lee EV, Feuerstein GZ, et al. Polymorphonuclear leukocyte infiltration into cerebral focal ischemic tissue: Myeloperoxidase activity assay and histologic verification. J Neurosci Res. 1991 Jul;29(3):336–345. doi: 10.1002/jnr.490290309. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Chopp M, Zhang RL, Bodzin G, Chen Q, Rusche JR, et al. Anti-CD11b monoclonal antibody reduces ischemic cell damage after transient focal cerebral ischemia in rat. Ann Neurol. 1994 Apr;35(4):458–463. doi: 10.1002/ana.410350414. [DOI] [PubMed] [Google Scholar]
- 4.Chen MP, Tsai JC, Chung FM, Yang SS, Hsing LL, Shin SJ, et al. Hypoadiponectinemia is associated with ischemic cerebrovascular disease. Arterioscler Thromb Vasc Biol. 2005 Apr;25(4):821–826. doi: 10.1161/01.ATV.0000157784.25920.a7. [DOI] [PubMed] [Google Scholar]
- 5.Chopp M, Zhang RL, Chen H, Li Y, Jiang N, Rusche JR. Postischemic administration of an anti-Mac-1 antibody reduces ischemic cell damage after transient middle cerebral artery occlusion in rats. Stroke. 1994 Apr;25(4):869–875. doi: 10.1161/01.str.25.4.869. discussion 875-6. [DOI] [PubMed] [Google Scholar]
- 6.Efstathiou SP, Tsioulos DI, Tsiakou AG, Gratsias YE, Pefanis AV, Mountokalakis TD. Plasma adiponectin levels and five-year survival after first-ever ischemic stroke. Stroke. 2005 Sep;36(9):1915–1919. doi: 10.1161/01.STR.0000177874.29849.f0. [DOI] [PubMed] [Google Scholar]
- 7.Grau AJ, Graf T, Hacke W. Altered influence of polymorphonuclear leukocytes on coagulation in acute ischemic stroke. Thromb Res. 1994 Dec;76(6):541–549. doi: 10.1016/0049-3848(94)90283-6. [DOI] [PubMed] [Google Scholar]
- 8.Grøgaard B, Schurer L, Gerdin B, Arfors KE. Delayed hypoperfusion after incomplete forebrain ischemia in the rat. The role of polymorphonuclear leukocytes. J Cereb Blood Flow Metab. 1989 Aug;9(4):500–505. doi: 10.1038/jcbfm.1989.73. [DOI] [PubMed] [Google Scholar]
- 9.Hallenbeck JM. Significance of the inflammatory response in brain ischemia. Acta Neurochir Suppl. 1996;66:27–31. doi: 10.1007/978-3-7091-9465-2_5. [DOI] [PubMed] [Google Scholar]
- 10.Hallenbeck JM, Dutka AJ, Tanishima T, Kochanek PM, Kumaroo KK, Thompson CB, et al. Polymorphonuclear leukocyte accumulation in brain regions with low blood flow during the early postischemic period. Stroke. 1986 Mar-Apr;17(2):246–253. doi: 10.1161/01.str.17.2.246. [DOI] [PubMed] [Google Scholar]
- 11.Hara H, Friedlander RM, Gagliardini V, Ayata C, Fink K, Huang Z, et al. Inhibition of interleukin 1beta converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc Natl Acad Sci U S A. 1997 Mar;94(5):2007–2012. doi: 10.1073/pnas.94.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuo Y, Kihara T, Ikeda M, Ninomiya M, Onodera H, Kogure K. Role of neutrophils in radical production during ischemia and reperfusion of the rat brain: Effect of neutrophil depletion on extracellular ascorbyl radical formation. J Cereb Blood Flow Metab. 1995 Nov;15(6):941–947. doi: 10.1038/jcbfm.1995.119. [DOI] [PubMed] [Google Scholar]
- 13.Matsuo Y, Onodera H, Shiga Y, Shozuhara H, Ninomiya M, Kihara T, et al. Role of cell adhesion molecules in brain injury after transient middle cerebral artery occlusion in the rat. Brain Res. 1994 Sep;656(2):344–352. doi: 10.1016/0006-8993(94)91478-8. [DOI] [PubMed] [Google Scholar]
- 14.Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004 Jan;24(1):29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 15.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002 Nov;106(22):2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 16.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, et al. Novel modulator for endothelial adhesion molecules: Adipocyte-derived plasma protein adiponectin. Circulation. 1999 Dec;100(25):2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 17.Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003 Dec;14(6):561–566. doi: 10.1097/00041433-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004 Dec;10(12):1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J Biol Chem. 2004 Jul;279(27):28670–28674. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 20.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005 Oct;11(10):1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki H, Abe K, Tojo SJ, Kitagawa H, Kimura K, Mizugaki M, et al. Reduction of ischemic brain injury by anti-P-selectin monoclonal antibody after permanent middle cerebral artery occlusion in rat. Neurol Res. 1999 Apr;21(3):269–276. doi: 10.1080/01616412.1999.11740930. [DOI] [PubMed] [Google Scholar]
- 22.Tao L, Jiao X, Gao E, Lau WB, Yuan Y, Lopez B, et al. Nitrative inactivation of thioredoxin-1 and its role in postischemic myocardial apoptosis. Circulation. 2006 Sep;114(13):1395–1402. doi: 10.1161/CIRCULATIONAHA.106.625061. [DOI] [PubMed] [Google Scholar]
- 23.Vasthare US, Heinel LA, Rosenwasser RH, Tuma RF. Leukocyte involvement in cerebral ischemia and reperfusion injury. Surg Neurol. 1990 Apr;33(4):261–265. doi: 10.1016/0090-3019(90)90046-r. [DOI] [PubMed] [Google Scholar]
- 24.Yatomi K, Miyamoto N, Komine-Kobayashi M, Liu M, Oishi H, Arai H, et al. Pathophysiological dual action of adiponectin after transient focal ischemia in mouse brain. Brain Res. 2009 Nov;1297:169–176. doi: 10.1016/j.brainres.2009.08.039. [DOI] [PubMed] [Google Scholar]
- 25.Zhang RL, Chopp M, Jiang N, Tang WX, Prostak J, Manning AM, et al. Anti-intercellular adhesion molecule-1 antibody reduces ischemic cell damage after transient but not permanent middle cerebral artery occlusion in the Wistar rat. Stroke. 1995 Aug;26(8):1438–1442. doi: 10.1161/01.str.26.8.1438. discussion 1443. [DOI] [PubMed] [Google Scholar]