Abstract
The role of chemotherapy in well differentiated neuroendocrine tumors (NET) has been questioned. It was recently demonstrated that everolimus and sunitinib have activity in low and intermediate grade pancreatic NET. The aim of this study was to evaluate the activity of capecitabine and oxaliplatin (CapOx) combination in treating NET in an unselected population. In this regard, we retrospectively evaluated 24 patients diagnosed with metastatic NET treated with CapOx at two Brazilian institutes that are reference centers in cancer care. Tumor response was measured by RECIST criteria. Median age at diagnosis was 56 years, 71% had ECOG 0 or 1, the majority of tumors were primary from pancreas (67%) followed by lung (17%), and 29% were functional. According to WHO classification criteria, 25% were grade 1, 37.5% grade 2 and 37.5% grade 3. Most patients received CapOx as second-line therapy, with a median of 6 cycles. Twenty-nine percent of patients had partial response by RECIST criteria. No association was observed between response rate and tumor grade, primary site or line of CapOx. The median time to progression was 9.8 months and median time to treatment failure was 12.1 months. Seventy-five percent of patients are alive at the time of this analysis; therefore, median overall survival was not reached. The CapOx combination was shown to be active in an unselected population with metastatic NET and may be a good platform for the incorporation of the newer molecular targeted agents being investigated for the treatment of this entity.
Key words: neuroendocrine tumors, chemotherapy, capecitabine, oxaliplatin
Introduction
Neuroendocrine tumors (NET) make up a broad spectrum of malignancies with different biological behavior, varying from indolent to highly proliferative and aggressive disease. They can arise from neuroendocrine cells throughout the body and may produce active peptides that cause hormonal syndromes.1
According to the SEER database, the incidence and prevalence of NET has increased substantially over the past 30 years, which may in part reflect the availability of better diagnostic techniques, advances in immunohistochemistry, new diagnostic biomarkers and a better understanding of this entity.1,2
Approximately 30% of patients present when symptoms become evident and are found to have distant metastatic disease. Prognostic factors include differentiation, grade, disease stage, and primary site.2 According to the recently published WHO grading system based on mitotic rate and Ki67 index, NET can be classified as: grade 1, <2 mitoses per 2 mm2 and/or Ki-67 index ≤2%; grade 2, 2-20 mitoses per 2 mm2 and/or Ki-67 index between 3% (intended as >2%) and 20%; or grade 3 with >20 mitoses per 2 mm2 and/or Ki-67 index >20%.3 The median overall survival (OS) of patients with metastatic NET is nearly three years for grade 1 tumors, approximately twenty-four months for grade 2, and in the range of ten months for grade 3.2
Due to the heterogeneity of disease biology, there is no standard approach to the management of metastatic NET. Therapeutic options include cytoreductive surgery, somatostatin analogs, peptide receptor radionuclide therapy (PRRT), liver-directed therapy such as arterial embolization, chemoembolization, radiofrequency ablation or cryotherapy and cytotoxic therapy.4 Biologics and chemotherapy agents that have been mostly evaluated in NET include interferon (IFN) associated with somatostatin analogs, temozolomide associated with thalidomide or capecitabine, dacarbazine, bevacizumab and fluorouracil. However, the only FDA approved cytotoxic combination is the fluorouracil, adriamicin and streptozotocin (FAS) regimen that demonstrated some activity in grade 1 or 2 pancreatic NET.5-7 For grade 3 disease, chemotherapy combinations based on cisplatin or carboplatin associated with etoposide (VP-16) or irinotecan (CPT-11) have been used in analogy to small cell carcinoma of the lung.8 Recently, sunitinib and everolimus have been approved for the treatment of grade 1 or 2 pancreatic NET, showing increased progression free survival (PFS) when compared to placebo.9,10
The majority of patients with metastatic NET are not curable and will eventually need chemotherapy in the course of their disease, either by presenting as an aggressive, poorly differentiated tumor or by transformation of a previous indolent, well differentiated neoplasia into a more aggressive carcinoma. Considering the few effective systemic therapeutic options available for this emerging population, and based on previous data showing encouraging results of the capecitabine and oxaliplatin combination in the metastatic NET scenario, we aimed to evaluate the activity of capecitabine and oxaliplatin (CapOx) in treating unselected patients with metastatic NET.11
Materials and Methods
Patients
We retrospectively evaluated 24 patients diagnosed with metastatic NET treated with CapOx at two Brazilian institutions: Hospital SírioLibanês (HSL) and Instituto do Câncer do Estado de São Paulo, Faculdade de Medicina, Universidade de São Paulo (ICESP) between November 2003 and February 2011. Approval for data collection and analysis was obtained from each institutional ethics committee. Patients were eligible if they had biopsy proven metastatic NET with measurable disease on computed tomography (CT) or magnetic resonance imaging (MRI), pathology description of the proliferative Ki67 index determined by immunostaining, had radiological evidence of disease progression and had received at least two cycles of CapOx.
Chemotherapy
The regimen consisted of oral capecitabine 2000 mg/m2 starting on Day 1 for 14 consecutive days, and intravenous 100 to 130 mg/m2 bolus oxaliplatin on Day 1, repeated every three weeks. Full blood counts and biochemistry were obtained before each chemotherapy cycle in all patients. Dose reductions were performed if grade 3 or 4 toxicity was observed. Toxicity information was collected retrospectively from the medical notes and classified according to the National Cancer Institute Common Toxicity Criteria Version 4.0. Only grade 2 or more adverse events reported in the patient chart were considered for analysis. Chemotherapy was continued until disease progression, unacceptable toxicity or patient intolerance.
Evaluation of tumor response
All patients were evaluated for response by complete physical examination and imaging (CT or MRI). The images were reviewed by the same radiologist (LTS). Tumor assessment was performed every 2 or 3 cycles of chemotherapy and toxicity was reported at each cycle as part of standard-of-care. Response to treatment was evaluated using the Response Evaluation Criteria In Solid Tumors (RECIST version 1.0):12 complete response (CR) was defined as the disappearance of all lesions, partial response (PR) was defined as at least a 30% reduction in the tumor load, progressive disease (PD) was defined as at least a 20% increase in the tumor load, and stable disease (SD) was defined as disease that showed neither sufficient shrinkage nor increase to qualify as PR or PD.
Statistical analysis
Descriptive statistics were used to report continuous and categorical variables. Time to event variables were analyzed by the Kaplan Meir method. Time to tumor progression (TTP) was defined as the time between the first dose of CapOx and documentation of radiological progression or death. Losses to follow-up were censored. We also calculated the time to treatment failure (TTF) which measured the interval from the first dose of Capox to the first dose of another systemic treatment. This end point was chosen as clinically valuable because some patients achieved long-term stable disease with CapOx and were re-challenged later with the same regimen. Median overall survival was not reached at the cut-off date for analysis (February 20th, 2011). The comparisons between response rate and tumor grade, primary site and line of CapOx were based on χ2 and Fisher’s exact tests, as appropriate. P<0.05 was considered statistically significant, after adjusting for multiple comparisons with the Bonferroni method. All analyses were carried out as intention-to-treat.
Results
We identified 32 patients with metastatic NET who were treated with CapOx. Out of the 32 patients, 24 (17 male and 7 female) met eligibility criteria. Four patients did not have images available for radiology review and 4 patients received one or two cycles of CapOx, and could not be evaluated for response at the cut-off date for this analysis.
The median age at the time of diagnosis was 56 years (range 23 to 73 years) and 71% of patients had ECOG performance status 0 or 1. The most common primary tumor site was pancreas (n=15, 63%), followed by lung (n=4, 17%). Liver was the main site of metastases (96%). Seven patients (29%) had functional syndrome: 1 VIPoma, 2 insulinomas and 4 had carcinoid syndrome. The majority of patients had grade 2 and 3 NET. Patient’s characteristics are listed in Table 1.
Table 1.
Patients characteristics.
| N. | % | |
|---|---|---|
| Sex | ||
| Male | 17 | 71 |
| Female | 7 | 29 |
| Performance status | ||
| ECOG 0 | 5 | 21 |
| ECOG 1 | 12 | 50 |
| ECOG ≥ 2 | 7 | 29 |
| Primary site | ||
| Pancreas | 15 | 63 |
| Lung | 4 | 17 |
| Small intestine | 2 | 8 |
| Unknown | 2 | 8 |
| Rectum | 1 | 4 |
| Functional syndrome | ||
| Carcinoid syndrome | 4 | 17 |
| Insulinoma | 2 | 8 |
| VIPoma | 1 | 4 |
| Grade | ||
| Grade 1 | 6 | 25 |
| Grade 2 | 9 | 37.50 |
| Grade 3 | 9 | 37.50 |
| Metastatic site | ||
| Liver | 23 | 96 |
| Lymph nodes | 18 | 75 |
| Peritoneum | 6 | 25 |
| Bone | 4 | 17 |
| Others | 5 | 21 |
Treatment
Ten patients (42%) had been previously treated locally: 7 patients underwent surgery (30%), 2 underwent hepatic artery embolization (8%) and one received chemoembolization (4%). CapOx was used as first-line therapy in 50% of patients. The median number of cycles was 6 (range 2 to 13). Out of the 24 patients treated with CapOx, 7 were kept in maintenance treatment after maximum response: 4 received capecitabine monotherapy, 2 everolimus and 1 somatostatin analog. Treatment characteristics are listed in Table 2.
Table 2.
Treatment charachteristics.
| N. | % | |
|---|---|---|
| Local treatment pre-CapOx | ||
| Surgery (primary site) | 1 | 4 |
| Metastasectomy (hepatic resection) | 6 | 25 |
| Embolization | 2 | 8 |
| Chemoembolization | 1 | 4 |
| Systemic treatment other than CapOx | ||
| Somatostatin analogs +/- INF | 11 | 46 |
| Cisplatin or carboplatin + VP-16 or CPT-11 | 8 | 33 |
| Capecitabine | 5 | 21 |
| Lutecium radioligand therapy | 5 | 21 |
| Everolimus | 5 | 21 |
| Others | 6 | 25 |
| Cisplatin or carboplatin + taxane | 2 | 8 |
| 5-FU + adriamicin + streptozotocin (FAS) | 2 | 8 |
| Line of CapOx | ||
| First | 12 | 50 |
| Second | 6 | 25 |
| Third or beyond | 6 | 25 |
| Maintenance treatment post-CapOx | ||
| Capecitabine | 4 | 17 |
| Everolimus | 2 | 8 |
| Somatostatin analogs | 1 | 4 |
Median n. cycles of CapOx 6 (range 2-13). INF, interferon; VP-16, cisplatin or carboplatin associated with etoposide; CPT-11, irinotecan; 5-FU, fluorouracil; FAS, fluorouracil + adriamicin + streptozotocin.
The CapOx regimen was generally well tolerated. Six patients (25%) experienced grade 3 toxicity and 15 patients (63%) had grade 2 toxicity, with the most common being peripheral neuropathy, hand-foot syndrome and diarrhea. Dose reduction was necessary in 8 patients (33%). Only one patient discontinued treatment due to side effects. There was no hospitalization or death associated to treatment. Toxicity profile associated with the CapOx regimen is described in Table 3.
Table 3.
Grade 2 and 3 toxicities associated with the CapOx regimen (total n. patients=24).
| Grade 2 (N) | % | Grade 3 (N) | % | Total n. | % of total | |
|---|---|---|---|---|---|---|
| Overall toxicity | ||||||
| Grade 2 | 15 | 63 | ||||
| Grade 3 | 6 | 25 | ||||
| CapOx dose reduction | 8 | 33 | ||||
| Hospitalizations | ||||||
| Disease-related | 4 | 17 | ||||
| Treatment-related | 0 | 0 | ||||
| Infection (not due to neutropenia) | 3 | 13 | ||||
| Main reported toxicities | ||||||
| Neuropathy | 7 | 29 | 2 | 8 | 9 | 37 |
| Hand-foot syndrome | 3 | 13 | 1 | 4 | 4 | 17 |
| Diarrhea | 1 | 4 | 2 | 8 | 3 | 12 |
| Hematologic toxicity (neutropenia) | 1 | 4 | 0 | 0 | 1 | 4 |
| Nausea | 2 | 8 | 0 | 0 | 2 | 8 |
| Asthenia | 1 | 4 | 0 | 0 | 1 | 4 |
| Oxaliplatin hypersensibility reaction | 0 | 0 | 1 | 4 | 1 | 4 |
Clinical outcomes
Out of the 24 patients who were available for analysis, 7 (29%) achieved a partial response (PR). The waterfall plot demonstrating the percentage of tumor size change following CapOx treatment is shown in Figure 1. Stable disease (SD) was the best response achieved by 17 (71%) patients. There was no significant statistical association between RR and either tumor grade, primary site or line of CapOx administration. Median TTP was 9.8 months (range 7.4 to 12.2 months; 8 patients censored) and median TTF was 12.1 months (range 6.9 to 17.2 months; 11 patients censored). Only 6 patients had died until the cutoff date for analysis, so median OS was not reached. Kaplan-Meier curves showing TTP and TTF are presented in Figures 2 and 3, respectively.
Figure 1.
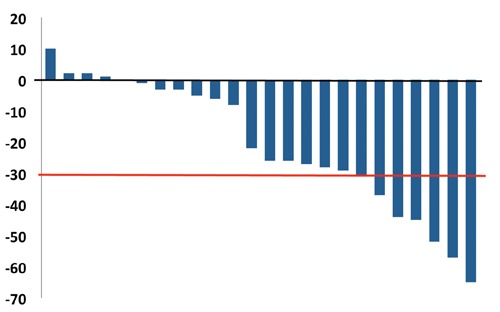
Waterfall plot demonstrating the percentage variation of tumor lesion size. Red line indicates 30% shrinkage (Partial response by RECIST criteria).
Figure 2.
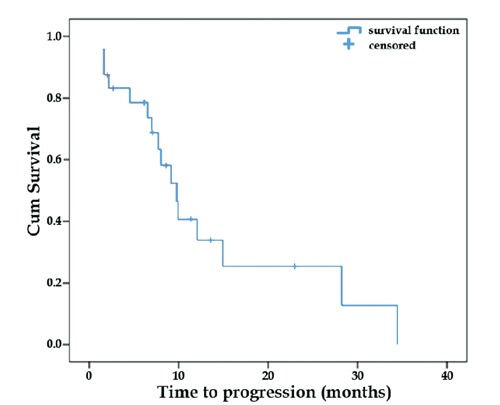
Time to tumor progression (TTP). Blue line demonstrates median ATP of 9.8 m (range 7.4-12.2 m). N. progressions=16; patients censored=8.
Figure 3.
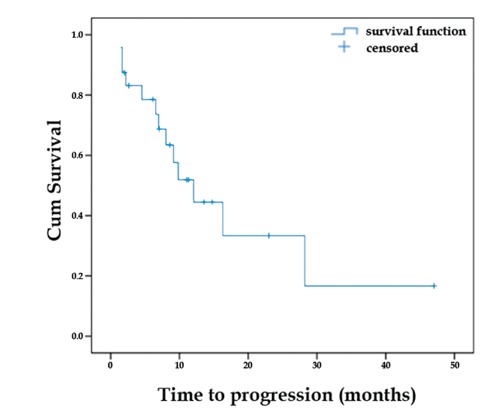
Time to treatment failure (TTF). Blue line demonstrates median TTP of 12.1 m (range 6.9-17.2 m). N. patients who failed CapOx=13; patients censored=11.
Discussion
Our analysis including 24 unselected patients with advanced NET treated with CapOx demonstrated that 29% achieved PR with this chemotherapy regimen. The TTP of 9.8 months and TTF of 12.1 months is compatible with an active regimen and is comparable with the current available therapeutic options.7,9,13,14
Somatostatin analogs alone or in combination with IFN can be used to manage hormonal symptoms, and it has recently also been shown to improve PFS in well differentiated, low volume midgut NET, but rarely results in tumor shrinkage.15,16 For more advanced grade 2 and 3 tumors, somatostatin analogs offer limited antitumor effect. Combinations including streptozotocin with fluorouracil and doxorubicin (FAS) have yielded variable results with RR varying from 6% to 39% for pancreatic NET, depending on the method used for response assessment.5,14,17 Dacarbazine has shown some degree of activity, with RR between 8% and 15% in carcinoid tumors and 33% in pancreatic islet cell carcinoma. However, concerns about toxicity have precluded its widespread use.18,19 The combination of temozolomide and thalidomide in 29 patients with metastatic NET demonstrated an objective RR of 25% and better toxicity profile than dacarbazine alone.20 More recently, retrospective data has shown promising activity of the combination of temozolomide and capecitabine in first-line treatment of G1 and G2 pancreatic NET, yielding a RR of 70% (21 in 30 patients) and a median PFS of 18 months, making the temozolomide combinations an attractive option for the treatment of NET.21 Other drugs combinations as bevacizumab and pegylated interferon alfa-2b or gemcitabine and oxaliplatin have shown RR of approximately 15%.7,13 Single agents such as docetaxel, paclitaxel, gemcitabine and pemetrexede have shown little, if any, activity in the metastatic NET scenario (RR 0-8%) and are not routinely recommended in clinical practice.22-25
In spite of the heterogeneous results obtained with the FAS regimen, it is still the most accepted chemotherapy combination for pancreatic NET. Streptozotocin is not commercially available in Brazil and is fairly toxic, which limits its use. In our study, the CapOx combination evaluated in 15 patients with pancreatic primary tumors resulted in 4 PR according to RECIST criteria (26.7%), which is comparable to the results of the FAS regimen.
The activity of the CapOx combination has been previously evaluated in a phase II Italian study including 40 patients with advanced NET.11 The population consisted of 13 poorly differentiated and 27 well-differentiated NET patients whose tumors had progressed after somatostatin analogs. The RR, evaluated according to the WHO criteria (decrease in 50% tumor burden),26 symptoms and changes in tumor marker, was 23% in the high-grade and 30% in the low-grade populations with a median TTP of 18 months and OS of 32 months. The authors concluded that while CapOx may be a good option for well-differentiated NET, it may not be a good choice for first-line treatment of high-grade NET when compared to historical data of patients treated with cisplatin and VP-16. However, the RR achieved with cisplatin and VP-16 combination has been inconsistent, varying according to the series between 14% and 67%.27,28 Our study included 9 patients with grade 3 NET: 6 received CapOx as first-line and 3 as second or further lines, with a median of 8 cycles. We observed 2 PR and 2 additional patients had at least 25% shrinkage in tumor load, suggesting CapOx may also be a salvage therapeutic option for poorly differentiated NET.
Another fluoropyrimidine-based regimen, FOLFOX (5-fluorouracil, leucovorin and oxaliplatin) associated with bevacizumab, was prospectively evaluated by Venook et al. in 13 patients with metastatic NET,29 showing similar results to the CapOx combination (RR of 20% for carcinoid tumors and 33% for pancreatic NET). However, significant toxicity associated with bevacizumab was observed, with one arterial thromboembolic event and one death due to intestinal perforation. Sunitinib, a multi-targeted tyrosine kinase inhibitor, and everolimus, an inhibitor of mammalian target of rapamycin (mTOR), were recently approved for treatment of low or intermediate grade pancreatic NET, showing a RR of approximately 10% and a median PFS of 11 months, which is comparable to our results. These targeted agents were tested in a population that had previously received local treatment or systemic therapy, making these drugs good options for patients who progress after chemotherapy, or as front-line therapy for asymptomatic patients with low disease burden.9,10
Considering the available options, our results demonstrate that the CapOx combination has promising activity in metastatic NET. This study is limited by its retrospective nature, the long period of patient recruitment and recognition that the data is still immature, with a high number of patients being censored and median OS not reached. In spite of this, the results are comparable with the published trial evaluating the same drug combination and has shown similar activity to the FAS and the temozolomide-based regimens.11
Conclusions
Metastatic NET is an heterogeneous and deadly disease, and due to the small patient population, data from large randomized trials are scarce. The CapOx regimen has shown to be active and well tolerated in an unselected population with metastatic NET and may be a good platform for the incorporation of the newer molecular targeted agents being investigated for the treatment of these tumors. Despite the need for longer follow up to better assess outcomes; the presented data support the inclusion of CapOx as an option in the management of metastatic NET.
Acknowledgments
Special thanks to the patients who participated in the study and their families. Contributions: RF and LT elaborated the spreadsheet and collected patient data; MS collected patient image studies for re-evaluation; LTS reviewed all images and evaluated response according to RECIST criteria; RPR performed the statistical analysis; RF reviewed the literature and wrote the manuscript; RPR and FPC performed the first review; PMH conceived the study and reviewed the final version.
References
- 1.Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 2008;9:61-72 [DOI] [PubMed] [Google Scholar]
- 2.Yao JC, Hassan M, Phan A, et al. One Hundred years after carcinoid: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72 [DOI] [PubMed] [Google Scholar]
- 3.Rindi G.The ENETS guidelines: the new TNM classification system. Tumori 2010;96:806-9 [DOI] [PubMed] [Google Scholar]
- 4.Fazio N, Cinieri S, Lorizzo K, et al. Biological targeted therapies in patients with advanced enteropancreatic neuroendocrine carcinomas. Cancer Treat Rev 2010;36:S87-S94 [DOI] [PubMed] [Google Scholar]
- 5.Kouvaraki MA, Ajani JA, Hoff P, et al. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol 2004;22:4762-71 [DOI] [PubMed] [Google Scholar]
- 6.Rivera E, Ajani JA.Doxorubicin, streptozocin, and 5-fluorouracil chemotherapy for patients with metastatic islet-cell carcinoma. Am J Clin Oncol 1998;21:36-8 [DOI] [PubMed] [Google Scholar]
- 7.Yao JC, Phan A, Hoff PM, et al. Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alfa-2b. J Clin Oncol 2008;26:1316-23 [DOI] [PubMed] [Google Scholar]
- 8.Strosberg JR, Coppola D, Klimstra DS, et al. The NANETS consensus guidelines for the diagnosis and management of poorly differentiated (high-grade) extrapulmonary neuroendocrine carcinomas. Pancreas 2010;39:799-800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raymond E, Dahan L, Raoul J-L, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. NEJM 2011;364:501-13 [DOI] [PubMed] [Google Scholar]
- 10.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. NEJM 2011;364:514-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bajetta E, Catena L, Procopio G, et al. Are capecitabine and oxaliplatin (XELOX) suitable treatments for progressing low-grade and high-grade neuroendocrine tumours? Cancer Chemother Pharmacol 2007;59:637-42 [DOI] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 2000;92:205-16 [DOI] [PubMed] [Google Scholar]
- 13.Cassier PA, Walter T, Eymard B, et al. Gemcitabine and oxaliplatin combination chemotherapy for metastatic well-differentiated neuroendocrine carcinomas. Cancer 2009;11:3392-9 [DOI] [PubMed] [Google Scholar]
- 14.McCollum AD, Kulke MH, Ryan DP, et al. Lack of Efficacy of streptozocin and doxorubicin in patients with advanced pancreatic endocrine tumors. Am J Clin Oncol 2004;27:485-8 [DOI] [PubMed] [Google Scholar]
- 15.Faiss S, Pape U-F, Böhmig M, et al. Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroen docrine gastroenteropancreatic tumors- The international lanreotide and interferon alfa study group. J Clin Oncol 2003;21:2689-96 [DOI] [PubMed] [Google Scholar]
- 16.Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID study group. J Clin Oncol 2009;27:4656-63 [DOI] [PubMed] [Google Scholar]
- 17.Cheng PNM, Saltz LB.Failure to confirm major objective antitumor activity for streptozocin and doxorubicin in the treatment of patients with advanced islet cell carcinoma. Cancer 1999;86:944-8 [PubMed] [Google Scholar]
- 18.Sun W, Lipsitz S, Catalano P, et al. Phase II/III study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: eastern cooperative oncology group study E1281. J Clin Oncol 2005;23:4897-904 [DOI] [PubMed] [Google Scholar]
- 19.Ramanathan RK, Cnaan A, Hahn RG, et al. Phase II trial of dacarbazine (DTIC) in advanced pancreatic islet cell carcinoma. Study of the Eastern Cooperative Oncology Group-E6282. Ann Oncol 2001;12:1139-43 [DOI] [PubMed] [Google Scholar]
- 20.Kulke MH, Stuart K, Enzinger PC, et al. Phase II study of temozolomide and thalidomide in patients with metastatic neuroendocrine tumors. J Clin Oncol 2006;24:401-6 [DOI] [PubMed] [Google Scholar]
- 21.Strosberg JR, Fine RL, Choi J, et al. Firstline chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011;117:268-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ansell SM, Pitot HC, Burch PA, et al. A Phase II study of high-dose paclitaxel in patients with advanced neuroendocrine tumors. Cancer 2001;91:1543-8 [DOI] [PubMed] [Google Scholar]
- 23.Kulke MH, Kim H, Stuart K, et al. A Phase II study of docetaxel in patients with metastatic carcinoid tumors. Cancer Invest 2004;22:353-9 [DOI] [PubMed] [Google Scholar]
- 24.Kulke MH, Kim H, Clark JW, et al. A Phase II trial of gemcitabine for metastatic neuroendocrine tumors. Cancer 2004;101:934-9 [DOI] [PubMed] [Google Scholar]
- 25.Chan J, Zhu A, Stuart K, et al. Phase II study of pemetrexed in patients with advanced neuroendocrine tumors. Cancer Chemother Pharmacol 2010;66:961-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller AB, Hoogstraten B, Staquet M, Winkler A.Reporting results of cancer treatment. Cancer 1981;47:207-14 [DOI] [PubMed] [Google Scholar]
- 27.Moertel CG, Kvols LK, O’Connell MJ, Rubin J.Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer 1991;68:227-32 [DOI] [PubMed] [Google Scholar]
- 28.Kulke M, Wu B, Ryan D, et al. A Phase II trial of irinotecan and cisplatin in patients with metastatic neuroendocrine tumors. Digest Dis Sci 2006;51:1033-8 [DOI] [PubMed] [Google Scholar]
- 29.Venook AP, Ko AH, Tempero MA, et al. Phase II trial of FOLFOX plus bevacizumab in advanced, progressive neuroendocrine tumors. ASCO Meeting 2008;26:15545 [Google Scholar]


