Abstract
The use of Toombak has been reported to play a major role in the etiology of oral cancer in Sudan. The cellular proliferative activity on the oral epithelium of 210 Toombak dippers was assessed by applying the micronuclei frequency, mean argyrophilic nucleolar organizer region (AgNOR) counts, Papanicolaou method, and 1% crystal violet stain. Participants were divided into 3 groups: 200 were apparently healthy individuals, 100 were Toombak users (cases), 100 were non-tobacco users (control) and 10 were patients with oral squamous cell carcinomas. Cytological atypia was identified among 4 (4%). Toombak users and was not found among the control group (P<0.04). The micronuclei frequencies were higher in Toombak users (1.026) than in the control group (0.356) (P<0.0001). The mean AgNOR counts in Toombak users (2.423) were higher than control group (1.303) (P<0.0001). Neither Toombak users nor control group showed mitotic figures in 1% crystal violet method. The results of this research showed that Toombak dipping is a high risk factor for increase in the cellular proliferation in the oral mucosa. The cytological proliferative marker methods used are useful for screening Toombak users.
Key words: micronuclei, Toombak, crystal violet, AgNOR, oral cancer
Introduction
Worldwide, more than 500,000 new cases of oral cancer are diagnosed each year. It has been established that there is a relationship between tobacco use and the development of oral cancer.1 Each part of the oral cavity is susceptible to cancer from tobacco smoking or chewing (snuffing) including the lip, tongue, palate, gum and cheek. The mouth offers non-invasive and repetitive examinations compared with other body sites. Thirty to eighty percent of the malignancies of the oral cavity arise from pre-malignant lesions, such as leukoplakia, erythroplakia and oral sub mucous fibrosis.2,3 Toombak (snuff) was introduced into Sudan approximately 400 years ago. Tobacco is used for the manufacture of the Toombak. The use of Toombak has been reported to play a major role in the etiology of oral cancer in Sudan. It was also suspected to be associated with neoplasm of salivary glands. Toombak dippers develop a clinically and histologically characteristic lesion at the site of dipping. The risk for cancer of the oral cavity among Toombak users was high (relative risk, RR 7.3-73.0 fold). The use of Toombak plays a significant role in the etiology of oral squamous cell carcinomas (OSCCs). Specifically, nitrosamines present in Toombak possibly act as principal carcinogen.4
Different methods have been used to detect the cytological changes and cellular proliferation.
The buccal micronucleus assay is a minimally invasive method for studying DNA damage, chromosomal instability and cell death. Many studies showed more applications for nuccal micronucleus assay on cancer, neurodegenerative diseases, regenerative diseases of human buccal mucosal tissue, and other cellular changes associated with increased risk of accelerated aging.5-9 Argyrophilic nucleolar organizer region (AgNOR) is a silver staining procedure to observe the nucleolar organizer region (NOR) of the nucleus that is suitable for prognostic assessments. In AgNOR, the high affinity acidic proteins for silver such as RNA-binding acidic protein (nucleolin) which is present in nucleolus and NOR-associated proteins (NORAPs) are bind to silver molecules.10-13 Numerous experiments have shown that the number of AgNORs are significantly higher in malignant tumors than in physiological, reactive and benign processes.14-17
Crystal violet is a basic dye that has a high affinity for the highly acidic chromatin of mitotic cells. The 1% crystal violet provides more clear identification for mitotic figures at lower magnification compared to routine Hematoxylin and Eosin-stain.18
The purpose of this study was to not only assess cytological changes induced by Toombak use, but also to test the applicability of using simple, cost-effective, selective and specific cytological techniques in the assessment of cellular proliferative activity among Toombak dippers.
Materials and Methods
A total of 210 participants (mean age 33 years, range 16-94 years) were included in this study of whom 200 were apparently healthy individuals: 100 were Toombak users (cases), 100 were non-tobacco users (controls), and 10 were patients (internal control group) with oral squamous cell carcinoma (OSCC). Ethical consent was obtained from the ethical committee of the Faculty Research Board and the participants.
Four cytological smears were made for each participant using a flat sterile wooden tongue depressor. The surface epithelium of buccal mucosa was scraped mainly at the dip site and the materials were prepared in clean grease-free frosted glass slides. All smears were fixed immediately by conventional fixative for Papanicolaou (Pap) smears (95% ethyl alcohol) while they were wet for 15 min.
Cytological interpretation in Pap smear
The presence of two or more of the following features indicated the presence of epithelial atypia: nuclear enlargement associated with increased nuclear cytoplasmic ratio; hyperchromatism, chromatin clumping with moderately prominent nucleolation; irregular nuclear borders; bi or multinucleation, increased keratinization; scantiness of the cytoplasm and variations in size and or shape of the cells and nuclei. For each of these features, three possible grades were provided (mild, moderate, severe).19,20
Buccal cell micronuclei
Fixed smears were treated for 1 min each in 50 and 20% ethanol and then washed for 2 min in distilled water. Smears were treated in 5 M hydrochloric acid for 30 min and then washed in running tap water for 3 min. Smears were drained but not allowed to dry out before being treated in room temperature Schiff’s reagent in the dark for 60 min. Smears were washed in running tap water for 5 min and rinsed well in distilled water for 1 min. Smears were stained for 30 s under 0.5% light green and rinsed well in distilled water for 2 min. Smears were allowed to air-dry, cleared in Xylene and mounted in DPX. Nuclei and Micronucleus are stained magenta, while the cytoplasm appears green. Slides were scored using a light microscope at 400x magnification.5 Micronuclei were scored only in buccal with uniformly stained nuclei. Cells with condensed chromatin or karyorrhexis cells were not scored for micronucleis (MNs). A total of 1000 buccal cells were scored in order to determine the frequency of MNs in a total of 1000 cells.21,22
Argyrophilic nucleolar organizer region
The component was made up of 50% silver nitrate solution, 2% gelatin in 1% formic acid. Smears were hydrated in 70% alcohol for 2 min, rinsed in distilled water and then incubated in freshly prepared working solution (2:1) for 45 min in a dark and moist area, then washed in distilled water, 5% sodium thiosulphate, dehydrated in two changes of absolute alcohol, cleared in two changes of Xylene, and mounted with DPX medium. All stained smears were examined by light microscope 100x lens magnification for the AgNOR quantitation. The NOR mean counts measured by counting the number of silver-stained dots per 50 nucleus for every smear, then the number obtained divided by 50.11,23
Crystal violet stain
Fixed smears were stained with 1% crystal violet prepared from stock crystal violet (powder) by distilled water for 1 min, and then smears were blotted in filter papers and cleared in two changes of Xylene and mounted with DPX medium. Mitotic cells are stained magenta and stand out distinctly against a light blue background of resting cells. Criteria to identify the mitotic cells are: the nuclear membrane must be absent, there must be clear, hairy extensions of nuclear material and two parallel or clearly separate chromosome clots.18 Mitotic figures were observed by light microscope under 400x magnification. Any mitotic figures presented are counted per 1000 buccal cells.
Statistical analysis
SPSS software package was applied throughout the statistical analysis of results of this study. Pearson’s χ2 test for statistical significance (P value) with 95% confidence level and confidence intervals were used.
Results
This retrospective case-control cohort study assessed oral epithelial proliferative activity by different cytological methods in 210 study subjects (100 Toombak users, 100 non-tobacco users and 10 oral cancer patients as an internal control). Age ranged from 16 to 94 years (mean 33 years). Cytological atypia was identified among 4 (4%) of the Toombak users and not found in the control group P<0.04. Of the 4 cases with cytological atypia, only one case was identified with a moderate degree of cytological atypia and the remaining 3 were categorized as having a mild degree of cytological atypia (Table 1). All cases with cytological atypia were present in the over 36-year old age group who had been smoking for more than 21 years. Thirty-two (32%) Toombak users were identified with keratinization; therefore, only 2 subjects from the control group were identified with keratinization (Table 1). Toombak dipping is a major risk factor for the occurrence of the keratinization in the oral mucosa. This was found to be statistically significant P<0.0001 (Figure 1). With regard to infection, 21 (21%) and 4 (4%) of the Toombak users were identified with bacterial infection and moniliasis, respectively. Three (3%) of the control group was also identified with bacteria. The inflammation was present in 13 (13%) and two (2%) of Toombak users and controls, respectively. Toombak users were more susceptible to infections and inflammation than the control group (P<0.0001). The mean NOR counts for Toombak users (2423) was higher than for the control group (1.303) (P<0.001) (Figure 2). The micronuclei frequencies mean per 1000 buccal cells was higher in the cases group (1026) than control group (0.356) (P<0.001) (Table 1). The mean NOR count and micronuclei frequencies were found to increase in line with the duration of exposure and increase in the frequency of Toombak use (P<0.0001) (Table 2). The highest mean NOR count and micronuclei frequencies were associated with keratinization (P<0.0001) (Figure 3). All 4 cases showing cytological atypia had mean NOR count over 2.1 and micronuclei frequency over 1.00 (P<0.0001). Neither Toombak users nor the control group had mitotic figures in 1% crystal violet technique.
Table 1.
Distribution of the study population by presence of atypia, keratinization, inflammation, infection, mean nucleolar organizer region count and micronuclei frequency.
| Variable | Category | Toombak users | Control | P | RR | ||||
|---|---|---|---|---|---|---|---|---|---|
| N. | % | N. | % | ||||||
| Atypia | Mild | 3 | 3 | 0 | 0 | <0.04 | 4% | ||
| Moderate | 1 | 1 | 0 | 0 | |||||
| Absent | 96 | 96 | 100 | 100 | |||||
| Keratinization presence | Present | 32 | 32 | 2 | 2 | <0.0001 | 16% | ||
| Absent | 68 | 68 | 98 | 98 | |||||
| Inflammation presence | Present | 13 | 13 | 2 | 2 | ||||
| Absent | 87 | 87 | 98 | 98 | <0.003 | 6.5% | |||
| Infection presence | Absent | 75 | 75 | 97 | 97 | <0.0001 | 8.3% | ||
| Bacteria | 21 | 21 | 3 | 3 | |||||
| Monilia | 4 | 4 | 0 | 0 | |||||
| Mean NOR count | Mean | 2.423 | 1.303 | <0.001 | - | ||||
| Micronuclei frequency | Mean | 1.026 | 0.356 | <0.001 | - | ||||
RR, relative risk; NOR, nucleolar organizer region.
Figure 1.
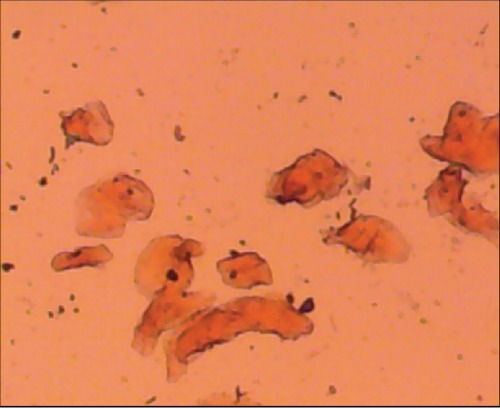
Photomicrograph of oral buccal cells from Toombak user dipping area showing the keratinization. Anucleated cells appeared in the field (Pap stain 100x).
Figure 2.
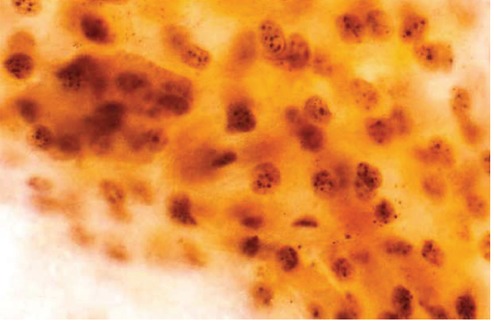
Photomicrograph of buccal cells from Toombak user showing more than 5 dots per nucleus (argyrophilic nucleolar organizer region stain 1000x).
Table 2.
The relationship between the argyrophilic nucleolar organizer region and mean micronuclei and duration, frequency of Toombak use.
| Variable | Duration/frequency | Mean AgNOR count | Mean micronuclei | P | |||
|---|---|---|---|---|---|---|---|
| <2 | 2.1+ | <1 | 1.1-2 | 2.1+ | |||
| Duration | <10 years | 25 | 19 | 37 | 7 | 0 | AgNOR <0.0001 |
| 11-20 years | 8 | 20 | 16 | 10 | 2MNI <0.0001 | ||
| >21 years | 2 | 26 | 6 | 10 | 12 | ||
| Frequency of Toombak use | <20 times/day | 14 | 4 | 17 | 1 | 0 | AgNOR <0.0001 |
| 21-30 | 9 | 27 | 23 | 5 | 8 | MNI <0.0001 | |
| 31-40 | 2 | 6 | 4 | 4 | 0 | ||
| 41+ | 10 | 28 | 15 | 17 | 6 | ||
AgNOR, argyrophilic nucleolar organizer region; MNI, mean micronuclei.
Figure 3.
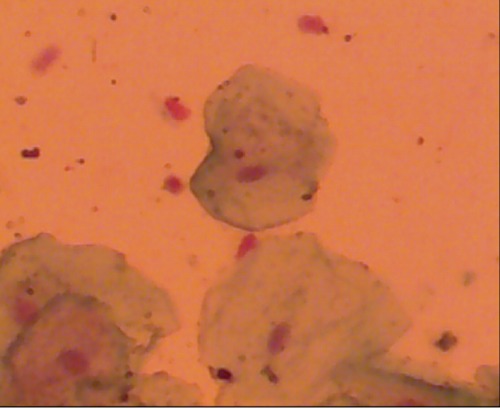
Photomicrograph of a buccal cell containing micronuclei from the dipping area (Fuelgen technique 100x).
Discussion
Oral cancer is one of the most common cancers worldwide. In Sudan, the use of Toombak plays a significant role in the etiology of OSCCs. Specifically, nitrosamines present in Toombak possibly act as principal carcinogen.4,24 For the screening and early detection of the pre-malignant and malignant lesions in high-risk groups, simple, reliable and cost-effective screening methods are urgently needed.25 In this study, the 4 cases found with cytological atypia (dyskaryosis) were among Toombak users. The use of Toombak was previously reported to induce cytological atypia and pre-malignant alterations of the oral cavity.26 Idris et al. found the high incidence of OSCCs and an equally high prevalence of potentially malignant oral mucosal lesions were strongly attributed to the habit of Toombak use in Sudan.27 Thirty-two (32%) subjects from the Toombak users in this study were identified with keratinization. Since keratinization with a nucleation may lead to pre-malignant lesion called leukoplakia, there is a strong association between cancers of the oral cavity and pharynx and Toombak use. The use of Toombak has shown to produce a variety of oral mucosal changes such as dysplasia and hyperkeratosis.28 Keratinization is directly proportionate to increase in the age of the Toombak users and the consequent prolonged exposure to Toombak use. With regard to infection and inflammatory conditions, Toombak users were more susceptible than controls, and the differences found were statistically significant (P<0.003). The erosions and exposure of the oral mucosa to the Toombak irritating substances are the major causative factors. The Toombak users had significantly higher AgNOR mean counts than the control group and this indicates there is an increase in cellular proliferation in Toombak dippers, a finding supported by a previous study.29 The duration, frequency and amount of Toombak use are directly proportionate to the AgNOR mean counts and this was found to be statistically significant. The AgNOR mean counts are increased with the presence of keratinization. Keratinization is a significant factor in the increased AgNOR counts. The inflammatory process can cause increases in the mean AgNOR counts of the Toombak users (P<0.006). A previous study also showed that a significant increase in AgNOR was observed in the linings of inflamed odontogenic keratocysts compared to non-inflamed lesions.30 Toombak users have significantly higher micronuclei frequency mean than the control group indicating that possible toxins in Toombak induce the micronuclei. This finding agrees with previous studies by Sellappa et al.31 and Ozkul et al.32 The micronuclei frequencies increase in line with an increase in the duration and frequency of Toombak use and this was found statistically significant (P<0.0001). Frequent use and prolonged exposure to Toombak are significant factors in the increase in micronuclei frequencies. Atypical changes in results of AgNOR, micronuclei and cytological examination show that cellular proliferation is significantly higher in Toombak users and that this might be attributed to the fact that production of a malignant cell requires cell proliferation and DNA activity. The mitotic figure was not observed in the Toombak users and control groups but was observed only in the internal control (oral cancer patients). This finding is supported by Ankle and Kale.18
Conclusions
Cytochemical methods such as micronuclei frequency and AgNOR counts are simple and cost-effective methods highly recommended for the assessment of cellular proliferative activity. Oral exfoliative cytology using Pap smear is useful in evaluation of epithelial atypia that is frequently encountered in pre-malignant and early malignant oral lesions. There is a need to standardize the cytological proliferative marker methods to provide rapid, inexpensive, quantitative, reproducible, technologically simple, and applicable monitoring and screening procedures for subjects who have been identified as being at high risk for developing oral cancer.
Acknowledgments
Our special thanks and appreciation to all those who helped us through their assistance and guidance in performing our research. We would like to thank the staff at the Department of Histopathology and Cytology Faculty of Medical Laboratory Sciences, Alneelain University and the staff of the research laboratory at Sudan University of Science and Technology. All sincere thanks to our colleagues at the Khartoum Teaching Hospital for their excellent assistance.
References
- 1.Stewart BW, Kleihues P.World cancer report. Lyon: IARC Press; 2008; p 3251 [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P.Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108 [DOI] [PubMed] [Google Scholar]
- 3.Taybos G.Oral changes associated with tobacco use. Am J Med Sci 2003;326:179-82 [DOI] [PubMed] [Google Scholar]
- 4.Ahmed HG, Mahgoob RM.Impact of Toombak dipping in the etiology of oral cancer: Gender-exclusive hazard in the Sudan. J Can Res Ther 2007;3:127-30 [DOI] [PubMed] [Google Scholar]
- 5.Thomas P, Harvey S, Gruner T, Fenech M.The buccal cytome and micronucleus frequency is substantially altered in Down’s syndrome and normal ageing compared to young healthy controls. Mutat Res 2007;638:37-47 [DOI] [PubMed] [Google Scholar]
- 6.Stich HF, Curtis JR, Parida BB.Application of the micronucleus test to exfoliated cells of high cancer risk groups: tobacco chewers. Int J Cancer 1982;30:553-9 [DOI] [PubMed] [Google Scholar]
- 7.Hazare VK, Goel RR, Gupta PC.Oral sub-mucous fibrosis, areca nut and pan masala use: a case-control study. Natl Med J India 1998;11:299. [PubMed] [Google Scholar]
- 8.Nersesyan A, Kundi M, Atefie K, et al. Effect of staining procedures on the results of micronucleus assays with exfoliated oral mucosa cells. Cancer Epidemiol Biomarkers Prev 2006;15:1835-40 [DOI] [PubMed] [Google Scholar]
- 9.Tolbert PE, Shy CM, Allen JW.Micronuclei and other nuclear anomalies in buccal smears: methods development. Mutat Res 1992;271:69-77 [DOI] [PubMed] [Google Scholar]
- 10.Boldy D, Crocker J, Ayres GJ.Application of the AgNOR method to cell imprints of lymphoid tissue. J Pathol 1989;157:75-9 [DOI] [PubMed] [Google Scholar]
- 11.Colecchia M, Leopardi O.Evaluation of AgNOR count in distinguishing benign from malignant mesothelial cells in pleural fluids. Pathol Res Pract 1992;166:53-60 [DOI] [PubMed] [Google Scholar]
- 12.Vajdovich P, Psáder R, Tóth ZA, Perge E.Use of the argyrophilic nucleolar region method for cytologic and histologic examination of the lymph nodes in dogs. Vet Pathol 2004;41:338-45 [DOI] [PubMed] [Google Scholar]
- 13.Mamaev NN, Medvedeva NV, Shust VF, et al. Nucleoli and AgNORs in Hodgkin’s disease. J Clin Pathol Mol Pathol 1997;50:149-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pillai KR, Sujathan K, Madhavan J, Abraham EK.Significance of silver-stained nucleolar organizer regions in early diagnosis and prognosis of oral squamous cell carcinoma: amultivariate analysis. In Vivo 2005;19:807-12 [PubMed] [Google Scholar]
- 15.Luthere E, Lindner Improvements in the silver-staining technique for nucleolar organizer regions (AgNOR). J Histochem Cytochem 1993;41:439-45 [DOI] [PubMed] [Google Scholar]
- 16.Torsten W, Remmerbach, Weidenbach H, et al. Diagnostic value of nucleolar organizer regions (AgNORs) in brush biopsies of suspicious lesions of the oral cavity. Anal Cell Pathol 2003;25:139-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ploton D, Menager M, Jeannersson P, et al. Improvement in the staining and in the visualization of the argyrophilic proteins of the nucleolar organizer region of the optical level. Histochem J 1986;18:5-14 [DOI] [PubMed] [Google Scholar]
- 18.Ankle MR, Kale AD, Charantimath S, Charantimath S.Comparison of staining of mitotic figures by haematoxylin and eosin-and crystal violet stains, in oral epithelial dysplasia and squamous cell carcinoma. Indian J Dent Res 2007;18:101-5 [DOI] [PubMed] [Google Scholar]
- 19.Koss LG.Diagnostic cytology and its histopathologic bases. 5th edition London: JB Lippincott Company; 2005. pp 716-720 [Google Scholar]
- 20.Bancroft J, Gamble D, MarilynTheory and practice of histological techniques. 5th ed.London: Churchill Livingstone; 2002. pp 523-524 [Google Scholar]
- 21.Holland N, Bolognesi C, Kirsch-Volders M, et al. The micronucleus assay in human buccal cells as a tool for biomonitoring DNA damage: the HUMN project perspective on current status and knowledge gaps. Mutat Res 2008;659:93-108 [DOI] [PubMed] [Google Scholar]
- 22.Casartelli G, Bonatti S, De Ferrari M, et al. Micronucleus frequencies in exfoliated buccal cells in normal mucosa, precancerous lesions and squamous cell carcinoma. Anal Quant Cytol Histol 2000;22:486-92 [PubMed] [Google Scholar]
- 23.Cabrini RL, Schwint AE, Mendez A, et al. Morphometric study of nucleolar organizer regions in human oral normal mucosa, papilloma and squamous cell carcinoma. J Oral Pathol Med 1992;21:275-9 [DOI] [PubMed] [Google Scholar]
- 24.Elbeshir EI, Abeen HA, Idris AM, Abbas K.Snuff dipping and oral cancer in Sudan: a retrospective study. Br J Oral Maxillofac Surg 1998;27:243-8 [DOI] [PubMed] [Google Scholar]
- 25.Shulman JD, Beach MM, Rivera-Hidalgo F.The prevalence of oral mucosal lesions in U.S. adults: data from the Third National Health and Nutrition Examination Survey 1988-1994. J Am Dent Assoc 2004;135:1279-86 [DOI] [PubMed] [Google Scholar]
- 26.Ahmed HG, Idris A M, Ibrahim SO.Study of oral epithelial atypia among Sudanese tobacco users by exfoliative cytology. Anticanc Res 2003;23:1943-5 [PubMed] [Google Scholar]
- 27.Idris M, Ahmed HM, Mukhtar BI, et al. Descriptive epidemiology of oral neoplasms in sudan 1970-1985 and the role of toombak. Int J Cancer 1995;61:155-8 [DOI] [PubMed] [Google Scholar]
- 28.Idris AM, Warnakulasuriya KAAS, Ibrahim YE, et al. Toombak-associated oral mucosal lesions in Sudanese show a low prevalence of epithelial dysplasia. J Oral Pathol Med 1996;25:5. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed HG, Babiker AA.Assessment of cytological atypia, AgNOR and nuclear area in epithelial cells of normal oral mucosa exposed to toombak and smoking. Rare Tumors 2009;1:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Paula AMB, Carvalhais JN, Domingues MG, et al. Cell proliferation markers in the odontogenic keratocyst: effect of inflammation. J Oral Pathol Med 2000;29:477-82 [DOI] [PubMed] [Google Scholar]
- 31.Ozkul Y, Donmez H, Erenmemisoglu A, et al. Induction of micronuclei by smokeless tobacco on buccal mucosa cells of habitual users. Mutagenesis 1997;12:285-7 [DOI] [PubMed] [Google Scholar]
- 32.Sellappa S, Balakrishnan M, Raman S, Palanisamy S.Induction of micronuclei in buccal mucosa on chewing a mixture of betel leaf, areca nut and tobacco. Oral Sci 2009;51:289-92 [DOI] [PubMed] [Google Scholar]


