Abstract
Epithelial Na+/H+ exchanger-3 (NHE3) transport is fundamental for renal and intestinal sodium reabsorption. Cytoplasmic protons are thought to serve as allosteric modifiers of the exchanger and to trigger its transport through protein conformational change. This effect presupposes an intracellular pH (pHi) dependence of NHE3 activity, although the biophysical and molecular basis of NHE3 pHi sensitivity have not been defined. NHE3, when complexed with the calcineurin homologous protein-1 (CHP1), had a shift in pHi sensitivity (0.4 units) toward the acidic side in comparison with NHE3 alone, as measured by oscillating pH electrodes combined with whole-cell patch clamping. Indeed, CHP1 interaction with NHE3 inhibited NHE3 transport in a pHi -dependent manner. CHP1 binding to NHE3 also affected its acute regulation. Intracellular perfusion of peptide from the CHP1 binding region (or pHi modification to reduce the CHP1 amount bound to NHE3) was permissive and cooperative for dopamine inhibition of NHE3 but reversed that of adenosine. Thus, CHP1 interaction with NHE3 apparently establishes the exchanger set point for pHi, and modification in this set point is effective in the hormonal stimuli–mediated regulation of NHE3. CHP1 may serve as a regulatory cofactor for NHE3 conformational change, dependent on intracellular protonation.—Babich V., Vadnagara K., Di Sole, F. The biophysical and molecular basis of intracellular pH sensing by the Na+/H+ exchanger-3.
Keywords: electroneutral transport, patch clamp, epithelial cells, G-protein-coupled receptors.
Variations in pH, both within and outside the cells, affect essential cellular processes (1, 2), because expression of several metabolic enzymes is altered by disturbances in acid-base homeostasis, and the function of plasma membrane channels and transporters is affected by variations in pH (1, 3). Therefore, the acid-base status is tightly regulated by a series of molecules including several plasma membrane proton (H+) extrusion systems, such as the Na+/H+ exchanger (NHE) protein family (1, 4, 5). Members of the family are found ubiquitously in most living organisms and are involved in a variety of vital cell functions (4, 6). In addition to maintaining acid-base homeostasis, they regulate functions such as cell volume and absorption of salt and water across various epithelia (4, 7, 8).
The mammalian NHE protein family catalyzes the net exchange of extracellular sodium (Na+) for intracellular H+ (4, 6). This exchange reaction is reversible, and, in physiological conditions, the constant driving force for H+ extrusion is provided by the inwardly directed Na+ gradient produced by Na+-K+-ATPase. Internal H+ finely regulates the exchanger's activity, because of the steep intracellular pH (pHi) dependence of the rate of exchange that allows NHE isoforms to respond to small changes in pHi. Thus, intracellular H+ serves as an allosteric modifier of the exchanger, and H+ sensing is predicted to occur at clusters of electrostatically coupled amino acids of the NHE isoforms' C termini (9–11). These basic properties of this family of proteins have been known since the 1980s (12), but the biophysical and molecular mechanisms that govern these functional features of the exchanger have not been well defined.
In mammals, the NHE protein family comprises 9 related gene products (NHE1–9), a cluster of distant NHE-related genes (NHA1 and NHA2) and a sperm-specific NHE gene product (4, 7, 13) that exhibit distinct patterns of cell expression and membrane localization specific to their functions (4, 7, 13). In the epithelium of the kidney and intestine, the apically located exchanger isoform 3 (NHE3) plays a fundamental role in sodium reabsorption and acid secretion (14, 15). Indeed, the NHE3 transport function is regulated by a wide range of hormonal stimuli, and its activity is central in a multiplicity of severe clinical conditions, such as hypertension, diarrhea, diabetic nephropathy, acute kidney injury, and heart failure (7, 16). A variety of interacting proteins with the NHE3 regulatory C-terminal cytoplasmic domain finely coordinate NHE3 transport (17, 18). One such binding partner is the calcineurin homologous protein (CHP).
CHP is part of the calcium (Ca2+) binding protein family characterized by an EF-hand structural motif (i.e., the Ca2+ binding region composed of the E and F helices joined by a loop; refs. 19–21). Thus far, the CHP subfamily comprises 3 known members, all of them targeting several members of the NHE subfamily (19, 22, 23) and serving as essential cofactors for NHE isoform activity, specifically NHE1–3 (19, 20, 22–28). CHP binds NHE isoforms at the predicted H+-sensing amino acid cluster and the CHP-NHE complex formation is essential for optimal NHE1 activity (23, 26, 27). Furthermore, CHP binding to NHE1 stimulates its biosynthetic processing and trafficking to the plasma membrane (22, 29). Specifically, CHP isoform 1 has been found either to modulate NHE3 constitutive transport or to mediate acute inhibition of NHE3's activity by the adenosine receptor subtype 1 (A1R; refs. 24, 25).
In this study, we investigated the seemingly contradictory functions of the CHP1-NHE3 complex, and the findings provided new insights into the molecular mechanisms that are activated by the complex. Using oscillating pH microelectrodes combined with whole-cell patch-clamping, we determined that CHP1, when bound to NHE3, controls its pHi sensitivity, indicating that CHP1 interaction with NHE3 may control its sensitivity to regulation by intracellular H+. Furthermore, changes in pHi sensitivity dependent on CHP1 binding affect the NHE3 response to specific hormonal stimuli, such as dopamine and adenosine. We established an acute vs. chronic effect of CHP1 expression on the NHE3 transport function, thus clarifying the dissimilar functions found for the CHP1-NHE3 complex. These results are valuable for deciphering the regulatory mechanisms that permit pHi sensing by NHE3 molecules.
MATERIALS AND METHODS
Chemicals
All chemicals were obtained from Sigma (St. Louis, MO, USA), with the exception of the cell culture reagents (DMEM/F12, DMEM, 0.1% trypsin/0.5 mM EGTA, FBS, penicillin-streptomycin solution, and Lipofectamine 2000), which were obtained from Invitrogen (Carlsbad, CA, USA).
Cell culture
Chinese hamster ovary (CHO) and opossum kidney (OK) proximal tubule-like cell lines were from the American Type Culture Collection (Manassas, VA, USA). The CHO cells were cultured on DMEM/F12 supplemented with 10% FBS, 50 IU/ml penicillin, and 50 μg/ml streptomycin. The cultures were incubated in a humidified 95% air/5% CO2 atmosphere at 37°C and subcultured weekly by trypsinization using 0.1% trypsin and 0.5 mM EGTA in PBS. The OK cells were cultured as described previously (24, 25). Both cell lines were transiently transfected by using Lipofectamine 2000, according to the manufacturer's instructions. The cells were maintained in serum-free medium for 48 h before the experiments. For patch-clamp experiments, the CHO and OK cells were trypsinized for 2–3 min and then resuspended in cell culture medium. The cells were used after trypsinization for 2–3 h, after which they were replaced by a new batch of trypsinized cells. The CHO cells were also used for CHP1 protein expression.
Isolation of RNA and RT-PCR
Total RNA was isolated from OK cells grown to confluence in 100 mm–diameter culture dishes with the RNeasy Plus Universal Mini Kit (Qiagen, Germantown, MD, USA), which includes nonenzymatic removal of genomic DNA. RNA samples (5 μg) were reverse transcribed with the RT2 First Strand Kit (Qiagen). Polymerase chain reaction was performed for 30 cycles with denaturation at 94°C (30 s), annealing at 55°C (30 s), and extension at 68°C (30 s), with OneTaq 2× Master Mix (New England BioLabs, Ipswich, MA, USA). Samples of PCR products (10 μl) were analyzed by electrophoresis on 2% agarose gel and visualized by UV light after staining with ethidium bromide. The following opossum NHE isoform-specific primers were used for PCR: NHE1 forward, 5′-GATGCTGTCACTGTCGTGCTG-3′, reverse 5′-GAAGAGCGGCTCGATGACC-3′; NHE2 forward, 5′-GACGGGCATCTACTTCAACATCT-3′, reverse, 5′-GTCTCCGTGCACTTCGTTTCTC-3′; NHE3 forward, 5′-TCCTTCACCCTCACCCCAA-3′, reverse, 5′-GTCTCCCATGATGCCACTGA-3′; NHE4 forward, 5′-TGGGAGTTTTTCACTTGCATTTTT-3′, reverse, 5′-TCCATCAGACGGGTATGAAGCT-3′; NHE5 forward, 5′-CGATGCAGTCACAGTGGTGC-3′, reverse, 5′-GGCTCGATGATACGGACCC-3′; and NHE8 forward, 5′-TGCATTTCTTGGTCTGTCCATC-3′, reverse, 5′-GGAATTGCTCCACGTAAACCAC-3′.
Patch-clamp experiments
Electrophysiological methods were as published previously (30–32). The cells were held in whole-cell configuration (30, 31). The holding potential in all experiments was kept at 0 mV, and the signals were recorded by Capmeter 6 software, with the amplifier Axon Axopatch 200B (Molecular Devices, Sunnyvale, CA, USA; refs. 30, 33).
Proton-selective microelectrodes
The pH microelectrodes were prepared as described previously (34). Briefly, proton-selective microelectrodes with tip diameters of 2–4 μm were pulled from borosilicate glass capillaries (outer diameter, 1.2 mm; World Precision Instruments, Sarasota, FL, USA). The electrodes were vapor silanized with bis(dimethylamino)-dimethyl silane, with the tip filled with hydrogen ionophore I cocktail B, and backfilled with 100 mM KCl (pH 7.0 with 10 mM HEPES). They were fixed on a holder connected to the high-impedance probe (1015 Ω) of an electrometer (FD-223; World Precision Instruments). Average electrode responses were 58 mV per unit of pH from pH 6.0 to 8.0.
Recording chamber, patch pipette oscillation, and cell membrane capacitance
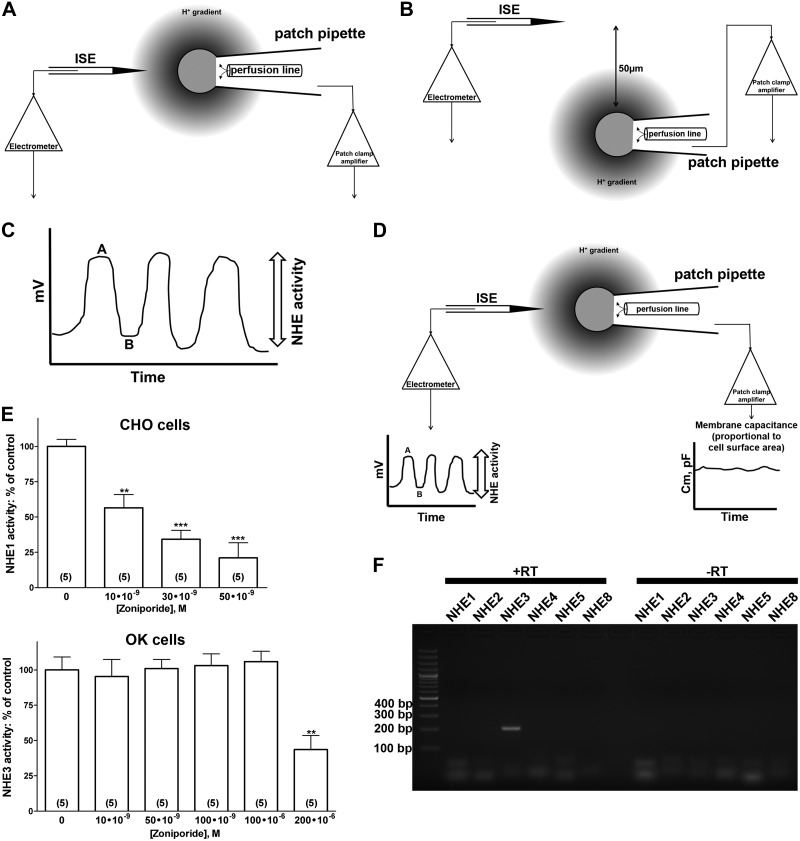
All recordings were performed at 35–37°C in a heated recording chamber (RC-26; Warner Instruments, Hamden, CT, USA) in the absence of HCO3−, pH microelectrode recording was as described by Fuster and colleagues (30, 31) and presented in Fig. 1. After the whole-cell patch-clamp configuration was obtained, the microscope stage was moved to a position with the cell in front of the proton-selective microelectrode (≈5–10 μm). A patch pipette then was manually moved laterally between 2 positions (position 1, Fig. 1A, and position 2, Fig. 1B), far enough apart to detect the entire proton gradient (standard movement between the 2 positions of 50 μm). Pipette perfusion via a line was performed (35) with capillaries placed 50–150 μm from the patch pipette tip (Fig. 1A, B, D). Free flow and adequate cell perfusion through the line were checked by perfusion of a dye before each series of experiments. A representation of the readout from the pH microelectrode (in millivolts) is shown in Fig. 1C, in which a cell was moved repetitively close to (point A) and away from (point B) the pH microelectrode. Oscillations from A to B are representative of NHE activity. Square wave perturbations (20 mV, 0.2 kHz) were used to monitor cell membrane capacitance (Cm) for all whole-cell capacitance recording (Fig. 1D), with cell parameters determined by the Capmeter 6 software (33). The readout in millivolts of the pH microelectrodes was translated to H+ flux (30, 31). Briefly, for the whole-cell recording, the cell shape was approximated as a sphere. The H+ flux (JH+) can be estimated from the difference in free H+ concentration between the cell surface and the bulk solution (ΔH+):
where DH+ and DB are the H+ and buffer diffusion coefficients, respectively; BT is the total buffer concentration; KD is the diffusion coefficient of the buffer; r is the cell radius; and ΔBH/ΔH is the steady-state concentration change of bound H+ for a small change in free H+. The flux carried by free H+ is negligible in most experiments. H+ fluxes were converted into current equivalents of the fluxes (i.e., JH+/Faraday) in picoamperes, so as to correlate the data with those in other studies (30, 31).
Figure 1.
H+ flux measurement and correlation between NHE activity and differences in potential recorded by a pH microelectrode. A) The initial experimental setting. The cell is held by the patch-clamp pipette in whole-cell configuration in front of the H+-selective microelectrode at a distance of 5–10 μm. In this position, the H+-selective microelectrode registers the free H+ concentration at the cell surface. B) The patch-clamp pipette is manually moved laterally for 50 μm. In this position, the H+-selective microelectrode registers free H+ concentration in the solution. C) Voltage differences recorded via the H+-selective microelectrode, corresponding to the 2 positions of a patch pipette with the cell in front of (point A) and far from (point B), the H+-selective microelectrode. Oscillations from A to B are representative of NHE activity. Data from 6–10 oscillations were averaged per condition for each cell. D) Complete recording settings. The Capmeter 6 software generates square-wave perturbations (20 mV, 0.2 kHz) that are used to monitor the Cm for the whole-cell capacitance recording in picofarads (pF). Also, the software registers voltage differences recorded by the H+-selective microelectrode. E) Inhibition of differences in potential recorded by the pH microelectrode after treatment of the cells with the specific NHE1 inhibitor zoniporide dihydrochloride in CHO and OK cells. ISE, ion-selective microelectrode (or H+-selective microelectrode). The results are expressed as a percentage of the control (0 nM of zoniporide). Numbers in parentheses: experiments performed in identical conditions. **P < 0.01, ***P < 0.001; ANOVA, compared with control. F) Detection by RT-PCR of the plasma membrane NHE isoforms expressed in OK cells. Total RNA was prepared from confluent OK cells. RT-PCR was performed with opossum NHE isoform–specific forward and reverse primers for NHE1–5, and NHE8 (see Materials and Methods for primer sequences and reaction conditions). The expected molecular size of the NHE3 transcript is 200 bp. +RT/−RT, RT reactions performed in the presence or absence of reverse transcriptase. Results are representative of 3 independent experiments. Lane 1: 100-bp ladder.
The correlation between NHE activity and differences in potential recorded by the pH microelectrode has been established by ion substitution (extracellular Na+ replaced by K+) and NHE inhibitors (31). We confirmed this correlation in our setting by using the selective NHE1 inhibitor zoniporide dihydrochloride, which was chosen for this set of experiments because there are no commercially available selective NHE3 inhibitors. Zoniporide dihydrochloride has high selectivity for NHE1, but low selectivity for other NHE isoforms (36, 37). CHO cells express endogenous NHE1 at the plasma membrane (30, 31, 38) and zoniporide dihydrochloride inhibited the differences in potential recorded by the pH microelectrode in CHO cells in a concentration-dependent manner (Fig. 1E). The calculated IC50 was ∼20 nM, which corresponded well to the published IC50 of zoniporide dihydrochloride for NHE1 (36). In OK cells, zoniporide dihydrochloride did not change the differences in potential recorded by the pH microelectrode until a concentration of 2 × 105 nM was reached (reduction of ∼50–60%; Fig. 1E), which matched well with the published IC50 of zoniporide dihydrochloride for NHE3 (36). These results also confirmed that OK cells do not express NHE1 and NHE2 at the cell surface.
Solutions
The pipette solution contained (in mM): 115 potassium aspartate (KAsp), 1 EGTA, 0.5 MgCl2, 10 Mg-ATP, 12.5 HEPES, 12.5 PIPES, 12.5 MOPS, and 12.5 MES (pH 6.0–7.5, adjusted with aspartic acid). Pipette solutions were heavily buffered. The free-Ca2+ concentration was maintained at 100 nM by the addition of CaCl2, with the amount being calculated by Maxchelator software (http://maxchelator.stanford.edu). The bath solution contained (in mM): 140 NaCl, 2 CaCl2, 2 MgCl2, and 0.1 Tris-HCl (pH 8.2).
Peptide synthesis
The sequence of the peptide from the CHP1 binding region and the mutant peptide (23) were synthesized in the Protein Chemistry Core at the University of Texas Southwestern Medical Center (Dallas, TX, USA). Peptide sequences correspond to the Didelphis marsupialis NHE3 sequence that binds to CHP1: NHE3478–500-wild type (WT), YGRKKRRQRRRGIEGRGPKLNEKLHGRAFDHILSAIEDIS, and NHE3478–500-4RR, YGRKKRRQRRRGIEGRGPKLNEKLHGRRRDHRRSAIEDIS. [The transactivator of transcription (TAT) peptide sequence of HIV is highlighted in italic; the mutations are shown in bold.] The peptides were dissolved in the pipette solution to a final concentration of 5 μM. Addition of the peptides did not modify the pH of the pipette solution (pH change, 0.001±0.0018, n=5, with vs. without peptide).
Plasmid constructs and short hairpin RNA (shRNA)
His-CHP1 was generated by digestion of enhanced green fluorescent protein (eGFP)-CHP1 (25) with AgeI/BsrGI restriction enzymes. eGFP cDNA was extracted, and a cDNA sequence corresponding to the His6 tag was substituted in the frame. Mutation of valine 185 to glutamine (V185Q) of CHP1 impaired CHP1's ability to bind NHE1 (28). The CHP1 mutant (His-CHP1-V185Q) was generated by site-directed mutagenesis, with His-CHP1 as the template and the following primer: 5′-GGTTTTGGAAAAAGTGGATCAAGAACAGAAGATGAGCATCCGATTTC-3′ (mutated nucleotides are shown in bold). Site-directed mutagenesis was performed with the QuikChange II kit (Agilent Technologies), according to the manufacturer's instructions. All constructs were sequence verified. Short hairpin (sh)RNA CHP1 (CHP-shRNA) was obtained by cloning the sequence used for CHP1-siRNA (24) into the MSCV-LTRmiR30-PIG vector (Open Biosystems, Huntsville, AL) via the XhoI and EcoRI restriction sites, according to the manufacturer's instructions. eGFP in the MSCV-LTRmiR30-PIG vector serves as a marker for CHP1-shRNA expression (eGFP-CHP1-shRNA; ref. 25). eGFP-CHP1-shRNA has been shown to decrease protein expression of endogenous CHP1 in OK cells by 80% (25). For the perfusion experiment, either His-CHP1 or His-CHP1-V185Q was expressed in CHO cells and purified by affinity chromatography with Ni-NTA columns (Qiagen). The purity of the protein was confirmed with silver-stained SDS-PAGE (not shown). After purification, the proteins were concentrated and dialyzed against the desired pipette solution (pH 6.0 or 6.5) using the Slide-A-Lyzer MINI Dialysis Device, 10K MWCO (Thermo-Fisher Scientific, Rockford, IL, USA).
Pull-down assay
OK cells, transiently transfected with His-CHP1 or His-CHP1-V185Q, were lysed in RIPA buffer (containing 100 mM NaCl, 20 mM imidazole, 1% Triton X-100, 0.5% Nonidet P-40, 50 mM Tris-HCl, and protease inhibitor cocktail) at various pHs (6.0, 6.5, 7.0, and 7.5) for 1 h at 4°C. The lysate from a separate pool of cells for each pH condition was centrifuged at 16,000 g for 30 min, the supernatant was retrieved, and the protein content of the supernatants was quantified by the Bradford method (Bio-Rad, Hercules, CA, USA). An equal amount of protein was equilibrated with Ni-NTA beads (Qiagen) at 4°C. The beads were then extensively washed with RIPA buffer at each specific pH. The proteins were eluted in SDS-PAGE loading buffer and subjected to SDS-PAGE. NHE3 was detected by monoclonal mouse anti-NHE3 (3H3) antibody (24, 25, 39). As a control for His-CHP1 binding to Ni-NTA, His-CHP1 (or His-CHP1-V185Q) was detected by Penta-His antibody (Qiagen).
Statistical analyses
The results are represented as means ± se. Quantitative differences between control and test conditions were assessed statistically by ANOVA. Values of P < 0.05 were considered to be statistically significant.
RESULTS
Overexpression of CHP1 had a dual effect on NHE3 transport
To characterize the role of CHP1 in the regulation of NHE3 transport by oscillating H+ selective electrodes, we used an OK epithelial cell line that maintains the characteristics of renal proximal tubule cells (40, 41). In particular, we selected this cell line because, of all the NHE protein family members, it exclusively expresses NHE3 (refs. 40–43 and Fig. 1F), therefore greatly simplifying the analysis and interpretation of the data. Furthermore, the OK cells used in this study express endogenous CHP1 (24).
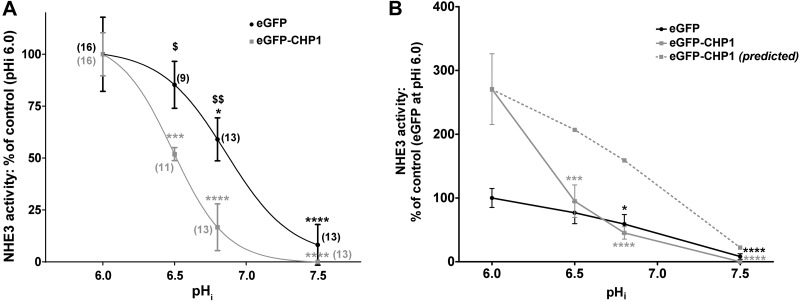
To obtain the maximum transport of NHE3, we maintained the cells in nearly zero-transport conditions (i.e., the bath solution contained 140 mM Na+, and the pipette solution was nominally Na+ free) at pHi 6.0 (30, 31). pHi was successively increased via the pipette perfusion line. Indeed, NHE3 reached peak transport at pHi 6.0, but rapidly declined with increasing pHi, becoming very low at pHi 7.5 (Fig. 2A, black line). The EC50 of transport was reached at pHi 6.86 ± 0.25; the Hill coefficient was ∼2. These data relate well to other results obtained by 22Na uptake (12). The overexpression of CHP1 tagged with eGFP in OK cells resulted in a shift in NHE3 transport response to pHi toward more acidic pHi without a significant change in the shape of the curve (Fig. 2A, gray line). The Hill coefficient was still ∼2, but the EC50 of transport in cells expressing eGFP-CHP1 was reached at a lower pHi (6.49±0.11) than that of cells transfected with eGFP alone. This significant shift in NHE3 toward a more acidic pHi indicates that the expression of CHP1 modifies the sensitivity to pHi of NHE3 transport. Indeed, eGFP-CHP1 expression induced a greater inhibition of NHE3 transport than did the control (eGFP alone) cells at a given pHi (Fig. 2A, gray line vs. black line).
Figure 2.
pHi dependence of NHE3 transport in OK cells. A) The cells were transiently transfected with eGFP-CHP1 (solid gray line) or eGFP (solid black line), and endogenous NHE3 transport was measured in single cells at increasing pHi of 6.0, 6.5, 7.0, and 7.5 with an extracellular pH of 8.2, 140 mM of extracellular Na+, and nominally no intracellular Na+. The eGFP-CHP1 trace (solid gray line) was normalized to the percentage of NHE3 activity at pHi 6.0 in cells expressing eGFP-CHP1. The eGFP trace (solid black line) was normalized to the percentage of NHE3 activity at pHi 6.0 in cells expressing eGFP. Lines in the plot represent the best fit of data to the Hill equation. B) eGFP-CHP1 (solid gray line) and eGFP (solid black line) traces were both normalized to the percentage of NHE3 activity at pHi 6.0 in cells expressing eGFP. Dotted gray line: predicted changes of NHE3 activity at increasing pHi after expression of eGFP-CHP1. Curve was calculated by dividing the percentage of NHE3 activity in cells expressing eGFP-CHP1 by that of eGFP alone at pHi 6.0. The resulting factor was then multiplied by values of NHE3 activity from pHi 6.5 to 7.5 of cells expressing eGFP alone. Solid circles or squares: average data of 9–16 individual experiments. Error bars: se. Numbers in parentheses: experiments performed in identical conditions. *P < 0.05, ***P < 0.001, ****P < 0.0001 vs. control; ANOVA. $P < 0.05, $$P < 0.01 for cells expressing eGFP alone vs. cells expressing eGFP-CHP1; ANOVA.
Overexpression of CHP1 in OK cells has been shown to activate rather than inhibit NHE3 transport in baseline conditions (referred to hereafter as baseline NHE3 transport). Activation is attributable to an increase in NHE3 protein abundance at the plasma membrane (25). Indeed, at pHi 6.0 eGFP-CHP1-expressing cells manifested a 3-fold greater rate of pH change than did cells expressing eGFP alone (Fig. 2B, solid gray line vs. solid black line). In support of these results, knockdown of endogenous CHP1 by shRNA led to a complete suppression of NHE3 activity at the same pHi of 6.0 (−87.88±16.69%, n=6, P < 0.01 compared with the control). However, if eGFP-CHP1 expression solely modifies baseline NHE3 transport, the curve of its activity at different pHi in CHP1-overexpressing cells would be shifted upward (Fig. 2B, dashed gray line, predicted curve), but would still be shaped similarly to that of the control cells (Fig. 2B, dashed gray line vs. solid black line). The shape of the curve obtained for NHE3 transport from CHP1-overexpressing cells was not similar to that of the one for the control cells—in particular, between pHi 6.0 and 7.0 (Fig. 2B, solid gray line vs. solid black line). Therefore, we postulated that CHP1 overexpression has a dual effect on NHE3 function: it modifies the set point of its transport toward a more acidic pHi and also increases baseline NHE3 transport at pHi 6.0.
NHE3 was inactivated by interaction with CHP1
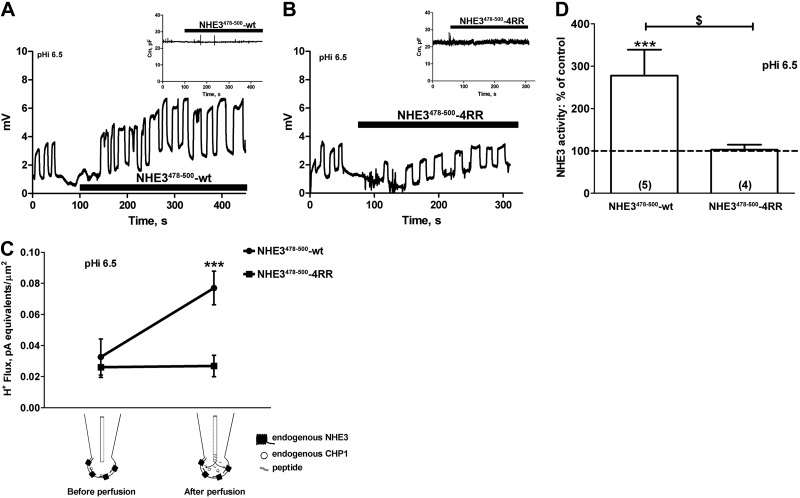
To analyze the mechanisms of the dual effect of CHP1 expression on NHE3 transport, we attempted to separate the two effects. We hypothesized that changes in NHE3 baseline transport—hence, in NHE3 protein expression—were the result of a long-term effect of CHP1, whereas changes in pHi sensitivity were the result of a short-term effect of CHP1 on NHE3. Indeed, perfusion via a patch pipette perfusion line of the peptide from the CHP1 binding region (NHE3478–500-WT), which competes for CHP1 interaction to NHE3 (Fig. 3C, diagram), led to an abrupt and sustained activation of NHE3 (Fig. 3A). pHi 6.5 was chosen for this set of experiments, because it is the pHi closest to the EC50 of NHE3 in cells expressing eGFP-CHP1 (Fig. 2A).
Figure 3.
Effect of NHE3478–500-WT peptide perfusion on NHE3 transport and Cm. A, B) Example of a recording set for NHE3 transport in a single OK cell before and after perfusion with the NHE3478–500-WT peptide (A) and its mutant, NHE3478–500-4RR (B). pHi was maintained at 6.5, and extracellular pH, extracellular Na+, and intracellular Na+ were as described in Fig. 2. Each oscillation represents the recording of NHE3 activity and resulted from the movement of the pH microelectrode close to (higher microvolts) and away from (lower microvolts) the plasma membrane of a single cell. Traces show a momentary stop in the recording of oscillation measurements immediately before and after perfusion of the peptides. Inset: effect on Cm (pF) of the perfusion of the NHE3478–500-WT or NHE3478–500-4RR peptides. C, D) Summary of the data expressed as H+ flux (C) and a percentage of the control (D). Cells were held in whole-cell configuration, and the peptide NHE3478–500-WT or NHE3478–500-4RR was perfused via a pipette line. Solid circles or squares: average data from individual experiments; bars and error bars: means ± se. Numbers in parentheses: experiments performed in identical conditions. ***P < 0.001 vs. control; ANOVA. $P < 0.05; ANOVA.
This increase in NHE3 due to the perfusion of NHE3478–500-WT was not caused by the exocytosis of NHE3 at the plasma membrane, given that the Cm (an index of cell surface; refs. 33, 44) did not change (Fig. 3A, inset). To confirm the specificity of this response, we also perfused the cells with the mutant peptide NHE3478–500-4RR, which binds CHP1 inefficiently (23). Perfusion with NHE3478–500-4RR neither modified the activity of NHE3 (Fig. 3B) nor caused a significant change in Cm (Fig. 3B, inset). These results are summarized in Figs. 3C, D, respectively, where the microvoltage signal was transformed in H+ flux (refs. 30, 31 and Fig. 3C), normalized to the cell membrane surface, and presented as a percentage of the control (Fig. 3D).
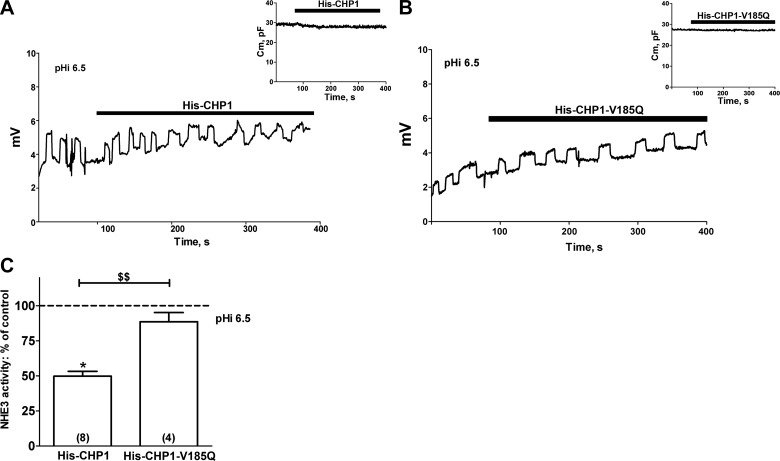
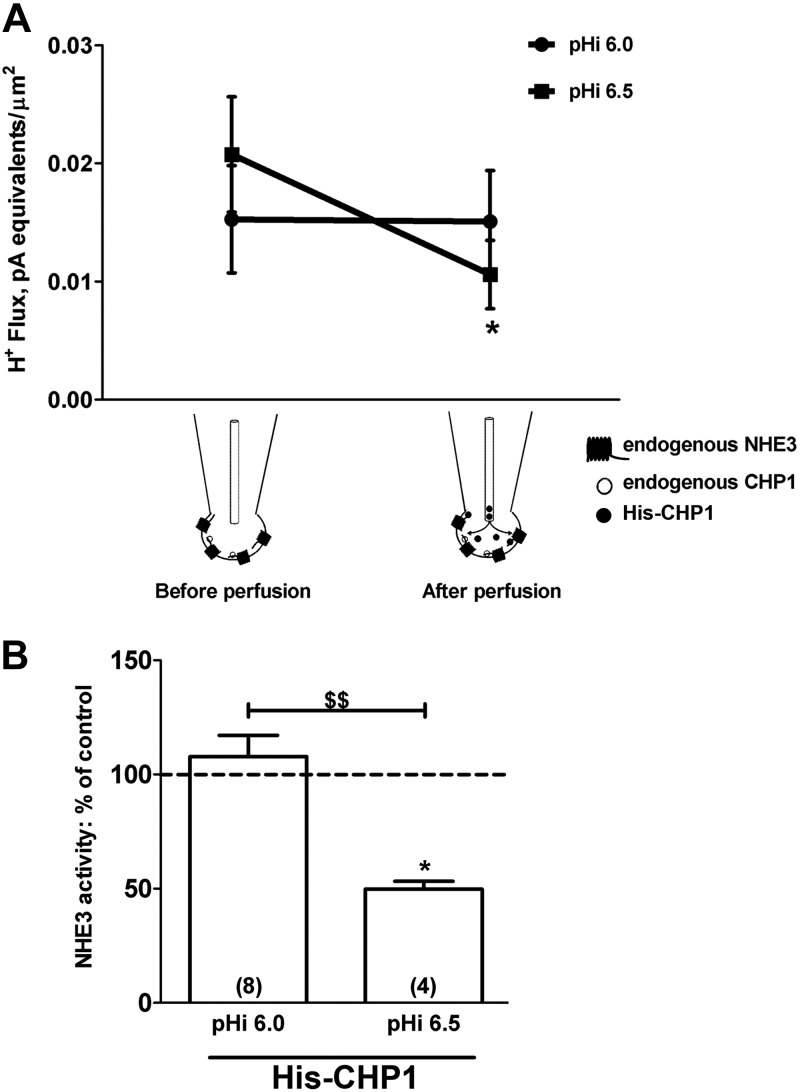
An opposite effect on NHE3 transport was measured during full-length CHP1 perfusion of the cells. Perfusion of His6-CHP1 at pHi 6.5 resulted in a significant (50%) inhibition of NHE3 activity (Fig. 4A, C) without changes in Cm (Fig. 4A, inset). In support of these findings, perfusion of the mutant V185Q of CHP1-His6 (His-CHP1-V185Q), which weakens the ability of CHP1 to bind NHE1 (28), neither affected NHE3 activity (Fig. 4B, C) nor induced changes in Cm (Fig. 4B, inset).
Figure 4.
Effect of CHP1 perfusion on NHE3 transport and Cm. A, B) Example of a recording set for NHE3 transport in a single OK cell before and after perfusion of purified His-CHP1 (A) and its mutant His-CHP1-V185Q (B). pHi was maintained at 6.5, and extracellular pH, extracellular Na+, and intracellular Na+ were as described in Fig. 2. Inset: effect of perfusion of either His-CHP1 or His-CHP1-V185Q on Cm (see Fig. 1). C) Summary of the data expressed as a percentage of the control. Bars and error bars: means ± se. Numbers in parentheses: experiments performed in identical conditions. *P < 0.05 vs. control; ANOVA. $$P < 0.01; ANOVA.
In summary, short-term modifications of CHP1's interaction with NHE3 altered NHE3 transport without changing its expression, thus supporting our hypothesis of a short- and long-term effect of CHP1 on the function of NHE3.
Binding of CHP1 to NHE3 was pH dependent
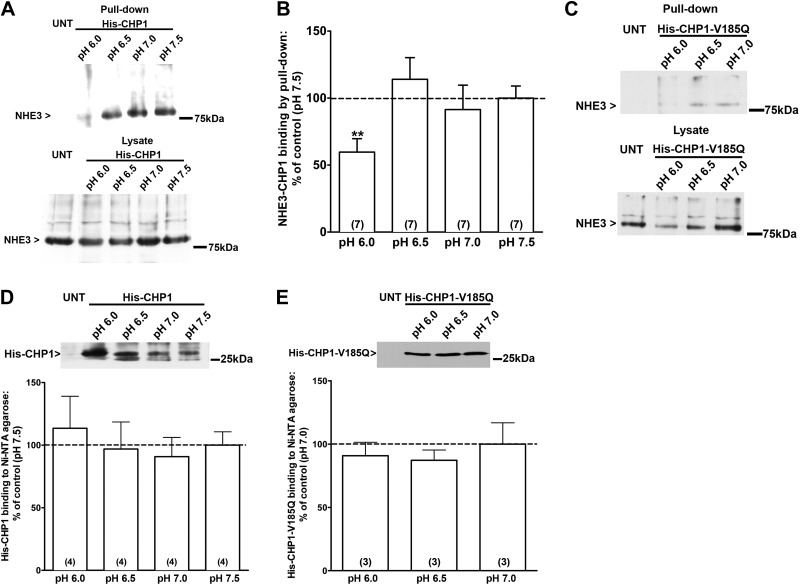
The overexpression of CHP1 affected NHE3 activity in a pHi-dependent manner (Fig. 2A); therefore, we next tested whether the interaction of CHP1 with NHE3 is pH dependent, by using OK cells transiently expressing His-CHP1 in pull-down experiments (Fig. 5). Endogenous NHE3 binds to His-CHP1 in a pH-dependent manner; indeed, at pH 6.0, there were fewer NHE3 molecules complexed with His-CHP1 than at pH 6.5 (Fig. 5A, top panel). OK cells transiently expressing the mutant form of CHP1 (His-CHP1-V185Q; ref. 28) were also subjected to a pull-down assay (Fig. 5C top panel). The NHE3 signal in the pull-down by His-CHP1-V185Q was weaker than the one in the pull-down by His-CHP1 (Fig. 5A vs. C, top panels). (The NHE3 signal in a pull-down assay by His-CHP1-V185Q was detectable only after overnight exposure of the film, whereas in the case of the pull-down by His-CHP1, it was visible after only a few minutes. pH dependence was still detected in this condition.) The amount of NHE3 present in the lysate of cells expressing either His-CHP1 or His-CHP1-V185Q was similar in the study condition, as well as the expression of His-CHP1 or His-CHP1-V185Q (Fig. 5A, C, bottom panels; D, E). Indeed, Mishima et al. (28) found no difference in the expression level between CHP1 and CHP1-V185Q. The formation of the NHE3-CHP1 complex was pH dependent and reached its maximum at pH 6.5 (Fig. 5B), a pH that matches that of the EC50 for the CHP1-dependent exchanger transport.
Figure 5.
pH dependence of CHP1-NHE3 binding. A) NHE3 total protein binding to CHP1 at increasing pH quantified by pull-down assay using the 3H3 anti-NHE3 antibody in untransfected (UNT) and His-CHP1-transfected cells, along with NHE3 total lysate in the same condition. B) Results are expressed as a percentage of NHE3-CHP1 binding at different pH vs. pH 7.5. C) Control pull-down of NHE3 total protein by His-CHP1-V185Q and total NHE3 lysate in the same conditions. D) His-CHP1 expression. Results are expressed as a percentage of His-CHP1 binding to Ni-NTA agarose at different pH vs. pH 7.5. E) His-CHP1-V185Q expression. Results are expressed as a percentage of His-CHP1 binding to Ni-NTA agarose at different pH vs. pH 7.0. Bars and error bars: mean ± se. Numbers in parentheses: experiments performed in identical conditions. **P < 0.01 vs. control; ANOVA.
This pH dependence in the formation of the NHE3-CHP1 complex was confirmed by measuring NHE3 transport at different pHi during His-CHP1 perfusion of the cells (Fig. 6). At pHi 6.0, perfusion of His-CHP1 via a patch pipette line did not have a significant effect on NHE3 activity (Fig. 6A), thus supporting the hypothesis of a short-term effect of CHP1 that is unrelated to NHE3 protein expression, whereas perfusion of His-CHP1 at pHi 6.5 had an inhibitory effect (Fig. 6A). The transport of NHE3 after His-CHP1 perfusion was significantly different at pHi 6.5 than at 6.0 (Fig. 6B).
Figure 6.
pH-dependent effect of CHP1 perfusion on NHE3 transport. A) Effect of His-CHP1 perfusion on NHE3 H+ flux at pHi 6.0 and 6.5. Cells were held in a whole-cell configuration, and His-CHP1 was perfused via a patch pipette line. B) Results are presented as a percentage of control NHE3 activity. Data before perfusion of His-CHP1 were normalized to data after perfusion at the indicated pHi. Bars and error bars: mean ± se. Numbers in parentheses: experiments performed in identical conditions. *P < 0.05 vs. control; ANOVA. $$P < 0.01; ANOVA.
In summary, the formation of the NHE3-CHP1 complex is pH dependent, reaching a maximum at pH 6.5 and maintaining its maximum at a higher pH. At a pH lower than 6.5, CHP1 binds less to NHE3 and does not influence NHE3 pHi sensitivity, but rather its baseline transport (Fig. 2B).
The CHP1-NHE3 complex was involved in the hormonal stimuli–dependent regulation of NHE3 transport
NHE3 is regulated acutely by a variety of stimuli (14, 16, 17, 45, 46). We investigated whether the CHP1-NHE3 complex is involved in the hormonal stimuli–mediated regulation of NHE3. We tested hormonal stimuli that, based on the current literature, acutely regulate NHE3 activity in OK cells, but their effect on NHE3 may or may not be associated with CHP1 (18, 24).
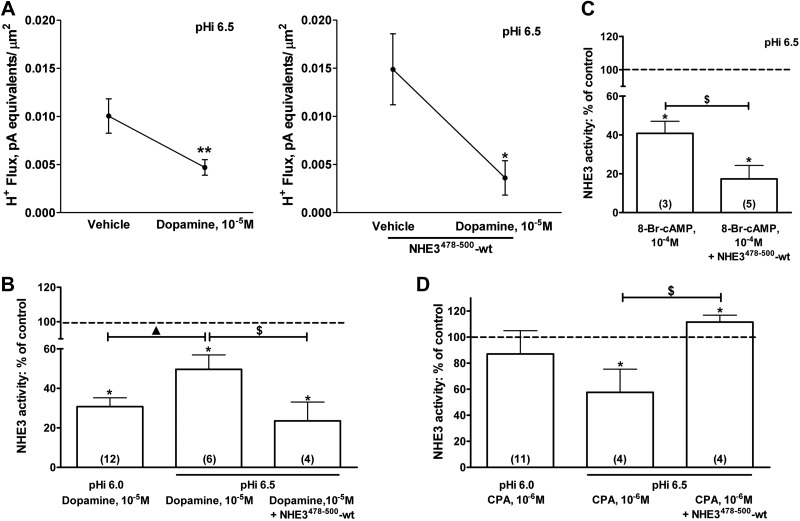
Activation of D1-type dopamine receptors leads to a decrease in NHE3 transport in OK cells (47, 48). We obtained results similar to those that had been previously reported using an oscillating H+-sensitive electrode; treatment with 10−5 M dopamine at pHi 6.5 resulted in the inhibition of NHE3 transport (Fig. 7A, left panel). In a similar set of experiments the NHE3478–500-WT peptide was perfused via a patch pipette line before dopamine treatment (Fig. 7A, right panel). The perfusion amplified the inhibition of NHE3 transport induced by dopamine, per se, at the pHi (Fig. 7A, right panel; B). These findings suggest that NHE3 bound to CHP1 is somehow unavailable for dopamine-mediated inhibition of NHE3. This assumption was supported by the fact that, at pHi 6.0, dopamine inhibited NHE3 transport more than at pHi 6.5 (60 vs. 50%, respectively; Fig. 7B). CHP1 bound NHE3 less at pHi 6.0 than at 6.5; therefore, the pHi -dependent change in the NHE3 response to dopamine treatment mimicked that of NHE3478–500-WT perfusion. Both effects resulted in a partial dissociation of the NHE3-CHP1 complex and in an enhancement of NHE3 inhibition by dopamine. Dopamine affects NHE3 activity in OK cells via an increase in intracellular cAMP level and activation of protein kinase A (PKA; refs. 47, 49). To confirm the data obtained by dopamine treatment, we mimicked its effect on NHE3 by activation of PKA. 8-Bromo-cAMP (8-br-cAMP), a PKA activator, was added to the OK cells, with or without perfusion with the NHE3478–500-WT peptide (Fig. 7C). 8-Br-cAMP inhibits NHE3 activity (50, 51) by 60% at pHi 6.5. Perfusion of the NHE3478–500-WT peptide before 8-Br-cAMP treatment resulted in an augmented PKA-mediated inhibition of NHE3 activity (∼80%). In summary, dissociation of CHP1 from NHE3 by the perfusion of the peptide NHE3478–500-WT or by lowering the pHi resulted in an enhanced inhibition of NHE3 transport by activation of dopamine and PKA.
Figure 7.
Effect of the CHP1-NHE3 complex formation on NHE3 acute regulation by specific hormonal stimuli. A, B) Effect of dopamine (10−5 M, 10 min) on NHE3 H+ flux with (A, right panel) and without (A, left panel) perfusion with the NHE3478–500-WT peptide at pHi 6.5; and on NHE3 H+ flux at pHi 6.0 and 6.5 (B, first and second bars) and on NHE3 H+ flux at pH 6.5, before and after perfusion with the NHE3478–500-WT peptide (B, second and third bars). Results are a percentage of the control of NHE3 activity. Data in the first and second bars were normalized by comparing dopamine 10−5 M-treated with vehicle-treated cells at pH 6.0 and 6.5. Data presented in the third bar were dopamine 10−5 M and peptide perfusion compared with vehicle and peptide perfusion. C) Effect of 8-Br-cAMP (10−4 M, 10 min) on NHE3 H+ flux, before and after perfusion with the NHE3478–500-WT peptide at pHi 6.5. Data were normalized as in B. D) Effect of CPA (10−6 M, 10 min), on NHE3 H+ flux at pHi 6.0 and 6.5 and reverse of CPA-mediated inhibition of NHE3 H+ flux at pHi 6.5 by perfusion with NHE3478–500-WT peptide. Data were normalized as in B. Results are presented as a percentage of the control of NHE3 activity. Circles, bars, and error bars: means ± se. Numbers in parentheses: experiments performed in identical conditions. *P < 0.05, **P < 0.01 vs. control; ANOVA. $P < 0.05; ANOVA. ▲P < 0.05; ANOVA.
Activation of A1R by a high concentration of the specific agonist N6-cyclopentyladenosine (CPA) inhibited NHE3 activity—this inhibition being associated with an increase in NHE3-CHP1 binding (24). We evaluated the effect of CHP1-NHE3 dissociation on A1R-dependent inhibition of NHE3, by perfusing the competitive peptide (NHE3478–500-WT) and lowering pHi (Fig. 7D). Perfusion of the NHE3478–500-WT peptide via a patch pipette line before CPA treatment resulted in a complete reversal of the NHE3 inhibition induced by CPA. A significant increase in NHE3 activity (∼110%; Fig. 7D) was measured in this condition. Treatment of 10−6 M CPA at pHi 6.0 did not affect NHE3 activity. However, treatment with CPA 10−6 M at pHi 6.5 resulted in a 50% decrease in NHE3 transport; inhibition of NHE3 activity was reversed by the A1R antagonist, 8-cyclopentyl-1,3-dipropylxanthine [CPX; 10−6 M, 5 min, pHi 6.5, −11.40±2.64%, n=3, nonsignificant (NS) vs. vehicle; pHi 6.5, CPX 10−6 M+CPA 10−6 M, −9.10±2.87%, n=3, NS vs. vehicle]. Of note was that A1R activation with a low dose of CPA (10−9 M) resulted in an increase in NHE3 activity as previously reported (50) that was significant only at pHi 6.0 (pHi 6.0, +59.80±19.63%, n=4, P<0.05 vs. vehicle and pHi 6.5, +27.00±18.22%, n=7, NS vs. vehicle).
In summary, the inhibition of NHE3 by a high concentration of A1R agonist was dependent on the formation of the NHE3-CHP1 complex. A decrease in the amount of CHP1 bound to NHE3 reduced the effect of A1R activation on NHE3 transport. In contrast, a decrease in the amount of CHP1 bound to NHE3 augmented the effect of the low concentration of the A1R agonist on NHE3, similar to the activation of dopamine receptor and PKA. The effect of the CHP1-NHE3 complex on the acute regulation of NHE3 depends on the specific hormonal stimulus and, in the case of A1R activation, on the concentration of the A1R agonist.
DISCUSSION
Regulation of the NHE protein family is attributable to a change in the affinity for intracellular H+. This pHi dependence inactivates the exchanger at pHi above a certain activity threshold—hence, above a set point—and then activates it rapidly as pHi falls below the set point. Specifically, NHE proteins are thought to be activated through conformational changes caused by intracellular protonation, and the exchanger possesses one or more cytoplasmic pH sensor sites that initiate the changes (12, 52). Indeed, histidine residues at the juxtamembrane cytoplasmic domain of NHE3 participate in the pHi sensitivity of the exchanger (9). pHi sensing is a common feature of the NHE protein family transport function, as C-terminal cytoplasmic regulatory domains involved in the set point of NHE-activation have also been identified for other exchangers (53, 54). In particular, sites of histidine residues devoted to NHE3 pHi sensitivity on its C terminus overlap with the region of CHP subfamily interaction.
Although evidence of allosteric regulatory sites has been collected for >20 yr, and several hypotheses on the modus operandi of an internal H+ sensor site have been proposed over the years, the molecular mechanism responsible for establishing the NHE3 set point remains to be defined experimentally. Validation is lacking because accurate measurement of pHi is sometimes difficult to perform, given the rather limited availability of experimental transport methods for the study of NHE activity. The limitations of typically used methods, such as 22Na+ uptake and fluorescent membrane permeants, includes moderated sensitivity, time resolution, and inadequate control of the intracellular concentration of H+. Measurements of NHE-induced H+ fluxes by pH microelectrodes during the recording in whole-cell patch-clamp experiments have been shown to be effective in overcoming some of these limitations (30, 31). Therefore, NHE3 transport was measured by taking advantage of this technical approach to the study of the molecular basis of NHE3 pHi sensing.
In this study, CHP1 expression increased transport of endogenous NHE3 in baseline conditions in epithelial cells, results that are well matched with previous observations on a CHP1-dependent activation of NHE3 activity in response to an increase in NHE3 protein expression (25). Nevertheless, CHP1 expression not only acted on NHE3 protein expression but also modified the NHE3 response to changes in pHi. A 50% inhibition of NHE3 activity was measured at pHi 6.9 in the control cells; the same inhibition was measured earlier at pHi 6.5 in cells expressing CHP1, with an acidic shift of pHi -dependence induced by CHP1 expression of 0.4 pHi units. Altogether, these results indicate that CHP1 not only increases NHE3 protein expression but also induces a change in NHE3 pHi sensitivity.
Next, we determined whether the association of CHP1 to NHE3 triggers the change in pHi sensitivity. Indeed, CHP1 binding to NHE3 induced the pH dependence of NHE3, in particular from pH 6.0 to 6.5. The amount of CHP1 bound to NHE3 increased with the elevation of pH, whereas inhibition of NHE3 was augmented as CHP1 interacted with NHE3. In contrast, preventing the formation of the CHP1-NHE3 complex by perfusion via a patch pipette line of the peptide NHE3478–500-WT at pHi 6.5 induced NHE3 activity. Purified CHP1 perfusion also affected NHE3 activity, confirming the results obtained with overexpressed CHP1, showing that there are NHE3 molecules available to be inhibited by exogenous CHP1. However, the CHP1 perfusion effect was significant, but smaller, compared with that of the NHE3478–500-WT peptide perfusion at the same pHi, thus supporting the biochemical finding that most NHE3 is bound to CHP1 at that pHi. In summary, an increase in binding of CHP1 to NHE3 between pHi 6.0 and 6.5 is proposed as the CHP1-dependent signal that alters the NHE3 set point for pHi. Nevertheless, an important additional effect of acidic pHi of altering the ability of CHP1 to inhibit NHE3 activity, over and above its effect on the CHP1-NHE3 association, cannot be ruled out based on the presented observations.
Perfusion of the peptide NHE3478–500-WT modified NHE3 activity but not its amount of surface protein (detected by cell capacitance measurement; Fig. 3A, inset). These findings revealed that short-term modification of the CHP1-NHE3 interaction does not affect the amount of NHE3 protein. In summary, short-term modification of the interaction seems to involve changes in the exchanger's pHi sensitivity, whereas long-term modification involves changes in NHE3's trafficking to the cell surface, possibly promoting its surface stability (22, 29, 55). Indeed, CHP-NHE interaction is thought to happen at an early stage of the transporter's biosynthesis (22). OK cells are estimated to have 10–15% of total NHE3 on the brush border and the rest of in the cytosol (55), a fact that supports the existence of CHP1-NHE3 pools (brush border vs. intracellular). The 50% of total NHE3 protein that was bound to CHP1 at pH 6.0 (Fig. 5B) comprised a significant portion of the intracellular CHP1-NHE3 pool that might be integral in regulating NHE3's trafficking rather than its surface activity.
In summary, these findings support a function for the CHP1-NHE3 complex that is more related to both NHE3 transport at baseline and its molecular functional conformation than to the stimulus-dependent regulatory function as for other NHE3 regulatory complexes (18). Whether this is actually the case or whether acute regulation of NHE3 by physiological stimuli is affected by the formation of the CHP1-NHE3 complex was further investigated. Specific hormonal stimuli were chosen based on their target regions on the NHE3 C terminus (i.e., the NHE3 C terminus distant from or close to the CHP1 binding sequence on NHE3; refs. 17, 18). For example, the D1-type dopamine receptor inhibits NHE3 transport in OK cells mainly via activation of the PKA signaling pathway (47, 49). The region on the NHE3 C terminus that is essential for the PKA response has been shown to be distant from that of CHP1 binding (17, 56). The inhibition of NHE3 transport by dopamine was confirmed by recording H+ fluxes at pHi 6.5. Perfusion with the NHE3478–500-WT peptide enhanced dopamine's inhibitory effect on NHE3, indicating that augmentation of NHE3 unbound to CHP1 increased the amount of NHE3 sensitive to inhibition by dopamine. In support of these observations, dopamine's effect on NHE3 activity was quantitatively greater, as the amount of CHP1 bound to NHE3 decreased from pHi 6.5 to 6.0. Similarly, cells treated with 8-Br-cAMP, to mimic the signaling pathway activated by dopamine, responded to perfusion with NHE3478–500-WT peptide, confirming that the NHE3 molecules not bound to CHP1 are sensitive to regulation by dopamine and activation of the PKA signaling cascade. In support of these observations, PKA activation does not interfere with the intrinsic transport function of NHE3 (57). Rather, phosphorylation of NHE3 by PKA has been implicated in NHE3's trafficking along the microvilli (58).
In contrast, CHP1's interaction with NHE3 is critical for the A1R-dependent control of NHE3 activity. The inhibition of NHE3 activity induced by a high concentration of the A1R agonist CPA was reversed by NHE3478–500-WT peptide perfusion, supporting the involvement of the CHP1-NHE3 complex in the regulation of NHE3 by A1R activation (24). The inhibitory effect of CPA was not only reversed by perfusion with the NHE3478–500-WT peptide, but a significant activation of NHE3 was measured in this condition. A1R activation has a dual mode of action on NHE3 transport in OK cells, with CPA <10−8 M activating NHE3 and CPA >10−8 M inhibiting its activity (50). Inhibition of NHE3 activity by a high concentration of CPA is mediated by activation of the intracellular signal pathways Ca2+/phospholipase C/protein kinase C (PKC) and is dependent on the formation of the CHP1-NHE3 protein complex (24, 50, 59). Inhibition of PKC not only blocks the negative regulation of NHE3 activity but also allows activation of NHE3 in the presence of a high concentration of CPA (50). Similarly, blocking CHP1 binding to NHE3 not only stopped the negative regulation of NHE3 transport but also allowed activation of NHE3 in the presence of a high concentration of CPA. These results showed that an increase in CHP1-NHE3 complex formation participates in the inhibitory, but not in the stimulatory, control of NHE3 induced by CPA, as reported for the effector PKC (50). Indeed, as the effect of dopamine on NHE3 activity was enhanced by reducing the formation of the CHP1-NHE3 complex, so was the effect of a low concentration of CPA on NHE3 activity.
In summary, modification of the amount of CHP1 bound to NHE3 alters the exchanger set point for pHi in baseline and stimulus-mediated conditions. CHP1 may serve as a regulatory molecule for NHE3 conformational changes due to intracellular protonation.
Acknowledgments
The authors thank Drs. Pedro A. Jose (University of Maryland School of Medicine, Baltimore, MD, USA), Orson W. Moe (University of Texas Southwestern Medical Center, Dallas, TX, USA), Donald W. Hilgemann (University of Texas Southwestern Medical Center), and Peter B. Gahan (King's College, London, UK) for valuable discussions.
This work was supported by U.S. National Institutes of Health grants R01HL092196-05 and R01DK041612, the Simmons Family Foundation, and the Charles and Jane Pak Center of Mineral Metabolism. F.D. was supported by a Carl W. Gottschalk Research Scholar Award from the American Society of Nephrology.
Footnotes
- 8-Br-cAMP
- 8-bromoadenosine 3′,5′-cyclic monophosphate
- A1R
- adenosine receptor subtype 1
- CHO
- Chinese hamster ovary
- CHP
- calcineurin homologous protein
- Cm
- cell membrane capacitance
- CPA
- N6-cyclopentyladenosine
- CPX
- 8-cyclopentyl-1,3-dipropylxanthine
- eGFP
- enhanced green fluorescent protein
- NHE
- Na+/H+ exchanger
- NHE1–9
- Na+/H+ exchanger isoform 1–9
- NS
- nonsignificant
- OK
- opossum kidney
- pHi
- intracellular pH
- PKA
- protein kinase A
- PKC
- protein kinase C
- shRNA
- short hairpin RNA
- WT
- wild type
REFERENCES
- 1. Brown D., Wagner C. A. (2012) Molecular mechanisms of acid-base sensing by the kidney. J. Am. Soc. Nephrol. 23, 774–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Srivastava J., Barber D. L., Jacobson M. P. (2007) Intracellular pH sensors: design principles and functional significance. Physiology (Bethesda) 22, 30–39 [DOI] [PubMed] [Google Scholar]
- 3. Webb B. A., Chimenti M., Jacobson M. P., Barber D. L. (2011) Dysregulated pH: a perfect storm for cancer progression. Nat. Rev. Cancer 11, 671–677 [DOI] [PubMed] [Google Scholar]
- 4. Orlowski J., Grinstein S. (2004) Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflügers Arch. 447, 549–565 [DOI] [PubMed] [Google Scholar]
- 5. Cardone R. A., Casavola V., Reshkin S. J. (2005) The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat. Rev. Cancer 5, 786–795 [DOI] [PubMed] [Google Scholar]
- 6. Brett C. L., Donowitz M., Rao R. (2005) Evolutionary origins of eukaryotic sodium/proton exchangers. Am. J. Physiol. Cell Physiol. 288, C223–239 [DOI] [PubMed] [Google Scholar]
- 7. Bobulescu I. A., Di Sole F., Moe O. W. (2005) Na+/H+ exchangers: physiology and link to hypertension and organ ischemia. Curr. Opin. Nephrol. Hypertens. 14, 485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zachos N. C., Tse M., Donowitz M. (2005) Molecular physiology of intestinal Na+/H+ exchange. Annu. Rev. Physiol. 67, 411–443 [DOI] [PubMed] [Google Scholar]
- 9. Cha B., Oh S., Shanmugaratnam J., Donowitz M., Yun C. C. (2003) Two histidine residues in the juxta-membrane cytoplasmic domain of Na+/H+ exchanger isoform 3 (NHE3) determine the set point. J. Membr. Biol. 191, 49–58 [DOI] [PubMed] [Google Scholar]
- 10. Ikeda T., Schmitt B., Pouyssegur J., Wakabayashi S., Shigekawa M. (1997) Identification of cytoplasmic subdomains that control pH-sensing of the Na+/H+ exchanger (NHE1): pH-maintenance, ATP-sensitive, and flexible loop domains. J. Biochem. 121, 295–303 [DOI] [PubMed] [Google Scholar]
- 11. Dibrov P., Murtazina R., Kinsella J., Fliegel L. (2000) Characterization of a histidine rich cluster of amino acids in the cytoplasmic domain of the Na+/H+ exchanger. Biosci. Rep. 20, 185–197 [DOI] [PubMed] [Google Scholar]
- 12. Aronson P. S., Nee J., Suhm M. A. (1982) Modifier role of internal H+ in activating the Na+-H+ exchanger in renal microvillus membrane vesicles. Nature 299, 161–163 [DOI] [PubMed] [Google Scholar]
- 13. Donowitz M., Ming Tse C., Fuster D. (2013) SLC9/NHE gene family, a plasma membrane and organellar family of Na+/H+ exchangers. Mol. Aspects Med. 34, 236–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bobulescu I. A., Moe O. W. (2009) Luminal Na+/H+ exchange in the proximal tubule. Pflügers Arch. 458, 5–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hayashi H., Szaszi K., Grinstein S. (2002) Multiple modes of regulation of Na+/H+ exchangers. Ann. N. Y. Acad. Sci. 976, 248–258 [DOI] [PubMed] [Google Scholar]
- 16. Girardi A. C., Di Sole F. (2012) Deciphering the mechanisms of the Na+/H+ exchanger-3 regulation in organ dysfunction. Am. J. Physiol. Cell Physiol. 302, C1569–C1587 [DOI] [PubMed] [Google Scholar]
- 17. Donowitz M., Li X. (2007) Regulatory binding partners and complexes of NHE3. Physiol. Rev. 87, 825–872 [DOI] [PubMed] [Google Scholar]
- 18. Donowitz M., Mohan S., Zhu C. X., Chen T. E., Lin R., Cha B., Zachos N. C., Murtazina R., Sarker R., Li X. (2009) NHE3 regulatory complexes. J. Exp. Biol. 212, 1638–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Di Sole F., Vadnagara K., Moe O. W., Babich V. (2012) Calcineurin homologous protein: a multifunctional Ca2+-binding protein family. Am. J. Physiol. Renal Physiol. 303, F165–F179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ammar Y. B., Takeda S., Hisamitsu T., Mori H., Wakabayashi S. (2006) Crystal structure of CHP2 complexed with NHE1-cytosolic region and an implication for pH regulation. EMBO J. 25, 2315–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Naoe Y., Arita K., Hashimoto H., Kanazawa H., Sato M., Shimizu T. (2005) Structural characterization of calcineurin B homologous protein 1. J. Biol. Chem. 280, 32372–32378 [DOI] [PubMed] [Google Scholar]
- 22. Zaun H. C., Shrier A., Orlowski J. (2008) Calcineurin B homologous protein 3 promotes the biosynthetic maturation, cell surface stability, and optimal transport of the Na+/H+ exchanger NHE1 isoform. J. Biol. Chem. 283, 12456–12467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pang T., Su X., Wakabayashi S., Shigekawa M. (2001) Calcineurin homologous protein as an essential cofactor for Na+/H+ exchangers. J. Biol. Chem. 276, 17367–17372 [DOI] [PubMed] [Google Scholar]
- 24. Di Sole F., Cerull R., Babich V., Quinones H., Gisler S. M., Biber J., Murer H., Burckhardt G., Helmle-Kolb C., Moe O. W. (2004) Acute regulation of Na/H exchanger NHE3 by adenosine A1 receptors is mediated by calcineurin homologous protein. J. Biol. Chem. 279, 2962–2974 [DOI] [PubMed] [Google Scholar]
- 25. Di Sole F., Babich V., Moe O. W. (2009) The calcineurin homologous protein-1 increases Na+/H+-exchanger 3 trafficking via ezrin phosphorylation. J. Am. Soc. Nephrol. 20, 1776–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pang T., Wakabayashi S., Shigekawa M. (2002) Expression of calcineurin B homologous protein 2 protects serum deprivation-induced cell death by serum-independent activation of Na+/H+ exchanger. J. Biol. Chem. 277, 43771–43777 [DOI] [PubMed] [Google Scholar]
- 27. Pang T., Hisamitsu T., Mori H., Shigekawa M., Wakabayashi S. (2004) Role of calcineurin B homologous protein in pH regulation by the Na+/H+ exchanger 1: tightly bound Ca2+ ions as important structural elements. Biochemistry 43, 3628–3636 [DOI] [PubMed] [Google Scholar]
- 28. Mishima M., Wakabayashi S., Kojima C. (2007) Solution structure of the cytoplasmic region of Na+/H+ exchanger 1 complexed with essential cofactor calcineurin B homologous protein 1. J. Biol. Chem. 282, 2741–2751 [DOI] [PubMed] [Google Scholar]
- 29. Zaun H. C., Shrier A., Orlowski J. (2012) N-myristoylation and Ca2+ binding of calcineurin B homologous protein CHP3 are required to enhance Na+/H+ exchanger NHE1 half-life and activity at the plasma membrane. J. Biol. Chem. 287, 36883–36895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fuster D., Moe O. W., Hilgemann D. W. (2008) Steady-state function of the ubiquitous mammalian Na/H exchanger (NHE1) in relation to dimer coupling models with 2Na/2H stoichiometry. J. Gen. Physiol. 132, 465–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fuster D., Moe O. W., Hilgemann D. W. (2004) Lipid- and mechanosensitivities of sodium/hydrogen exchangers analyzed by electrical methods. Proc. Natl. Acad. Sci. U. S. A. 101, 10482–10487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kang T. M., Markin V. S., Hilgemann D. W. (2003) Ion fluxes in giant excised cardiac membrane patches detected and quantified with ion-selective microelectrodes. J. Gen. Physiol. 121, 325–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang T. M., Hilgemann D. W. (2008) Ca-dependent nonsecretory vesicle fusion in a secretory cell. J. Gen. Physiol. 132, 51–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shcheynikov N., Kim K. H., Kim K. M., Dorwart M. R., Ko S. B., Goto H., Naruse S., Thomas P. J., Muallem S. (2004) Dynamic control of cystic fibrosis transmembrane conductance regulator Cl−/HCO3− selectivity by external Cl−. J. Biol. Chem. 279, 21857–21865 [DOI] [PubMed] [Google Scholar]
- 35. Hilgemann D. W., Lu C. C. (1998) Giant membrane patches: improvements and applications. Methods Enzymol. 293, 267–280 [DOI] [PubMed] [Google Scholar]
- 36. Marala R. B., Brown J. A., Kong J. X., Tracey W. R., Knight D. R., Wester R. T., Sun D., Kennedy S. P., Hamanaka E. S., Ruggeri R. B., Hill R. J. (2002) Zoniporide: a potent and highly selective inhibitor of human Na+/H+ exchanger-1. Eur. J. Pharmacol. 451, 37–41 [DOI] [PubMed] [Google Scholar]
- 37. Guzman-Perez A., Wester R. T., Allen M. C., Brown J. A., Buchholz A. R., Cook E. R., Day W. W., Hamanaka E. S., Kennedy S. P., Knight D. R., Kowalczyk P. J., Marala R. B., Mularski C. J., Novomisle W. A., Ruggeri R. B., Tracey W. R., Hill R. J. (2001) Discovery of zoniporide: a potent and selective sodium-hydrogen exchanger type 1 (NHE-1) inhibitor with high aqueous solubility. Bioorg. Med. Chem. Lett. 11, 803–807 [DOI] [PubMed] [Google Scholar]
- 38. Rotin D., Grinstein S. (1989) Impaired cell volume regulation in Na+-H+ exchange-deficient mutants. Am. J. Physiol. 257, C1158–C1165 [DOI] [PubMed] [Google Scholar]
- 39. Bobulescu I. A., Quinones H., Gisler S. M., Di Sole F., Hu M. C., Shi M., Zhang J., Fuster D. G., Wright N., Mumby M., Moe O. W. (2010) Acute Regulation of renal Na+/H+ exchanger NHE3 by dopamine: role of protein phosphatase 2A. Am. J. Physiol. Renal Physiol. 298, F1205–F1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Helmle-Kolb C., Montrose M. H., Murer H. (1990) Parathyroid hormone regulation of Na+/H+ exchange in opossum kidney cells: polarity and mechanisms. Pflügers Arch. 416, 615–623 [DOI] [PubMed] [Google Scholar]
- 41. Helmle-Kolb C., Montrose M. H., Stange G., Murer H. (1990) Regulation of Na+/H+ exchange in opossum kidney cells by parathyroid hormone, cyclic AMP and phorbol esters. Pflügers Arch. 415, 461–470 [DOI] [PubMed] [Google Scholar]
- 42. Helmle-Kolb C., Counillon L., Roux D., Pouyssegur J., Mrkic B., Murer H. (1993) Na/H exchange activities in NHE1-transfected OK-cells: cell polarity and regulation. Pflügers Arch. 425, 34–40 [DOI] [PubMed] [Google Scholar]
- 43. Azarani A., Goltzman D., Orlowski J. (1995) Parathyroid hormone and parathyroid hormone-related peptide inhibit the apical Na+/H+ exchanger NHE-3 isoform in renal cells (OK) via a dual signaling cascade involving protein kinase A and C. J. Biol. Chem. 270, 20004–20010 [DOI] [PubMed] [Google Scholar]
- 44. Fine M., Llaguno M. C., Lariccia V., Lin M. J., Yaradanakul A., Hilgemann D. W. (2011) Massive endocytosis driven by lipidic forces originating in the outer plasmalemmal monolayer: a new approach to membrane recycling and lipid domains. J. Gen. Physiol. 137, 137–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Albrecht F. E., Xu J., Moe O. W., Hopfer U., Simonds W. F., Orlowski J., Jose P. A. (2000) Regulation of NHE3 activity by G protein subunits in renal brush-border membranes. Am. J. Physiol. 278, R1064–R1073 [DOI] [PubMed] [Google Scholar]
- 46. Xu J., Li X. X., Albrecht F. E., Hopfer U., Carey R. M., Jose P. A. (2000) Dopamine1 receptor, Gsα, and Na+-H+ exchanger interactions in the kidney in hypertension. Hypertension 36, 395–399 [DOI] [PubMed] [Google Scholar]
- 47. Wiederkehr M. R., Di Sole F., Collazo R., Quinones H., Fan L., Murer H., Helmle-Kolb C., Moe O. W. (2001) Characterization of acute inhibition of Na/H exchanger NHE-3 by dopamine in opossum kidney cells. Kidney Int. 59, 197–209 [DOI] [PubMed] [Google Scholar]
- 48. Li X. X., Albrecht F. E., Robillard J. E., Eisner G. M., Jose P. A. (2000) Gβ regulation of Na/H exchanger-3 activity in rat renal proximal tubules during development. Am. J. Physiol. 278, R931–R936 [DOI] [PubMed] [Google Scholar]
- 49. Hu M. C., Fan L., Crowder L. A., Karim-Jimenez Z., Murer H., Moe O. W. (2001) Dopamine acutely stimulates Na+/H+ exchanger (NHE3) endocytosis via clathrin-coated vesicles: dependence on protein kinase A-mediated NHE3 phosphorylation. J. Biol. Chem. 276, 26906–26915 [DOI] [PubMed] [Google Scholar]
- 50. Di Sole F., Cerull R., Petzke S., Casavola V., Burckhardt G., Helmle-Kolb C. (2003) Bimodal acute effects of A1 adenosine receptor activation on Na+/H+ exchanger 3 in opossum kidney cells. J. Am. Soc. Nephrol. 14, 1720–1730 [DOI] [PubMed] [Google Scholar]
- 51. Moe O. W. (1999) Acute regulation of proximal tubule apical membrane Na/H exchanger NHE-3: role of phosphorylation, protein trafficking, and regulatory factors. J. Am. Soc. Nephrol. 10, 2412–2425 [DOI] [PubMed] [Google Scholar]
- 52. Wakabayashi S., Hisamitsu T., Pang T., Shigekawa M. (2003) Kinetic dissection of 2distinct proton binding sites in Na+/H+ exchangers by measurement of reverse mode reaction. J. Biol. Chem. 278, 43580–43585 [DOI] [PubMed] [Google Scholar]
- 53. Wakabayashi S., Bertrand B., Ikeda T., Pouyssegur J., Shigekawa M. (1994) Mutation of calmodulin-binding site renders the Na+/H+ exchanger (NHE1) highly H+-sensitive and Ca2+ regulation-defective. J. Biol. Chem. 269, 13710–13715 [PubMed] [Google Scholar]
- 54. Wakabayashi S., Fafournoux P., Sardet C., Pouyssegur J. (1992) The Na+/H+ antiporter cytoplasmic domain mediates growth factor signals and controls “H+-sensing”. Proc. Natl. Acad. Sci. U. S. A. 89, 2424–2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Matsushita M., Sano Y., Yokoyama S., Takai T., Inoue H., Mitsui K., Todo K., Ohmori H., Kanazawa H. (2007) Loss of calcineurin homologous protein-1 in chicken B lymphoma DT40 cells destabilizes Na+/H+ exchanger isoform-1 protein. Am. J. Physiol. Cell Physiol. 293, C246–C254 [DOI] [PubMed] [Google Scholar]
- 56. Cabado A. G., Yu F. H., Kapus A., Lukacs G., Grinstein S., Orlowski J. (1996) Distinct structural domains confer cAMP sensitivity and ATP dependence to the Na+/H+ exchanger NHE3 isoform. J. Biol. Chem. 271, 3590–3599 [DOI] [PubMed] [Google Scholar]
- 57. Kocinsky H. S., Dynia D. W., Wang T., Aronson P. S. (2007) NHE3 phosphorylation at serines 552 and 605 does not directly affect NHE3 activity. Am. J. Physiol. Renal Physiol. 293, F212–F218 [DOI] [PubMed] [Google Scholar]
- 58. Inoue B. H., dos Santos L., Pessoa T. D., Antonio E. L., Pacheco B. P., Savignano F. A., Carraro-Lacroix L. R., Tucci P. J., Malnic G., Girardi A. C. (2012) Increased NHE3 abundance and transport in renal proximal tubule of rats with heart failure. Am. J. Physiol. 302, R166–R174 [DOI] [PubMed] [Google Scholar]
- 59. Di Sole F., Casavola V., Mastroberardino L., Verrey F., Moe O. W., Burckhardt G., Murer H., Helmle-Kolb C. (1999) Adenosine inhibits the transfected Na+-H+ exchanger NHE3 in Xenopus laevis renal epithelial cells (A6/C1). J. Physiol. 515, 829–842 [DOI] [PMC free article] [PubMed] [Google Scholar]