Abstract
Gastric cancer is the second leading cause of cancer-related deaths worldwide. In advanced and metastatic gastric cancer, the conventional chemotherapy with limited efficacy shows an overall survival period of about 10 months. Patient specific and effective treatments known as personalized cancer therapy is of significant importance. Advances in high-throughput technologies such as microarray and next generation sequencing for genes, protein expression profiles and oncogenic signaling pathways have reinforced the discovery of treatment targets and personalized treatments. However, there are numerous challenges from cancer target discoveries to practical clinical benefits. Although there is a flood of biomarkers and target agents, only a minority of patients are tested and treated accordingly. Numerous molecular target agents have been under investigation for gastric cancer. Currently, targets for gastric cancer include the epidermal growth factor receptor family, mesenchymal-epithelial transition factor axis, and the phosphatidylinositol 3-kinase-AKT-mammalian target of rapamycin pathways. Deeper insights of molecular characteristics for gastric cancer has enabled the molecular classification of gastric cancer, the diagnosis of gastric cancer, the prediction of prognosis, the recognition of gastric cancer driver genes, and the discovery of potential therapeutic targets. Not only have we deeper insights for the molecular diversity of gastric cancer, but we have also prospected both affirmative potentials and hurdles to molecular diagnostics. New paradigm of transdisciplinary team science, which is composed of innovative explorations and clinical investigations of oncologists, geneticists, pathologists, biologists, and bio-informaticians, is mandatory to recognize personalized target therapy.
Keywords: Stomach neoplasms, Therapeutics, Biological markers, Gene expression, Sequence analysis
Introduction
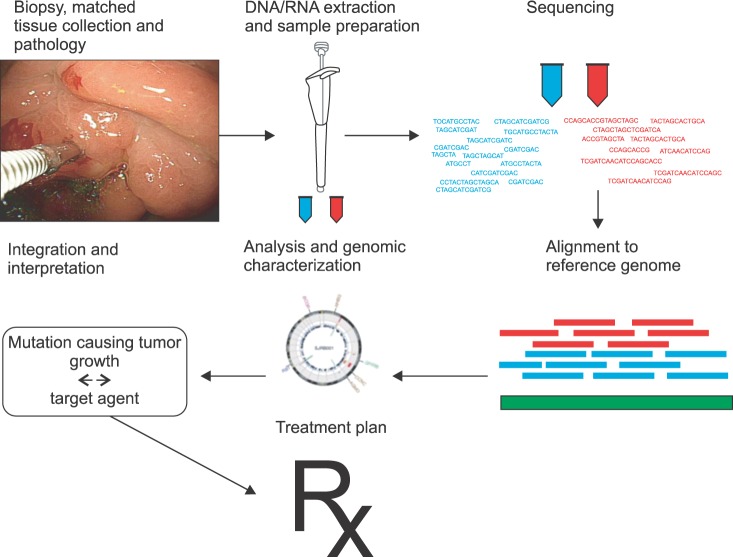
The personalized cancer therapy target aberrations that drive tumor growth and survival, by administering the right drug combination for the right person. Advances in high-throughput technologies such as microarray and next generation sequencing for gene or protein expression profiles and oncogenic signaling pathways have reinforced the discovery of treatment targets and predictive biomarkers. Because of the dramatic advances in genome-scale technologies and analytical tools, the personalized cancer therapy has been attracted oncologists' attention since the 2000s. To exploit informative biomarker is also obligatory to develop target treatment.1 The DNA-based markers include mutations, single nucleotide polymorphisms (SNPs), chromosomal aberrations, changes in DNA copy number, differential methylation. The RNA-based biomarkers include overexpressed or underexpressed transcripts and microRNAs. The protein markers include growth factors, cell surface receptors, phosphorylation states, and peptides released by tumors into serum. In 1990s, the Human Genome Project that firstly sequenced a human genome, consumed $2,700,000,000 and was completed after 15 years,2 however, only $1,000 whole genome sequencing is currently available. The era of personal genome sequencing has accelerated personalized target treatment (Fig. 1). However, there are numerous challenges from cancer target discovery to practical clinical benefit. Though the flood of biomarkers and target agents, only a minority of patients are tested for biomarkers and treated accordingly.
Fig. 1.
Fitting the cancer treatment to different patients genome.
Gastric cancer is the second leading cause of cancer-related deaths worldwide.3 Surgery is the only curative treatment strategy and conventional chemotherapy has shown limited efficacy for advanced gastric cancer showing an overall survival of about 10 months. Gastric cancer is a highly heterogeneous disease where even similar clinical and pathologic features,4 and there are various endogenous and exogenous causes. Helicobacter pylori infection is important exogenous causes for intestinal type of gastric cancer and influences the phenotype differences of gastric cancer.5,6 Germline mutations and deletions of E-cadherin (CDH1) are the underlying genetic defect in 45% of hereditary diffuse gastric cancer.7 Many genetic polymorphisms are also found to be associated with predisposition to gastric cancer development, including cyclin D1, epidermal growth factor receptor (EGFR), p16INK4A, p21WAF1/CIP1, prostate stem cell antigen, etc.8
Recently, molecular subtypes of gastric cancer have been suggested through analysis of gene or protein expression profiles and oncogenic signaling pathways.9-14 The molecular diversity causes clinical heterogeneity. Though gastric cancers are molecular biologically heterogeneous disease, treatment strategy is generally determined by clinical stage without considering molecular characteristics. Detailed molecular characterization of the patient's tumor will enable tailored therapies to improve outcomes and decrease toxicity.
Trastuzumab, a monoclonal antibody against human EGFR 2 (HER2; also known as ERBB2) is the firstly approved molecular target agent for gastric cancer. In Trastuzumab for Gastric Cancer (ToGA) trial, 594 patients with gastric cancer showing overexpression of HER2 protein were randomly assigned to chemotherapy (capecitabine/fluorouracil plus cisplatin) or chemotherapy in combination with trastuzumab.15 Trastuzumab extended median overall survival from 11.1 months to 13.8 months (hazard ratio [HR] 0.74; P=0.0046). ToGA trial satisfacted primary objective and was referenced in the guideline for cancer treatment (National Comprehensive Cancer Network guideline). On the other hand, another phase III, randomized clinical trial evaluated the clinical benefit of bevacizumab, a monoclonal antibody against vascular endothelial growth factor-A, in 770 gastric cancer patients without considering biomarker.16 The AVAGAST study showed that adding bevacizumab to chemotherapy in patients with advanced gastric cancer improved progression-free survival and tumor response rate but not overall survival (12.1 vs. 10.1 months; HR 0.87; P=0.1002). The lesson from the two studies is that identifying tumors most sensitive to target agent is requisite to realize personalized target treatment.
Molecular Targets and Clinical Trials in Gastric Cancer
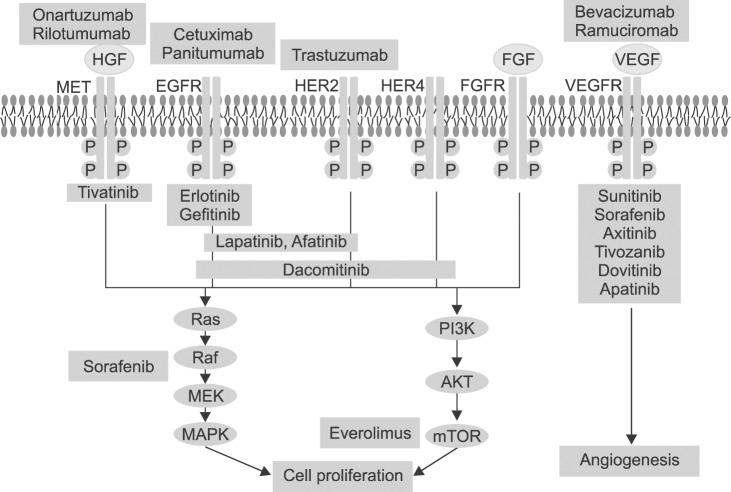
Advances in high-throughput cancer genome sequencing, genome-wide profiling technologies, and clinical proof-of-concept, are affecting the development and approval of target agents. Multiple key regulatory signaling pathways have been identified as key drivers of cancer through genetic and epigenetic aberrations. For gastric cancer, key drivers include EGFR family, fibroblast growth factor receptor (FGFR), hepatocyte growth factor (HGF) mesenchymal-epithelial transition factor (c-MET) axis, and the phosphatidylinositol 3-kinase (PI3K) AKT mammalian target of rapamycin (mTOR) and RAS/RAF/MEK/mitogen-activated protein kinase pathways (Fig. 2). EGFR family includes EGFR (ErbB1), ErbB2 (HER2), ErbB3 (HER3), and ErbB4 (HER4). The activated EGFR family consequently stimulates cell proliferation. EGFR overexpression has been reported in 30~50% of gastric cancer and correlated with poor prognosis.17 HER2 overexpression, appearing in 6~35% of gastric cancer, was also associated with poor clinical outcome,18 however, the high rate of intratumoral heterogeneity of HER2 expression in gastric cancer should be considered to predict prognosis.19 The c-MET was overexpression in 10~40% gastric cancer and activates proliferation and invasion of cancer cells after binding to HGF.20,21 Aberrant FGFR accelerates cancer growing and especially FGFR2 was reported to be amplified in 9% of gastric cancer.22 The activation of PI3K/AKT/mTOR signaling pathway is correlated with poor prognostic cancer and has been studied as a treatment target.23 Angiogenesis plays an important role in cancer development, growth, and survival, and VEGFs and receptors have been spotlighted as treatment targets. Histone deacetylase24 and Poly (ADP-ribose) polymerase (PARP; a family of proteins involved in a number of cellular processes involving DNA repair and programmed cell death) also have been investigated as treatment targets of gastric cancer.25
Fig. 2.
Target agents for cellular signaling pathway in gastric cancer. HGF = hepatocyte growth factor; MET = mesenchymal-epithelial transition; EGFR = epidermal growth factor receptor; FGFR = fibroblast growth factor receptor; VEGFR = vascular endothelial growth factor receptor.
Up to date, numerous phase 2/3 trials have evaluated molecular target agents for gastric cancer (Fig. 2) and phase 3 trials were listed in Table 1.15,16,26-30 Table includes not only completed trials, but also still recruiting or unpublished trials. As shown in table, the clinical benefit of target agents were demonstrated in only limited trials for trastuzumab and ramucirumab. Furthermore, only a few trials investigated biomarker for inclusion. Because the survival benefit of several target agents was not demonstrated, researchers have strained to search for predictive biomarker retrospectively.31
Table 1.
Recent phase III clinical trials investigating target agents for gastric cancer
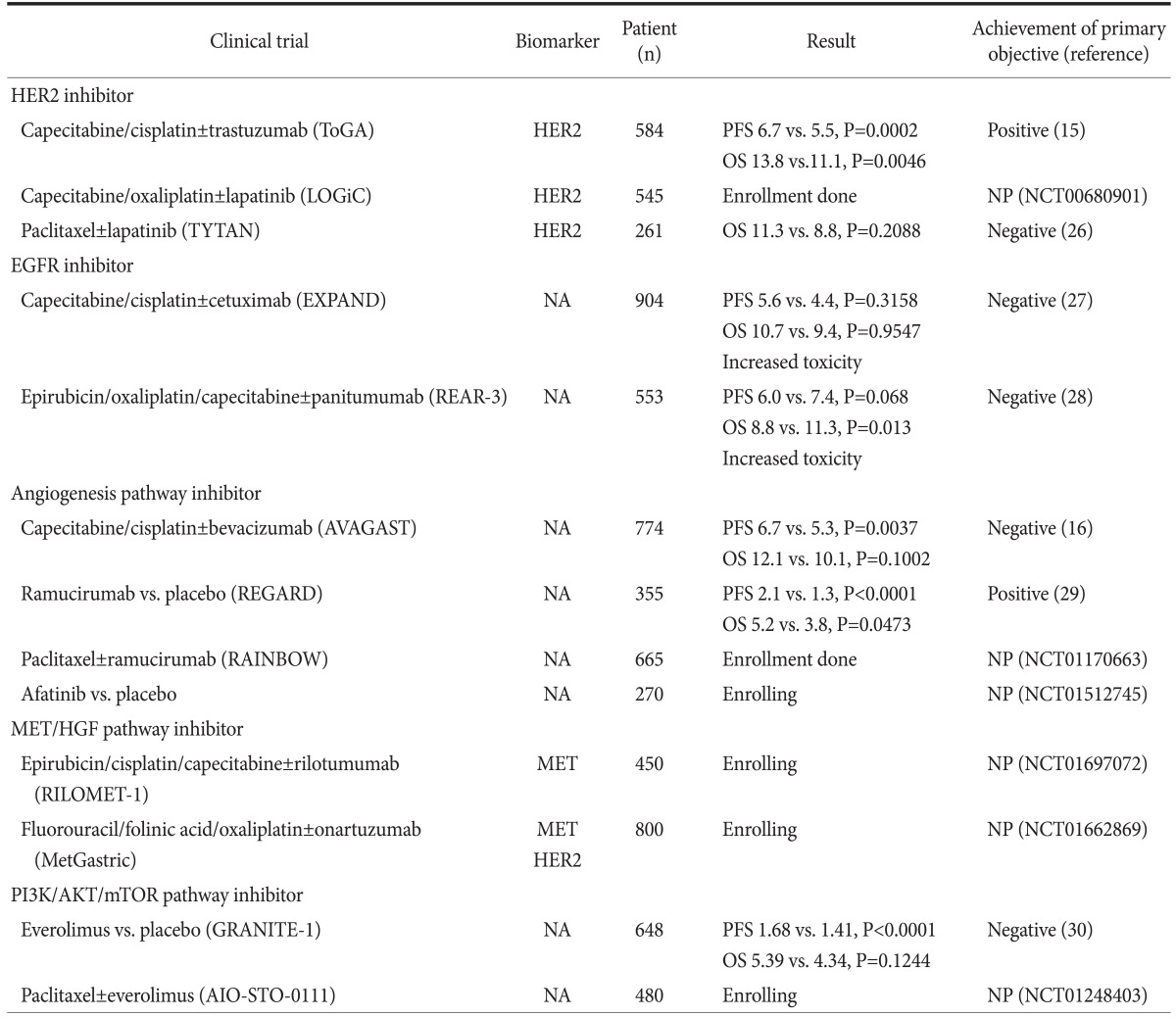
ToGA = Trastuzumab for Gastric Cancer; PFS = progression free survival; OS = overall survival; NP = not published; NCT = ClinicalTrials.gov Identifier; EGFR = epidermal growth factor receptor; NA = not applicable; MET = mesenchymal-epithelial transition factor; HGF = hepatocyte growth factor.
Molecular Diagnostic Approaches Using Genome Sequencing and Genome-Wide Profiling Technologies for Gastric Cancer
Deeper insight into molecular characteristics of gastric cancer has enabled the molecular classification of gastric cancer, the diagnosis of gastric cancer, the prediction of prognosis, the recognition of gastric cancer driver genes, and the discovery of potential therapeutic targets.
Since 2000s, the gene expression profile analysis of gastric cancer has been performed in relatively large sample size.11 The gene expression patterns in 90 primary gastric cancers, 14 metastatic gastric cancers, and 22 non-neoplastic gastric tissues were analyzed using cDNA microarrays representing about 30,300 genes. Gastric cancers were distinguished from non-neoplastic gastric tissues by characteristic gene expression patterns (2,565 genes; p≤0.001, false discovery rate 0.13%). The expression level IGF-2 and PLA2G2A were significantly correlated with patient survival. There was a diversity of gene expression patterns in gastric cancer, reflecting variation in properties of tumor as it is called heterogeneity.
It was suggested that distinct gastric cancer subtypes may be distinguished by gene expression analysis.32 Gastric cancer may be classified into 3 distinct subtypes-proximal, diffuse, and distal gastric cancer-based on histopathologic and anatomic criteria. From 36 patients with gastric cancer, 4~6 targeted biopsies of the primary tumor were obtained. Macrodissection was carried out and HG-U133A GeneChip (Affymetrix, Santa Clara, CA, USA) was used for cDNA expression analysis. Using supervised analysis, a classifier was built to distinguish the 3 gastric cancer subtypes. Gene set analysis identified several pathways that were differentially regulated in each gastric cancer subtype. These preliminary data suggested a new classification of gastric cancer for improving our understanding of disease biology and identification of unique molecular drivers for each gastric cancer subtype.
The practical biomarker predicting relapse of gastric cancer after surgical treatment was investigated.33 Microarray technologies (HumanHT-12 Expression BeadChip; Illumina, San Diego, CA, USA) were used to generate and analyze gene expression profiling data from 65 gastric cancer patients. Two subgroups of gastric cancer based on different gene expression pattern were strongly associated with the prognosis. A scoring system based on the expression of six genes (CTNNB1, EXOCS3, TOP2A, LBA1, CCL5, and LZTR1) was developed and predicted independently the likelihood of relapse after curative resection. Prognostic characteristics of the risk score may not be sufficient to change current clinical practice. However, biomarker study in breast cancer showed that 21-gene prognostic marker was approved as predictive marker for standard adjuvant chemotherapy.34 Thus, in future study, risk score could also be applied to clinical practice of gastric cancer.
To identify the molecular underpinnings of gastric cancer, an RNA-sequencing approach (SOLiD whole-transcriptome sequencing and small RNA-sequencing; Applied Biosystems, Foster City, CA, USA) was applied to 24 gastric tumor and 6 noncancerous specimens, generating 680 million informative short reads to quantitatively characterize the entire transcriptome of gastric cancer.35 A multilayer analysis was then developed to identify multiple types of transcriptional aberrations associated with different stages of gastric cancer, including differentially expressed mRNAs, recurrent somatic mutations, and key differentially expressed miRNAs. The central metabolic regulator AMP-activated protein kinase (AMPK) was identified as a potential functional target and translational relevance of AMPK was proved as a potential therapeutic target for early-stage gastric cancer in Asian patients.
Recent exome sequencing (SureSelect Human All Exon Kit v1 [Agilent Technology, Santa Clara, CA, USA] and Illumina GAIIx sequencer [Illumina]) of 15 gastric adenocarcinoma identified key tumorigenic events in a subset of gastric cancers.36 Frequently mutated genes in the gastric cancer were TP53, PIK3CA, and ARID1A and the most enriched biological pathway of the frequently mutated genes was cell adhesion. A prevalence screening confirmed mutations in FAT4, a cadherin family gene, in 5% of gastric cancers and FAT4 genomic deletions in 4% of gastric tumors. Frequent mutations in chromatin remodeling genes (ARID1A, MLL3 and MLL) occurred in 47% of the gastric cancers. ARID1A mutations were detected in 8% of tumors, which were associated with concurrent PIK3CA mutations and microsatellite instability. In functional assays, both FAT4 and ARID1A exert tumor-suppressor activity. Somatic inactivation of FAT4 and ARID1A may thus be key tumorigenic events in a subset of gastric cancers.
The most prevalent molecular targets in gastric cancer were identified through SNP profiling.22 Using Affymetrix SNP 6.0 arrays (Affymetrix), copy number alterations were profiled in 233 gastric cancers (193 primary tumors, 40 cell lines) and 98 gastric non-malignant samples. Twenty two recurrent focal alterations (13 amplifications and nine deletions) included both known targets (FGFR2, ERBB2) and also novel genes (KLF5, GATA6) in gastric cancer. The study identified five distinct gastric cancer subgroups, defined by the genomic alterations FGFR2 (9% of tumors), KRAS (9%), EGFR (8%), ERBB2 (7%) and MET (4%). This study implies that the five gastric cancer subgroups may be potentially treatable by target therapies.
A new class of small non-coding RNAs-microRNAs-are composed of 19~25 nucleotides and bind to mRNAs of potentially hundreds of genes, resulting in degradation of target mRNAs and inhibition of translation. Numerous microRNAs are expressed aberrantly and correlate with tumorigenesis, progression, and prognosis of various tumors. Three-hundred fifty-three gastric samples were analysed using Ohio State University custom microRNA microarray chip (OSU_CCC version 3.0; ArrayExpress [European Bioinformatics Institute, Cambridge, UK]).37 In paired samples of non-tumour mucosa and cancer, 22 microRNAs were upregulated and 13 were downregulated in gastric cancer. The two histological subtypes (diffuse and intestinal) of gastric cancer showed different microRNA signatures. MiR-125b, miR-199a, and miR-100 were progression-related. Low expression of let-7g and miR-433 and high expression of miR-214 were associated with unfavourable outcome in overall survival. The study suggested that microRNAs are useful biomarker for progression and prognosis of gastric cancer.
Integrative Analysis of Omics Information for Gastric Cancer
We systematically analyzed the expression profiles of mRNA, microRNA, and protein simultaneously to identify molecular biologic differences between good- and poor-prognosis patient subgroups. Array technologies were used to generate about 25,000 annotated genes, 1,100 microRNA, and 124 protein expression profiles of 65 patients with gastric cancer. Unsupervised clustering revealed distinctive subtypes with clear differences in overall gene expression patterns.33 Two major subtypes were highly associated with prognosis. 2,755 genes displayed a poor prognosis-specific gene expression pattern. Subsequently, microRNA expression data were compared between two prognostic subtypes and four microRNAs were uniquely dysregulated (P<0.05) between two subtypes. On the other hand, protein expression was highly variable compared with mRNA and microRNA expression. The expression levels of 48 proteins were significantly distinct (P<0.05) between cancer and non-cancer tissues. Through integrative analysis of multiple omics information, we perceived molecular diversity of gastric cancer and prospected both affirmative potentials of and the hurdles against molecular diagnostics.
Conclusions
The effect of molecular diagnostics is apparent in everywhere of cancer research. However, its application to the clinic is still limited. Although integrative molecular characterization of cancer can generate the statistical evidence for new candidate cancer targets for therapy and diagnosis, converting these into therapeutic agents and biomarkers will require deep insights to the biologic mechanism of action. New paradigm of transdisciplinary team science, which composed of innovative exploration and clinical investigation of oncologists, geneticists, pathologists, biologists, and bioinformaticians, is mandatory to realize personalized target therapy.
References
- 1.Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer. 2005;5:845–856. doi: 10.1038/nrc1739. [DOI] [PubMed] [Google Scholar]
- 2.Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Shah MA, Ajani JA. Gastric cancer--an enigmatic and heterogeneous disease. JAMA. 2010;303:1753–1754. doi: 10.1001/jama.2010.553. [DOI] [PubMed] [Google Scholar]
- 5.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 6.Park DW, Lee KJ, Jin SH, Lee JH, Min JS, Park SH, et al. Phenotypic differences of gastric cancer according to the Helicobacter pylori infection in Korean patients. J Gastric Cancer. 2010;10:168–174. doi: 10.5230/jgc.2010.10.4.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, et al. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402–405. doi: 10.1038/32918. [DOI] [PubMed] [Google Scholar]
- 8.Study Group of Millennium Genome Project for Cancer. Study Group, Sakamoto H, Yoshimura K, Saeki N, Katai H, Shimoda T, et al. Genetic variation in PSCA is associated with susceptibility to diffuse-type gastric cancer. Nat Genet. 2008;40:730–740. doi: 10.1038/ng.152. [DOI] [PubMed] [Google Scholar]
- 9.Tay ST, Leong SH, Yu K, Aggarwal A, Tan SY, Lee CH, et al. A combined comparative genomic hybridization and expression microarray analysis of gastric cancer reveals novel molecular subtypes. Cancer Res. 2003;63:3309–3316. [PubMed] [Google Scholar]
- 10.Kim B, Bang S, Lee S, Kim S, Jung Y, Lee C, et al. Expression profiling and subtype-specific expression of stomach cancer. Cancer Res. 2003;63:8248–8255. [PubMed] [Google Scholar]
- 11.Chen X, Leung SY, Yuen ST, Chu KM, Ji J, Li R, et al. Variation in gene expression patterns in human gastric cancers. Mol Biol Cell. 2003;14:3208–3215. doi: 10.1091/mbc.E02-12-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee HS, Cho SB, Lee HE, Kim MA, Kim JH, Park do J, et al. Protein expression profiling and molecular classification of gastric cancer by the tissue array method. Clin Cancer Res. 2007;13:4154–4163. doi: 10.1158/1078-0432.CCR-07-0173. [DOI] [PubMed] [Google Scholar]
- 13.Tan IB, Ivanova T, Lim KH, Ong CW, Deng N, Lee J, et al. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology. 2011;141:476–485. doi: 10.1053/j.gastro.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ooi CH, Ivanova T, Wu J, Lee M, Tan IB, Tao J, et al. Oncogenic pathway combinations predict clinical prognosis in gastric cancer. PLoS Genet. 2009;5:e1000676. doi: 10.1371/journal.pgen.1000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastrooesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 16.Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968–3976. doi: 10.1200/JCO.2011.36.2236. [DOI] [PubMed] [Google Scholar]
- 17.Lieto E, Ferraraccio F, Orditura M, Castellano P, Mura AL, Pinto M, et al. Expression of vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) is an independent prognostic indicator of worse outcome in gastric cancer patients. Ann Surg Oncol. 2008;15:69–79. doi: 10.1245/s10434-007-9596-0. [DOI] [PubMed] [Google Scholar]
- 18.Tanner M, Hollmén M, Junttila TT, Kapanen AI, Tommola S, Soini Y, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273–278. doi: 10.1093/annonc/mdi064. [DOI] [PubMed] [Google Scholar]
- 19.Yang J, Luo H, Li Y, Li J, Cai Z, Su X, et al. Intratumoral heterogeneity determines discordant results of diagnostic tests for human epidermal growth factor receptor (HER) 2 in gastric cancer specimens. Cell Biochem Biophys. 2012;62:221–228. doi: 10.1007/s12013-011-9286-1. [DOI] [PubMed] [Google Scholar]
- 20.Nakajima M, Sawada H, Yamada Y, Watanabe A, Tatsumi M, Yamashita J, et al. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer. 1999;85:1894–1902. doi: 10.1002/(sici)1097-0142(19990501)85:9<1894::aid-cncr3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 21.Lee J, Seo JW, Jun HJ, Ki CS, Park SH, Park YS, et al. Impact of MET amplification on gastric cancer: possible roles as a novel prognostic marker and a potential therapeutic target. Oncol Rep. 2011;25:1517–1524. doi: 10.3892/or.2011.1219. [DOI] [PubMed] [Google Scholar]
- 22.Deng N, Goh LK, Wang H, Das K, Tao J, Tan IB, et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut. 2012;61:673–684. doi: 10.1136/gutjnl-2011-301839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu G, Wang J, Chen Y, Wang X, Pan J, Li G, et al. Overexpression of phosphorylated mammalian target of rapamycin predicts lymph node metastasis and prognosis of chinese patients with gastric cancer. Clin Cancer Res. 2009;15:1821–1829. doi: 10.1158/1078-0432.CCR-08-2138. [DOI] [PubMed] [Google Scholar]
- 24.Claerhout S, Lim JY, Choi W, Park YY, Kim K, Kim SB, et al. Gene expression signature analysis identifies vorinostat as a candidate therapy for gastric cancer. PLoS One. 2011;6:e24662. doi: 10.1371/journal.pone.0024662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Virág L, Szabó C. The therapeutic potential of poly (ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- 26.Bang YJ. A randomized, open-label, phase III study of lapatinib in combination with weekly paclitaxel versus weekly paclitaxel alone in the second-line treatment of HER2 amplified advanced gastric cancer (AGC) in Asian population: Tytan study. J Clin Oncol. 2012;30(suppl 34):abstr 11. doi: 10.1200/JCO.2013.53.6136. [DOI] [PubMed] [Google Scholar]
- 27.Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, et al. Arbeitsgemeinschaft Internistische Onkologie and EXPAND Investigators. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490–499. doi: 10.1016/S1470-2045(13)70102-5. [DOI] [PubMed] [Google Scholar]
- 28.Waddell TS, Chau I, Barbachano Y, Gonzalez de Castro D, Wotherspoon A, Saffery C. A randomized, multicenter trial of epirubicin, oxaliplatin, and capecitabine (EOC) plus panitumumab in advanced esophagogastric cancer (REAL3) J Clin Oncol. 2012;30(suppl):abstr LBA4000. [Google Scholar]
- 29.Fuchs CS, Tomasek J, Cho JY, Dumitru F, Passalacqua R, Goswami C, et al. REGARD: a phase III, randomized, double-blinded trial of ramucirumab and best supportive care (BSC) versus placebo and BSC in the treatment of metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma following disease progression on first-line platinum- and/or fluoropyrimidine-containing combination therapy. J Clin Oncol. 2012;30(suppl 34):abstr LBA5. [Google Scholar]
- 30.Van Cutsem E, Yeh KH, Bang YJ, Shen L, Ajani JA, Bai YX, et al. Phase III trial of everolimus (EVE) in previously treated patients with advanced gastric cancer (AGC): GRANITE-1. J Clin Oncol. 2012;30(suppl 4):abstr LBA3. [Google Scholar]
- 31.Van Cutsem E, de Haas S, Kang YK, Ohtsu A, Tebbutt NC, Ming Xu J, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol. 2012;30:2119–2127. doi: 10.1200/JCO.2011.39.9824. [DOI] [PubMed] [Google Scholar]
- 32.Shah MA, Khanin R, Tang L, Janjigian YY, Klimstra DS, Gerdes H, et al. Molecular classification of gastric cancer: a new paradigm. Clin Cancer Res. 2011;17:2693–2701. doi: 10.1158/1078-0432.CCR-10-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho JY, Lim JY, Cheong JH, Park YY, Yoon SL, Kim SM, et al. Gene expression signature-based prognostic risk score in gastric cancer. Clin Cancer Res. 2011;17:1850–1857. doi: 10.1158/1078-0432.CCR-10-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, nodenegative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 35.Kim YH, Liang H, Liu X, Lee JS, Cho JY, Cheong JH, et al. AMPKα modulation in cancer progression: multilayer integrative analysis of the whole transcriptome in Asian gastric cancer. Cancer Res. 2012;72:2512–2521. doi: 10.1158/0008-5472.CAN-11-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zang ZJ, Cutcutache I, Poon SL, Zhang SL, McPherson JR, Tao J, et al. Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nat Genet. 2012;44:570–574. doi: 10.1038/ng.2246. [DOI] [PubMed] [Google Scholar]
- 37.Ueda T, Volinia S, Okumura H, Shimizu M, Taccioli C, Rossi S, et al. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol. 2010;11:136–146. doi: 10.1016/S1470-2045(09)70343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]