Abstract
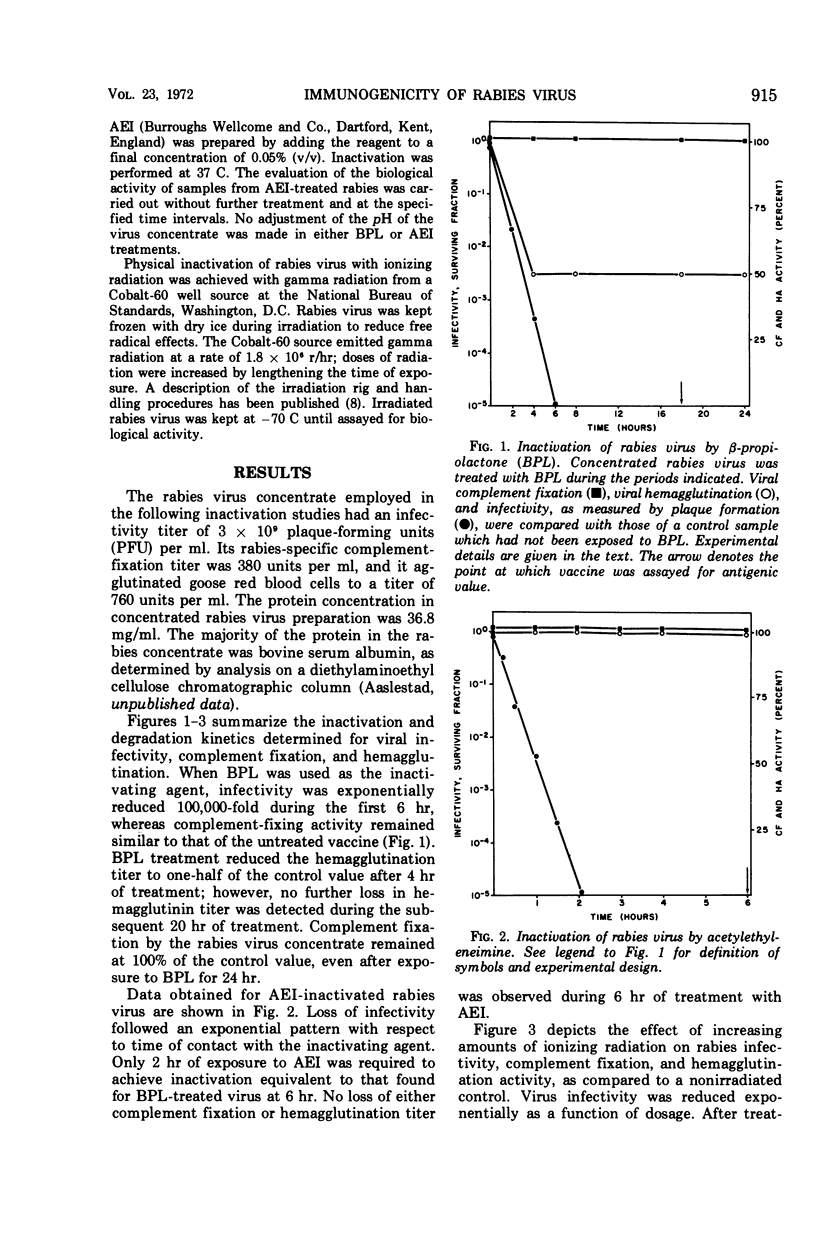
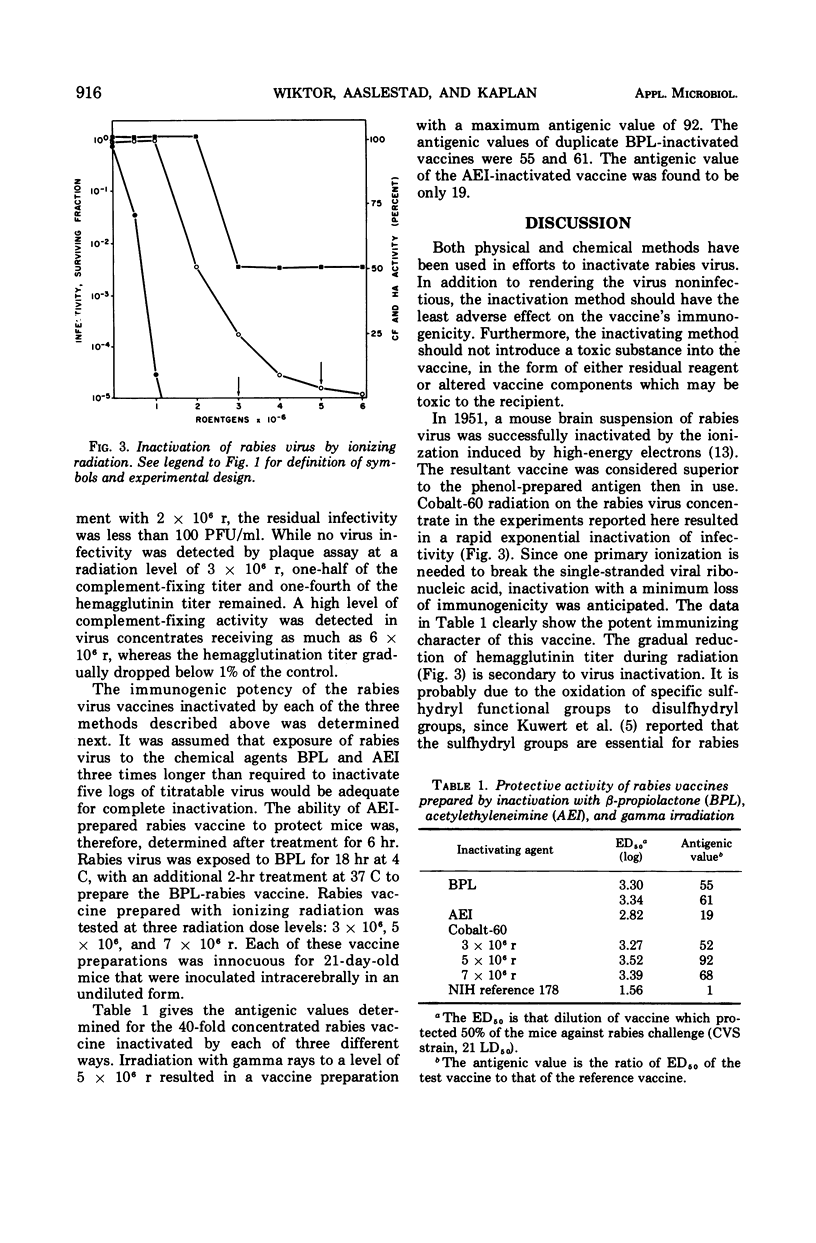
Ionizing radiation, β-propiolactone, and acetylethyleneimine were compared for their ability as virus-inactivating agents for the preparation of rabies vaccine. Each agent reduced viral infectivity exponentially; ionizing radiation also destroyed viral hemagglutinin. The vaccine prepared by ionizing radiation was equal or superior to that prepared by β-propiolactone in its ability to protect mice from rabies infection. The acetylethyleneimine-treated vaccine was a less potent immunogen.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crick J., Brown F. Viral subunits for rabies vaccination. Nature. 1969 Apr 5;222(5188):92–92. doi: 10.1038/222092a0. [DOI] [PubMed] [Google Scholar]
- Gruber J. Purification, concentration, and inactivation of Venezuelan equine encephalitis virus. Appl Microbiol. 1970 Sep;20(3):427–432. doi: 10.1128/am.20.3.427-432.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISSLING R. E., REESE D. R. ANTI-RABIES VACCINE OF TISSUE CULTURE ORIGIN. J Immunol. 1963 Sep;91:362–368. [PubMed] [Google Scholar]
- Kuwert E., Wiktor T. J., Sokol F., Koprowski H. Hemagglutination by rabies virus. J Virol. 1968 Dec;2(12):1381–1392. doi: 10.1128/jvi.2.12.1381-1392.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- POLLEY J. R. Factors influencing inactivation of infectivity and hemagglutinin of influenza virus by gamma radiation. Can J Microbiol. 1961 Aug;7:535–541. doi: 10.1139/m61-063. [DOI] [PubMed] [Google Scholar]
- Reitman M., Tribble H. R. Inactivation of Venezuelan Equine Encephalomyelitis Virus by gamma-Radiation. Appl Microbiol. 1967 Nov;15(6):1456–1459. doi: 10.1128/am.15.6.1456-1459.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlumberger H. D., Wiktor T. J., Koprowski H. Antigenic and immunogenic properties of components contained in rabies virus-infected tissue culture fluids. J Immunol. 1970 Aug;105(2):291–298. [PubMed] [Google Scholar]
- Sedwick W. D., Wiktor T. J. Reproducible plaquing system for rabies, lymphocytic choriomeningitis,k and other ribonucleic acid viruses in BHK-21-13S agarose suspensions. J Virol. 1967 Dec;1(6):1224–1226. doi: 10.1128/jvi.1.6.1224-1226.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol F., Kuwert E., Wiktor T. J., Hummeler K., Koprowski H. Purification of rabies virus grown in tissue culture. J Virol. 1968 Aug;2(8):836–849. doi: 10.1128/jvi.2.8.836-849.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAUB F. B., FRIEDEMANN U., BRASCH A., HUBER W. High intensity electrons as a tool for preparation of vaccines. I. Preparation of rabies vaccine. J Immunol. 1951 Nov;67(5):379–384. [PubMed] [Google Scholar]
- Wiktor T. J., Sokol F., Kuwert E., Koprowski H. Immunogenicity of concentrated and purified rabies vaccine of tissue culture origin. Proc Soc Exp Biol Med. 1969 Jul;131(3):799–805. doi: 10.3181/00379727-131-33981. [DOI] [PubMed] [Google Scholar]


