Abstract
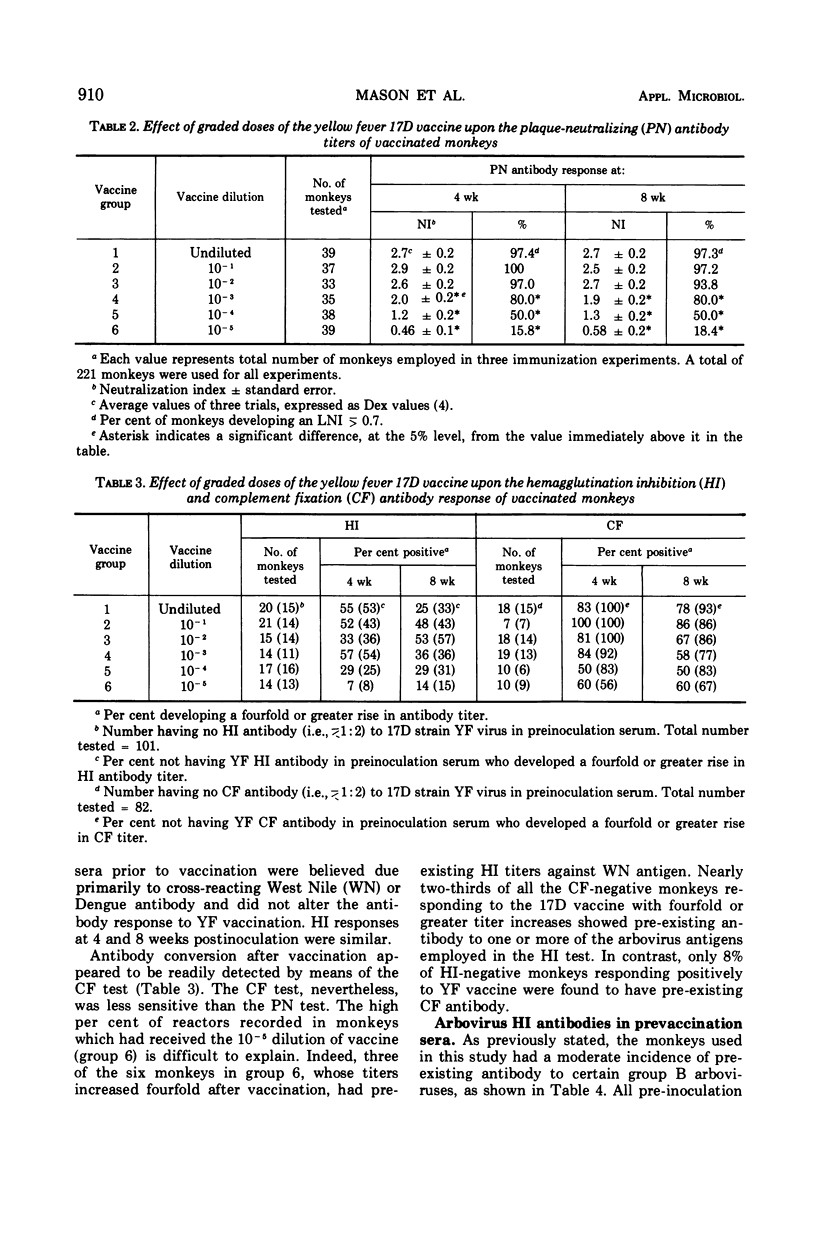
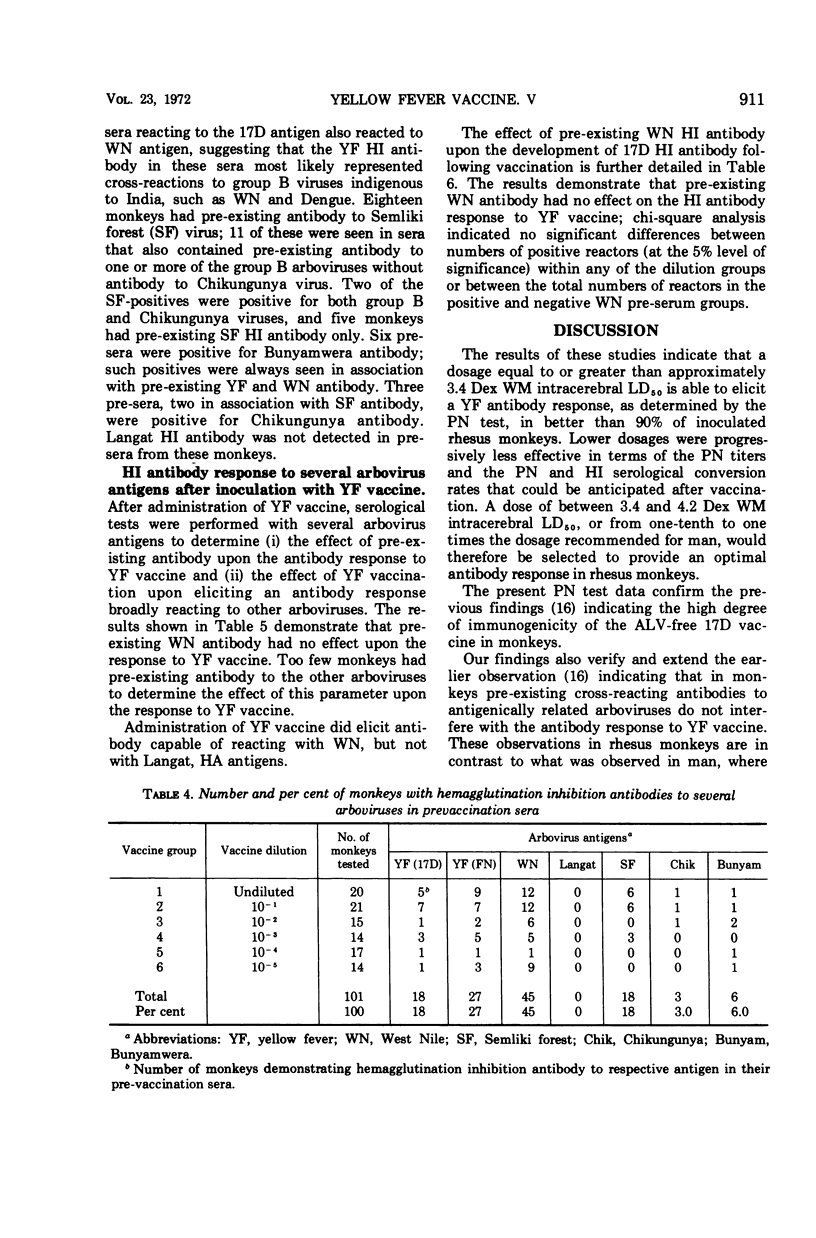
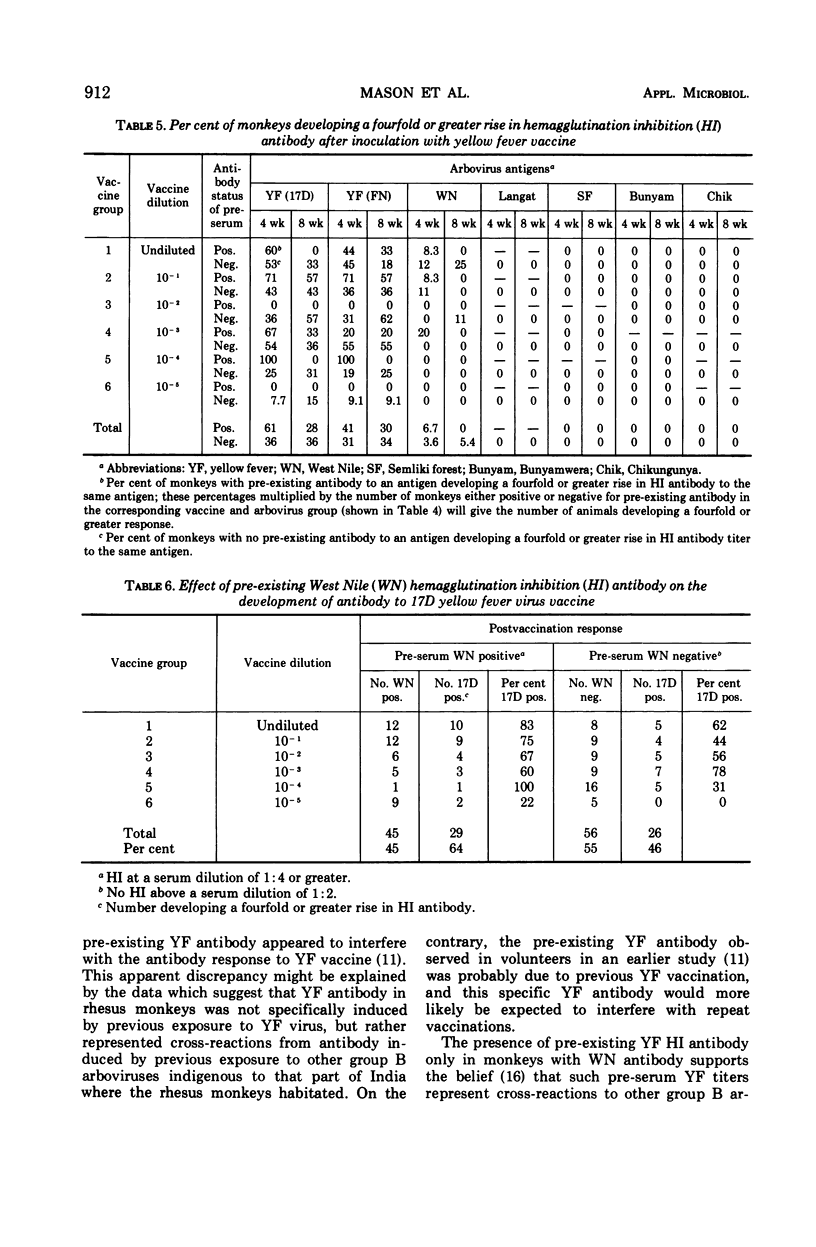
A dosage equal to or greater than approximately 3.4 Dex (decimal exponent, log10) weanling mouse intracerebral 50% lethal dose (LD50) was sufficient to elicit a yellow fever antibody response, as determined by the plaque neutralization (PN) test, in better than 90% of vaccinated rhesus monkeys. Lower dosages were progressively less effective in terms of PN titers and the PN and hemagglutination-inhibition serological conversion rates observed. A dose of between 3.4 and 4.2 Dex weanling mouse intracerebral LD50, or one-tenth to one times the dosage recommended for man, provided an optimal antibody response in monkeys. In rhesus monkeys, in contrast to the findings for man, pre-existing yellow fever antibody did not interfere with the antibody response to yellow fever vaccine. The PN test was felt to be a more sensitive and specific indicator of yellow fever antibody in rhesus monkeys after vaccination than the hemagglutination inhibition or complement fixation tests.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper C. C. A yellow fever vaccine free from avian leucosis viruses. J Hyg (Lond) 1967 Dec;65(4):505–513. doi: 10.1017/s0022172400046040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Spector S. L., Tauraso N. M. Yellow fever virus. II. Factors affecting the plaque neutralization test. Appl Microbiol. 1969 Nov;18(5):736–743. doi: 10.1128/am.18.5.736-743.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector S., Tauraso N. M. Yellow fever virus. I. Development and evaluation of a plaque neutralization test. Appl Microbiol. 1968 Nov;16(11):1770–1775. doi: 10.1128/am.16.11.1770-1775.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAURASO N., SHELOKOV A. PROTECTION AGAINST JUNIN VIRUS BY IMMUNIZATION WITH LIVE TACARIBE VIRUS. Proc Soc Exp Biol Med. 1965 Jul;119:608–611. doi: 10.3181/00379727-119-30251. [DOI] [PubMed] [Google Scholar]
- Tauraso N. M., Coultrip R. L., Legters L. J., Richman A. V., Rosenberg D. M., Savadge T. O., Shelokov A., Spector S. L., Trimmer R. W. Yellow fever vaccine. IV. Reactogenicity and antibody response in volunteers inoculated with a vaccine free from contaminating avian leukosis viruses. Proc Soc Exp Biol Med. 1972 Feb;139(2):439–446. doi: 10.3181/00379727-139-36161. [DOI] [PubMed] [Google Scholar]
- Tauraso N. M., Spector S. L., Jahnes W. G., Shelokov A. Yellow fever vaccine. I. Development of a vaccine seed free from contaminating avian leukosis viruses. Proc Soc Exp Biol Med. 1968 Apr;127(4):1116–1120. doi: 10.3181/00379727-127-32885. [DOI] [PubMed] [Google Scholar]
- Tauraso N. M., Spector S. L., Kirschstein R. L., Seligmann E. B., Jr, Farber J. F. Yellow fever vaccine. II. Antigenicity and neurovirulence of a vaccine seed free from avian leukosis virus. Appl Microbiol. 1969 Jun;17(6):866–870. doi: 10.1128/am.17.6.866-870.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISKRANTZ L. Effects of medial temporal lesions on taste preference in the monkey. Nature. 1960 Sep 3;187:879–880. doi: 10.1038/187879b0. [DOI] [PubMed] [Google Scholar]


