Abstract
Extrapulmonary poorly differentiated neuroendocrine carcinoma (PDNEC) is a rare and highly aggressive neoplasm for which the optimal chemotherapy remains unclear. The objective of this study was to evaluate the outcomes of patients with PDNEC treated with cisplatin and irinotecan (IP) and perform a review of the literature. From 2008 to 2012, patients with advanced PDNEC (Ki67≥20%) who received the IP combination were selected for analysis. Radiologic responses were determined through Response Evaluation Criteria In Solid Tumors criteria. Twenty-eight patients were included. The median age at diagnosis was 57 years and the most common presentation was pancreatic PDNEC. Twenty-five patients (89%) received chemotherapy with cisplatin and irinotecan and three received carboplatin and irinotecan. Forty-six percent of the patients achieved objective response and the median time to tumor progression was 3.7 months. The median overall survival was 11.7 months. Thirteen patients (46%) had treatment interruptions or dose reductions due to grade 3/4 toxicity. This retrospective cohort of advanced extrapulmonary PDNEC patients suggests that the IP combination is feasible and resulted in similar response rate and median survival to other treatments previously reported.
Key words: neuroendocrine carcinoma, neuroendocrine tumors, cisplatin, irinotecan
Introduction
Extrapulmonary neuroendocrine tumors (EPNET) encompass a wide group of neoplasms with heterogeneous characteristics defined by the presence of cells with secretory granules and the capability of producing polypeptide hormones and biologically active agents. The spectrum of these tumors range from well differentiated subtypes with little potential for metastatization and low mitotic rate, to highly aggressive lesions. High grade neoplasms have been recently classified as poorly differentiated neuroendocrine carcinomas (PDNEC),1,2 based on morphology and immunohistochemistry staining for Ki67 in more than 20% of the cells in the area of highest nuclear labeling. PDNEC represent a rare entity, with approximately 1000 new cases per year in the United States, and, despite the recent advances, still associated with limited prognosis and extremely low cure rates.2-4 Albeit other primary sites were reported, 35-55% of all extra-pulmonary NEC arise from the gastrointestinal tract and are metastatic at the time of diagnosis.3-6
In contrast to well-differentiated tumors, patients with advanced PDNEC often respond to cytotoxic chemotherapy, and diverse treatment protocols have been evaluated in this setting. However, due to its rarity and shifting classification, the lack of large-scale prospective trials has limited the definition of a gold standard chemotherapy schedule. Based on the similarity, both in behavior and histopathological characteristics, chemotherapy regimens originally developed for small cell lung cancer (SCLC) have been tested, showing different grades of activity. Platinum based doublets, mostly combinations with etoposide, have shown response rates in extrapulmonary PDNEC ranging from 36% to 67% and median survival times of approximately 6 to 8 months.7-11 However, the high incidence of adverse effects, mainly hematological, represents a major concern associated with regimens based on cisplatin and etoposide and has limited the widespread use of this approach.
Following the same rationale, cisplatin in combination with irinotecan was tested in patients with advanced extrapulmonary PDNEC in small phase II trials, with response rate achieving approximately 40% to 50% and no activity in patients with well-differentiated neuroendocrine tumors.12-14 However, the efficacy and safety of this combination has not been evaluated in a non-selected population of PDNEC patients. Our objective was to retrospectively evaluate the outcomes of patients with metastatic extrapulmonary PDNEC treated with cisplatin plus irinotecan and present a review of the currently available literature on systemic treatment of PDNEC.
Materials and Methods
We conducted a retrospective analysis of patients with extrapulmonary PDNEC treated at two major cancer centers located in the city of São Paulo, Brazil (Hospital Sírio-Libanês and Instituto do Câncer do Estado de São Paulo) between 2008 and 2012. Patients were identified from the hospitals’ administrative databases and relevant information retrieved from electronic medical record. Patients with NET, irrespective of the final classification and grading, were screened for eligibility criteria.
Eligible patients were required to have a histologically confirmed diagnosis of extrapulmonary poorly differentiated neuroendocrine carcinoma and Ki67 proliferative index of 20% or higher when evaluated by immunhistochemistry staining for M1B1 antibody (Ki67). Patients with unknown primary and concurrent metastatic disease to multiple sites, including the lungs, were excluded from the analysis, since NEC of pulmonary origin could not be ruled out in these situations. According to the institution’s protocols, the cisplatin and irinotecan regimen was recommended for those patients with a performance status of 0 to 2 by Southwest Oncology Group Criteria and adequate renal and hematologic function. The use of prior chemotherapy regimens other than cisplatin and irinotecan was not an exclusion criterion.
The treatment regimen consisted of irinotecan 60 mg/m2 and cisplatin 30 mg/m2 delivered intravenously on days 1 and 8 repeated at 3-week intervals for at least two cycles or irinotecan 60 mg/m2 and carboplatin AUC 4 if renal dysfunction was documented by the time of diagnosis. All patients received 1000 mL of 0.9% saline, 20 mg of dexamethasone and a 5HT3 antagonist prior to chemotherapy. Standard protocol pre-medication also included 0.5 mg of atropine to prevent irinotecaninduced diarrhea. This regimen was considered an alternative to cisplatin and etoposide and recommended based on the attending physician’s discretion.
Radiologic evaluation was performed at baseline and every two to three cycles using computed tomography (CT) or magnetic resonance imaging (MRI) scans. Partial and complete radiologic responses were determined retrospectively according to the Response Evaluation Criteria in Solid Tumors – RECIST (v1.1) criteria, on those patients whose images could be retrieved. Date of progression was defined either by the date of consultation with attending physician, in the case of clinical deterioration, or on the date of imaging tests (whenever a CT or MRI was available). Due to the retrospective nature of this report, only grade 2 or greater toxic effects were captured from patients’ medical records and they were classified according to the National Cancer Institute’s Common Toxicity Criteria for Adverse Events, version 3.0.
Literature search was conducted through Medline from date inception to November, 2012. We used the search terms neuroendocrine carcinoma, neuroendocrine tumors, chemotherapy and poorly differentiated neuroendocrine carcinomas.
Statistical analysis
Analysis was performed under descriptive objectives using frequency percentage tables, with calculation of descriptive measures. Overall survival (OS) was measured from the date of initial treatment to the date of death from any cause, with the date of last follow up being censored. Time to disease progression (TTP) was defined from the beginning of treatment until radiologic progression, clinical deterioration not associated with treatment-related adverse effects or death from any cause. Both OS and TTP curves were estimated using the Kaplan-Meier method.
Statistical analyses were performed using MedCalc software version 11.3.1.0 (Maria - kerke, Belgium).
Results
From January 2008 to October 2012, 28 patients with extrapulmonary PDNEC received irinotecan combined with either cisplatin or carboplatin and were considered eligible. Patients’ characteristics are described in Table 1. Metastatic PDNEC from unknown primary site (n=6) and pancreatic PDNEC (n=6) were the most common presentations. All patients had metastatic disease, and distant dissemination occurred most frequently to the liver (75%) and lymph nodes (61%). Twenty-five patients (89%) received chemotherapy with cisplatin and irinotecan and three received carboplatin and irinotecan due to renal function impairment at diagnosis. The median number of cycles per patient was 4.5 (range 1-14). With respect to safety, twelve patients (42.8%) had at least one treatment delay and thirteen patients (46.4%) had dose reductions due to toxicity. The most frequently reported treatment-related grade 2 or higher adverse events were nausea (42%), diarrhea (39%), neutropenia (21.4%), anemia (17.8%) and elevation in serum creatinine concentration (9.1%). One patient developed grade 4 diarrhea and one additional patient had grade 4 thrombocytopenia. There were no treatment-related deaths.
Table 1.
Baseline characteristics of the study participants.
| Characteristic | Number (%) |
|---|---|
| Age years Median (range) | 57 (35-76) |
| Sex | |
| Male | 19 (67.8) |
| Female | 9 (32.1) |
| Primary site | |
| Pancreas | 6 (21.4) |
| Small intestine | 4 (14.2) |
| Colon | 3 (10.7) |
| Stomach | 3 (10.7) |
| Rectum | 2 (7.1) |
| Prostate | 1 (3.7) |
| Nasopharynx | 1 (3.7) |
| Retroperitoneum | 1 (3.7) |
| Unknown primary | 6 (21.4) |
| Ki-67 | |
| Median (range) | 80 (20-90) |
| Site of metastases | |
| Liver | 21 (75) |
| Lymph nodes | 17 (60.7) |
| Lung | 4 (14.3) |
| Peritoneum | 3 (10.7) |
| Other | 4 (14.3) |
After a median follow up of 9.2 months, thirteen patients (46.4% overall, 50% of the evaluable patients) achieved objective radiological response according to RECIST criteria. Overall, the median time to tumor progression was 3.7 months (range 1.2-12.0 months). Eighteen patients (64.2%) had died at the time of the analysis and median overall survival was 11.7 months. Eighteen patients received second-line therapies after progression on irinotecan combinations, and the most frequently recommended regimens were cisplatin or carboplatin with etoposide or taxane-based combinations. Nine patients received at least three lines of treatment (Figure 1).
Figure 1.
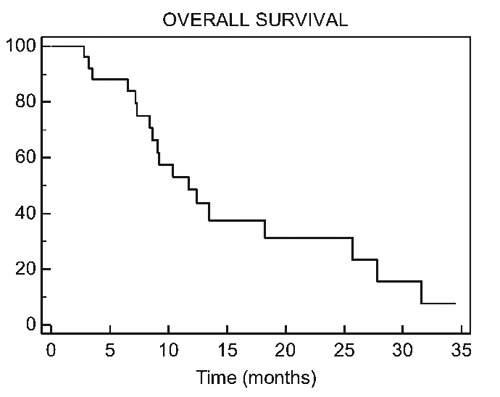
Overall survival.
Discussion
Extra-pulmonary PDNEC still represent a rare entity with dismal prognosis. In this retrospective analysis of 28 patients with metastatic extrapulmonary PDNEC, platinum analogs and irinotecan doublets yielded an overall response rate of 50%. The median time to tumor progression was 3.7 months (range 1.2-12.0) and median OS was 11.7 months (range 0.6-34.5). Our data suggest that platinum analogs and irinotecan (IP) is an active regimen, associated with an acceptable toxicity profile.
Patients with early stage poorly differentiated neuroendocrine carcinomas benefit from surgical excision followed or not by adjuvant chemotherapy, which corresponds to the only currently available curative approach.4 Nevertheless, the high rate of locally advanced or metastatic disease by the time of diagnosis frequently limits the use of this modality, resulting in a median overall survival of less than one year.2,5,15 Despite the remarkable advances in the management of neuroendocrine tumors with the use of sunitinib and everolimus, only patients with well and moderately differentiated NET were included in the prospective trials that approved these new agents.16-18 Therefore, for this selected group of patients with PDNEC, the backbone of the treatment of neuroendocrine carcinomas still relies on combinations cytotoxic agents.19 In a recent series of more than 300 individuals with gastrointestinal PDNEC, median survival for patients treated with palliative chemotherapy was 11 months, in contrast to 1 month for patients receiving best supportive care only.2
The early chemotherapy combinations adopted for patients with PDNEC was an extrapolation from small cell lung carcinoma (SCLC), mostly platinum-based doublets. Trials with cisplatin and etoposide produced response rates of up to 86% in SCLC.20 The EP combination resulted in high antitumor activity in patients with extrapulmonary NET, including carcinomas.9,21 In the retrospective study published by Sorbye and colleagues, approximately 78% of the patients received either cisplatin or carboplatin with etoposide. Response rates were 31% and 30% for cisplatin and carboplatin, respectively, and median overall survival intervals were 12 and 11 months.2 Moertel et al. evaluated 45 patients with neuroendocrine tumors treated with 130 mg/m2 of etoposide and 45 mg/m2 of cisplatin.8 Response rate, measured in the pre RECIST era, among 18 patients with anaplastic neuroendocrine tumors was 67%, the median interval to progression was 11 months and median OS was 19 months. However, patients had many different types of NET, hematologic toxicity was universal, and 66% of the patients experienced clinically relevant nephrotoxicity. In a series of 36 previosuly treated patients with metastatic poorly differentiated or rapidly progressing neuroendrocrine tumors (5 atypical foregut and 4 poorly differentiated pancreatic tumors), etoposide at a dose of 100 mg/m2 and cisplatin at a dose of 45 mg/m2 resulted in an overall response rate of 50-56% and a median survival of 19 months. An incidence of grade 3-4 neutropenia of 64% and high nephrotoxicity were also associated with this regimen.7 This sometimes restraining incidence of adverse effects was not only attibutable to patients with PDNEC and was uniformly reported among patients with SCLC. High dose reductions and discontinuation due to toxicity, mainly hematological, were also related to the cisplatin and etoposide regimen.8,9,20,22
After promising results and an acceptable toxicity profile reported by phase II trials,23 the combination of cisplatin and irinotecan (IP) was also evaluated in patients with SCLC and was associated with reduced hematological toxicity when compared to the EP regimen; nevertheless, two large scale phase III trials that failed to confirm a survival benefit of IP over EP.24,25 Based on these results, the efficacy and safety of the combination of irinotecan and cisplatin was also addressed in patients with extrapulmonary PDNEC. There are five studies reporting the activity of IP in PDNEC patients. In a retrospective study published by Hou et al., fourteen patients with metastatic extrapulmonary PDNEC treated with irinotecan 50-60 mg/m2 and cisplatin 25-30 mg/m2 on days 1 and 8 of 21-day cycles achieved a 43% response rate, including 1 complete response and 5 (36%) partial responses.12 A phase II trial of 20 patients with previously untreated advanced extrapulmonary PDNEC reported two (11%) complete responses and 9 (47%) partial responses, with a median TTP of 4 months.13 Okita et al. reported 12 patients with gastric PDNEC treated with IP. The authors observed a 75% response rate with a median progression free survival (PFS) of nearly 7 months.26 Nakano et al. also reported an overall response rate of 50%, PFS of 4.8 months and 1-year overall survival of 67% in patients treated with IP.27 Untreated patients had a better outcome, with a response rate of 64% and PFS of 7.3 months. A large series of patients with PDNEC treated with platinum-based combinations was presented by Yamagushi and colleagues.28 This report included 258 individuals with tumors arising from the gastrointestinal tract or the hepato-biliary-pancreatic system. The most commonly used regimen was irinotecan plus cisplatin (62%), followed by etoposide plus cisplatin (18%). Response rates were 50% and 27%, the median PFS were 5.2 and 4.0 months, and the median OS were 13.0 and 7.3 months, respectively.
Our data show similar response rates (50% among evaluable patients and 46% in the intention to treat population) to previous studies, even though our study population had a median Ki-67 of 80% with very aggressive behavior. In the series published by Sorbye and colleagues, a Ki-67 cut-off value of <55% was a strong prognostic factor, associated with a median survival of 14 months, versus 10 months for patients with Ki-67>55% (P<0.001).2
Other alternative chemotherapy regimens, based on oxaliplatin, capecitabine or taxanes have been reported. Bajetta and colleagues treated 40 patients, including 13 with PDNEC, with the combination of capecitabine and oxaliplatin (CapOx). Clinical benefit was achieved in 30% of the patients with PDNEC, with a median PFS of 4 months and median OS of only 5 months.29 The same regimen was retrospectively evaluated in an unselected population of 24 patients with NET (37.5% with PDNEC), yielding an ORR of 29% and median PFS of 9.8 months.30 In a phase II trial with 78 untreated patients with PDNEC, the combination of paclitaxel, carboplatin and etoposide showed a response rate of 53%, including a complete response rate of 15%. However, the median PFS and OS were only 7.5 and 14.5 months, respectively. Toxicity was extreme in this trial with 82% of patients reporting grade 3/4 neutropenia and fifteen patients (19%) required hospitalization.31 A summary of chemotherapy outcomes in PDNEC is presented in Table 2.
Table 2.
Active regimens for extra-pulmonary poorly differentiated neuroendocrine carcinomas.
| Author | Year | Regimen | N. of pts | Histology | ORR | mPFS (months) | mOS (months) |
|---|---|---|---|---|---|---|---|
| Moertel et al. | 1991 | EP | 18 | PDNEC | 67% | 11 | 19 |
| Mitry et al. | 1999 | EP | 41 | PDNEC | 41.5% | 8.9 | 15 |
| Fjallskog et al. | 2001 | EP | 36 | Mixed - NET | 55% | NI | 19 |
| Sorbye et al. | 2012 | EP | 129 | PDNEC | 31% | 4.0 | 1.0 |
| Hou et al. | 2003 | IP | 14 | PDNEC | 43% | NI | NR |
| Mani et al. | 2008 | IP | 20 | PDNEC | 58% | 4 | NI |
| Okita et al. | 2011 | IP | 37 | Gastric NET | 75% | 7.1 | 22.6 |
| Nakano et al. | 2012 | IP | 50 | PDNEC | 50% | 4.8 | NR |
| Yamagugchi et al. | 2012 | IP/EP | 206 | PDNEC | 50/27% | 5.2/4 | 13/7.3 |
| Hainsworth et al. | 2006 | PCE | 78 | PDNEC | 53% | 7.5 | 14.5 |
| Bajetta et al. | 2007 | CapOx | 13 | PDNEC | 23% | 4.0 | 5.0 |
| Ferrarotto et al. | 2011 | CapOx | 24 | NET/PDNEC | 29% | 9.8 | NR |
EP, etoposide and platin; IP, irinotecan and platin; PCE, ,paclitaxel, carboplatin, etoposide; CapOx, capecitabine and oxaliplatin; PDNEC, poorly differentiated neuroendocrine carcinomas; NET, neuroendocrine tumors; ORR, overal response rate; mPFS, median progression free survival; mOS, median overall survival; NI, not informed; NR, not reached.
Even though toxicity was a limiting issue in our series, most grade 3 adverse events were manageable and the proportion of patients experiencing significant toxicity was similar to those reported in the literature with platinbased combinations. In accordance with the data from phase III trials with SCLC patients, nausea and diarrhea represented the most frequent non-hematological toxic effects. Thirteen patients (46.4%) had delayed or decreased dose of chemotherapy due to grade 3 adverse events, which could result in decreased efficacy of the cytotoxic therapy. However, the fact that few grade 4 adverse events (7%) and no treatment-related deaths were observed in this retrospective series is in line with previously reported data, suggesting a more favorable toxicity profile related to irinotecan-based combinations. In the two previously mentioned studies addressing the use of the same combination for patients with unselected PDNEC, treatment was well tolerated and no grade 5 toxicities were reported.19,20 In contrast, in the series reported by Okita et al. including patients with gastric PDNEC, the rates of grade 3 or 4 neutropenia and diarrhea were 58% and 17%, respectively.25 Limitations of the present study should be noted. First, this was retrospective study and, yet encouraging, our findings resulted from a small number of cases. Second, considering that this was a registry-based study, small variations in the protocols, the quality of supportive care and patients’ clinical conditions could have led to biases. Lastly, the lack of available data regarding the access to further lines of treatment limit considerations regarding overall survival.
The impact of chemotherapy on the outcome of patients with advanced PDNEC is consistent and supports the use of platinum-based regimens. Unfortunately, the limited activity provides only short lasting clinical benefit to many patients.2
The development and conduction of well-controlled trials of cytotoxic chemotherapy in PDNEC in an unmet need. Because of its rarity, clinical trials should be preferably conducted by collaborative groups. Additionally, molecular biomarkers of sensitivity or resistance to treatment could help individualize patient candidates for different regimens. Future studies may consider the role of excision repair cross-complementing (ERCC) gene family expression in the outcome and resistance to chemotherapy in small cell lung cancer has been recently suggested, and could be further evaluated in patients with PDNEC treated with cisplatin combinations.
Conclusions
The IP regimen seems to be an active regime and should be considered an alternative treatment option for PDNEC. Like other commonly used regimens in this setting, the time to progression and overall survival are still of short duration. The toxicity profile of IP regimen seems favorable. The fact that few grade 4 toxicities and no treatment related-deaths were reported suggests that this combination is feasible. However, the best cytotoxic regimen is yet to be defined and further trials are demanded, especially addressing the role of prognostic and predictive biomarkers.
References
- 1.Bosman F, Carneiro F, Hruban R, et al. WHO classification of tumours of the digestive system. Lyon: IARC Press; 2010 [Google Scholar]
- 2.Sorbye H, Welin S, Langer SW, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol 2013;24:152-60 [DOI] [PubMed] [Google Scholar]
- 3.Remick SC, Ruckdeschel JC.Extrapulmonary and pulmonary small-cell carcinoma: tumor biology, therapy, and outcome. Med Pediatr Oncol 1992;20:89-99 [DOI] [PubMed] [Google Scholar]
- 4.Brenner B, Tang LH, Klimstra DS, et al. Small cell carcinomas of the gastrointestinal tract: a review. J Clin Oncol 2004;22:2730-9 [DOI] [PubMed] [Google Scholar]
- 5.Yao JC, Eisner MP, Leary C, et al. One hundred years after carcinoid: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72 [DOI] [PubMed] [Google Scholar]
- 6.Modlin IM, Sandor A.An analysis of 8305 cases of carcinoid tumors. Cancer 1997;79:813-29 [DOI] [PubMed] [Google Scholar]
- 7.Fjallskog ML, Granberg DP, Welin SL, et al. Treatment with cisplatin and etoposide in patients with neuroendocrine tumors. Cancer 2001;92:1101-7 [DOI] [PubMed] [Google Scholar]
- 8.Moertel CG, Kvols LK, O Connell MJ, et al. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Cancer 1991;68:227-32 [DOI] [PubMed] [Google Scholar]
- 9.Mitry E, Baudin E, Ducreux M, et al. Treatment of poorly differentiated neuroendocrine tumors with etoposide and cisplatin. Br J Cancer 1999;81:1351-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rougier P, Mitry E.Chemotherapy in the treatment of neuroendocrine malignant tumors. Digestion 2000;62 (Suppl 1):73-8 [DOI] [PubMed] [Google Scholar]
- 11.Hainsworth JD, Thompson DS, Urba WJ, et al. Phase II trial of paclitaxel, carboplatin, and etoposide in advanced poorly differentiated neuroendocrine carcinoma: a Minnie Pearl Cancer Research Network study. J Clin Oncol 2006;24:3548-54 [DOI] [PubMed] [Google Scholar]
- 12.Hou Z, Elasmar A, Lozano R, et al. A pilot study of irinotecan plus cisplatin in patients with metastatic high-grade neuroendocrine carcinoma. Proc Am Soc Clin Oncol 2003;22:375 [Google Scholar]
- 13.Mani M, Shroff RT, Jacobs C, et al. A phase II study of irinotecan and cisplatin for metastatic or unresectable high grade neuroendocrine carcinoma. J Clin Oncol 2008;26S:15550 [Google Scholar]
- 14.Kulke MH, Wu B, Ryan DP, et al. A phase II trial of irinotecan and cisplatin in patients with metastatic neuroendocrine tumors. Dig Dis Sci 2006;51:1033. [DOI] [PubMed] [Google Scholar]
- 15.Arnold R.Endocrine tumours of the gastrointestinal tract. Introduction: definition, historical aspects, classification, staging, prognosis and therapeutic options. Best Pract Res Clin Gastroenterol 2005;19:491-505 [DOI] [PubMed] [Google Scholar]
- 16.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raymond E, Dahan L, Raoul JL, et al. Sunitinib Malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501-13 [DOI] [PubMed] [Google Scholar]
- 18.Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumors associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet 2011;378:2005-12 [DOI] [PubMed] [Google Scholar]
- 19.Bezerra JE, Riechelamann RP, Hoff PM.Poorly differentiated neoendocrine tumors. In: Yao JC, Hoff PM, Hoff AO.Neuroendocrine tumors. 1st ed.New York: Humana Press; 2011 [Google Scholar]
- 20.Mascaux C, Paesmans M, Berghmans T, et al. A systematic review of the role of etoposide and cisplatin in the chemotherapy of small cell lung cancer with methodology assessment and meta-analysis. Lung Cancer 2000;30:23-36 [DOI] [PubMed] [Google Scholar]
- 21.Morant R, Bruckner HW.Complete remission of refractory small cell carcinoma of the pancreas with cisplatin and etoposide. Cancer 1989;64:2007-9 [DOI] [PubMed] [Google Scholar]
- 22.Einhorn LH, Crawford J, Birch R, et al. Cisplatin plus etoposide consolidation following cyclophosphamide, doxorubicin and vincristine in limited small cell lung cancer. J Clin Oncol 1988;6:451-6 [DOI] [PubMed] [Google Scholar]
- 23.Kudoh S, Fujiwara Y, Takada Y, et al. Phase II study of irinotecan combined with cisplatin in patients with previously untreated small-cell lung cancer. J Clin Oncol 1998;16:1068-7 [DOI] [PubMed] [Google Scholar]
- 24.Hanna N, Bunn PA, Langer C, et al. Randomized Phase iii trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol 2006;24:2038-43 [DOI] [PubMed] [Google Scholar]
- 25.Lara PN, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensivestage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol 2009;27:2530-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okita NT, Kato K, Takahari D, et al. Neuroendocrine tumors of the stomach: chemotherapy with cisplatin plus irinotecan is effective for gastric poorly-differentiated neuroendocrine carcinoma. Gastric Cancer 2011;14:161-5 [DOI] [PubMed] [Google Scholar]
- 27.Nakano K, Takahashi S, Yuasa T, et al. Feasibility and efficacy of combined cisplatin and irinotecan chemotherapy for poorly differentiated neuroendocrine carcinomas. Jpn J Clin Oncol 2012;42:697-703 [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi T, Machida N, Kasuga A, et al. Multicenter retrospective analysis of systemic chemotherapy in poorly differentiated neuroendocrine carcinoma of the digestive system. J Clin Oncol 2012;30:27422184379 [Google Scholar]
- 29.Bajetta E, Catena L, Procopio G, et al. Are capecitabine and oxaliplatin (XELOX) suitable treatments for progressing low-grade and hogh-grade neuroendocrine tumors? Cancer Chemother Pharmacol 2007;59:637-42 [DOI] [PubMed] [Google Scholar]
- 30.Ferrarotto R, Testa L, Riechelmann RP, et al. Combination of capecitabine and oxaliplatin (CAPOX) is an effective option for the treatment of neuroendocrine tumors (NET). Poster presentation session. Meeting of the European Society of Medical Oncology, 2011, Stockholm, Sweden [Google Scholar]
- 31.Hainsworth JD, Spigel DR, Litchy S, et al. Phase II trial of paclitaxel, parboplatin and etoposide in advanced poorly differentiated neuroendocrine carcinoma: a Minnie Pearl Cancer Research Network Study. J Clin Oncol 2006;24:3548. [DOI] [PubMed] [Google Scholar]


