Abstract
Purpose
To critically review and summarize the literature comparing the results of surgery via an anterior approach and that via a posterior approach for the treatment of thoracolumbar burst fractures to identify the better approach.
Methods
In this meta-analysis, we conducted electronic searches of MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials and other databases using the search terms “thoracolumbar fractures”, “anterior”, “posterior”, “controlled clinical trials”. Relevant journals or conference proceedings were also searched manually. Data extraction and quality assessment were in accordance with Cochrane Collaboration guidelines. The analysis was performed on individual patient data from all the trials that met the selection criteria. Sensitivity analysis was performed when there was significant heterogeneity. Results were expressed as risk difference for dichotomous outcomes and mean difference for continuous outcomes with 95 % confidence interval.
Results
Four randomized clinical trials and three controlled clinical trials comparing the results of the anterior versus posterior approach in the treatment of thoracolumbar burst fractures were retrieved; these studies included 179 and 152 patients in the anterior and posterior approach groups, respectively. There were no differences in terms of neurological recovery, return to work, complications and Cobb angle between the two groups. The anterior approach was associated with longer operative time, greater blood loss and higher cost than the posterior approach.
Conclusions
The posterior approach may be more effective than the anterior approach. However, more high-quality, randomized controlled trials are required to compare these approaches and guide clinical decision-making.
Level of Evidence Level II, therapeutic study. See the Guidelines for Authors for a complete description of level of evidence.
Keywords: Anterior approach, Posterior approach, Thoracolumbar burst fractures, Meta-analysis
Introduction
The majority of spine fractures occur in the thoracolumbar region, with burst fractures accounting for 10–20 % of cases, presumably because in this region, the relatively immobile thoracic spine connects with the relatively mobile lumbar spine [1, 2]. The burst fracture is an injury characterized by anterior vertebral body height loss, fracture of the posterior wall or retropulsion bone fragments into the spinal canal [3]. The clinical features of burst fractures include acute back pain, restricted motion and neurological deficits such as motor or sensory changes and sphincter disturbances. Radiographic signs of vertebral instability include widening of the interspinous and interlaminar distances, translation of more than 2 mm, kyphosis of more than 20°, dislocation, height loss of more than 50 % and articular process fractures [4, 5].
Although many studies have obtained good results with nonoperative treatment of thoracolumbar burst fractures [6–8], most authors agree that surgical treatment is required for symptomatic, unstable burst fractures [9, 10]. Surgical intervention can decompress neural elements, restore vertebral body height, correct angular deformity and stabilize the spine. Stabilization of these injuries has many advantages such as early mobilization and the potential for neurological improvement. However, controversies exist over the specific approach to be used for surgical treatment. Surgeons today have the option of either an anterior or a posterior approach. Some authors recommend posterior instrumentation with or without decompression because of the excellent results obtained in terms of spinal stability, anatomical alignment, postoperative neurological improvement and low patient morbidity [11–13]. In contrast, advocates of the anterior approach cite predictable decompression of the spinal canal, improvement in postoperative neurological function and no significant increase in surgical morbidity [14–16]. Both the anterior and posterior approaches are associated with satisfactory results as well as some complications. Treatment of thoracolumbar injury depends on many clinical factors such as patient age, extent of spinal canal compromise, sagittal index, anterior body height, degree of integrity of posterior elements and presence of neurological deficit [17]. All these parameters affect the decision regarding the surgical approach, with each parameter being an indication for one or the other approach. Therefore, no consensus has been reached about the ideal treatment approach [18, 19].
Studies that have compared the outcomes of thoracolumbar burst fractures treated via an anterior or a posterior approach have reported ambiguous results. Moreover, some doctors prefer a specific approach for a given fracture. Therefore, the objective of this study was to systematically review relevant randomized controlled trials to clarify the differences in these two approaches.
Materials and methods
Search strategy
Electronic searches of MEDLINE (1950–present), EMBASE (1980–present), the Cochrane Central Register of Controlled Trials (most recent edition) and other internet databases were performed to identify trials according to the Cochrane Collaboration guidelines. We used the following search terms and different combinations of MeSH (Medical Subject Heading) terms and textual words: “thoracolumbar fractures”, “anterior”, “posterior”, “controlled clinical trials”. Manual searches, including those of reference lists of all included studies, were used to identify trials that the electronic search may have failed to identify. Two reviewers (Xu and Fu) independently assessed the titles and abstracts of all reports identified by the electronic and manual searches. There was no restriction on language. When inclusion was unclear based on abstracts, full-text articles were retrieved. Any disagreements were resolved through discussion.
Selection criteria and quality assessment
Trials with the following characteristics were included: (1) randomized, quasi-randomized or controlled clinical trials, (2) patients without confirmed pathological thoracolumbar burst fractures based on computed tomography (CT) and plain radiographs, (3) comparison of the anterior and posterior approaches of surgical management and (4) full-text articles. We excluded articles that were duplicate reports of earlier trials or post hoc analyses of randomized controlled trial data and articles whose full text we were unable to acquire. To assess the methodological quality of the included studies, we used a modification of the generic evaluation tool used by the Cochrane Bone, Joint and Muscle Trauma Group [20]. The methodological quality of each trial was scored from 0 to 24. Disagreements were resolved by consensus or consultation with the senior reviewer.
Data extraction
Two authors independently extracted data from the included articles. Information regarding the study design, patient demographics, inclusion and exclusion criteria, interventions, outcomes, follow-up duration and rate of lost to follow-up duration for each treatment group was extracted. Data were managed using the Review Manager (RevMan) 5.1 software (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). We attempted to contact authors for supplementary information when the reported data were inadequate.
Data analysis and statistical methods
The meta-analysis was undertaken using RevMan 5.1 for Windows (Cochrane Collaboration, Oxford, UK). We assessed the statistical heterogeneity of each study by using a standard Chi square test (statistical heterogeneity was considered significant at P > 0.1) and the I2 statistic [21]. An I2 value of 50 % or higher was considered to indicate substantial heterogeneity. When heterogeneity existed, pooled data were meta-analyzed using a random-effects model [22]. Otherwise, a fixed-effects model was used for the analysis. Risk difference (RD) and 95 % confidence interval (CI) were calculated for dichotomous outcomes, while mean difference (MD) and 95 % CI were used for continuous outcomes.
Results
Study characteristics
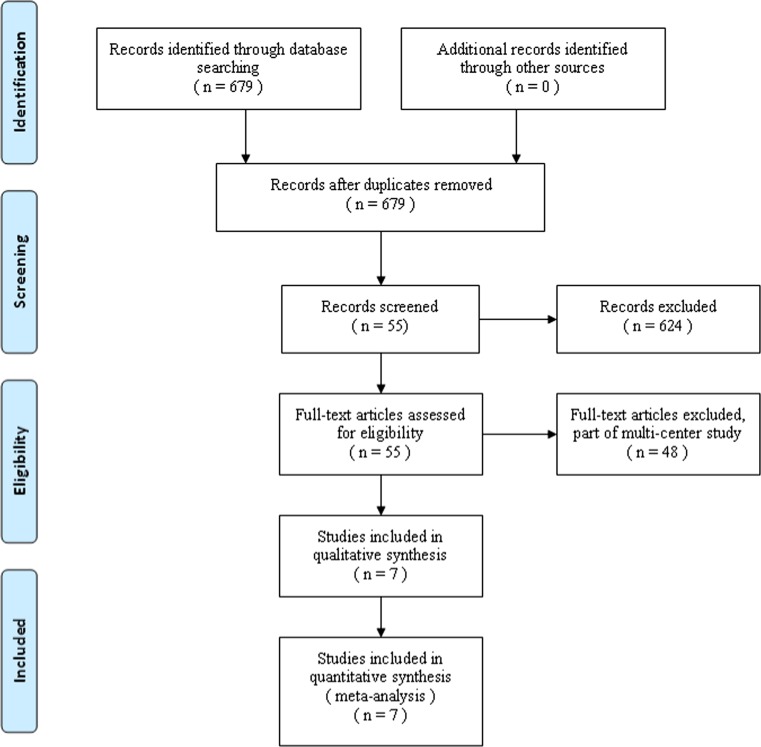
Figure 1 shows a flow chart of the study selection and inclusion process. The search strategy identified 679 citations, of which seven satisfied the pre-defined inclusion criteria for data extraction and meta-analysis [23–29] (Table 1). These studies involved 179 patients in the anterior approach group and 152 patients in the posterior approach group. The majority of the included trials were small studies with between 25 and 63 participants. Their quality-assessment scores ranged from 10 to 22. Three studies had a score of <14. The patients’ characteristics were comparable within each study group. Individual patient data were available from these articles. This did not include data for those lost to follow-up. There were no between-group differences in gender, mechanism of injury, level of fracture or length of follow-up.
Fig. 1.
Flow chart showing identification and selection of cases
Table 1.
Characteristics of the included studies
| Study | Group | Number | Age (years) | Gender | Fracture level | Lost | Follow-up duration (months) | QAS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | ~T10 | T11 | T12 | L1 | L2 | L3~ | |||||||
| Lin et al. [29] | Anterior | 32 | 38 | 14 | 18 | 0 | 3 | 10 | 14 | 5 | 0 | 0 | 45 (24–72) | 22 |
| Posterior | 32 | 39 | 16 | 16 | 0 | 2 | 9 | 17 | 4 | 0 | 0 | |||
| Stancic [25] | Anterior | 13 | 36 | 7 | 6 | 0 | 0 | 1 | 10 | 2 | 0 | 0 | >12 | 20 |
| Posterior | 12 | 35 | 8 | 4 | 0 | 0 | 2 | 9 | 2 | 0 | ||||
| Sasso et al. [28] | Anterior | 40 | 37 | 29 | 11 | 0 | 38 | 2 | 3 | 31 (9–89) | 13 | |||
| Posterior | 13 | 34 | 10 | 3 | 0 | 0 | 4 | 6 | 2 | 1 | 0 | |||
| Wood et al. [26] | Anterior | 22 | 38 | 12 | 8 | 0 | 1 | 1 | 10 | 8 | 0 | 2 | 44 (24–108) | 21 |
| Posterior | 21 | 42 | 13 | 5 | 0 | 0 | 4 | 11 | 3 | 0 | 3 | |||
| Esses [23] | Anterior | 18 | 34 | 25 | 15 | 1 | 0 | 5 | 22 | 9 | 3 | 2 | 20 (12–34) | 19 |
| Posterior | 22 | 0 | ||||||||||||
| Hitchon et al. [27] | Anterior | 38 | 42 | 26 | 12 | 0 | 1 | 13 | 18 | 6 | 0 | 0 | 33 (6–96) | 10 |
| Posterior | 25 | 42 | 19 | 6 | 0 | 1 | 3 | 12 | 9 | 0 | 0 | |||
| Danisa et al. [24] | Anterior | 16 | 35 | 11 | 5 | 0 | 0 | 1 | 13 | 2 | 0 | 0 | 27 (6–54) | 12 |
| Posterior | 27 | 38 | 19 | 8 | 0 | 0 | 8 | 16 | 3 | 0 | 0 | |||
Results of data analysis
Radiographic evaluation
The Cobb angle was reported in all studies, except for the study by Stancic et al. [25]. Wood et al. [26] and Esses et al. [23] compared canal encroachment. At the final follow-up, canal encroachment had improved to a greater extent in the anterior approach group than in the posterior approach group (MD, −8.2; 95 % CI −17.33 to 0.92; P = 0.08; Fig. 2). However, no between-group difference was found in the Cobb angle (MD, −0.06; 95 % CI −0.62 to 0.51; P = 0.84; Fig. 3).
Fig. 2.
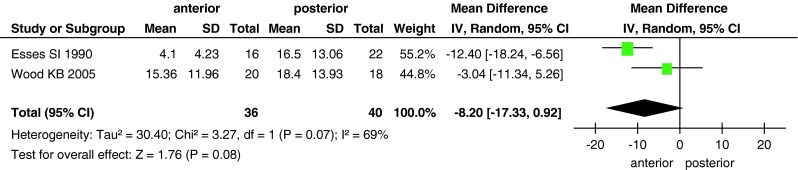
Forest plot showing canal encroachment in the two groups
Fig. 3.
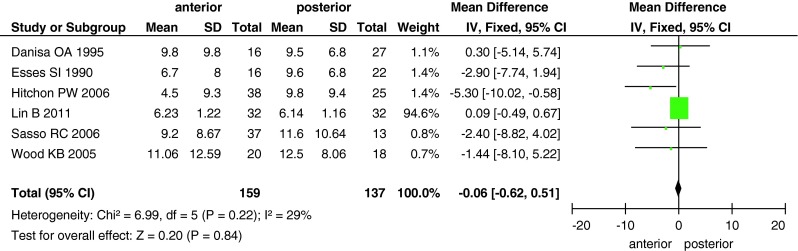
Forest plot showing the Cobb angle in the two groups
Frankel score
Although five studies evaluated neurological recovery, we could obtain data from only four studies, in which a total of 194 patients (107 in the anterior approach group and 87 in the posterior approach group) were examined for neurological function using Frankel scores. We could not use the information from the study by Lin et al. [29], because we used the improvement in Frankel scores (by one or more grades) to calculate the effects of the two surgical approaches. No significant differences were detected between the anterior and posterior approach groups (RD, 0.06; 95 % CI −0.07 to 0.19; P = 0.38; Fig. 4). In addition, Lin et al. [29] did not find a significant difference between the anterior and posterior approach groups.
Fig. 4.
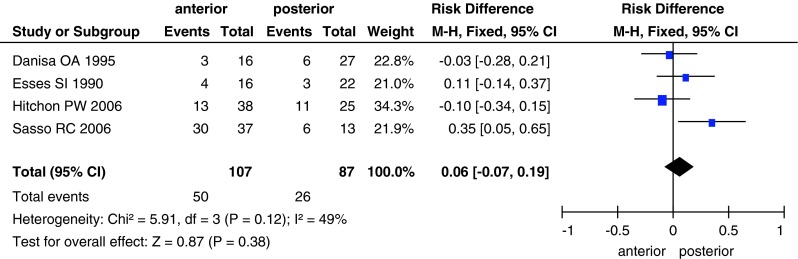
Forest plot showing Frankel scores in the two groups
Return to work
A total of 163 patients (84 in the anterior approach group and 79 in the posterior approach group) returned to work (Fig. 5). There was no significant between-group difference (RD, −0.02; 95 % CI −0.16 to 0.13; P = 0.8) in the number of patients who returned to work.
Fig. 5.
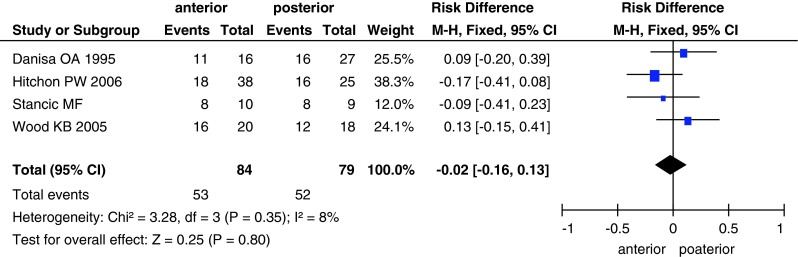
Forest plot showing the number of patients who returned to work in the two groups
Operative data and cost
The differences in blood loss, operative time and cost between the two groups are shown in Figs. 6, 7, 8. Data on blood loss were available from three trials with a total of 145 patients (68 in the anterior approach group and 77 in the posterior approach group). There was significant heterogeneity (I2 = 82 %) among these studies, and analysis with the random-effects model revealed significant between-group differences (MD, 310.67; 95 % CI 26.38–594.96; P = 0.03). Operative time was reported in three studies with 165 patients, and it significantly differed between the two surgical approaches (MD, 17.17; 95 % CI 5.78–28.57; P = 0.003). Only two studies reported the surgical cost for the two groups; the cost was higher in the anterior approach group than in the posterior approach group (MD, 15,530; 95 % CI 6,720–24,340; P = 0.0006).
Fig. 6.
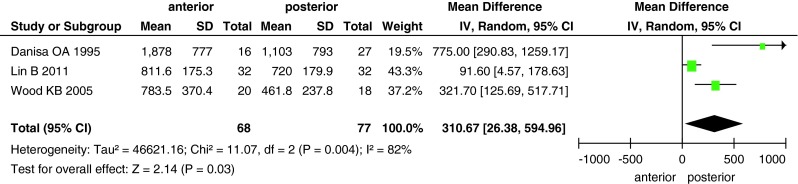
Forest plot showing blood loss in the two groups
Fig. 7.
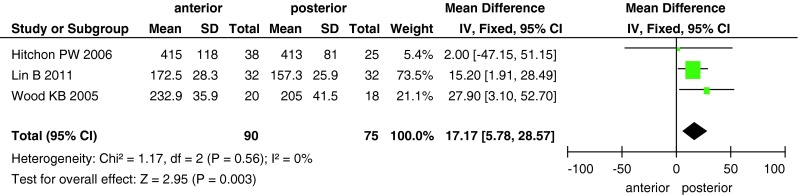
Forest plot showing operative time in the two groups
Fig. 8.
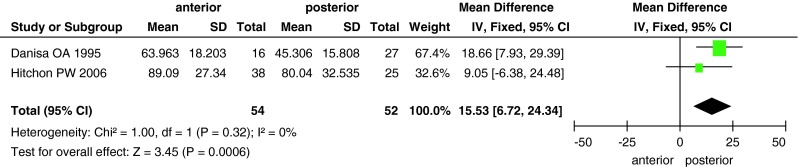
Forest plot showing surgical cost in the two groups
Complications
The incidence of complications was documented in all studies with a total of 331 patients. There was significant heterogeneity (I2 = 97 %) among the studies; however, this disappeared after the exclusion of two studies (Lin et al. [29] and Wood et al. [26]; I2 = 3 %, Fig. 9). We used the random-effects model for the analysis, and found no significant between-group difference in the incidence of complications (RD, −0.06; 95 % CI −0.15 to 0.02; P = 0.13). Among the reported complications, four cases of deep wound infections were recorded in three studies [24, 26, 27], and all four cases occurred in the posterior approach group.
Fig. 9.
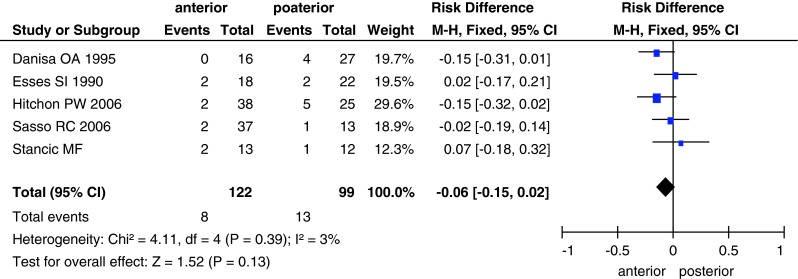
Forest plot showing complications in the two groups
Discussion
Thoracolumbar burst fractures can be treated via different approaches: anterior, posterior or a combination of these two approaches in some special cases. Theoretically, the anterior approach offers some benefits such as better canal decompression [16, 30]. This approach provides better exposure of the fractured vertebrae, enabling a more thorough decompression [10]. In contrast, the posterior approach can only support indirect decompression [31]. Therefore, good canal remodeling after canal encroachment occurred in the anterior approach group just as all the included studies concerning this parameter. Our meta-analysis showed that canal remodeling was better in the anterior approach group than in the posterior approach group at the final follow-up. Such remodeling has also been reported in other articles [32–34].
Interestingly, although some authors have reported that compared to the posterior approach, the anterior approach provides better decompression of thoracolumbar fractures, which facilitates neurological recovery [35], others have found no significant differences [26, 28]. The relationship between the extent of canal encroachment and neurological function has been studied; although concerns have been raised regarding inadequate spinal canal remodeling after treatment via the posterior approach, there is no evident association between the percentage of canal encroachment and clinical symptoms [36, 37].
Generally, the most important purpose of the surgical management of thoracolumbar fractures is to minimize the change in the patients’ lives. Our review showed that although the anterior approach was associated with better canal remodeling, it was not associated with a greater improvement in Frankel scores or a higher incidence of return to work. This is similar to reports that recovery of neurological function did not depend on the extent of spinal decompression and canal encroachment [37, 38]. Among the seven included studies, only Wood et al. [26] reported pain scores on a 10-point visual analog pain scale; the results showed that there was no significant difference in pain reduction between the two groups.
On critical evaluation of the included trials, we found that Lin et al. [29] reported many more complications in the anterior approach group than in the posterior group, including twenty-seven cases of hemopneumothorax, two cases of respiratory tract infection, three cases of intercostal neuralgia and thirteen cases of abdominal distension and constipation. In the study conducted by Wood et al. [26], there were seventeen “events” in the posterior approach group, including six cases of instrument removal, two cases of wound dehiscence, two cases of instrumentation/bone failure, two urinary tract infections, two cases of instrument breakage, one deep wound infection, one case of pseudarthrosis and one case of seroma. Because these two studies markedly differed from the other included studies, sensitivity analysis after the removal of these studies showed a significant reduction in the heterogeneity among studies, from I2 97–3 %. After the exclusion of these two studies, our results showed that the incidence of complications did not differ between the two approaches. Deep wound infection is a catastrophic complication that necessitates implant removal. Only three studies reported this complication, and all four of the reported cases were in the posterior approach group, which suggests that deep wound infection is more common after surgery via a posterior approach. Unfortunately, we do not know why the smaller incision of the posterior approach was associated with a higher incidence of deep wound infection.
In addition, we found significant differences in operative time, blood loss and cost between the two groups. The anterior approach group was associated with longer operative times, greater blood loss and higher costs; thus, use of the posterior approach could potentially decrease the risks associated with long operative times and greater blood loss and transfusion.
We acknowledge some limitations of the literature and our review. First, although several relevant trials have been published, the majority were small and of low quality. Few comparative trials satisfied our inclusion criteria, including three articles with quality-assessment scores of below 14. As a result, we could not perform subgroup analysis because the information required was unavailable. Second, the heterogeneity of the study populations in terms of complications and therapeutic options posed additional challenges in evaluating the individual therapeutic options. This clinical heterogeneity, combined with the small sample sizes of the included studies, resulted in high I2 values for our pooled results for complications, blood loss and Cobb angle. Third, the use of variable outcome measures and suboptimal reporting, often at nonstandardized intervals, further undermined informed decision-making. Lastly, we must mention that the selection of the surgical approach for the treatment of thoracolumbar fractures should be individualized because many factors influence this choice.
Conclusion
The results of this review showed that in the surgical management of thoracolumbar burst fractures, the anterior approach was not significantly superior to the posterior approach in terms of recovery of neurological function and return to work and that it was disadvantageous in terms of operative time, blood loss and cost. To some extent, the posterior approach was better than the anterior approach. However, the quality of the studies included was not satisfactory. Therefore, selection of the appropriate approach must be made cautiously and on a case-by-case basis. More high-quality, randomized controlled trials are required to guide the selection of the surgical approach in patients with thoracolumbar burst fractures.
Acknowledgments
The authors are grateful for the financial support of the Project of National Natural Science Foundation of China (No. 11072021) and Wu Jie Ping Medical Foundation (No. 320.6750.11017).
Conflict of interest
Each author certifies that he has no commercial associations that might pose a conflict of interest with the submitted article.
Footnotes
G. J. Xu and Z. J. Li equally contributed to the study.
X. Fu and X. L. Ma equally contributed to the corresponding author.
References
- 1.Muller U, Berlemann U, Sledge J, Schwarzenbach O. Treatment of thoracolumbar burst fractures without neurologic deficit by indirect reduction and posterior instrumentation: bisegmental stabilization with monosegmental fusion. Eur Spine J. 1999;8(4):284–289. doi: 10.1007/s005860050175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magerl F, Aebi M, Gertzbein SD, Harms J, Nazarian S. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3(4):184–201. doi: 10.1007/BF02221591. [DOI] [PubMed] [Google Scholar]
- 3.Oner FC, Ramos LM, Simmermacher RK, Kingma PT, Diekerhof CH, Dhert WJ, Verbout AJ. Classification of thoracic and lumbar spine fractures: problems of reproducibility. A study of 53 patients using CT and MRI. Eur Spine J. 2002;11(3):235–245. doi: 10.1007/s00586-001-0364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersilge CA, Emery SE. Thoracolumbar burst fracture: evaluating stability. Semin Ultrasound CT MR. 1996;17(2):105–113. doi: 10.1016/S0887-2171(96)90010-4. [DOI] [PubMed] [Google Scholar]
- 5.Cho WS, Chung CK, Jahng TA, Kim HJ. Post-laminectomy kyphosis in patients with cervical ossification of the posterior longitudinal ligament: does it cause neurological deterioration? J Korean Neurosurg Soc. 2008;43(6):259–264. doi: 10.3340/jkns.2008.43.6.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai LY, Jiang LS, Jiang SD (2008) Conservative treatment of thoracolumbar burst fractures: a long-term follow-up results with special reference to the load sharing classification. Spine (Phila Pa 1976) 33(23):2536–2544 [DOI] [PubMed]
- 7.Post RB, van der Sluis CK, Leferink VJ, Ten DH. Long-term functional outcome after type A3 spinal fractures: operative versus non-operative treatment. Acta Orthop Belgica. 2009;75(3):389–395. [PubMed] [Google Scholar]
- 8.Thomas KC, Bailey CS, Dvorak MF, Kwon B, Fisher C. Comparison of operative and nonoperative treatment for thoracolumbar burst fractures in patients without neurological deficit: a systematic review. J Neurosurg Spine. 2006;4(5):351–358. doi: 10.3171/spi.2006.4.5.351. [DOI] [PubMed] [Google Scholar]
- 9.Heary RF, Salas S, Bono CM, Kumar S. Complication avoidance: thoracolumbar and lumbar burst fractures. Neurosurg Clin N Am. 2006;17(3):377–388. doi: 10.1016/j.nec.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Kim HS, Lee SY, Nanda A, Kim JY, Park JO, Moon SH, Lee HM, Kim HJ, Wei H, Moon ES. Comparison of surgical outcomes in thoracolumbar fractures operated with posterior constructs having varying fixation length with selective anterior fusion. Yonsei Med J. 2009;50(4):546–554. doi: 10.3349/ymj.2009.50.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cresswell TR, Marshall PD, Smith RB. Mechanical stability of the AO internal spinal fixation system compared with that of the Hartshill rectangle and sublaminar wiring in the management of unstable burst fractures of the thoracic and lumbar spine. Spine (Phila Pa 1976) 1998;23(1):111–115. doi: 10.1097/00007632-199801010-00022. [DOI] [PubMed] [Google Scholar]
- 12.Parker JW, Lane JR, Karaikovic EE, Gaines RW. Successful short-segment instrumentation and fusion for thoracolumbar spine fractures: a consecutive 41/2-year series. Spine (Phila Pa 1976) 2000;25(9):1157–1170. doi: 10.1097/00007632-200005010-00018. [DOI] [PubMed] [Google Scholar]
- 13.Sjostrom L, Karlstrom G, Pech P, Rauschning W. Indirect spinal canal decompression in burst fractures treated with pedicle screw instrumentation. Spine (Phila Pa 1976) 1996;21(1):113–123. doi: 10.1097/00007632-199601010-00026. [DOI] [PubMed] [Google Scholar]
- 14.Carl AL, Tranmer BI, Sachs BL. Anterolateral dynamized instrumentation and fusion for unstable thoracolumbar and lumbar burst fractures. Spine (Phila Pa 1976) 1997;22(6):686–690. doi: 10.1097/00007632-199703150-00022. [DOI] [PubMed] [Google Scholar]
- 15.Dai LY, Jiang LS, Jiang SD. Anterior-only stabilization using plating with bone structural autograft versus titanium mesh cages for two- or three-column thoracolumbar burst fractures: a prospective randomized study. Spine (Phila Pa 1976) 2009;34(14):1429–1435. doi: 10.1097/BRS.0b013e3181a4e667. [DOI] [PubMed] [Google Scholar]
- 16.Kaneda K, Taneichi H, Abumi K, Hashimoto T, Satoh S, Fujiya M. Anterior decompression and stabilization with the Kaneda device for thoracolumbar burst fractures associated with neurological deficits. J Bone Joint Surg Am. 1997;79(1):69–83. doi: 10.2106/00004623-199701000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Dai LY, Jiang SD, Wang XY, Jiang LS (2007) A review of the management of thoracolumbar burst fractures. Surg Neurol 67(3):221–231 [DOI] [PubMed]
- 18.Tasdemiroglu E, Tibbs PA. Long-term follow-up results of thoracolumbar fractures after posterior instrumentation. Spine (Phila Pa 1976) 1995;20(15):1704–1708. doi: 10.1097/00007632-199508000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Verlaan JJ, Diekerhof CH, Buskens E, van der Tweel I, Verbout AJ, Dhert WJ, Oner FC. Surgical treatment of traumatic fractures of the thoracic and lumbar spine: a systematic review of the literature on techniques, complications, and outcome. Spine (Phila Pa 1976) 2004;29(7):803–814. doi: 10.1097/01.BRS.0000116990.31984.A9. [DOI] [PubMed] [Google Scholar]
- 20.Handoll HH, Gillespie WJ, Gillespie LD, Madhok R. The Cochrane Collaboration: a leading role in producing reliable evidence to inform healthcare decisions in musculoskeletal trauma and disorders. Indian J Orthop. 2008;42(3):247–251. doi: 10.4103/0019-5413.41849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 23.Esses SI, Botsford DJ, Kostuik JP. Evaluation of surgical treatment for burst fractures. Spine (Phila Pa 1976) 1990;15(7):667–673. doi: 10.1097/00007632-199007000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Danisa OA, Shaffrey CI, Jane JA, Whitehill R, Wang GJ, Szabo TA, Hansen CA, Shaffrey ME, Chan DP. Surgical approaches for the correction of unstable thoracolumbar burst fractures: a retrospective analysis of treatment outcomes. J Neurosurg. 1995;83(6):977–983. doi: 10.3171/jns.1995.83.6.0977. [DOI] [PubMed] [Google Scholar]
- 25.Stancic MF, Gregorovic E, Nozica E, Penezic L. Anterior decompression and fixation versus posterior reposition and semirigid fixation in the treatment of unstable burst thoracolumbar fracture: prospective clinical trial. Croat Med J. 2001;42(1):49–53. [PubMed] [Google Scholar]
- 26.Wood KB, Bohn D, Mehbod A (2005) Anterior versus posterior treatment of stable thoracolumbar burst fractures without neurologic deficit: a prospective, randomized study. J Spinal Disord Tech 18(Suppl):S15–S23 [DOI] [PubMed]
- 27.Hitchon PW, Torner J, Eichholz KM, Beeler SN. Comparison of anterolateral and posterior approaches in the management of thoracolumbar burst fractures. J Neurosurg Spine. 2006;5(2):117–125. doi: 10.3171/spi.2006.5.2.117. [DOI] [PubMed] [Google Scholar]
- 28.Sasso RC, Renkens K, Hanson D, Reilly T, McGuire RJ, Best NM. Unstable thoracolumbar burst fractures: anterior-only versus short-segment posterior fixation. J Spinal Disord Tech. 2006;19(4):242–248. doi: 10.1097/01.bsd.0000211298.59884.24. [DOI] [PubMed] [Google Scholar]
- 29.Lin B, Chen ZW, Guo ZM, Liu H, Yi ZK. Anterior approach versus posterior approach with subtotal corpectomy, decompression, and reconstruction of spine in the treatment of thoracolumbar burst fractures: a prospective randomized controlled study. J Spinal Disord Tech. 2011 doi: 10.1097/BSD.0b013e3182204c53. [DOI] [PubMed] [Google Scholar]
- 30.Falavigna A, Righesso NO, Polesso MA, Franceschini PR (2007) Anterior approach in patients with traumatic compression fracture type of thoracolumbar spine (T11–L2). Arq Neuropsiquiatr 65(3B):906–911 [DOI] [PubMed]
- 31.Kuner EH, Kuner A, Schlickewei W, Mullaji AB. Ligamentotaxis with an internal spinal fixator for thoracolumbar fractures. J Bone Joint Surg Br. 1994;76(1):107–112. [PubMed] [Google Scholar]
- 32.Ha KI, Han SH, Chung M, Yang BK, Youn GH. A clinical study of the natural remodeling of burst fractures of the lumbar spine. Clin Orthop Relat Res. 1996;323:210–214. doi: 10.1097/00003086-199602000-00029. [DOI] [PubMed] [Google Scholar]
- 33.Allain J. Anterior spine surgery in recent thoracolumbar fractures: an update. Orthop Traumatol Surg Res. 2011;97(5):541–554. doi: 10.1016/j.otsr.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Scapinelli R, Candiotto S. Spontaneous remodeling of the spinal canal after burst fractures of the low thoracic and lumbar region. J Spinal Disord. 1995;8(6):486–493. doi: 10.1097/00002517-199512000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Shi R, Liu H, Zhao X, Liu X, Gong Q, Li T, Liu L, Zeng J, Song Y. Anterior single segmental decompression and fixation for Denis B type thoracolumbar burst fracture with neurological deficiency: thirty-four cases with average twenty-six month follow-up. Spine (Phila Pa 1976) 2011;36(9):E598–E605. doi: 10.1097/BRS.0b013e3181e04b8f. [DOI] [PubMed] [Google Scholar]
- 36.Dai LY, Wang XY, Jiang LS (2007) Neurologic recovery from thoracolumbar burst fractures: is it predicted by the amount of initial canal encroachment and kyphotic deformity? Surg Neurol 67(3):232–237, 238 [DOI] [PubMed]
- 37.Tropiano P, Huang RC, Louis CA, Poitout DG, Louis RP. Functional and radiographic outcome of thoracolumbar and lumbar burst fractures managed by closed orthopaedic reduction and casting. Spine (Phila Pa 1976) 2003;28(21):2459–2465. doi: 10.1097/01.BRS.0000090834.36061.DD. [DOI] [PubMed] [Google Scholar]
- 38.McLain RF (2004) Functional outcomes after surgery for spinal fractures: return to work and activity. Spine (Phila Pa 1976) 29(4):470–477, Z6 [DOI] [PubMed]