Abstract
Purpose
Clinical stage of gastric cancer is currently assessed by computed tomography. Accurate clinical staging is important for the tailoring of therapy. This study evaluated the accuracy of clinical N staging using stomach protocol computed tomography.
Materials and Methods
Between March 2004 and November 2012, 171 patients with gastric cancer underwent preoperative stomach protocol computed tomography (Jeju National University Hospital; Jeju, Korea). Their demographic and clinical characteristics were reviewed retrospectively. Two radiologists evaluated cN staging using axial and coronal computed tomography images, and cN stage was matched with pathologic results. The diagnostic accuracy of stomach protocol computed tomography for clinical N staging and clinical characteristics associated with diagnostic accuracy were evaluated.
Results
The overall accuracy of stomach protocol computed tomography for cN staging was 63.2%. Computed tomography images of slice thickness 3.0 mm had a sensitivity of 60.0%; a specificity of 89.6%; an accuracy of 78.4%; and a positive predictive value of 78.0% in detecting lymph node metastases. Underestimation of cN stage was associated with larger tumor size (P<0.001), undifferentiated type (P=0.003), diffuse type (P=0.020), more advanced pathologic stage (P<0.001), and larger numbers of harvested and metastatic lymph nodes (P<0.001 each). Tumor differentiation was an independent factor affecting underestimation by computed tomography (P=0.045).
Conclusions
Computed tomography with a size criterion of 8 mm is highly specific but relatively insensitive in detecting nodal metastases. Physicians should keep in mind that computed tomography may not be an appropriate tool to detect nodal metastases for choosing appropriate treatment.
Keywords: Stomach neoplasms; Neoplasm staging; Technology, radiologic
Introduction
Although the incidence of gastric cancer has been declining in most industrialized nations, it remains the most prevalent form of cancer in East Asian countries.1,2 In Korea, the incidence of early gastric cancer (EGC) has increased due to improvements in diagnostic methods and changes in the concept of routine health examinations.2 Early detection has led to the introduction of various tailored and limited therapies for EGC, enhancing survival outcomes.3 Less invasive treatment methods of resection include endoscopic and laparoscopic treatment.3,4 Accurate preoperative clinical staging has therefore become very important in determining treatment plans and predicting patient outcomes.
The most important prognostic factors in gastric cancer include depth of invasion, lymph node (LN) involvement, and distant metastases.5,6 In addition, adequate lymphadenectomy is important in predicting treatment outcomes.7 Patients without LN metastasis should be spared aggressive procedures to improve their quality of life. Therefore, preoperative knowledge of LN status would be helpful in planning the optimal extent of gastric resection and lymphadenectomy. This study was designed to assess the diagnostic accuracy of stomach protocol computed tomography (S-CT) for cN staging. Because undertreatment can be caused by underestimation of preoperative cN staging, the risk factors associated with underestimation of LN status were analyzed.
Materials and Methods
1. Patient selection
From March 2004 to November 2012, a total of 284 patients diagnosed with gastric adenocarcinoma underwent laparoscopic or open gastrectomy at Jeju National University Hospital (Jeju, Korea). Patients examined by conventional computed tomography (CT) without the stomach protocol were excluded, as were patients who underwent endoscopic resection. Endoscopic resection was indicated in our hospital for patients with tumors confined to the mucosa, <2 cm in diameter; classified as well or moderately differentiated adenocarcinoma; and with no evidence of LN or distant metastases on abdominal CT or endoscopic ultrasonography (EUS). A total of 171 patients, who underwent S-CT preoperatively within 3 weeks from the operation, were included in this study. The specimens obtained by surgical resection were histopathologically evaluated, and the findings were used as reference standards for N staging. Histologic staging was based on the 7th edition of American Joint Committee on Cancer (AJCC) staging system of gastric cancer. In addition, each LN tier was classified according to Japanese Gastric Cancer Association guidelines.4
The authors' criteria for cN staging were adopted after review of published articles.8-10 Most of these patients were discussed in preoperative inter-department weekly conferences, which determined clinical stage by S-CT. Clinical determination of N stage was reviewed by two radiologists, blinded to each other's findings and the pathology data, with the final diagnosis determined by consensus.
Patients' medical records were reviewed retrospectively. Demographic, tumor and clinical characteristics were analyzed to determine the diagnostic value of clinical N staging and the risk factors associated with underestimation of LN status. This study was approved by the institutional review board of Jeju National University Hospital (JEJUNUH 2013-05-022).
2. Clinical N staging
S-CT was performed with a 16 row multi-detector row computed tomography (MDCT) scanner (Sensation 16, Siemens Medical Systems, Erlangen, Germany) after administration of 10 mg of butyl scopolamine (Buscopan, Boehringer Ingelheim, Seoul, Korea) and two packs of effervescent granules.11 The scanning protocol was: 16×0.75 mm detector configuration; rotation time 0.5 seconds; slice thickness 1 mm; pitch 1.25; and kVp and mAs of 120 and 160. Images were reconstructed at intervals of 0.7 mm for 3-dimensional (3D) imaging and 3 mm for clinical interpretation. CT images were obtained 70 sec after injection of 120 ml of nonionic contrast material (iopromide, Ultravist370; Schering, Berlin, Germany) at a rate of 3~4 ml/s. During CT scanning, the patients were placed in a 30° right anterior oblique position for better distension of the upper half of the stomach, followed by placement in the supine position for appropriate distension of the lower half of the stomach. CT images were reconstructed using coronal and sagittal multiplanar reformations as well as axial images and 3D surface-shaded volume-rendering techniques. Reconstructed images and transverse 2D images were used to determine clinical stages.
cN stage on S-CT was determined using the following criteria: LN metastasis was considered present if the short-axis diameter of any LNs was ≥8 mm (Fig. 1 and 2), if there was a cluster of three or more perilesional nodes regardless of size, or if the LNs showed strong enhancement (>100 HU) (Fig. 3). LNs with central necrosis and perinodal infiltration were also considered indicative of LN metastasis, irrespective of size. Based on the number of metastatic nodes in CT images, clinical N stage was classified according to the 7th edition of the AJCC staging system.
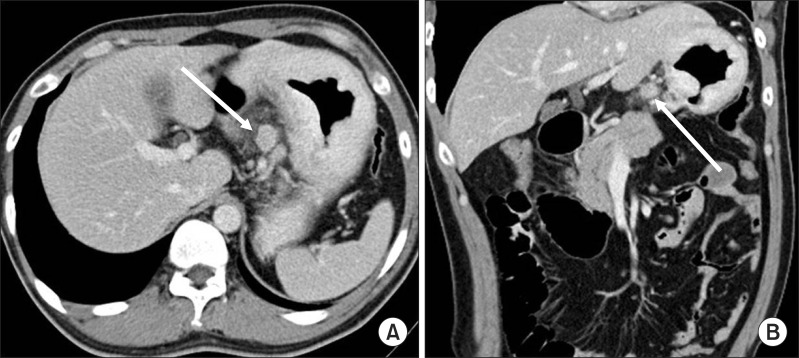
Fig. 1.
Clinical N staging using stomach protocol computed tomography: A 15 mm sized lymph node (arrows) is seen along the left gastric artery (A, B). This was proven metastatic lymph node by pathological examination.
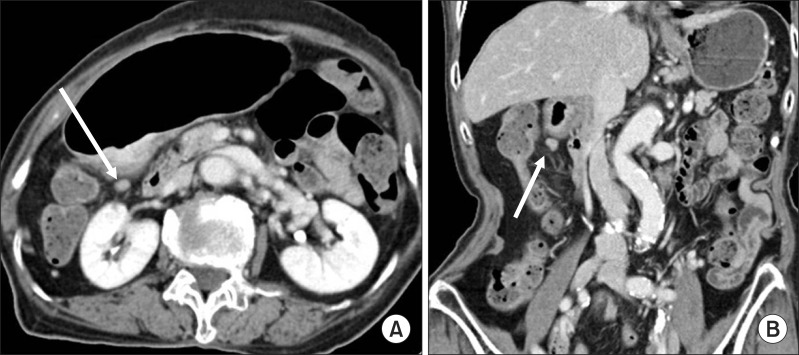
Fig. 2.
Clinical N staging using stomach protocol computed tomography: Contrast enhanced computed tomography shows a small lymph node along the lesser curvature (arrows in A, B). The size of this lymph node was 8.3 mm in short diameter on axial image. However, it was no evidence of metastasis by pathological examination.
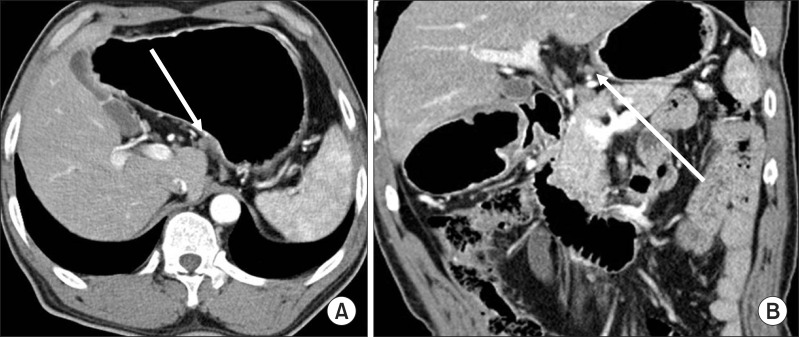
Fig. 3.
Clinical N staging using stomach protocol computed tomography: Contrast-enhanced computed tomography shows a 5.3 mm sized lymph node in infrapyloric area (arrows in A, B). The computed tomography number of this lymph node was measured about 115 HU on the average. This was revealed metastatic lymph node by pathological examination.
3. Statistical analysis
Quantitative results are expressed as mean±standard deviation. Ordinal variables were compared using independent t-tests or ANOVA with Tukey's test, and categorical variables were compared using Fisher's exact tests. Multivariate logistic regression analysis was used to determine the factors leading to underestimation. All statistical analyses were performed using the PASW Statistics 18.0 software program (IBM Co., Armonk, NY, USA), with P-values less than 0.05 considered statistically significant.
Results
The clinical and pathologic characteristics of the 171 included patients are summarized in Table 1. The study cohort consisted of 105 men and 66 women, of mean age 62.9±12.0 years. Mean tumor size was 4.0±3.4 cm, and mean body mass index was 23.19±3.26 kg/m2. Final diagnosis included 103 EGCs and 68 advanced gastric cancers (AGCs) lesions. Of the 171 patients, 97 (56.7%) underwent laparoscopic gastrectomy and 74 (43.3%) underwent gastrectomy under laparotomy; nine (5.3%) underwent total gastrectomy and 162 (94.7%) underwent distal subtotal gastrectomy. Eleven patients with stage IV gastric cancer underwent palliative gastric resection with lymphadenectomy more than D1+.
Table 1.
Demographic features of 171 gastric cancer lesions
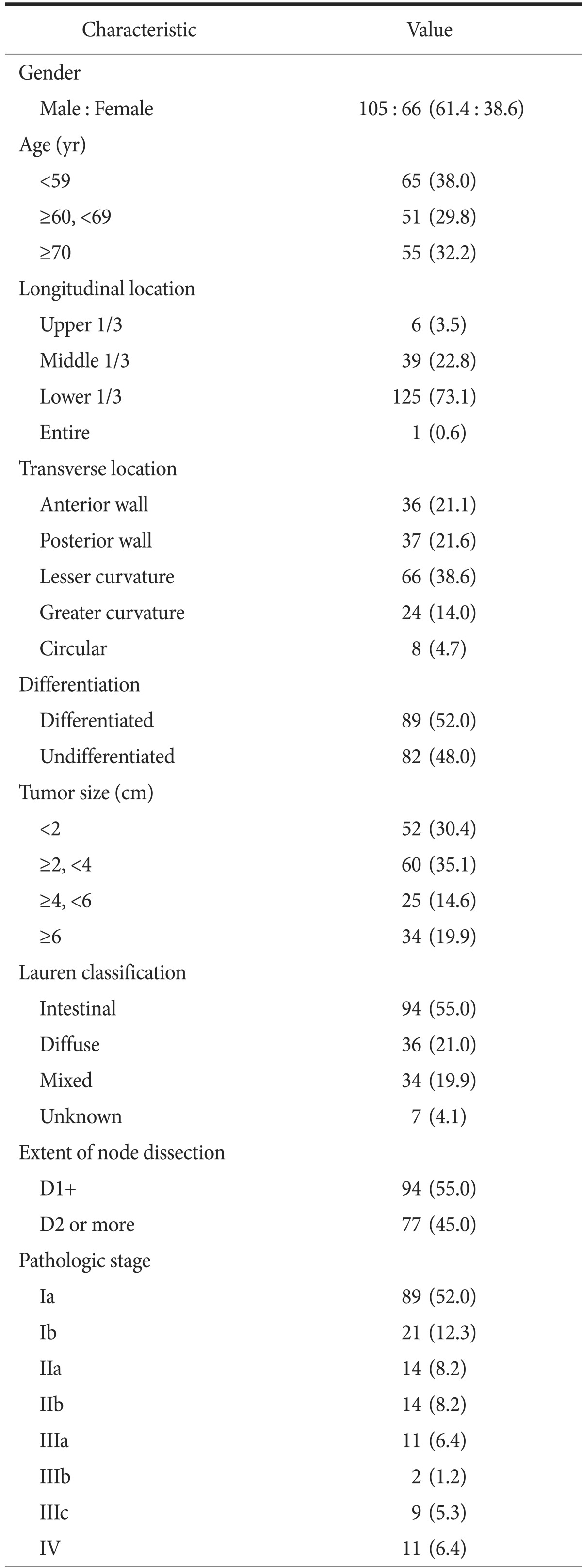
Values are presented as number (%).
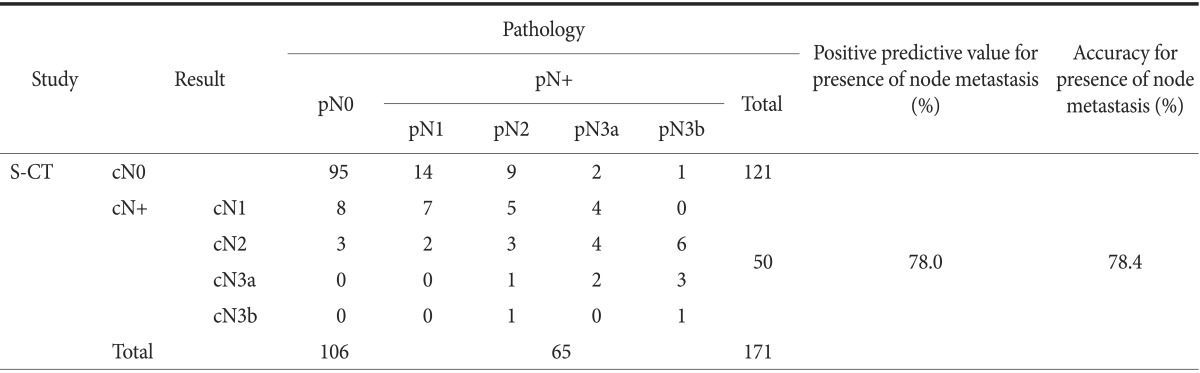
cN stage estimated by S-CT was matched with pathologic result (Table 2). The overall accuracy rate of detailed N staging by S-CT was 63.2%. S-CT indicated that 95 (89.6%) of the 106 pN0 lesions were cN0, with 11 (10.4%) being overestimated. Of the 65 pN1 or higher lesions, 48 (73.8%) were underestimated and four (6.2%) were overestimated. Overall, S-CT had a sensitivity of 60.0%, a specificity of 89.6%, an accuracy of 78.4%, and a positive predictive value of 78.0% in determining the presence of LN metastasis.
Table 2.
Results of stomach protocol computed tomography (S-CT) in preoperative determination of N stage
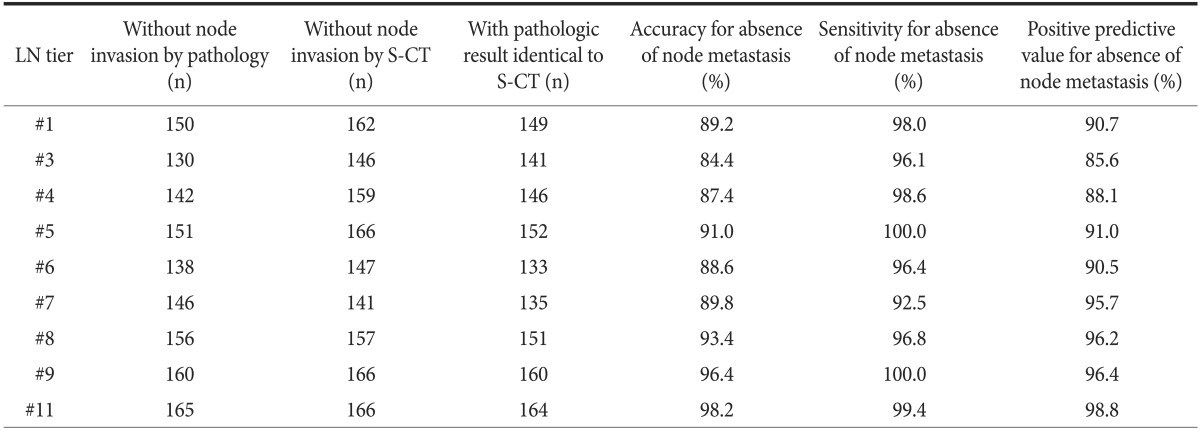
The presence of metastasis for each LN tier, as estimated by S-CT, was compared with the pathologic results (Table 3). Four patients had pathologic information about the total number of metastatic LNs but not about the status of each LN tier and were excluded from this analysis. The accuracy of S-CT in determining the presence of metastasis in each LN tier ranged from 84.4% to 98.2%, and the positive predictive value ranged from 85.6% to 98.8%. S-CT showed relatively low accuracy and positive predictive value (<90% each) for the perigastric LNs around the lesser or greater curvature of LNs 3 and 4, but had higher accuracy and positive predictive value (>90% each) for LNs 1, 5, 6, 7, 8, 9, and 11.
Table 3.
Results of N staging according to lymph node tier (n=167)
LN = lymph node; S-CT = stomach protocol computed tomography.
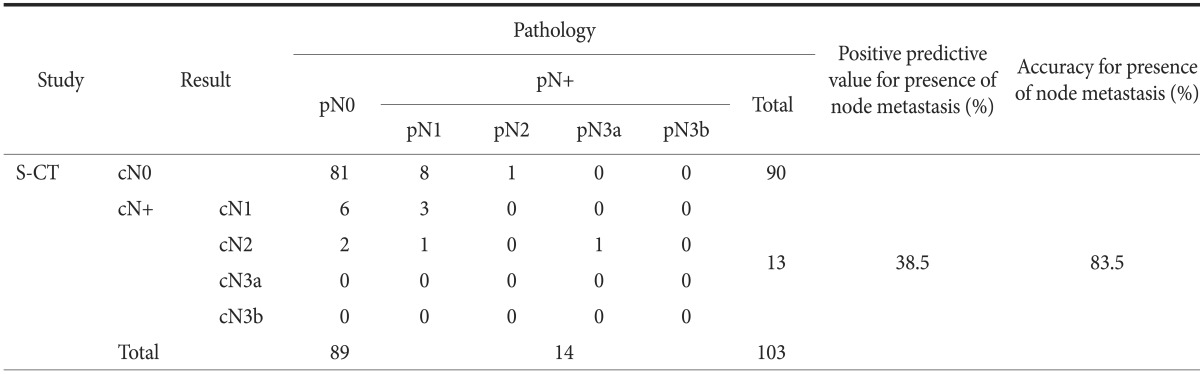
When clinical staging with S-CT for LN metastasis was compared with histological nodal staging in patients with EGC, the overall accuracy for detailed cN staging was found to be 81.6% (Table 4). Of the 89 pN0 lesions, 81 (91.0%) were cN0 on S-CT, whereas 8 (9.0%) were overestimated. Of the 14 lesions staged as pN1 or higher, 10 (71.4%) were underestimated and 1 (7.1%) was overestimated. Overall, S-CT had a sensitivity of 35.7%, a specificity of 91.0%, an accuracy of 83.5%, and a positive predictive value of 38.5% in identifying LN metastasis in patients with EGCs.
Table 4.
Results of stomach protocol computed tomography (S-CT) in preoperative determination of the presence of node metastasis for early gastric cancer
Of the 171 patients, 48 (28.1%) showed overstaging, indicating a more advanced pN stage than the results of S-CT (Table 5). When compared with patients with pN stage identical to cN stage and patients downstaged, the pathologic findings of overstaged patients were associated with undifferentiated (P=0.003) and diffuse (P=0.020) types. These patients had tumors of more advanced pathologic stage (P<0.001) and larger size (P<0.001). In addition, higher numbers of metastatic and harvested LNs (P<0.001 each) were associated with overstaging. Multivariate analysis showed that tumor differentiation (P=0.045) was an independent factor affecting overstaging by S-CT, whereas age, gender, body mass index, and tumor location were not.
Table 5.
Clinicopathologic features associated with overstaging of clinical stage by S-CT
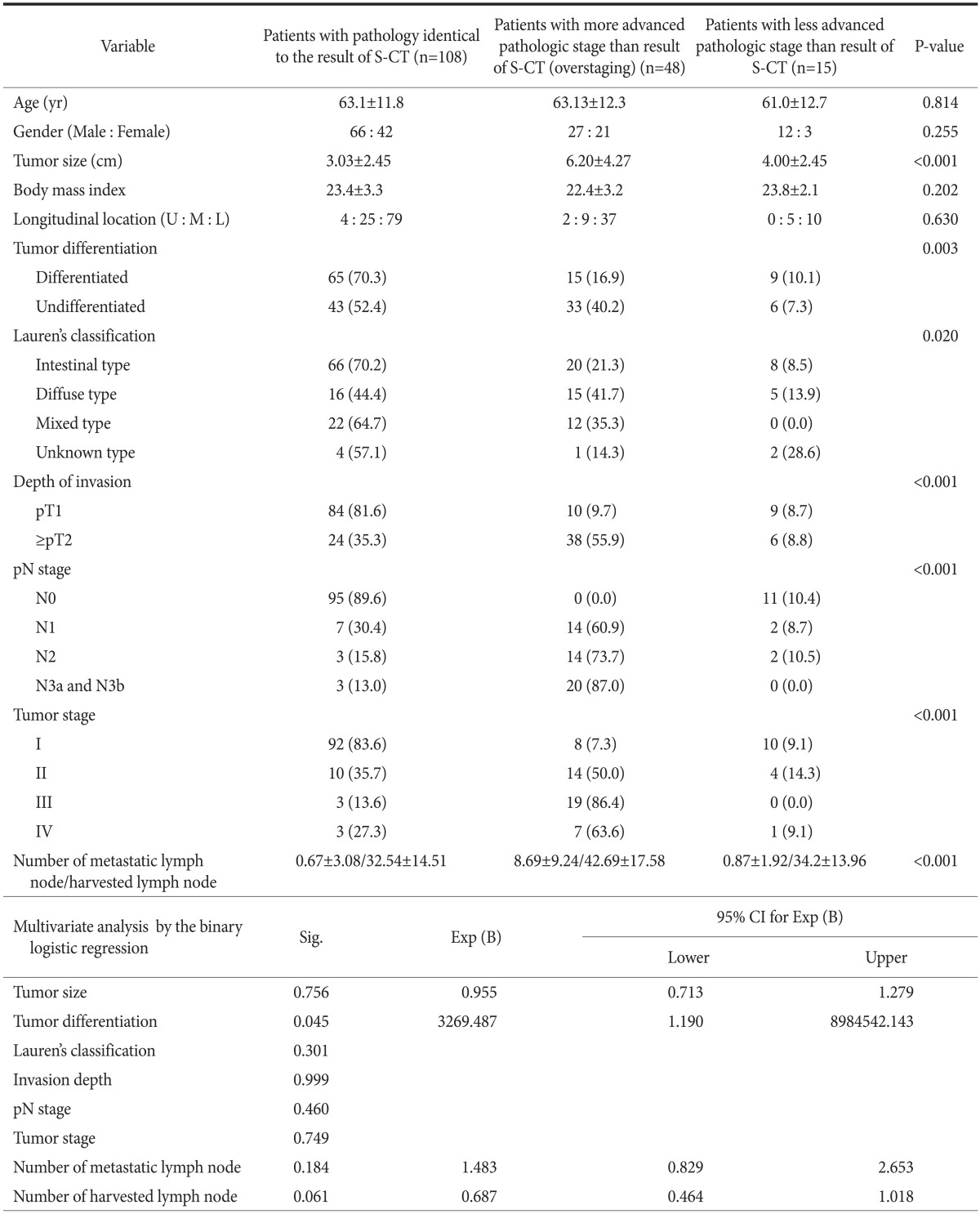
Values are presented as mean±standard deviation or number (%). S-CT = stomach protocol computed tomography; U = upper 1/3; M = middle 1/3; L = lower 1/3; Sig. = significance; Exp (B) = odds ratio; CI = confidence interval.
Discussion
cN staging is as critical as cT staging in determining the optimal type or extent of surgery.5-7 Several imaging modalities have been utilized to evaluate the cN stage of gastric cancers. Although the diagnostic accuracies of EUS, MDCT, magnetic resonance imaging, and 18F-fluoro-2-deoxyglucose positron emission tomography in assessing LN status have been evaluated in patients with gastric cancer,12-15 none of these techniques has shown both high sensitivity and high specificity in the preoperative detection of LN metastasis.16 MDCT remains the most widely used diagnostic tool for cT and cN staging. Thinner slices of MDCT have been associated with improved diagnosis of LN metastasis,17 and MDCT with multi-planar reformatted and 3D images has improved the accuracy of T and N staging in patients with gastric cancer.8,9 However, most investigations have found that the sensitivity and specificity of MDCT ranged widely from 60% to 90%.13,16 When the criteria defined all identifiable LNs as metastatic LNs, a high sensitivity and a relatively low specificity were revealed.18 Whereas, a relatively low sensitivity and a high specificity were revealed in the criteria of size more than 8 mm.8,16 The less than satisfactory diagnostic accuracy of MDCT for cN staging indicates the need to develop imaging modalities with both high sensitivity and high specificity.
Although cN staging by MDCT has been based on the size, attenuation values, and shapes of LNs, the criteria have varied.8,9,12-17 These discrepancies are likely due to the high frequency of microscopic tumor involvement in small-sized LNs and the poor differentiation between reactive and metastatic involvement of largesized LNs. For example, metastases have been found in 22.9% of undetected LNs and in about 60% of LNs >10 mm in size; furthermore, since at least 50% of the detected LNs were oval-shaped, shape alone was not a specific criterion to define infiltration.19 Another histologic study investigating the correlation between LN size and metastatic infiltration found that benign LNs of clinically relevant size are significantly more frequently observed in gastric cancer patients than in asymptomatic healthy individuals, as well as being more frequent in advanced than in EGC.20 These findings indicate that the diagnostic accuracy of MDCT for cN staging can be influenced by the cN stage criteria.
We found that CT images of slice thickness 3.0 mm could diagnose the presence of LN metastasis with a sensitivity of 60.0%, a specificity of 89.6%, an accuracy of 78.4%, and a positive predictive value of 78.0%. However, its sensitivity and specificity for the presence of LN metastasis in patients with EGC were 35.7% and 91.0%, respectively. Our MDCT results were similar to those of previous reports, except that we observed lower sensitivity.13,16 Yan et al.21 demonstrated that the diagnostic sensitivity of MDCT for determining LN metastasis of gastric carcinoma in patients with ≥4 metastatic LNs was higher than that in those with <4 (94.9% vs. 73.1%, P=0.000). We speculate that this low sensitivity results from high proportion (85.7%) of N1 among pN stages with LN metastasis in present study. In addition, our criteria may be too strict for EGC frequently with small or normal-sized metastatic nodes. S-CT had similar diagnostic accuracy among LN tiers, except that its sensitivity and specificity for LNs 3 and 4 were lower than for other LN tiers. LN tiers 3 and 4 are wider than the other LN tiers, suggesting the possibility of overlap with the gastric wall.
S-CT had an overall accuracy of 56.5% for cT stage and 63.2% for cN stage in our institute.11 Differentiation between EGC and AGC is important in deciding the extent of surgery. CT images could diagnose EGC lesions with a sensitivity of 90.3%, a specificity of 83.1%, an accuracy 87.5%, and a positive predictive value of 89.4%.11 Therefore, S-CT is highly specific but relatively insensitive for detecting nodal metastases, but is more sensitive in predicting invasion depth beyond the gastric muscular layer in our institute.
Underestimation of cN staging was more frequent in the lesions staged as pN1 or higher. Part of the lesions (71.4%) were underestimated in present study. Our result was similar to that (72.8%) of previous report.5 Few studies have assessed the risk factors for false prediction of LN status in patients with gastric cancer. The present study found that the underestimation of cN stage was significantly associated with larger tumor size, undifferentiated type, diffuse type, more advanced pathologic stage, and higher numbers of metastatic and harvested LNs. These underestimations are likely due to the more infiltrative growth pattern of tumors with these risk factors and the high frequency of microscopic tumor involvement of small-sized LNs. The associations between higher numbers of metastatic and harvested LNs and underestimation of cN stage may be due to the use of too strict criteria for small LNs, because overestimation was less frequent than underestimation. Because tumor differentiation was an independent factor affecting underestimation, surgeon considers the possibility of more advanced pN stage in the tumor with poor differentiation for avoidance of incomplete lymphadenectomy.
This study had several limitations. First, it was a retrospective study of patients evaluated over 8 years. Second, the histology of false-negative and false-positive LNs was not examined in detail. Future prospective studies, including a histologic analysis of lesionby-lesion matching of each LN, seem to be necessary. Third, 60.2% of the patients in this study had EGC and 62.0% were of pN0 stage, introducing a selection bias. In addition, LN tiers 2 and 12 beyond the extent of D1+ lymphadenectomy were not examined, because of a high proportion of EGC and a small number of metastasis to relevant LN tiers.
In conclusion, MDCT is highly specific but relatively insensitive in detecting nodal metastases in our criteria. Although the diagnostic accuracy of cN staging by MDCT is not poor, its sensitivity of 60% presents problems when using it to make therapeutic decisions. Improvements in imaging equipment and techniques will be essential in overcoming the drawbacks of this method, and rigorous criteria should be developed to improve the clinical usefulness of MDCT.
References
- 1.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Jung KW, Park S, Kong HJ, Won YJ, Boo YK, Shin HR, et al. Cancer statistics in Korea: incidence, mortality and survival in 2006-2007. J Korean Med Sci. 2010;25:1113–1121. doi: 10.3346/jkms.2010.25.8.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim YW, Yoon HM, Eom BW, Park JY. History of minimally invasive surgery for gastric cancer in Korea. J Gastric Cancer. 2012;12:13–17. doi: 10.5230/jgc.2012.12.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 5.Park SR, Kim MJ, Ryu KW, Lee JH, Lee JS, Nam BH, et al. Prognostic value of preoperative clinical staging assessed by computed tomography in resectable gastric cancer patients: a viewpoint in the era of preoperative treatment. Ann Surg. 2010;251:428–435. doi: 10.1097/SLA.0b013e3181ca69a7. [DOI] [PubMed] [Google Scholar]
- 6.Jeong JY, Kim MG, Ha TK, Kwon SJ. Prognostic factors on overall survival in lymph node negative gastric cancer patients who underwent curative resection. J Gastric Cancer. 2012;12:210–216. doi: 10.5230/jgc.2012.12.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Son T, Hyung WJ, Lee JH, Kim YM, Kim HI, An JY, et al. Clinical implication of an insufficient number of examined lymph nodes after curative resection for gastric cancer. Cancer. 2012;118:4687–4693. doi: 10.1002/cncr.27426. [DOI] [PubMed] [Google Scholar]
- 8.Lee IJ, Lee JM, Kim SH, Shin CI, Lee JY, Kim SH, et al. Diagnostic performance of 64-channel multidetector CT in the evaluation of gastric cancer: differentiation of mucosal cancer (T1a) from submucosal involvement (T1b and T2) Radiology. 2010;255:805–814. doi: 10.1148/radiol.10091313. [DOI] [PubMed] [Google Scholar]
- 9.Hur J, Park MS, Lee JH, Lim JS, Yu JS, Hong YJ, et al. Diagnostic accuracy of multidetector row computed tomography in T- and N staging of gastric cancer with histopathologic correlation. J Comput Assist Tomogr. 2006;30:372–377. doi: 10.1097/00004728-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Chen CY, Hsu JS, Wu DC, Kang WY, Hsieh JS, Jaw TS, et al. Gastric cancer: preoperative local staging with 3D multidetector row CT--correlation with surgical and histopathologic results. Radiology. 2007;242:472–482. doi: 10.1148/radiol.2422051557. [DOI] [PubMed] [Google Scholar]
- 11.Kim TH, Kim JJ, Kim SH, Kim BS, Song HJ, Na SY, et al. Diagnostic value of clinical T staging assessed by endoscopy and stomach protocol computed tomography in gastric cancer: the experience of a low-volume institute. J Gastric Cancer. 2012;12:223–231. doi: 10.5230/jgc.2012.12.4.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee HH, Lim CH, Park JM, Cho YK, Song KY, Jeon HM, et al. Low accuracy of endoscopic ultrasonography for detailed T staging in gastric cancer. World J Surg Oncol. 2012;10:190. doi: 10.1186/1477-7819-10-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn HS, Lee HJ, Yoo MW, Kim SG, Im JP, Kim SH, et al. Diagnostic accuracy of T and N stages with endoscopy, stomach protocol CT, and endoscopic ultrasonography in early gastric cancer. J Surg Oncol. 2009;99:20–27. doi: 10.1002/jso.21170. [DOI] [PubMed] [Google Scholar]
- 14.Kang BC, Kim JH, Kim KW, Lee DY, Baek SY, Lee SW, et al. Value of the dynamic and delayed MR sequence with Gd-DTPA in the T-staging of stomach cancer: correlation with the histopathology. Abdom Imaging. 2000;25:14–24. doi: 10.1007/s002619910003. [DOI] [PubMed] [Google Scholar]
- 15.Yun M, Lim JS, Noh SH, Hyung WJ, Cheong JH, Bong JK, et al. Lymph node staging of gastric cancer using (18)F-FDG PET: a comparison study with CT. J Nucl Med. 2005;46:1582–1588. [PubMed] [Google Scholar]
- 16.Kwee RM, Kwee TC. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer. 2009;12:6–22. doi: 10.1007/s10120-008-0492-5. [DOI] [PubMed] [Google Scholar]
- 17.Shinohara T, Ohyama S, Yamaguchi T, Muto T, Kohno A, Kato Y, et al. Clinical value of multidetector computed tomography in detecting lymph node metastasis of early gastric cancer. Eur J Surg Oncol. 2005;31:743–748. doi: 10.1016/j.ejso.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Stabile Ianora AA, Pedote P, Scardapane A, Memeo M, Rotondo A, Angelelli G. Preoperative staging of gastric carcinoma with multidetector spiral CT. Radiol Med. 2003;106:467–480. [PubMed] [Google Scholar]
- 19.Morgagni P, Petrella E, Basile B, Mami A, Soro A, Gardini A, et al. Preoperative multidetector-row computed tomography scan staging for lymphatic gastric cancer spread. World J Surg Oncol. 2012;10:197. doi: 10.1186/1477-7819-10-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park HS, Kim YJ, Ko SY, Yoo MW, Lee KY, Jung SI, et al. Benign regional lymph nodes in gastric cancer on multidetector row CT. Acta Radiol. 2012;53:501–507. doi: 10.1258/ar.2012.120054. [DOI] [PubMed] [Google Scholar]
- 21.Yan C, Zhu ZG, Yan M, Zhang H, Pan ZL, Chen J, et al. Value of multidetector-row computed tomography in the preoperative T and N staging of gastric carcinoma: a large-scale Chinese study. J Surg Oncol. 2009;100:205–214. doi: 10.1002/jso.21316. [DOI] [PubMed] [Google Scholar]