Abstract
Study design
This is a prospective observational study.
Purpose
The aim of this study was to determine whether the combination of thoracoscopically assisted corpectomy with posterior percutaneous transpedicular instrumentation in prone position achieves treatment goals in burst thoracic or thoracolumbar fractures and minimizes the associated morbidities.
Methods
Between December 2007 and December 2008, 26 patients with acute burst spinal fractures were operated upon in our hospital. Those patients underwent posterior percutaneous stabilization plus anterior thoracoscopically assisted corpectomy and fusion in prone position. Clinical and radiological outcomes of these patients were evaluated after a minimum follow-up period of 2 years. The Oswestry Disability Index (ODI) combined with clinical examination was used for clinical evaluation. Plain X-ray in two views was used for the radiological evaluation.
Results
The mean operative time was 248 min. The average blood loss was 765 ml. Ten patients had preoperative neurological deficits ranging from Frankel A to D. One patient did not show any neurological improvement at the final follow-up. The mean ODI at final follow-up was about 7. The mean preoperative kyphosis angle was 25.58°, improved to 9.2° postoperatively and to 13.8° at the final follow-up. No cases of implant failure were reported at the final follow-up.
Conclusions
Minimal invasive spinal techniques including thoracoscopic decompression and fusion and short segment posterior percutaneous instrumentation showed good clinical outcomes and can be considered as alternative to open procedures with decreased rates of morbidities in managing burst thoracic and thoracolumbar fractures.
Keywords: Thoracoscopically assisted, Thoracolumbar fractures, Corpectomy, Percutaneous
Introduction
Success in diagnosis and management of thoracolumbar fractures is dependent on an accurate assessment of spinal stability, a concept that is defined at least in part by the integrity of the spine and its supporting structures, as well as the neurologic status of the patient [1].
Evolved technologies and implants, improved imaging, a better understanding of fracture and implant biomechanics, and the introduction of a variety of new anterior and posterior fixation devices permit surgeons to plan definitive stabilizing procedures for any fracture pattern, allowing rapid mobilization and return to function. The goal of treatment “operative or otherwise” remains to protect neural elements, restore or maintain neurologic function, prevent or correct segmental collapse and deformity, prevent spinal instability and pain, permit early ambulation and return to function, and restore normal spinal mechanics [2].
Surgical treatment restores sagittal alignment, corrects translational deformities, and restores canal dimensions more reliably than does cast treatment. Finally, surgical decompression more reliably restores neurologic function and decreases rehabilitation time [3–6]. The spinal cord must be decompressed at the site of compression if there is intent of relieving the source of pressure [7].
As the anterior approach permits unobstructed visualization of the thecal sac, it remains the most reliable method for achieving a thorough decompression and is ideal for the patient with incomplete neurologic deficit who demonstrates significant canal occlusion on axial imaging studies. Anterior procedures also are indicated for the stabilization of burst fractures with substantial vertebral body comminution in which anterior column reconstruction using load-sharing strut grafts or other interbody devices to correct a collapsed kyphotic segment is necessary. The widely accepted indications for anterior surgery currently include retropulsed fragments occupying >67 % of the total canal area, extensive comminution of the vertebral column in conjunction with a kyphotic deformity >30°, and a delay in surgical treatment of more than 4 days [8, 9]. In addition, any traumatic disk herniations causing symptomatic compression of the spinal cord or nerve roots are best managed with an anterior approach [1]. Added to these indications is a disk injury with subsequent degeneration and apoptosis leading to progressive kyphosis [10].
Approach-related morbidity of conventional thoracotomy or thoraco-phreno-lumbotomy such as pain syndromes, “postthoracotomy syndrome”, relaxation of the abdominal wall, or intercostal neuralgia can reach a substantial extent, thus reducing the benefits of an anterior approach. Since the thoracolumbar junction is the location most commonly affected in spine fractures, the morbidity of opening the chest is additionally increased by the required detachment of the diaphragm [11].
Minimal invasive thoracoscopic approaches allow gaining the advantages of anterior decompression and reconstruction of the anterior column with less approach-related morbidity while preserving the broad, direct view and unobstructed surgical access to the entire ventral surfaces of the spine and spinal cord. Thoracoscopy has several advantages (i.e., minimal muscular incisions, no rib retraction, and minimal rib resection) that both thoracotomy and costotransversectomy lack. Complex dissections of the spine, such as spinal cord decompression, reconstruction, and instrumentation, can be performed using thoracoscopy. Unlike costotransversectomy, thoracoscopy offers a direct, complete view of the entire ventral surface of the spinal cord [12].
Thoracoscopy requires several new skills, psychomotor strategies, and perceptions of the anatomy that differ substantially from open surgery. Portals provide narrow windows of restricted access through the chest wall. Trajectories are restricted and confined, based on the position and trajectory of the portals. The “learning curve” for acquiring these psychomotor and technical skills for thoracoscopy is long [12].
Conventional, open dorsal instrumentation of the thoracolumbar spine requires extensive tissue dissection leading to denervation of paravertebral muscles as well as muscle and soft tissue ischemia potentially contributing to some cases of failed fracture stabilization [13]. Physical compression by soft tissue retractors during surgery induces time-dependent muscular histological damage via increased intramuscular pressure [14]. Furthermore, conventional approach to the spine is associated with extensive blood loss, risk of wound infection and prolonged hospitalization [15].
The combination of minimal invasive surgical techniques allows gaining the advantages of these techniques and avoiding the morbidities related to the open approaches. The aim of this study was to test whether the expected advantages of combining two minor access procedures achieve treatment goals in patients who require posterior stabilization and anterior column reconstruction for thoracic and thoracolumbar fractures, while avoiding inferior results in fracture treatment and/or new technique-associated disadvantages.
Materials and methods
Between December 2007 and December 2008, 26 patients (5 females and 21 males) with acute burst spinal fractures were operated upon in our hospital using thoracoscopically assisted corpectomy and posterior percutaneous transpedicular instrumentation. They were available for a minimum follow-up period of 2 years.
The mean age at operation was 50.5 years. Regarding the type of trauma, 18 patients (69 %) were falls from heights, 5 patients (19 %) sustained road traffic accidents (RTA), and 3 patients (11.5 %) sustained other types of trauma. The thoracolumbar junction was the most affected segment with 16 patients (61.5 %) fractured between T10 and L1 (Table 1).
Table 1.
Distribution of patients according to fractured level
| Frequency | Percent | |
|---|---|---|
| T1–T4 | 1 | 3.8 |
| T5–T9 | 8 | 30.8 |
| T10–L1 | 16 | 61.5 |
| L2 | 1 | 3.8 |
| Total | 26 | 100 |
Eight patients had associated injuries involving head, chest, or extremities. Two patients were polytraumatized; in those patients, percutaneous spinal instrumentation was done as a damage control procedure followed later on with the anterior thoracoscopically assisted corpectomy. Twelve patients were operated in two separate operative sessions. Ten patients had neurological deficits ranging from Frankel B to Frankel D. The indications for corpectomy were not different from those mentioned above including burst thoracic or thoracolumbar fractures with a retropulsed fragment with spinal canal stenosis >50 % or kyphosis >30° one day after trauma, extensive comminution of the anterior column or failure to achieve adequate correction using posterior percutaneous instrumentation in fresh fractures. Most of the cases included in this study were Type A3 (46.2 %) according to Magerl/AO classification with deficient comminuted anterior column.
After general anesthesia using a single-lumen endotracheal tube, the patient is positioned in the prone position. Sterilization and draping were done taking care that the anterior axillary line is in the sterile area on the side where the approach will be done. The iliac crest should be accessible for possible graft harvesting.
Posterior percutaneous instrumentation
Posterior percutaneous transpedicular instrumentation was used in all cases. Pedicle screws were placed under the control of two image intensifiers in two perpendicular planes.
A ten-gauge vertebroplasty needles were inserted bilaterally through the pedicles of the targeted spinal levels percutaneously using techniques identical to those employed during vertebroplasty and kyphoplasty procedures.
A modified technique as described by Wiesner et al. [16] was used for the insertion of needles. The image intensifier is oriented in a perfect anteroposterior direction.
Once the tip of the needle has been advanced into the anteromedial portion of the vertebral body, the stylet of the needle is removed and replaced by a guidewire. After insertion of all needles, the rest of the procedure is continued under control of image intensifier in the lateral plane only.
After insertion of the guidewire, the needle is removed and skin incision is done. A metal sheath with its central dilator is inserted, through this sheath the pedicle is opened, and then tapped using cannulated instruments. The cannulated polyaxial screw (Expedium-LISS) is then inserted, and the guidewire is removed. After insertion of all screws, the position of them is checked using the C-arm in both anteroposterior and lateral views. Short screws were used for the pedicles of the fractured vertebra. The rods are then applied usually after completion of the anterior procedure and tightened to the screws; compression is also applied when needed. The incisions are then closed, closing the deep fascia, subcutaneous tissues, and adhesive strips are then applied.
Thoracoscopic corpectomy
The thoracoscopic surgical technique included two incisions: the first is about 2.5 cm minithoracotomy done in the mid-axillary line and the second is about 1 cm in the posterior axillary line for the 30° thoracoscopy optic. Cooperation with the anesthetist to momentary deflate the lung during the first few minutes of the approach is mandatory [17, 18].
The aimed level is determined and checked radiographically. The pre-vertebral parietal pleura is incised and pealed using a blunt ball-tipped hooked dissector. For lesions below T12, the vertebral attachment of the diaphragm is minimally disinserted in a caudal direction using Cobb periosteal elevator. The segmental vessels can be identified, ligated and cut. The disk spaces above and below the vertebra to be removed were identified, incised, cleaned thoroughly, and the endplates of the vertebrae above and below are scraped. Corpectomy is done, and anterior column reconstruction is performed using tricortical iliac graft (2 cases) or an expandable (X-Tenz) vertebral body replacement cage filled with corpectomy bone material (22 cases) or filled with cement (2 severely osteoporotic cases) (Fig. 1). Spinal canal decompression was done in all cases.
Fig. 1.
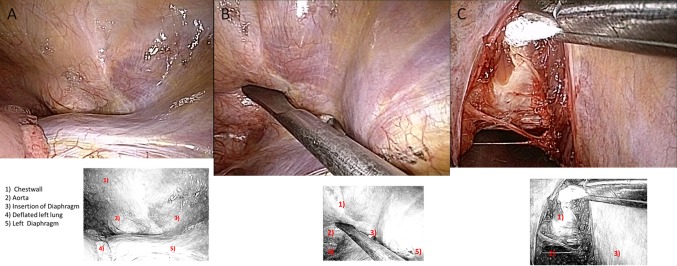
Thoracoscopic view of the thoracolumbar junction through a left-sided thoracoscopy. a Left thoracolumbar junction after deflation of the lung, b palpation of the anterior spinal border, and c all prevertebral structures securely shielded by a maleable blade
Spinal canal decompression in prone position is a demanding procedure. After thorough discectomy of the adjacent disks, loose bony fragments are removed. Starting from the adjacent disk space using a 90° hooked ball-tipped dissector, protruded fragments are mobilized and levered out of the spinal canal. Should this not suffice due to solid impaction or due to necessity of removal of the entire posterior wall, the posterior longitudinal ligament is opened from both disk spaces to have a clear orientation about the spinal canal. There is no need to resect bone of the pedicle for visualization purpose. The vertebral body is osteotomized leaving a thin shell of the posterior and anterior walls intact to create a central cavity. This thin posterior shell is then removed using either a side-cutting rongeur or a 90° angled curette making sure that the direction of delivery of bony fragments is toward anterior (i.e., following gravity). Epidural bleeding, that occurs as in open technique, follows the same direction, thus does not obstruct the direct thoracoscopic vision and does not require suction at the spot of cord decompression. Complete corpectomy is not the aim of the procedure, so just enough of the vertebral body is removed to safely decompress the spinal canal and to get a space for the vertebral body replacement cage. Care is taken to preserve the nutrient vessels of the anterior fourth of the body. This part is pushed en bloc anteriorly during insertion of the cage and then re-positioned next to the open cage like a vascularized flap. In two cases of severe osteoporosis or avascular necrosis of the fractured vertebra, the cage was filled with bone cement instead of resected bone.
At the end of the operation, the pre-vertebral pleura is closed, the thoracic cavity is inspected and an intercostal tube is inserted. The posterior instrumentation is then completed (Fig. 2).
Fig. 2.
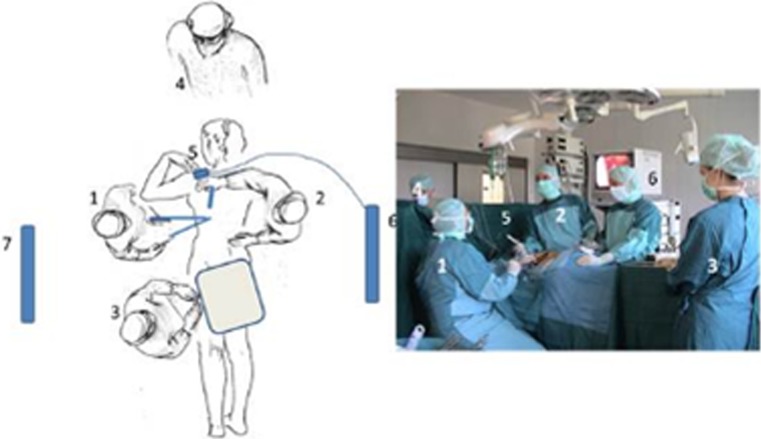
Intraoperative setup for thoracoscopic surgery to the anterior spine from left side in prone position: 1 position of the surgeon (sitting), 2 position of first assistant, 3 scrub nurse, 4 anesthetist, 5 thoracoscope, 6 and 7 videomonitors
The follow-up protocol in this study included subjective patient satisfaction indicated by Oswestry Disability Index (ODI) and clinical examination (range of motion, local tenderness, scar condition and neurological examination). Radiologically all patients had anteroposterior and lateral X-rays to evaluate fusion, position of the implants, metal failure, loosening, Cobb angle and sagittal index at the operated segment.
Fusion was evaluated according to modified Brantigan–Steffee classification [19]; these criteria include the denser and more mature bone in fusion area than originally achieved during surgery, no interspace between the cage and the vertebral body, and mature bony trabeculae bridging in fusion area. If one of the three criteria was not met, we classified the patient as being in a non-fusion state. Although Brantigan cages were radiolucent and those used in this study were titanium that made radiological evaluation relatively difficult, we used these Brantigan–Steffee criteria to evaluate fusion. There is no available satisfactory classification for radiological fusion especially in cases after corpectomy and vertebral body replacement cages.
Results
The mean total operative time was 248 ± 63 min; the mean operative time for anterior surgery was 141 ± 42 min; and the mean operative time for posterior surgery was 103 ± 34 min. The mean total blood loss was 765 ± 466 ml.
Regarding the patients’ distribution according to the fracture types using AO classification system, the A3 fractures were the most common type encountered (Table 2).
Table 2.
Preoperative, postoperative and final follow-up clinical and radiographic evaluation parameters
| Patient‘s number | Age | Fractured level | Fracture type | Preop. kyphosis | Postop. kyphosis | Final kyphosis | Final ODI | Anterior implant |
|---|---|---|---|---|---|---|---|---|
| 1 | 52 | T12 | A1.3 | 26 | 11 | 16 | 7 | X-Tenz |
| 2 | 56 | T5, 6 | A3.3 | 38 | 16 | 19 | 0 | X-Tenz |
| 3 | 47 | T12, L1 | A3.2 | 56 | 7 | 7 | 16 | X-Tenz |
| 4 | 72 | L1 | A1.3 | 2 | −7 | 19 | 33 | X-Tenz |
| 5 | 47 | L1 | A3.3 | 16 | −3 | 16 | 8 | X-Tenz |
| 6 | 47 | T2 | B2.3 | 30 | 22 | 26 | 5 | X-Tenz |
| 7 | 54 | L1 | A3.3 | 28 | 12 | 12 | 2 | X-Tenz |
| 8 | 76 | T8 | B2.3 | 35 | 22 | 25 | 9 | X-Tenz-Cement |
| 9 | 69 | L1 | A3.2 | 24 | 3 | 5 | 8 | X-Tenz |
| 10 | 68 | L1 | B1.2 | 20 | −6 | 1 | 23 | X-Tenz |
| 11 | 52 | T6 | A3.3 | 30 | 20 | 25 | 7 | X-Tenz |
| 12 | 58 | L1 | A3.3 | 20 | −11 | −6 | 9 | X-Tenz |
| 13 | 20 | L1 | C2.1 | 24 | 10 | 11 | 15 | X-Tenz |
| 14 | 20 | T12 | A3.2 | 19 | 4 | 5 | 6 | X-Tenz |
| 15 | 77 | L1 | A3.3 | 20 | 6 | 30 | 1 | X-Tenz-Cement |
| 16 | 59 | T7 | A2.3 | 24 | 13 | 13 | 5 | X-Tenz |
| 17 | 26 | T12 | A3.2 | 25 | −7 | 1 | 2 | X-Tenz |
| 18 | 71 | T4 | A1.3 | 43 | 30 | 30 | 10 | X-Tenz |
| 19 | 45 | T6 | B2.3 | 31 | 20 | 37 | 2 | X-Tenz |
| 20 | 30 | T12 | B2.3 | 11 | 8 | 8 | 4 | X-Tenz |
| 21 | 58 | T7, 8 | C1.3 | 38 | 21 | 20 | 8 | X-Tenz |
| 22 | 25 | T6 | B3.2 | 29 | 16 | 20 | 3 | X-Tenz |
| 23 | 45 | T8 | A2.3 | 25 | 15 | 20 | 12 | Graft |
| 24 | 68 | L1 | A1.3 | 19 | 14 | 16 | 11 | X-Tenz |
| 25 | 28 | L1 | A3.3 | 10 | 1 | 5 | 0 | Graft |
| 26 | 44 | T12 | A3.3 | 22 | 2 | 6 | 1 | X-Tenz |
| Mean | 50.5 | – | – | 25.576 | 9.192 | 14.884 | 7.96 | – |
| Mean* | – | – | – | 21.882 | 3.882 | 10.470 | 8.88 | – |
* Mean values after exclusion of patients with affected thoracic levels above T9
The ODI was used for clinical evaluation of the patients at the final follow-up. It ranged from 0 to 33 with an average of 7.96. No local tenderness was detected in any patient, and all showed excellent scar condition. Nine patients showed neurological improvement by one or more Frankel grade.
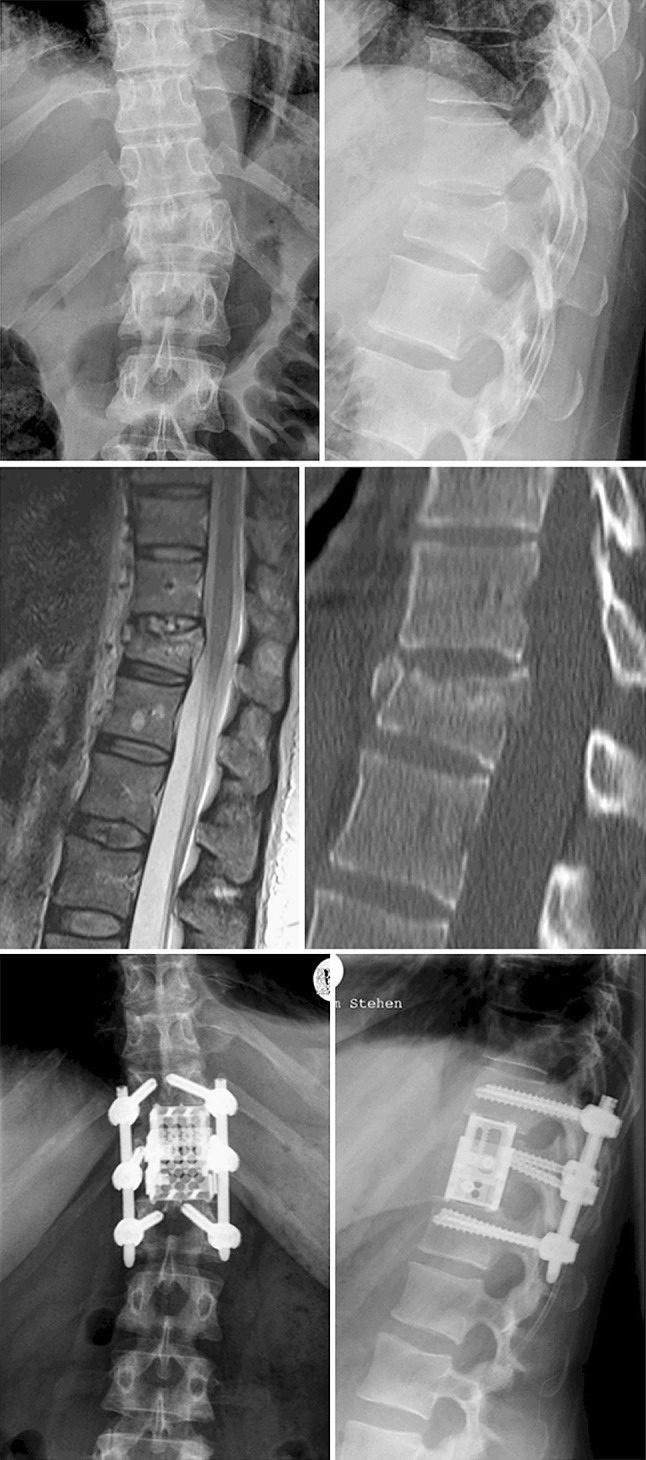
At 2 years follow-up, radiographic fusion was detected in 23 patients (88.5 %), 2 patients had cement-filled cages, and 1 patient did not meet the three criteria of Brantigan and also did not show any clinical symptoms or implant failure either anterior or posterior (Fig. 3).
Fig. 3.
A case of incomplete burst fracture of T12 treated with thoracoscopic corpectomy and percutaneous instrumentation with the 2 years follow-up X-rays
The average preoperative, postoperative and final follow-up radiographic measurements are shown in Table 3.
Table 3.
Preoperative, postoperative and final follow-up radiographic evaluation parameters
| N | Preoperative | Postoperative | Final follow up | P value | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | Std. deviation | Mean | Std. deviation | Mean | Std. deviation | |||
| Kyphosis Cobb angle | 26 | 25.58° | 10.98 | 9.19 | 10.63 | 14.88 | 10.43 | 0.001 significant |
| Sagittal index | 26 | 12.16 | 6.3 | 0.54 | 2.766 | 2.9 | 2.132 | 0.004 significant |
One patient had superficial wound healing problem. There were no cases of metal failure or loosening of the instrumentation. At the 2 years follow-up, there was no reoperation or relevant adjacent segment degeneration in this series.
Discussion
The treatment of spine injuries aims at prevention and limitation of neurological injury as well as restoration of spinal stability to regain a pain-free stable spinal column. Other issues include deformity correction, minimizing motion loss and rapid rehabilitation to long-term unrestricted activity. These goals should be accomplished with the introduction of as little additional risk or morbidity as possible [20].
Many studies reported on the use of video-assisted thoracoscopic surgery in the management of thoracolumbar fractures, but in all of these either anterior instrumentation systems were used or an open posterior stabilization was done. We combined the anterior spinal decompression and reconstruction of the anterior column through a minimal invasive thoracoscopic approach in prone position with the posterior percutaneous transpedicular stabilization. It is quiet difficult to compare our results with similar studies because to our knowledge there are no available studies that combine the two above-mentioned techniques.
Thoracolumbar region is the most commonly affected part of vertebral column by traumatic fractures reaching 68.8 % [21] and 80 % [22, 23] in some studies, followed by the thoracic and then the lumbar region [21].
In this study, 61.5 % of cases had fractures between T10 and L1, the second most commonly affected region of the vertebral column was between T5 and T9 representing 30.8 %. This reflects the importance of thoracoscopic techniques as a valuable option in treatment of these injuries.
Due to the presence of 30.8 % of cases in the normally kyphotic region between T5 and T9, the mean postoperative Cobb angle, and the degree of achieved correction were adversely affected compared to similar studies. Table 2 shows the mean Cobb angle calculated for the whole cohort of patients and that after exclusion of cases with fractures above the level of T9. This is why the use of sagittal index—although still needs clarification and validation—to evaluate the radiographic results is recommended. Reviewing the literature, we did not find the use of sagittal index as a radiographic parameter for evaluation of results of treatment of thoracolumbar fractures to be common. The main advantage of it is that it compared the measured posttraumatic kyphosis against an established baseline. This process transformed the measured angle from an absolute value, into a relative one. The result was a more useful parameter, which could be used to guide surgical indications, as well as the amount of desirable correction [24].
Value of prone position
Traditionally, the lateral decubitus position has been used for performing video-assisted thoracoscopic approaches. King et al. [25] and Lieberman et al. [26] mentioned that the prone position offered the following advantages during performing the surgical technique.
Prone position saves time required for re-positioning, sterilization and draping the patient. It facilitates reduction of associated kyphosis simply due to body weight and maintains it intraoperatively. It also allows the great vessels to fall forward, exposing an area of areolar tissue between them and the anterior longitudinal ligament, so that the risk of vascular injuries might be minimized. By virtue of prone positioning, the back–front combined approach could be simultaneously performed thus eliminating a need to stage the procedure.
In the prone position, the blood and debris (disk or bony fragments) fall anteriorly away from the spine and are removed by suction and forceps at the end of the procedure before inflating the lung. This saves the time required for repeated suction and clearing the operative field near the cord.
Surgeons operating in the lateral decubitus position claim that it would be time saving in case of vascular injury to do open thoracotomy to control bleeding without the need to re-position and re-drap the patient. We never faced this problem in fracture treatment and recommend the strict adherence to the above-described thoracoscopic technique to minimize the incidence of vascular injury and in the very rare case, if it happens to compress the site of bleeding, with a piece of gauze till re-positioning and re-draping for open thoracotomy.
The senior author (H.B.) prior to this series had performed more than 1,000 thoracoscopically assisted spine procedures. After 3 years of experience with lateral decubitus, prone position had been utilized since 1996 in more than 900 cases successfully and without the need of conversion to open thoracotomy. Therefore, it seems advisable to start this technique in an adapted infrastructure in presence of an experienced tutor.
Posterior instrumentation
Although percutaneous instrumentation is a demanding technique requiring a long learning curve, it is recommended for fracture stabilization as it is associated with minimal blood loss, paraspinal muscle trauma and approach-related morbidities without any significant decrease in safety compared to open technique.
Some drawbacks of percutaneous instrumentation have been detected. It does not allow placement of cross-links, which would be the precondition for stabilization of longer-ranging and seriously unstable segments. This did not present any disadvantage for us as we have always instrumented the pedicles of the fractured vertebra with short pedicle screws to allow for better biomechanical stability of the construct. In comparison to fixed mono-axial implants, the system has limited capability for closed reduction. Although compression handles allow for distraction and compression of the instrumented segment, the polyaxial screw design directs compression/distraction forces to the posterior column only. Therefore, excessive re-position maneuvers are not feasible and sufficient reduction of the fracture should be achieved using optimized posture and manual reduction, including axial leg tension or direct sagittal manipulation of the injured segment. We did not apply the rods posteriorly except after finishing the anterior approach, and thanks to the expandable character of the cage used we did not meet problems regarding reduction or correction of kyphosis. The anterior implant was placed, expanded to the distance needed to correct the kyphotic deformity and then fixed in this position. The rods are then applied posteriorly and compression of the posterior elements was applied. Another disadvantage of percutaneous instrumentation is that it has limited ability to correct three-dimensional deformities which are usually not the case in fractured spine.
Thoracoscopic corpectomy
Thoracoscopy has greater technical demands in terms of the required equipment and surgical expertise. Gaining appropriate experience involves a large investment of time and effort on the part of the surgical team and operating support staff. Experience with the open technique is one of the demands for performing any procedure endoscopically [18].
The aim of minimal invasive surgery was to minimize physical trauma to patients and achieving maximal therapeutic benefits and maximal safety. This means also to reduce operative and postoperative morbidities. The clinical comparison demonstrated the advantages of reduced early postoperative pain, improved shoulder girdle function, reduced impairment in the early postoperative pulmonary functions and shortened ICU stay [27, 28].
Khoo et al. [22] summarized the advantages and disadvantages of VATS. Advantages of VATS treatment of thoracic fractures include the following: (1) small intercostal incisions without the need for rib resection or rib retractors; (2) excellent direct intraoperative visualization of the abnormality; (3) treatment of multi-segmental abnormality without the need for additional rib resection; and (4) significantly reduced injury to the chest wall (5). The magnified anterolateral view afforded during thoracoscopic visualization outstrips even that of standard open thoracotomy because it places the operative viewing distance within a few centimeters of the abnormality. Furthermore, the surgeon’s view of the operative field is not obscured by either his or her hands or the surgical instruments, thus allowing for improved continuous surveillance of the procedure. Disadvantages of VATS procedures include a slightly increased anesthetic complexity and an extremely long operating learning curve for the surgeon and the operative team.
In this study of thoracoscopically assisted treatment of thoracolumbar fractures, thoracoscopy was associated with fewer complications compared to studies that used open thoracotomies. We also used the normal single-lumen endotracheal tube; this decreased the anesthetic complexity required for double-lumen endotracheal intubation.
Meticulous preoperative evaluation and planning is the keystone for successful treatment of thoracic and thoracolumbar trauma. Our experience demonstrates that minimal invasive thoracoscopic technique combined with percutaneous posterior instrumentation represents a safe and effective treatment option for thoracic and thoracolumbar spinal fractures. The main limitations of the present study are the small number of cases and the absence of a control group.
Conflict of interest
None.
References
- 1.Whang PG, Vaccaro AR. Thoracolumbar fractures: anterior decompression and interbody fusion. J Am Acad Orthop Surg. 2008;16:424–431. doi: 10.5435/00124635-200807000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Lewandrowski K-U, McLain RF (2004) Thoracolumbar fractures: evaluation, classification and treatment. Adult and paediatric spine, 3rd edition. Lippincott Williams and Wilkins, Philadelphia
- 3.Mumford J, Weinstein JN, Spratt KF, et al. Thoracolumbar burst fractures. The clinical efficacy and outcome of non-operative management. Spine. 1993;18:955–970. doi: 10.1097/00007632-199306150-00003. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs RR, Asher MA, Snider RK. Thoracolumbar spinal injuries. A comparative study of recumbent and operative treatment in 100 patients. Spine. 1980;5:463–477. doi: 10.1097/00007632-198009000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Bradford DS, McBride GG (1987) Surgical management of thoracolumbar spine fractures with incomplete neurologic deficits. Clin Orthop 218:201–216 [PubMed]
- 6.Clohisy JC, Akbarnia BA, Bucholz RD, et al. Neurologic recovery associated with anterior decompression of spine fractures at the thoracolumbar junction (T12–L1) Spine. 1992;17:S325–S330. doi: 10.1097/00007632-199208001-00019. [DOI] [PubMed] [Google Scholar]
- 7.White AAI, Panjabi MM. Clinical biomechanics of the spine. Philadelphia: JB Lippincott; 1978. [Google Scholar]
- 8.Bohlman HH, Kirkpatrick JS, Delamarter RB, et al. Anterior decompression for late pain and paralysis after fractures of the thoracolumbar spine. Clin Orthop Relat Res. 1994;300:24–29. [PubMed] [Google Scholar]
- 9.McCullen G, Vaccaro AR, Garfin SR. Thoracic and lumbar trauma: rationale for selecting the appropriate fusion technique. Orthop Clin North Am. 1998;29:813–828. doi: 10.1016/S0030-5898(05)70050-X. [DOI] [PubMed] [Google Scholar]
- 10.Heyde CE, Tschoeke SK, Hellmuth M, et al. Trauma induces apoptosis in human thoracolumbar intervertebral discs. BMC Clin Pathol. 2006;6:5. doi: 10.1186/1472-6890-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balabhadra RSV, Kim DH, Potulski M, et al. Thoracoscopic decompression and fixation (MACS-TL), endoscopic spine surgery and instrumentation. New york: Thieme Medical Publishers; 2004. pp. 180–198. [Google Scholar]
- 12.Dickmann CA, Rosenthal DJ, Perin NI. Thoracoscopic spine surgery, general principles of thoracoscopy. New York: Thieme; 1999. pp. 7–18. [Google Scholar]
- 13.Sihvonen T, Herno A, Paljδrvi L, et al. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine. 1993;18:575–581. doi: 10.1097/00007632-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Gejo R, Matsui H, Kawaguchi Y, et al. Serial changes in trunk muscle performance after posterior lumbar surgery. Spine. 1999;24:1023–1028. doi: 10.1097/00007632-199905150-00017. [DOI] [PubMed] [Google Scholar]
- 15.Rechtine GR, Bono PL, Cahill D, et al. Postoperative wound infection after instrumentation of thoracic and lumbar fractures. J Orthop Trauma. 2001;15:566–569. doi: 10.1097/00005131-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Wiesner L, Kothe R, Ruther W. Anatomical evaluation of two different techniques for the percutaneous insertion of pedicle screws in the lumbar spine. Spine. 1999;24:1599–1603. doi: 10.1097/00007632-199908010-00015. [DOI] [PubMed] [Google Scholar]
- 17.Boehm H, El-saghir H. Minimal invasives ventrales release und endoskopische ventrale instrumentation bei Skoliosen. Orthopaede. 2000;29:535–542. doi: 10.1007/s001320050492. [DOI] [PubMed] [Google Scholar]
- 18.El-Meshtawy M (2003) Value of endoscopic spinal surgery in management of thoracic and thoracolumbar kyphosis. M.D. thesis submitted for partial fulfillment of M.D. of Orthopaedic surgery. Assiut University, pp 542–575
- 19.Brantigan JW, Steffee AD. A carbon fiber implant to aid interbody lumbar fusion. Two-year clinical results in the first 26 patients. Spine. 1993;18:2106–2107. doi: 10.1097/00007632-199310001-00030. [DOI] [PubMed] [Google Scholar]
- 20.Vaccaro AR, Kim DH, Brodke DS, et al. Diagnosis and management of thoracolumbar spine fractures. J Bone Jt Surg Am. 2003;85:2456–2470. [Google Scholar]
- 21.Reinhold M, Knop C, Beisse R, et al. Operative treatment of traumatic fractures of the thorax and lumbar spine. Part II: surgical treatment and radiological findings. Unfallchirurg. 2009;112:149–167. doi: 10.1007/s00113-008-1538-1. [DOI] [PubMed] [Google Scholar]
- 22.Khoo LT, Beisse R, Potulski M. Thoracoscopic-assisted treatment of thoracic and lumbar fractures: a series of 371 consecutive cases. Neurosurgery. 2002;51(Suppl 2):104–117. [PubMed] [Google Scholar]
- 23.Siebenga J, et al. Treatment of traumatic thoracolumbar spine fractures: a multicenter prospective randomized study of operative versus nonsurgical treatment. Spine. 2006;31(25):2881–2890. doi: 10.1097/01.brs.0000247804.91869.1e. [DOI] [PubMed] [Google Scholar]
- 24.Keynan O, Fisher CG, Vaccaro A, et al. Radiographic measurement parameters in thoracolumbar fractures: a systematic review and consensus statement of the spine trauma study group. Spine. 2006;31(5):156–165. doi: 10.1097/01.brs.0000201261.94907.0d. [DOI] [PubMed] [Google Scholar]
- 25.King AG, Mills TE, Loe WA, et al. Video-assisted thoracoscopic surgery in the prone position. Spine. 2000;18:2403–2406. doi: 10.1097/00007632-200009150-00022. [DOI] [PubMed] [Google Scholar]
- 26.Lieberman IH, Salo PT, Orr D, Kraetschmer B. Prone position endoscopic trans-thoracic release simultaneous with posterior instrumentation for spinal deformity. Spine. 2000;25:2251–2257. doi: 10.1097/00007632-200009010-00017. [DOI] [PubMed] [Google Scholar]
- 27.Landreneau RJ, Hazelrigg SR, Mack MJ, et al. Postoperative pain-related morbidity. Video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg. 1993;56:1285–1289. doi: 10.1016/0003-4975(93)90667-7. [DOI] [PubMed] [Google Scholar]
- 28.Regan JJ, Ben-Yishay A, Mack MJ. Video-assisted thoracoscopic excision of herniated thoracic disc: description of technique and preliminary experience in the first 29 cases. J Spinal Disord. 1998;11:183–191. doi: 10.1097/00002517-199806000-00001. [DOI] [PubMed] [Google Scholar]